Introduction
Breast cancer is one of the most common diseases
worldwide (1), so developing
effective breast cancer therapies is important. While surgery is
the mainstay of treatment for most breast cancers, there are
limitations to the benefits that surgery can provide, especially in
patients with advanced disease. Therefore, the principal
developments in cancer therapy are expected to be provided by drug
therapy.
5-Fluorouracil (5-FU) has been a key drug for many
other cancers (2), and its
importance in breast cancer treatment has also been increasing
recently in both the adjuvant setting (3,4) and
metastatic setting (5). There are
three speculated mechanisms of action for 5-FU: incorporation into
RNA (6), incorporation into DNA
(7), and inhibition of DNA de
novo synthesis by inhibiting thymidine synthase (TS) (8). Among these speculated mechanisms, the
inhibition of TS has received the most focus because many
chemotherapeutic drugs similarly inhibit TS (9,10),
and some drugs enhancing the inhibition of TS have been developed
(11,12).
Fluoro-deoxyuridine monophosphate (FdUMP) is the key
molecule synthesized from 5-FU in cancer cells. It inhibits TS by
forming a ternary complex composed of TS, FdUMP and
5,10-methylenetetrahydrofolate (CH2THF) (2). This metabolism is associated with the
enzymes involved in the synthesis of deoxythymidine monophosphate
(dTMP), which is necessary for the synthesis of DNA. There are two
speculated pathways for synthesizing FdUMP: i) 5-FU is converted to
5-fluorouridine monophosphate (FUMP) by orotate
phosphoribosyltransferase (OPRT) and then converted to FdUMP by
several enzymes, including ribonucleotide reductase (RR), in a
process known as the ‘OPRT-RR pathway’ derived from the de
novo pathway of dTMP synthesis; or ii) 5-FU is converted to
fluoro-deoxyuridine (FdU) by thymidine phosphorylase (TP) and then
converted to FdUMP by thymidine kinase (TK), in a process known as
the ‘TP-TK pathway’ derived from the salvage pathway of dTMP
synthesis. These mechanisms are illustrated in Fig. 1A and B.
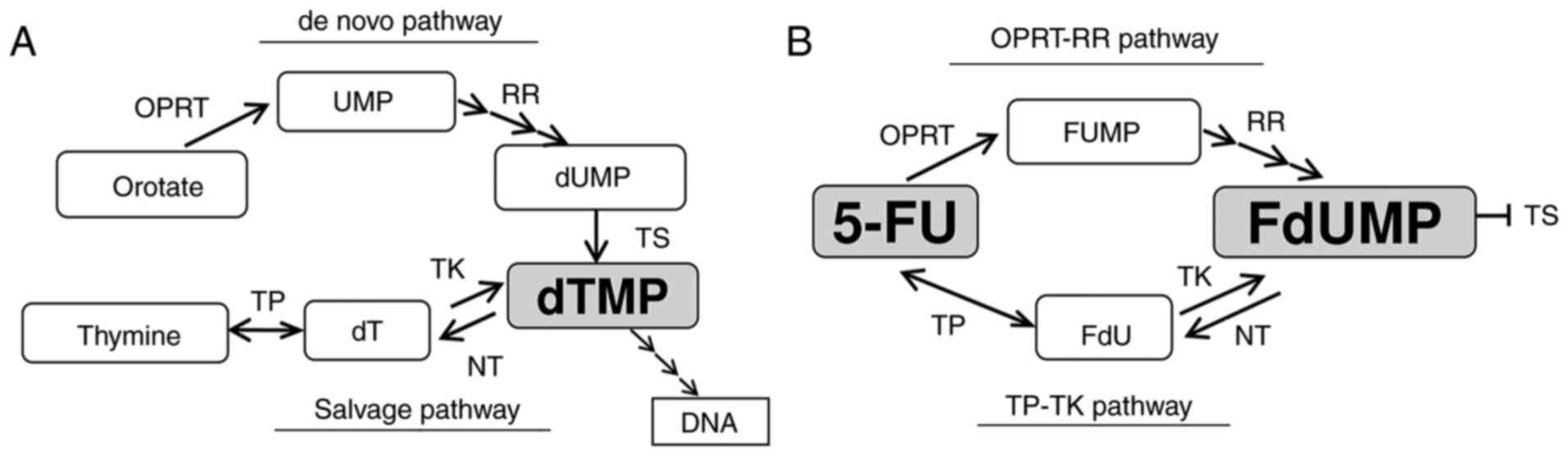 | Figure 1.Diagram of the thymidylate (dTMP)
synthesis and 5-FU metabolism. (A) The synthesis pathway of dTMP.
(B) The metabolism of 5-FU. 5-FU, 5-fluorouracil; dT, thymidine;
dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine
monophosphate; FdU, fluoro-deoxyuridine; FdUMP, fluoro-deoxyuridine
monophosphate; FUMP, fluoro-uridine monophosphate; NT,
nucleotidase; OPRT, orotate phosphoribosyl transferase; RR,
ribonucleotide reductase; TK, thymidine kinase; TP, thymidine
phosphorylase; TS, thymidylate synthase; UMP, uridine
monophosphate. |
Long-term treatment with 5-FU or other anti-cancer
drugs causes drug resistance, and overcoming resistance to 5-FU is
important for improving breast cancer treatment. However, the
changes in the metabolisms caused by the acquisition of resistance
to 5-FU in breast cancer cells has never been clearly described.
