Introduction
Programmed cell death-1 (PD-1), also termed CD279,
is a key co-inhibitory receptor expressed on T cells that functions
as a T cell checkpoint and plays a vital role in inhibiting cancer
cell proliferation (1–3). The binding of PD-1 to programmed
death-ligand 1 (PD-L1) on activated T cells inhibits antitumor
immunity and mediates immune escape (4–7).
Moreover, treatment with PD-1 inhibitors suppresses the PD-1/PD-L1
interaction and restores the T cell-mediated antitumor immune
responses, which promotes the killing of cancer cells (8). In recent years, PD-1 inhibitor
therapy has been regarded as a promising strategy for various types
of cancer, which is mainly administrated as a monotherapy,
monotherapy plus chemotherapy, or monotherapy plus targeted therapy
(5,9–11).
Although the aforementioned therapeutic approaches open new
horizons in tumor immunotherapy, the number of prognostic markers
for the assessment of PD-1 inhibitor efficacy remains insufficient
(12).
Immunological adverse events may occur during PD-1
inhibitor treatment, among which thyroid dysfunction is one of the
most common (13,14). Thyroid dysfunction is easily
ignored due to its asymptomatic nature or milder symptoms compared
with the severe symptoms of cancer itself (15). In addition, certain studies have
shown that thyroid dysfunction during PD-1 inhibitor treatment
correlates with prolonged survival in cancer. For example, it has
been reported that patients who develop thyroid dysfunction during
pembrolizumab treatment for non-small cell lung cancer (NSCLC)
exhibit improved overall survival (OS) (16); patients with renal cell carcinoma,
metastatic melanoma, and NSCLC who acquire overt thyroid
dysfunction during nivolumab or pembrolizumab treatment also
exhibit a satisfactory survival profile (17).
However, since the use of PD-1 inhibitors has been
approved in China for only three years, relevant data are very
limited or even unavailable. Therefore, the aim of the present
study was to investigate the incidence of thyroid dysfunction and
its relationship with progression-free survival (PFS) in Chinese
patients with cancer in a clinical setting. In addition, key
indices of thyroid function were assessed during PD-1 inhibitor
treatment in these patients.
Materials and methods
Patients
A total of 72 patients who were treated in Baotou
Tumor Hospital and Bayannur City Hospital between March 2018 and
July 2020 were enrolled in the present study. The inclusion
criteria used were the following: i) Use of PD-1 inhibitors for
tumor treatment; ii) availability of the data from thyroid function
assessment during treatment with PD-1 inhibitors; iii) availability
of PFS data; and iv) pathological diagnosis of cancer. The patients
were excluded from the present study according to the following
criteria: i) Lack of available clinical characteristic data; ii)
history of thyroid diseases or known thyroid dysfunction prior to
treatment with PD-1 inhibitors. Written informed consent was
obtained from all patients. The present study was approved by the
Internal Review Boards of Baotou Center Hospital (approval no.
2018-4).
Data collection
The clinical characteristics of the patients were
collected from their medical records. The collected clinical data
were as follows: i) Demographic characteristics, which included
age, sex, and history of smoking; ii) cancer type, which included
lung cancer, malignant melanoma, and other types; iii) disease
characteristics, including tumor-node-metastasis (TNM) stage and
brain metastasis; iv) tumor markers; v) biochemical indexes; and
vi) treatment, including PD-1 inhibitors (pembrolizumab, nivolumab,
toripalimab, camrelizumab, and sintilimab) and combined treatment.
In addition, PFS was estimated based on the follow-up data, and the
final date of follow-up was November 10, 2020.
Thyroid dysfunction assessment
According to the clinical records, serum samples
were collected prior to every second administration (every 4 or 6
weeks). The samples were used to perform serum thyroid function
tests and assess the levels of free triiodothyronine (FT3), free
thyroxine (FT4), and thyrotropin (TSH). Thyroid dysfunction was
evaluated at the first occurrence of thyroid function abnormality
on the basis of the serum thyroid function tests. Thyroid
dysfunction was defined as hyperthyroidism, subclinical
hyperthyroidism, hypothyroidism, and subclinical hypothyroidism in
accordance with a previous study (18). Hyperthyroidism was defined as a
decreased TSH level, and an elevated FT3 and/or FT4 level;
subclinical hyperthyroidism was defined as suppressed TSH with
normal FT3 and/or FT4 levels. Hypothyroidism was defined as an
increased TSH level and a decreased FT3 and/or FT4 level;
subclinical hypothyroidism was defined by a TSH level above the
upper limit of the reference range with an FT3 and/or FT4 level
within the reference range. The reference ranges for FT3, FT4 and
TSH were 2.3-4.0 pg/ml, 12–24 pmol/l, and 0.27-4.2 µIU/ml,
respectively.