Therefore, in the present study, we focused on the changes in the
metabolism of 5-FU and the synthesis of dTMP to elucidate the
mechanism underlying resistance to 5-FU using 5-FU-resistant breast
cancer cell lines.
Materials and methods
Drugs
5-FU was kindly provided by Kyowa Hakko. Tipiracil
(TP inhibitor), hydroxyurea (RR inhibitor) and raltitrexed (TS
inhibitor) were purchased from Sigma-Aldrich.
Cell lines and cell culture
MCF-7 and MDA-MB-231 cells obtained from ATCC were
cultured in Dulbecco's modified Eagle's medium (D5921-500ML;
Sigma-Aldrich; Merck KGaA) with 5% fetal bovine serum (10270-106;
Thermo Fisher Scientific), L-Glutamine (073-05391; FUJIFILM Wako
Pure Chemical Corporation), and Non-essential amino acid
(M7145-100ML; Sigma-Aldrich; Merck KGaA). MCF-7/5-FUR and
MDA-MB-231/5-FUR cells are 5-FU-resistant cell lines established in
our institute by continuously exposing MCF-7 or MDA-MB-231 cells to
5-FU over a few years. The initial concentration of 5-FU was 0.1
µM, and it was increased 2-fold once cell growth was confirmed, up
to 10 µM at 37°C. These cells were maintained in medium containing
2 µM of 5-FU and cultured in drug-free medium for at least 2 weeks
before experiments to eliminate the effects of 5-FU. The cell lines
were incubated in a humidified atmosphere of 5% CO2 at
37°C.
Western blot analyses and
antibodies
The cells were lysed in RIPA buffer (Sigma-Aldrich;
Merck KGaA) for 15 min on ice. The protein concentration of the
lysates was measured using a Bio-Rad Protein Assay Dye Reagent
Concentrate (Bio-Rad Laboratories, Inc.). The cell lysates were
boiled in sample buffer solution (FUJIFILM Wako Pure Chemical
Corporation). Total cell protein extracts (7 µg/lane) were
separated by 10% SDS-PAGE using SuperSep™ ACE (FUJIFILM Wako Pure
Chemical Corporation) and transferred onto polyvinyl difluoride
(PVDF) membranes (EMD Millipore). The membranes were blocked with
PVDF blocking reagent (Toyobo Co., Ltd.) for 1 h. The membranes
were then incubated with primary antibodies, such as anti-OPRT
antibody (kindly provided by Taiho Pharmaceutical Company
(https://www.taiho.co.jp/en/); 1:10,000),
RRM1 (D12F12) XP Rabbit mAb #8637 (Cell Signaling Technology;
1:5,000), rabbit polyclonal to thymidine phosphorylase (ab69120)
(Abcam; 0.4 µg/ml), anti-thymidine kinase 1 (EPR3193) antibody
(ab76495) (Abcam; 1:50,000), dNT-1 (C-10): sc-390041 (Santa Cruz
Biotechnology; 1:100), anti-thymidylate synthase, clone TS106
(MAB4130) (EMD Millipore; 1:5,000) or GAPDH (D16H11) XP Rabbit mAb
#5174 (Cell Signaling Technology; 1:5,000) for 2 h at room
temperature. The primary antibodies were diluted with Can Get
Signal Solution 1 (Toyobo Co., Ltd.). The membranes were then
washed with Dako Washing Buffer (Agilent Technologies, Inc.) and
incubated with Goat anti-Mouse IgG, Peroxidase Conjugated, heavy
chain + light chain (AP124P) (EMD Millipore) or Goat anti-Rabbit
IgG, Peroxidase Conjugate (AP132P) (EMD Millipore) diluted to
1:25,000 with Can Get Signal Solution 2 (Toyobo Co., Ltd.) for 1 h
at room temperature. Immunoreactive proteins were visualized with
the ImmunoStar LD reagent (FUJIFILM Wako Pure Chemical
Corporation), and images were captured using a GeneGnome HR system
(Syngene Europe). Western blot analysis was repeated at least three
times.
3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H tetrazolium bromide
(MTT) assay for the effects of 5-FU, raltitrexed or tipiracil
A total of 5×103 cells were seeded into
each well of 96-well plates and cultured for 24 h. The cells were
treated with 5-FU, raltitrexed or tipiracil for 72 h, and the
medium was replaced with 100 µl of a 0.5 mg/ml solution of MTT
(Sigma-Aldrich; Merck KGaA). The plates were then incubated for 4 h
at 37°C. The MTT solution was replaced with 100 µl of dimethyl
sulfoxide (FUJIFILM Wako Pure Chemical Corporation), and the
absorbance at 540 nm was measured using a Sunrise Rainbow RC-R
(Tecan Group Ltd. Männedorf, Switzerland). Eight wells were used
for each condition.
Statistical analyses
The mean half maximal inhibitory concentration
(IC50) values were calculated based on each result of
MTT assays using the Graphpad Prism 9 software program (GraphPad
Software, Inc.) presented as the mean, 95% confidence interval
(CI). The results of western blot analysis were semi-quantified by
dividing by the GAPDH expression and normalized by dividing by an
appropriate normalizer and then presented as the mean ± standard
error (SE). The significance of differences was determined by
two-group comparisons using unpaired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Sensitivities to 5-FU and changes in
the expression of the enzymes for 5-FU metabolism in breast cancer
cells
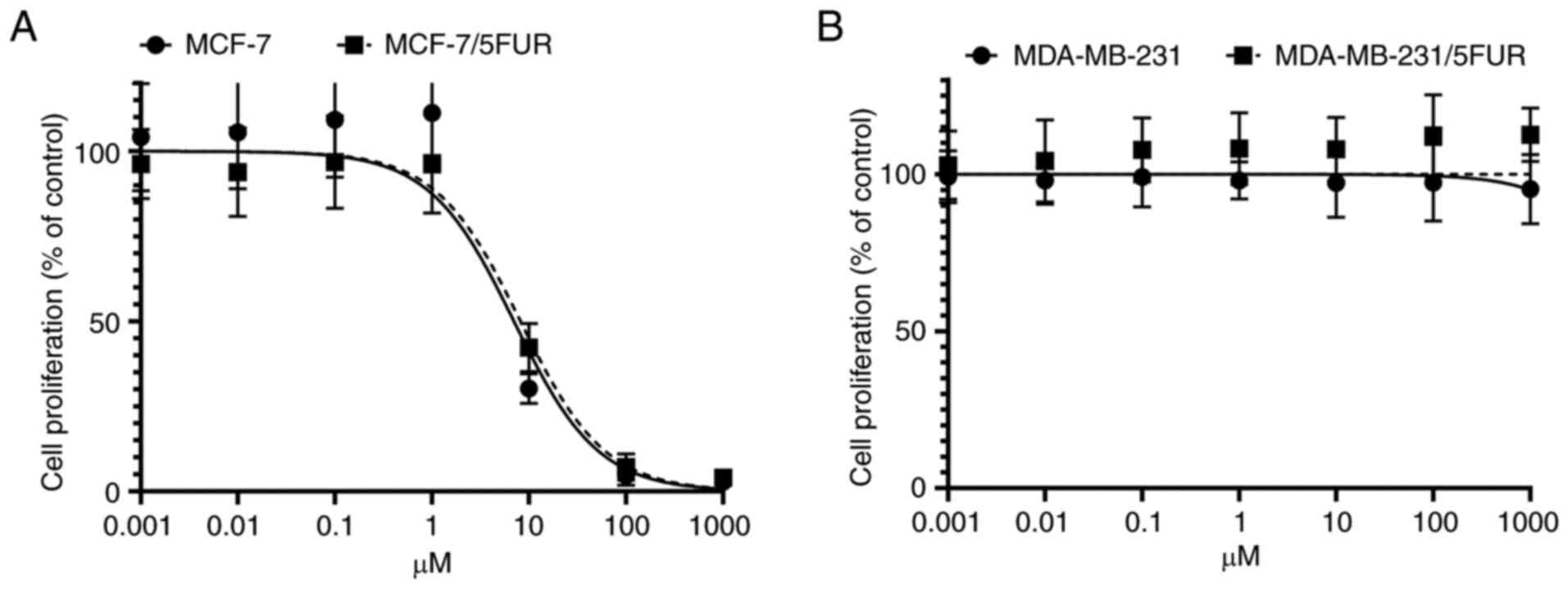
MCF-7/5-FUR cells showed an IC50 of 140.2
(95% CI: 104.3-188.8) µM, which represented a 36.6-fold increased
resistance compared with parental MCF-7 cells (IC50:
4.79 µM, 95% CI: 4.12-5.55) (Fig.
2A). A western blot analysis showed decreased OPRT (1.21±0.024
to 0.51±0.12, P<0.05), RR (1.19±0.026 to 0.39±0.0022, P<0.01)
and TK (0.94±0.077 to 0.46±0.063, P=0.051) levels and increased NT
(0.40±0.11 to 2.86±0.66, P=0.09) and TS (0.21±0.047 to 4.26±0.97,
P=0.07) levels in MCF-7/5-FUR cells compared with parental MCF-7
cells (Fig. 2B).