Statistical analysis
SPSS 26.0 (IBM Corp.) and GraphPad Prism 7.02
(GraphPad Software Inc.) were used for data analysis and graph
production. The comparisons of thyroid dysfunction and clinical
characteristics were performed using an unpaired Student's t-test,
and a χ2 and Wilcoxon rank-sum tests. PFS was analyzed
using Kaplan-Meier curves and compared with a log-rank test between
the different groups of patients. PFS was defined as the time from
the first day of administration of immunotherapeutic drugs to the
date of the first documentation of disease progression, loss of
follow-up, or patient death. The patients who were lost to
follow-up were censored at the last visit date. The prognostic
factors were determined by the multivariate Cox proportional-hazard
regression model analysis. All tests were two-sided, and a
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics
A total of 72 patients with cancer who received PD-1
inhibitors were recruited. Their clinical characteristics are shown
in Table I. In brief, the mean age
of these patients was 59.6±12.7 years. Among them, 47 (65.3%)
patients were males and 25 (34.7%) were females. With respect to
cancer type, 45 (62.5%) patients had lung cancer, 12 (16.7%)
presented with malignant melanoma, and 15 (20.8%) had other types
of cancer. The mean levels of FT3, FT4 and TSH were 4.23±0.92
pg/ml, 16.51±3.47 pmol/l, and 4.36±4.75 µIU/ml, respectively.
Moreover, the number of patients who received PD-1 inhibitor
monotherapy, PD-1 inhibitors plus chemotherapy, or PD-1 inhibitors
plus targeted therapy were 42 (58.3%), 18 (25.0%), and 12 (16.7%),
respectively. The detailed administration schedule of the PD-1
inhibitors is shown in Table
SI.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| A, Demographic
characteristics |
|---|
|
|---|
| Parameter | Value |
|---|
| Mean age ± SD,
years | 59.6±12.7 |
| Sex, n (%) |
|
| Male | 47 (65.3) |
|
Female | 25 (34.7) |
| Ethnic group, n