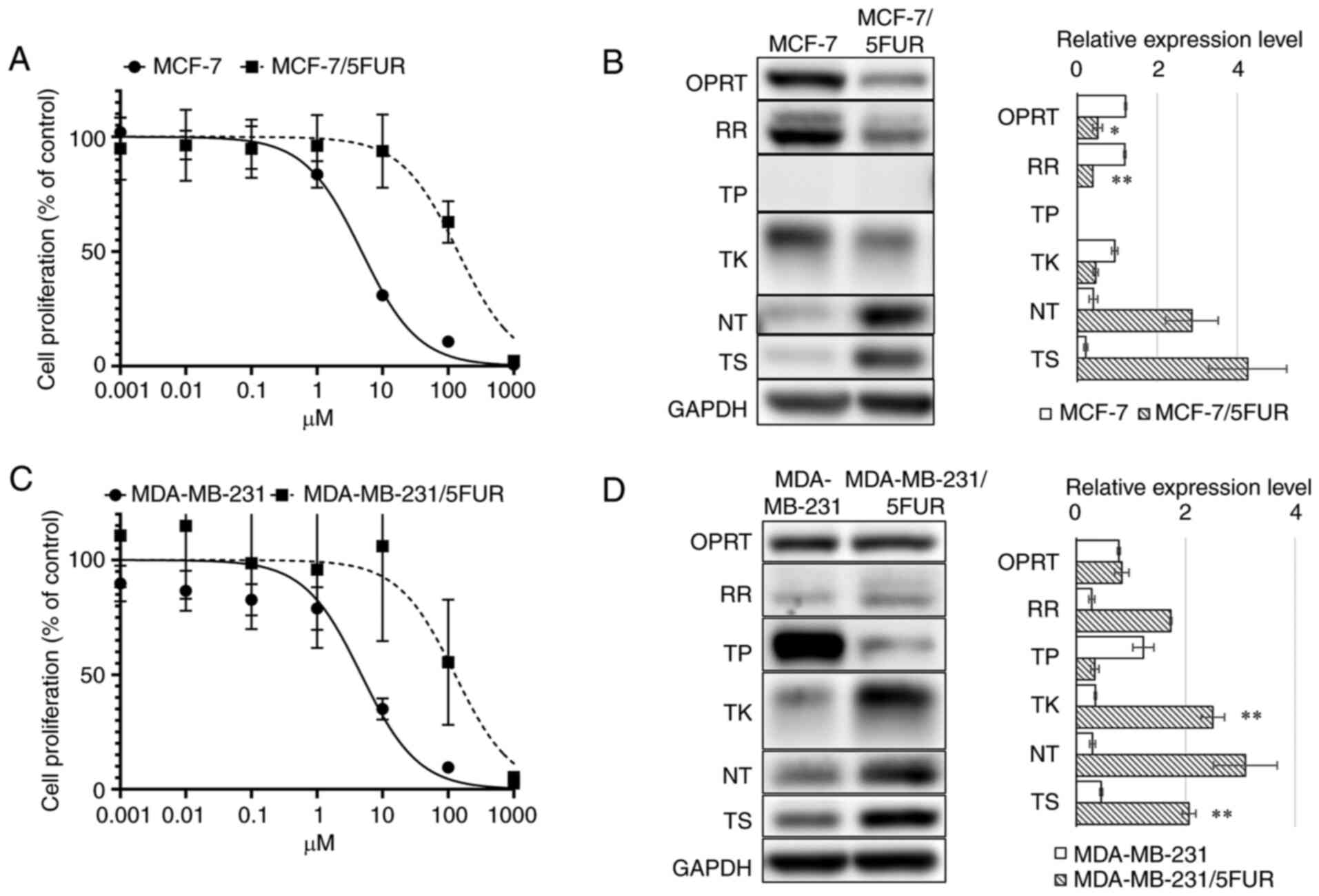 | Figure 2.Metabolism for 5-FU and sensitivities
to 5-FU. An MTT assay for 5-FU (A) in MCF-7 and MCF-7/5-FUR cells,
and (C) in MDA-MB-231 and MDA-MB-231/5-FUR cells. A western blot
analysis of the enzymes involved in 5-FU metabolism (B) in MCF-7
and MCF-7/5-FUR cells, and (D) in MDA-MB-231 and MDA-MB-231/5-FUR
cells. 5-FU, 5-fluorouracil; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; NT, nucleotidase; OPRT, orotate phosphoribosyl
transferase; RR, ribonucleotide reductase; TK, thymidine kinase;
TP, thymidine phosphorylase; T, thymidylate synthase. *P<0.05,
**P<0.01 vs. MDA-MB-231. |
MDA-MB-231/5-FUR cells showed resistance to 5-FU
(IC50: 127.3 µM, 95% CI: 66.9-247.0), which represented
a 15.8-fold increased resistance compared with parental MDA-MB-231
cells (IC50: 4.73 µM, 95% CI: 3.49-6.35) (Fig. 2C). A western blot analysis showed
decreased TP (01.23±0.19 to 0.34±0.075, P=0.068) levels and
increased TK (0.35±0.018 to 2.50±0.21, P<0.01), NT (0.30±0.053
to 3.09±0.58, P=0.05) and TS (0.46±0.026 to 2.06±0.12, P<0.01)
levels in MDA-MB-231/5-FUR cells compared with parental MDA-MB-231
cells (Fig. 2D).
These results indicated that these 5-FU-resistant
cells showed different changes in the metabolism of 5-FU after the
acquisition of resistance to 5-FU.