(%) |
|
| Han | 72 (100.0) |
|
Others | 0 (0.0) |
| History of smoking, n
(%) | 24 (33.3) |
|
| B, Cancer
type |
|
| Parameter | Value |
|
| Lung cancer, n
(%) | 45 (62.5) |
| ADC | 20 (27.8) |
| SCC | 17 (23.6) |
| SCLC | 8 (11.1) |
| Malignant melanoma, n
(%) | 12 (16.7) |
| Othersa, n (%) | 15 (20.8) |
|
| C, Disease
characteristics |
|
| Parameter | Value |
|
| TNM stage, n (%) |
|
|
I/II/III | 15 (20.8) |
| IV | 57 (79.2) |
| Brain metastases, n
(%) |
|
| Yes | 19 (26.4) |
| No | 53 (73.6) |
|
| D, Tumor
markers |
|
| Parameter | Value |
|
| Median CEA (IQR),
ng/ml | 3.6 (2.0-6.8) |
| Median CA125 (IQR),
U/ml | 17.4 (11.0-58.1) |
| Median SCCA (IQR),
ng/ml | 1.8 (1.2-18.4) |
| Median NSE (IQR),
ng/ml | 24.1 (10.8-62.5) |
| Median LDH (IQR),
U/l | 189.5
(163.5-236.0) |
|
| E, Thyroid
function indexes |
|
| Parameter | Value |
|
| Mean FT3 ± SD,
pg/ml | 4.23±0.92 |
| Mean FT4 ± SD,
pmol/l | 16.51±3.47 |
| Mean TSH ± SD,
µIU/ml | 4.36±4.75 |
|
| F,
Treatment |
|
| Parameter | Value |
|
| PD-1 inhibitor
monotherapy, n (%) | 42 (58.3) |
| PD-1 inhibitor plus
chemotherapy, n (%) | 18 (25.0) |
| PD-1 inhibitor plus
targeted therapy, n (%) | 12 (16.7) |
Thyroid dysfunction incidence
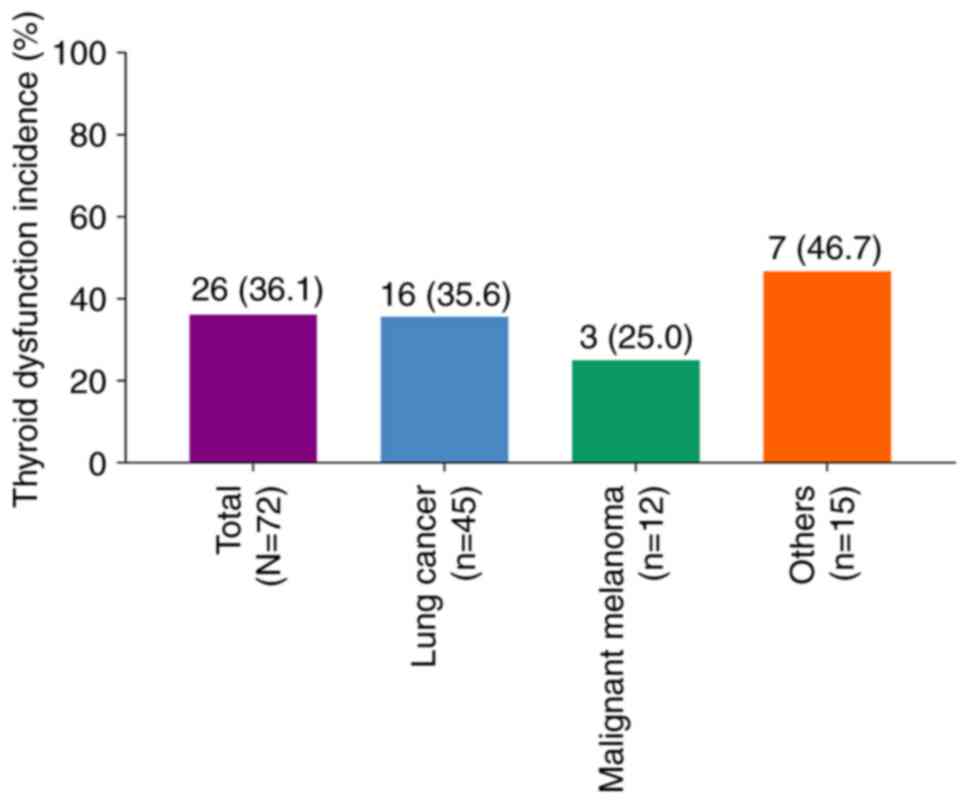
In total, 26 (36.1%) patients developed thyroid
dysfunction, which were all hypothyroidism. The incidence of
thyroid dysfunction was 35.6% in patients with lung cancer, 25.0%
in patients with malignant melanoma, and 46.7% in patients with
other types of cancer (Fig. 1).
Moreover, 23 patients received L-thyroxine for the treatment of
hypothyroidism.
Association between clinical
characteristics and the incidence of thyroid dysfunction
Subsequently, the association between clinical
characteristics and thyroid dysfunction was examined in patients
who received PD-1 inhibitor therapy. The results indicated that the
levels of cancer antigen 125 were downregulated in patients with
thyroid dysfunction (P=0.037). However, none of the other examined
characteristics differed between patients who developed thyroid
dysfunction and those who did not (all P>0.05; Table II).
 | Table II.Clinical characteristics by thyroid
dysfunction status groups. |
Table II.
Clinical characteristics by thyroid
dysfunction status groups.
|
| Thyroid
dysfunction |
|
|---|
|
|
|
|
|---|
| Items | No (n=46) | Yes (n=26) | P-value |
|---|
| Mean age ± SD,
years | 57.5±14.4 | 63.3±8.1 | 0.062 |
| Sex, n (%) |
|
| 0.309 |
|
Male | 32 (69.6) | 15 (57.7) |
|
|
Female | 14 (30.4) | 11 (42.3) |
|
| History of smoking,
n (%) |
|
| 0.386 |
|
Yes | 17 (37.0) | 7 (26.9) |
|
| No | 29 (63.0) | 19 (73.1) |
|
| Disease type, n
(%) |
|
| 0.503 |
| Lung
cancer | 29 (63.0) | 16 (61.5) |
|
|
Malignant melanoma | 9 (19.6) | 3 (11.5) |
|
|
Others | 8 (17.4) | 7 (26.9) |
|
| Lung cancer, n
(%) |
|
| 0.992 |
|
ADC | 13 (44.8) | 7 (43.8) |
|
|
SCC | 11 (37.9) | 6 (37.5) |
|
|
SCLC | 5 (17.2) | 3 (18.8) |
|
| TNM stage, n
(%) |
|
| 0.119 |
|
I/II/III | 7 (15.2) | 8 (30.8) |
|
| IV | 39 (84.8) | 18 (69.2) |
|
| Brain metastases, n
(%) |
|
| 0.699 |
|
Yes | 13 (28.3) | 6 (23.1) |
|
| No | 33 (71.7) | 20 (76.9) |
|
| Median CEA (IQR),
ng/ml | 3.6 (2.1-6.8) | 3.5 (2.0-5.7) | 0.548 |
| Median CA125 (IQR),
U/ml | 27.0
(13.8-90.3) | 12.0
(6.7-17.1) | 0.037 |
| Median SCCA (IQR),
ng/ml | 1.6 (1.0-43.5) | 1.8 (1.4-18.4) | 0.498 |
| Median NSE (IQR),
ng/ml | 24.9
(8.7-57.9) | 14.4
(11.1-66.4) | 0.935 |
| Median LDH (IQR),
U/l | 192.0
(136.8-236.8) | 185.5
(168.5-204.3) | 0.836 |
| Treatment, n
(%) |
|
| 0.835 |
| PD-1
inhibitor monotherapy | 28 (60.9) | 14 (53.8) |
|
| PD-1
inhibitor plus chemotherapy | 11 (23.9) | 7 (26.9) |
|
| PD-1
inhibitor plus targeted therapy | 7 (15.2) | 5 (19.2) |
|
Association of thyroid dysfunction
with PFS
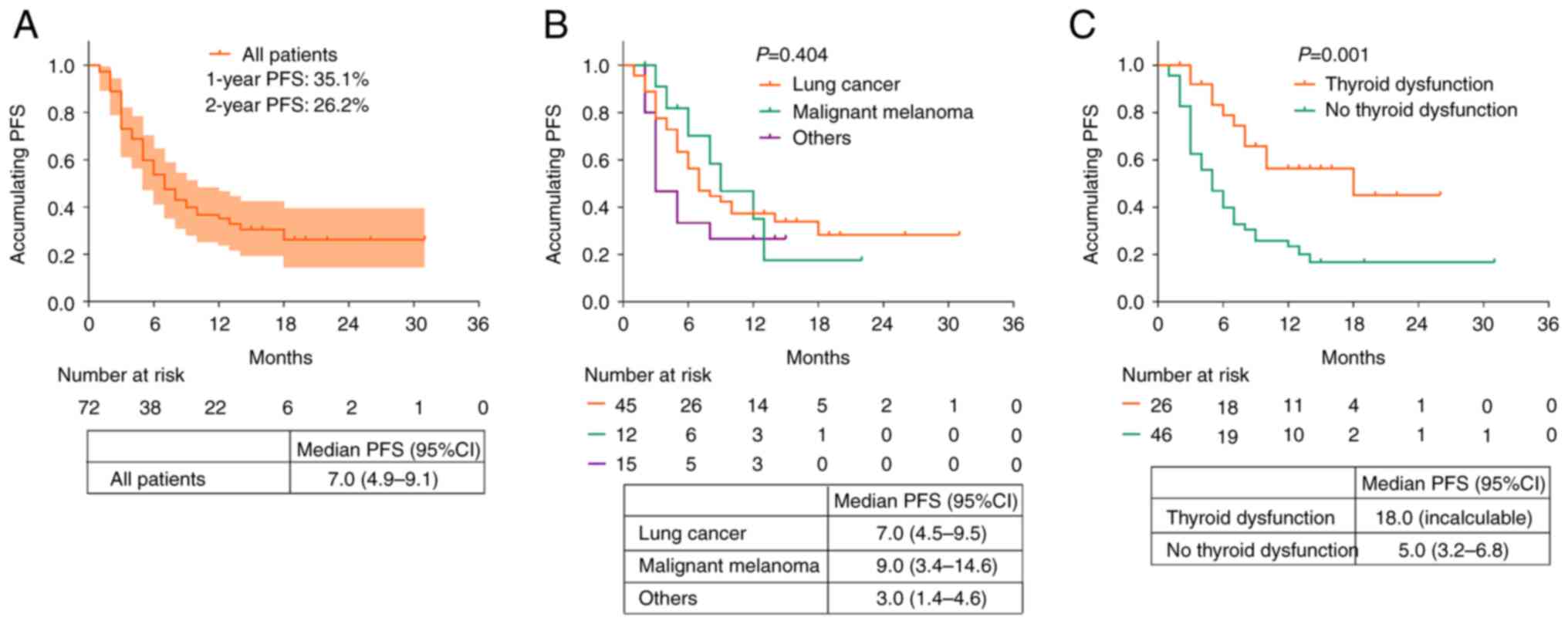
The median PFS of all patients who received PD-1
inhibitors was 7.0 [95% confidence interval (CI), 4.9-9.1] months.