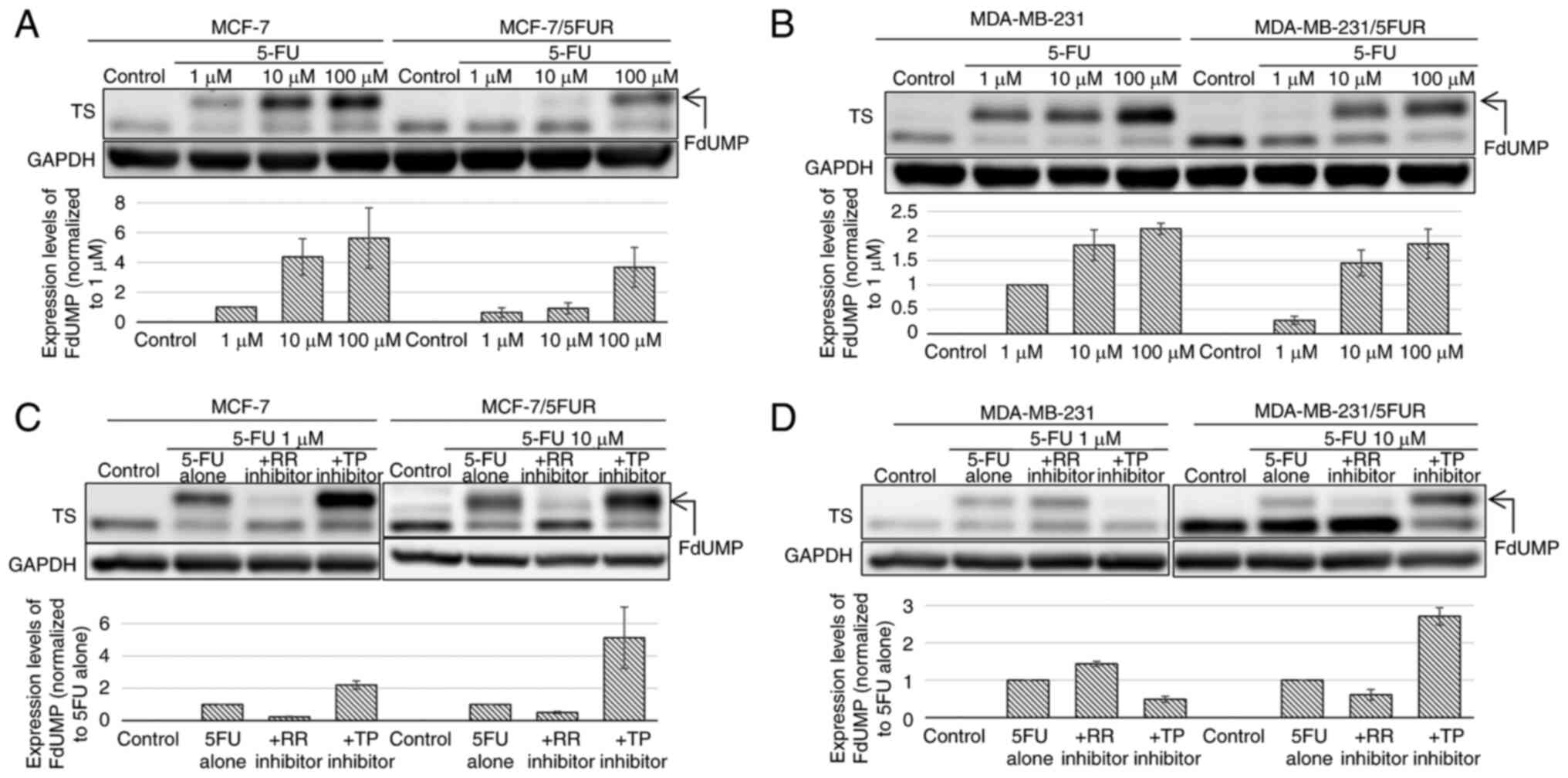
Changes in the amount of FdUMP after
treatment with 5-FU and the synthesis pathway of FdUMP after the
acquisition of resistance to 5-FU
After treatment with 5-FU, the upper band of TS was
detected on a western blot analysis, representing ternary complexes
composed of TS, FdUMP and CH2THF; the density of the
upper band was correlated with the intracellular concentration of
FdUMP (13). In both
5-FU-resistant cell lines, the upper band of TS was decreased
compared with the parental cell line: MCF-7/5-FUR (−79.1% at 1 µM)
and MDA-MB-231/5-FUR (−20.2% at 1 µM) (Fig. 3A and B). These results indicated
that the synthesis of FdUMP from 5-FU decreased on acquiring
resistance to 5-FU.
Next, we investigated the changes in the amount of
FdUMP after treatment with 5-FU combined with an RR or TP inhibitor
to clarify through which pathway FdUMP was synthesized. In MCF-7
and MCF-7/5-FUR cells, the upper band of TS was decreased when 5-FU
was combined with 1,000 µM of an RR inhibitor (MCF-7: −76.7%,
MCF-7/5-FUR: 50.0%), which indicated that FdUMP was synthesized
through the OPRT-RR pathway in these cells (Fig. 3C). The upper band of TS was
decreased by 51.0% when 5-FU was combined with 1 µM of a TP
inhibitor in MDA-MB-231 cells and was decreased by 39.1% when
combined with an RR inhibitor in MDA-MB-231/5-FUR cells, which
indicated that the synthesis pathway of FdUMP changed from the
TP-TK pathway to the OPRT-RR pathway on the acquisition of
resistance to 5-FU by these cells (Fig. 3D).
Interestingly, the upper band of TS was increased
when 5-FU was combined with a TP inhibitor in MCF-7/5-FUR cells
(+412%) as well as in MDA-MB-231/5-FUR cells (+171%), which
suggested that synthesized FdUMP was reduced through the TP-TK
pathway, and such reduction was inhibited by the TP inhibitor in
these cells.
The survival of MADMB231 and
MDA-MB-231/5-FUR cells without TS activity, with no products
derived from the de novo pathway detected in these cells
TS is a target enzyme of FdUMP and essential for the
synthesis of dTMP through the de novo pathway (14). Therefore, the inhibition of TS
leads to cell death if the cell is dependent on de novo
synthesis of dTMP.
In MCF-7 and MCF-7/5-FUR cells, the IC50
for the TS inhibitor was 7.00 nM (95% CI: 4.99-8.90) and 8.19 nM
(95% CI: 6.50-10.47), respectively (Fig. 4A). The cell growth of MDA-MB-231
and MDA-MB-231/5-FUR cells was not affected despite the presence of
more than 1,000 nM of a TS inhibitor (Fig. 4B). These results indicated that
MDA-MB-231 and MDA-MB-231/5-FUR cells were not dependent on the
de novo pathway, with the dTMP in these cells being
synthesized mainly through the salvage pathway.