In addition, the 1- and 2-year PFS rates of these patients were
35.1 and 26.2%, respectively (Fig.
2A). Moreover, the median PFS (95% CI) values of patients with
lung cancer, malignant melanoma, and other types of cancer were 7.0
(4.5-9.5) months, 9.0 (3.4-14.6) months, and 3.0 (1.4-4.6) months,
respectively. There were no significant differences in the PFS of
patients with different cancer types (P>0.05; Fig. 2B).
The patients with thyroid dysfunction had a longer
PFS (median, 18.0 months; 95% CI, not reached) compared with those
without thyroid dysfunction (median, 5.0 months; 95% CI: 3.2-6.8
months) (P=0.001; Fig. 2C).
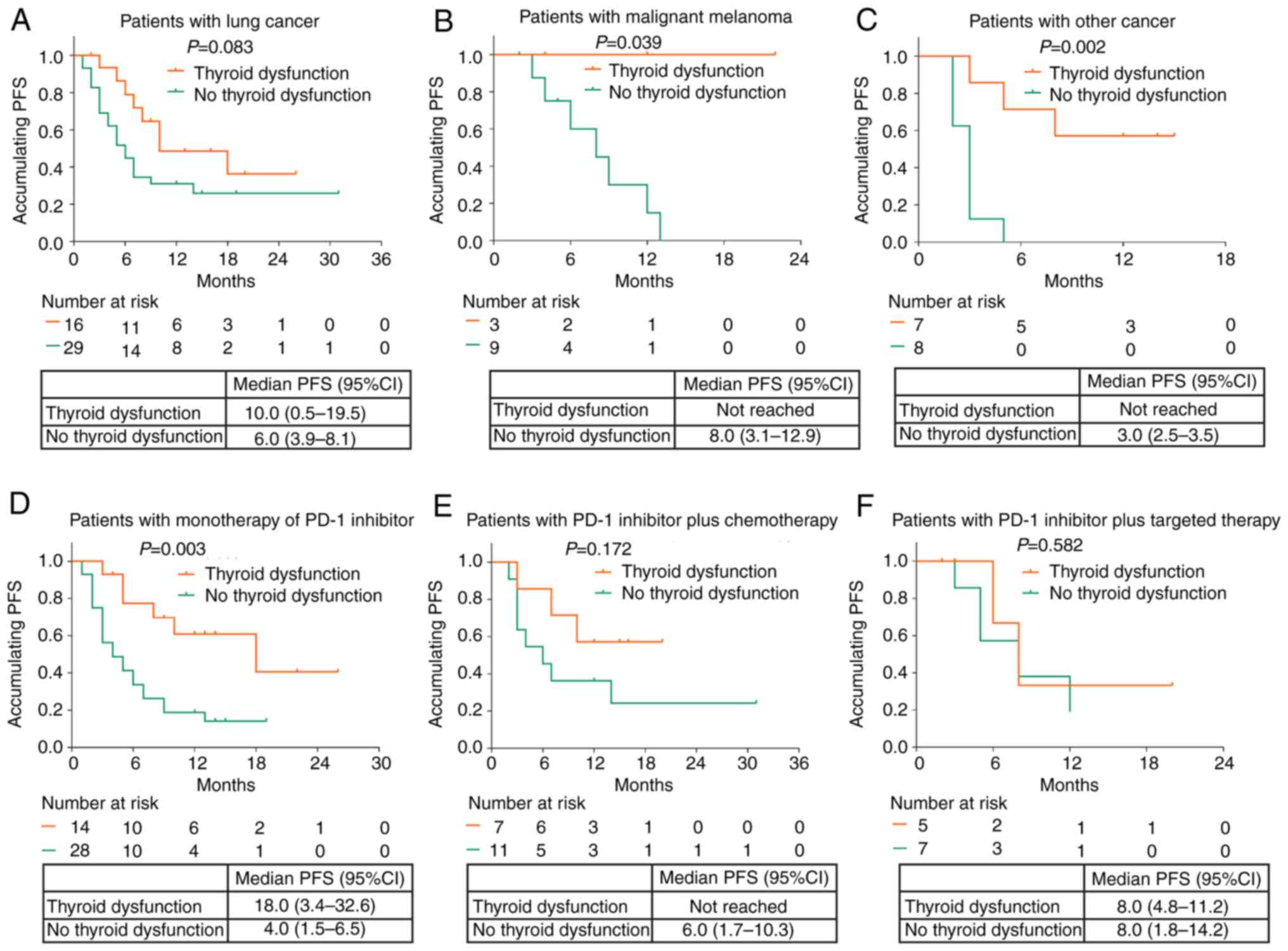
Furthermore, subgroup analysis demonstrated that thyroid
dysfunction was associated with longer PFS in patients with
malignant melanoma (P=0.039) and other cancer types (P=0.002), but
not in patients with lung cancer (P=0.083; Fig. 3A-C). Moreover, thyroid dysfunction
was associated with improved PFS in patients who received
monotherapy with PD-1 inhibitors (P=0.003; Fig. 3D). However, no association was
found between thyroid dysfunction and PFS in patients who received
PD-1 inhibitors plus chemotherapy (P=0.172; Fig. 3E) or those who received PD-1
inhibitors plus targeted therapy (P=0.582; Fig. 3F).
Factors affecting PFS
Univariate Cox regression analysis indicated that
thyroid dysfunction was the only factor associated with a longer
PFS [yes vs. no; P=0.003; hazard ratio (HR)=0.350]. In addition,
multivariate Cox regression analysis indicated that thyroid
dysfunction (yes vs. no; P=0.001; HR=0.260) served as an
independent factor for satisfactory PFS, while monotherapy with
PD-1 inhibitors compared with PD-1 inhibitor plus chemotherapy or
targeted therapy (P=0.015; HR=2.231) served as an independent
factor for unsatisfactory PFS (Table
III).
 | Table III.Cox proportional-hazards regression
analysis of factors affecting PFS. |
Table III.
Cox proportional-hazards regression
analysis of factors affecting PFS.
|
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|---|
| Parameter | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Thyroid dysfunction
(yes vs. no) | 0.003 | 0.350
(0.177-0.691) | 0.001 | 0.260
(0.115-0.585) |
| Age (vs. ≥70
years) |
|
|
|
|
| ≥70
years | Reference |
| Reference |
|
| 60-69
years | 0.088 | 2.506
(0.873-7.195) | 0.098 | 2.740
(0.831-9.038) |
| 50-59
years | 0.088 | 2.404
(0.878-6.585) | 0.069 | 3.110
(0.916-10.566) |
| <50
years | 0.966 | 0.978
(0.348-2.747) | 0.754 | 1.203
(0.377-3.836) |
| Sex (male vs.
female) | 0.510 | 1.223
(0.672-2.225) | 0.209 | 1.627
(0.762-3.472) |
| History of smoking
(yes vs. no) | 0.306 | 0.724
(0.391-1.343) | 0.077 | 0.539
(0.272-1.069) |
| Cancer type (vs.
others) |
|
|
|
|
|
Others | Reference |
| Reference |
|
| Lung
cancer | 0.238 | 0.656
(0.326-1.321) | 0.110 | 0.501
(0.215-1.168) |
|
Malignant melanoma | 0.302 | 0.606
(0.234-1.568) | 0.053 | 0.344
(0.117-1.013) |
| TNM stage (IV vs.
I/II/III) | 0.185 | 1.675
(0.781-3.595) | 0.460 | 1.402
(0.572-3.434) |
| Brain metastases
(yes vs. no) | 0.345 | 1.352
(0.722-2.531) | 0.391 | 1.395
(0.652-2.986) |
| PD-1 inhibitor
monotherapy (yes vs. no) | 0.361 | 1.315
(0.730-2.369) | 0.015 | 2.231
(1.172-4.247) |
Discussion
The conclusions of the present study can be
summarized as follows: i) The incidence of thyroid dysfunction was
36.1%; ii) thyroid dysfunction was associated with longer PFS in
patients with malignant melanoma and other cancer types, as well as
in patients who received PD-1 inhibitor monotherapy; moreover,
thyroid dysfunction served as an independent factor for
satisfactory PFS.