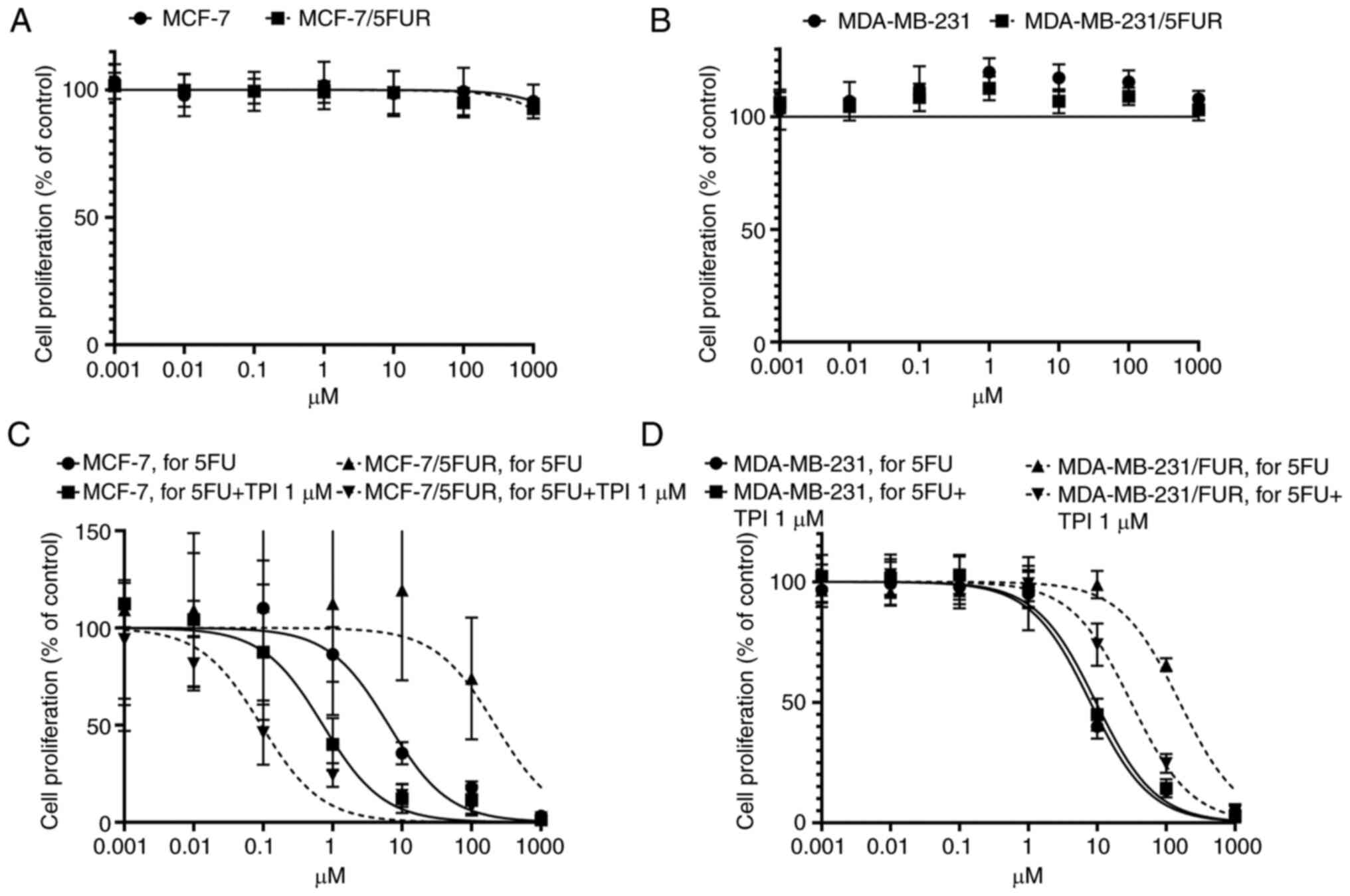
Reversal of 5-FU resistance in both
5-FU-resistant cell lines by a TP inhibitor
The TP inhibitor alone did not exert any
cytotoxicity in either cell line on an MTT assay (Fig. 5A and B). Interestingly, resistance
to 5-FU in 5-FU-resistant cells was completely reversed in
MCF-7/5-FUR cells (IC50: 219.9 µM, 95% CI: 90.44-555.0
to 0.934 µM, 95% CI: 0.059-0.148) and decreased in MDA-MB-231/5-FUR
cells (IC50: 157.3 µM, 95% CI: 132.8-186.5 to 30.98 µM,
95% CI: 25.6-37.6) when 1 µM of a TP inhibitor was combined with
5-FU, although the IC50s in parental MCF-7 and
MDA-MB-231 cells were not so changed (MCF-7: IC50: 6.41
µM, 95% CI: 4.50-9.10 to 0.724 µM, 95% CI: 0.337-1.54, MDA-MB231:
7.95 µM, 95% CI: 0.811-0.990 to 9.60 µM, 95% CI: 8.12-11.4,
respectively) (Fig. 5C and D).
These results suggested that the inhibition of TP
could reverse resistance to 5-FU in 5-FU-resistant cells, although
the TP inhibitor itself did not show any cytotoxic effect.
Discussion
In the present study, we clarified that the
metabolism of 5-FU differed in each cell line, and the mechanism
underlying the resistance to 5-FU also differed in each
5-FU-resistant cancer cell line. In addition, we found that
resistance to 5-FU was reversed by the inhibition of TP by a TP
inhibitor in both 5-FU-resistant cell lines.