With respect to thyroid dysfunction incidence in
cancer patients who received PD-1 inhibitors, a previous study
demonstrated that among patients who received PD-1 inhibitors, 9
(7.5%) had melanoma and 5 (7.1%) lung cancer, whereas only 2 (6.5%)
patients with renal cell carcinoma developed thyroid dysfunction
(16). In another previous study
involving 150 patients with cancer who received PD-1 inhibitor
treatment, 25 out of 150 (16.7%) patients experienced thyroid
dysfunction during treatment (19). In the present study, it was shown
that 26 (36.1%) patients developed thyroid dysfunction after PD-1
inhibitor treatment. The incidence of thyroid dysfunction was 35.6%
in patients with lung cancer, 25.0% in patients with malignant
melanoma and 46.7% in patients with other cancer types. Compared
with previous studies, the incidence of thyroid dysfunction was
relatively high. Possible explanations could include the following:
i) The definitions of thyroid dysfunction differed between our
study and previous ones (16,19);
ii) the patient demographics differed between the studies; or iii)
come patients in the present study received PD-1 inhibitors
combined with chemotherapy or targeted therapy, which may have
aggravated thyroid injury. However, the current study showed that
there was no association between baseline characteristics of cancer
patients and thyroid dysfunction. A possible explanation might be
that thyroid dysfunction is closely related to PD-1 inhibitor
treatment, but not the characteristics of cancer patients. However,
another study suggests that the incidence of thyroid dysfunction is
higher in patients with head and neck cancer treated with
chemotherapy (20); thus the
correlation of characteristics with thyroid dysfunction needs to be
further verified.
With regards to the association between thyroid
dysfunction and the survival outcome in patients with cancer who
received PD-1 inhibitors, a previous study revealed that patients
with cancer who were treated with pembrolizumab and developed
thyroid dysfunction exhibited a higher median OS than those without
thyroid dysfunction (40 months vs. 14 months) (21). Another study indicated that
patients with NSCLC and thyroid dysfunction who received PD-1
inhibitors exhibited significantly higher OS and PFS than those
without thyroid dysfunction (22).
In addition, patients with NSCLC, renal cell carcinoma and
metastatic melanoma who received PD-1 inhibitors and acquired overt
thyroid dysfunction exhibited improved OS and PFS than those
without thyroid dysfunction (17).
In the present study, general, thyroid dysfunction was associated
with a longer PFS in Chinese patients receiving PD-1 inhibitors,
which was similar to previous studies focusing of Caucasian cancer
patients receiving PD-1 inhibitors (23,24).
Thus, it could be deduced that the prognostic value of thyroid
dysfunction was not affected by ethnicity, although further studies
should verify this hypothesis. Moreover, our study also found that
thyroid dysfunction was an independent factor for a higher PFS in
Chinese patients who received PD-1 inhibitors. This could be
explained by the fact that patients with thyroid dysfunction
presented with a higher susceptibility to autoimmunity. This may
affect antitumor treatment through an autoimmune-mediated pathway
and improve the therapeutic effects, contributing to a higher PFS
(17). In addition, based on
subgroup analysis, it was found that the prognostic value of
thyroid dysfunction was high in patients with melanoma and other
cancer types and in patients who received PD-1 inhibitor
monotherapy. Possible explanations include the fact that the
majority of enrolled patients with NSCLC received combined therapy,
which attenuated the predictive value of thyroid dysfunction with
regard to the therapeutic effect of the PD-1 inhibitors.
Despite the aforementioned findings, certain
limitations are apparent in the present study. Firstly, the sample
size was small, which may lead to limited representativeness of the
results. However, the use of PD-1 inhibitors has been recently
approved in China and this sample size was relatively large under
this circumstance. Secondly, the mechanism of thyroid dysfunction
during PD-1 inhibitor treatment was not investigated. Therefore,
further in vivo and in vitro experiments are
required. Thirdly, the association of thyroid dysfunction with
prognosis in cancer patients without the treatment of PD-1
inhibitors could be investigated in the future.
In conclusion, the present study revealed that
thyroid dysfunction occurred in 36.1% of patients with cancer who
underwent PD-1 inhibitor treatment and was associated with
prolonged PFS, notably in those who received PD-1 inhibitor
monotherapy. These findings suggest that thyroid dysfunction may
serve as a potential prognostic marker to guide patient management.
Further studies with larger sample sizes should be conducted to
verify these findings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Wu Jieping Medical
Foundation (grant no. 320.6750.2020-19-37).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HB and YG contributed to the conception and design
of the study. YW contributed to performing the experiments. ZW
contributed to data acquisition and analysis. YW and ZW contributed
to the preparation of the manuscript. HB and YG confirm the
authenticity of all the raw data. All authors contributed to the
review of the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Internal
Review Boards of Baotou Center Hospital (approval no. 2018-4).
Written informed consent was obtained from all patients
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang Y, Chen M, Nie H and Yuan Y: PD-1
and PD-L1 in cancer immunotherapy: Clinical implications and future
considerations. Hum Vaccin Immunother. 15:1111–1122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020. View Article : Google Scholar
|
|
3
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
4
|
Kythreotou A, Siddique A, Mauri FA, Bower
M and Pinato DJ: Pd-L1. J Clin Pathol. 71:189–194. 2018. View Article : Google Scholar
|
|
5
|
Ni JM and Ni AP: Landscape of PD-1/PD-L1
regulation and targeted immunotherapy. Chin Med Sci J. 33:174–182.