In MCF-7 cells, FdUMP is synthesized only through
the OPRT-RR pathway and reduced through the TP-TK pathway. After
the acquisition of resistance to 5-FU, the synthesis of FdUMP was
decreased by decreased OPRT, leading to resistance to 5-FU. To
maintain a sufficient supply of dTMP, MCF-7/5-FUR cells seemed to
increase the reduction of FdUMP, which inhibited the synthesis of
dTMP through the de novo pathway by decreased TK and
increased NT levels. These hypotheses are illustrated in Fig. 6A and B.
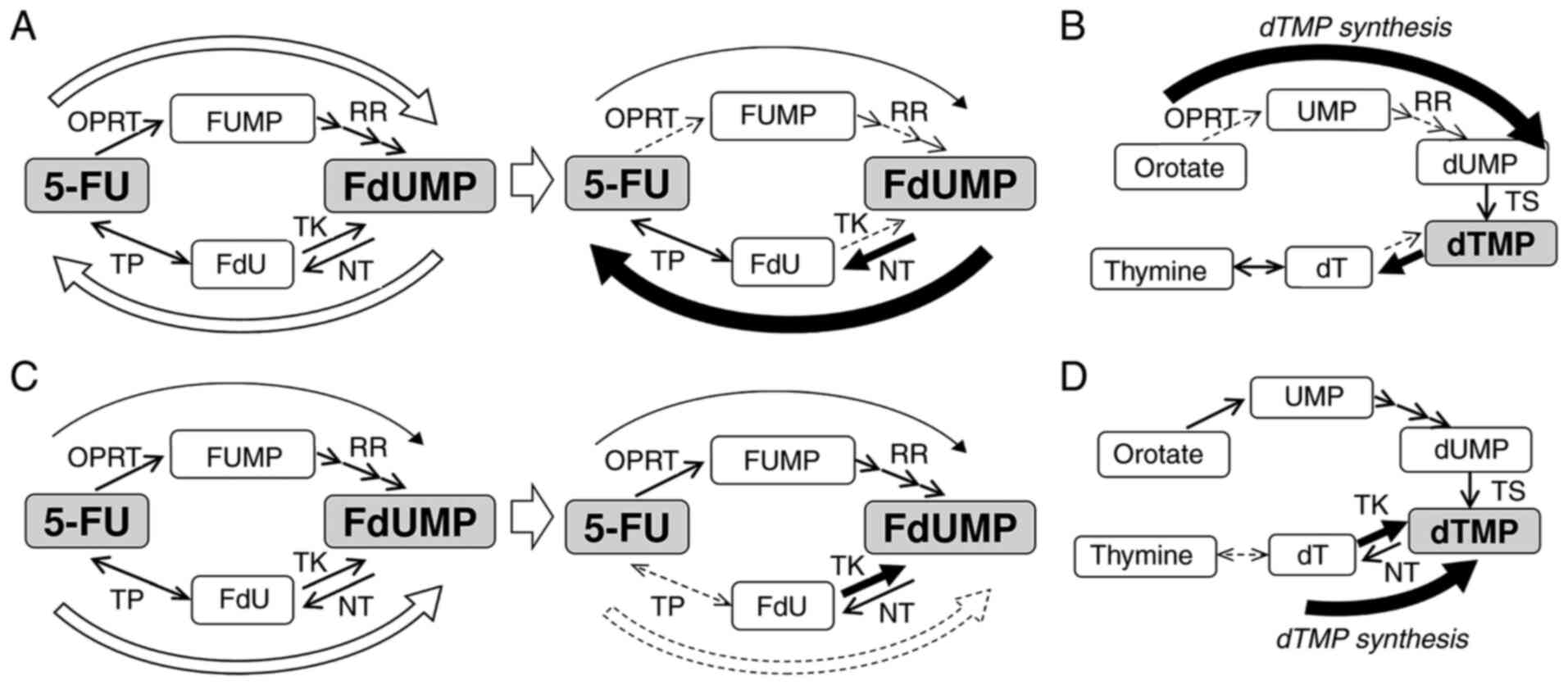 | Figure 6.Diagram for the mechanism underlying
resistance to 5-FU and the synthesis of dTMP in 5-FU-resistant
breast cancer cells. (A) In MCF-7 cells, FdUMP is synthesized
through the OPRT-RR pathway and reduced through the TP-TK pathway.
After the acquisition of resistance to 5-FU, synthesis of FdUMP was
decreased, and reduction of FdUMP was increased. As described in
the results, (B) in MCF-7 and MCF-7/5UR cells, dTMP is synthesized
only through the de novo pathway. (C) In MDA-MB-231 cells, FdUMP
was synthesized mainly through the TP-TK pathway, although some
FdUMP was synthesized through the OPRT-RR pathway. After the
acquisition of resistance to 5-FU, the synthesis of FdUMP through
the TP-TK pathway disappeared. (D) In MDA-MB-231 and
MDA-MB-231/5-FUR cells, the synthesis of dTMP occurred mainly
through the salvage pathway. 5-FU, 5-fluorouracil; dT, thymidine;
dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine
monophosphate; FdU, fluoro-deoxyuridine; FdUMP, fluoro-deoxyuridine
monophosphate; FUMP, fluoro-uridine monophosphate; NT,
nucleotidase; OPRT, orotate phosphoribosyl transferase; RR,
ribonucleotide reductase; TK, thymidine kinase; TP, thymidine
phosphorylase; TS, thymidylate synthase; UMP, uridine
monophosphate. |
However, in MDA-MB-231 cells, FdUMP was synthesized
mainly through the TP-TK pathway, although some FdUMP was
synthesized through the OPRT-RR pathway. After the acquisition of
resistance to 5-FU, the synthesis of FdUMP through the TP-TK
pathway disappeared due to the extremely decreased TP levels. The
synthesis of dTMP in these cells was mainly dependent on the
salvage pathway, and the expression of TK seemed to be increased to
supply sufficient dTMP through the salvage pathway. These
hypotheses are illustrated in Fig. 6C
and D.