2018.PubMed/NCBI
|
|
6
|
Gou Q, Dong C, Xu H, Khan B, Jin J, Liu Q,
Shi J and Hou Y: PD-L1 degradation pathway and immunotherapy for
cancer. Cell Death Dis. 11:9552020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie W, Medeiros LJ, Li S, Yin CC, Khoury
JD and Xu J: PD-1/PD-L1 pathway and its blockade in patients with
classic hodgkin lymphoma and non-hodgkin large-cell lymphomas. Curr
Hematol Malig Rep. 15:372–381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Song X, You L, Zheng L, Zhang T and Zhao Y: PD-1/PD-L1 and
immunotherapy for pancreatic cancer. Cancer Lett. 407:57–65. 2017.
View Article : Google Scholar
|
|
11
|
Hayashi H and Nakagawa K: Combination
therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol.
25:818–830. 2020. View Article : Google Scholar
|
|
12
|
Salmaninejad A, Valilou SF, Shabgah AG,
Aslani S, Alimardani M, Pasdar A and Sahebkar A: PD-1/PD-L1
pathway: Basic biology and role in cancer immunotherapy. J Cell
Physiol. 234:16824–16837. 2019. View Article : Google Scholar
|
|
13
|
D'Andrea G, Lassalle S, Guevara N, Mograbi
B and Hofman P: From biomarkers to therapeutic targets: The promise
of PD-L1 in thyroid autoimmunity and cancer. Theranostics.
11:1310–1325. 2021. View Article : Google Scholar
|
|
14
|
Manolis AA, Manolis TA, Melita H and
Manolis AS: Subclinical thyroid dysfunction and cardiovascular
consequences: An alarming wake-up call? Trends Cardiovasc Med.
30:57–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chalan P, Di Dalmazi G, Pani F, De Remigis
A, Corsello A and Caturegli P: Thyroid dysfunctions secondary to
cancer immunotherapy. J Endocrinol Invest. 41:625–638. 2018.
View Article : Google Scholar
|
|
16
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017. View Article : Google Scholar
|
|
17
|
Basak EA, van der Meer JWM, Hurkmans DP,
Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, Bins S, Joosse A,
Koolen SLW, Debets R, et al: Overt thyroid dysfunction and
anti-thyroid antibodies predict response to anti-PD-1 immunotherapy
in cancer patients. Thyroid. 30:966–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolffenbuttel BHR, Wouters HJCM, Slagter
SN, van Waateringe RP, Muller Kobold AC, van Vliet-Ostaptchouk JV,
Links TP and van der Klauw MM: Thyroid function and metabolic
syndrome in the population-based LifeLines cohort study. BMC Endocr
Disord. 17:652017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakakida T, Ishikawa T, Uchino J, Chihara
Y, Komori S, Asai J, Narukawa T, Arai A, Kobayashi T, Tsunezuka H,
et al: Clinical features of immune-related thyroid dysfunction and
its association with outcomes in patients with advanced
malignancies treated by PD-1 blockade. Oncol Lett. 18:2140–2147.
2019.
|
|
20
|
Endo K, Masatani T, Tsuji A, Kondo S,
Wakisaka N, Murono S and Yoshizaki T: Thyroid dysfunction after
intra-arterial chemotherapy for hypopharyngeal and laryngeal
cancer. Auris Nasus Larynx. 42:231–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Breimer LH, Nousios P, Olsson L and
Brunnström H: Immune checkpoint inhibitors of the PD-1/PD-L1-axis
in non-small cell lung cancer: Promise, controversies and
ambiguities in the novel treatment paradigm. Scand J Clin Lab
Invest. 80:360–369. 2020. View Article : Google Scholar
|
|
22
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar
|
|
23
|
Chmielewska I, Dudzińska M, Szczyrek M,
Świrska J, Wojas-Krawczyk K and Zwolak A: Do endocrine adverse
events predict longer progression-free survival among patients with
non-small-cell lung cancer receiving nivolumab? PLoS One.
16:e02574842021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frelau A, Jali E, Campillo-Gimenez B,
Pracht M, Porneuf M, Dinulescu M, Edeline J, Boussemart L and
Lesimple T: Prognostic impact of thyroid dysfunctions on
progression-free survival in patients with metastatic melanoma
treated with anti-PD-1 antibodies. Melanoma Res. 31:208–217. 2021.
View Article : Google Scholar : PubMed/NCBI
|