There have been many reports on the association
between resistance to 5-FU and the changes in the expression of the
enzymes that lead to the decreased synthesis of FdUMP. For example,
decreased OPRT is a predictor of resistance to 5-FU (15–17).
However, such changes in the enzymes may also decrease the
synthesis of dTMP, which is vital for cell growth. Therefore, the
acquisition of resistance to 5-FU is usually accompanied by changes
in the enzymes increasing the synthesis of dTMP, which has never
been clearly reported previously. In the present study, the
reduction of FdUMP through the TP-TK pathway in MCF-7/5-FUR cells
seemed to contribute to an increase in the synthesis of dTMP
through de novo synthesis, and the increased TK levels in
MDA-MB-231/5-FUR cells also seemed to contribute to an increase in
the synthesis of thymidylate through salvage synthesis. Based on
this hypothesis, all enzymes related to the metabolism of 5-FU and
dTMP should be examined when we investigate the mechanism of
5-FU.
Tipiracil, the TP inhibitor used in the present
study, has already been applied as a component of TAS-102, an
anti-cancer drug used practically in metastatic colorectal cancer
(18) and gastric cancer (19), and its efficacy and safety in daily
practice has already been established. Therefore, combination
therapy with 5-FU and a TP inhibitor would be easy to incorporate,
and we believe it will be a promising therapy for breast
cancer.
We previously reported the mechanism underlying the
resistance to 5-FU, focusing on the metabolism of 5-FU and FdUMP
using other 5-FU-resistant cell lines. In the present study, in the
5-FU-resistant MKN45/F2R cells established at our institution,
resistance to 5-FU was almost completely reversed by the inhibition
of TP like MCF-7/5FUR cells, although sensitivity to 5FU was not
changed by the inhibition of TP in parental MKN45 cells, unlike
MCF-7 cells (20,21). In contrast, the resistance to 5-FU
in 5-FU-resistant SW48 and LS174T colon cancer cells, which were
also established in our institution, was not reversed by the
inhibition of TP although FdUMP in these cells was synthesized
through the TP-TK pathway, like the MDA-MB-231/5FUR cells in the
present study (22). To our
knowledge, this is the first report describing the enhancement of
the efficacy of 5-FU in parental cells and the reversal of
resistance to 5-FU in cells in which FdUMP was synthesized through
the TP-TK pathway. These results suggest that a predictive marker
of the efficacy of TP inhibitor should be established.
Several limitations associated with the present
study warrant mention. In this study, an in vivo experiment
was not conducted, so the reversal of resistance to 5-FU by a TP
inhibitor in vivo is unclear. In addition, predictive
markers of the efficacy of TP inhibitors must be developed in order
to apply combination therapy with 5-FU and a TP inhibitor in daily
practice, as described above. Furthermore, we did not perform
high-throughput sequencing or metabolomic analysis for the cells,
and the mutational statuses, gene expression profiles and metabolic
profiles in parental cells and 5FU-resistant cells were not
compared.
In conclusion, we elucidated the differences in the
mechanisms underlying resistance to 5-FU among cell lines. In
addition, we observed the reversal of resistance of 5-FU in
5-FU-resistant cells by treatment with a TP inhibitor. Further
investigations regarding the mechanism underlying resistance to
5-FU in other 5-FU-resistant cell lines and predictive markers for
the reversal of resistance to 5-FU by TP inhibitors are required.
Such combination therapy involving 5-FU and a TP inhibitor will
hopefully be able to be applied in clinical practice in the
future.
Acknowledgements
Taiho Pharmaceutical Company (https://www.taiho.co.jp/en/) kindly provided us with
their original antibody.
Funding
This work was supported by the Japan Society for the Promotion
of Science Grants-in-Aid for Scientific Research (grant no.
JP19K09090).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RM and MF designed the study. RM carried out the
study, and wrote the manuscript. RM and MF confirm the authenticity
of all the raw data. JU, YT and YN participated in the design and
helped draft the manuscript. MF represented our division, and
supervised the study and the writing of the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
CH2THF
|
5,10-methylenetetrahydrofolate
|
|
CI
|
confidence interval
|
|
dT
|
thymidine
|
|
dTMP
|
deoxythymidine monophosphate
|
|
dUMP
|
deoxyuridine monophosphate
|
|
FdU
|
fluoro-deoxyuridine
|
|
FdUMP
|
fluoro-deoxyuridine monophosphate
|
|
FUMP
|
5-fluorouridine monophosphate
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
IC50
|
half maximal inhibitory
concentration
|
|
MTT
|
3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H tetrazolium
bromide
|
|
NT
|
nucleotidase
|
|
OPRT
|
orotate phosphoribosyl transferase
|
|
PBS
|
phosphate-buffered saline
|
|
RR
|
ribonucleotide reductase
|
|
SE
|
standard error
|
|
TK
|
thymidine kinase
|
|
TP
|
thymidine phosphorylase
|
|
TS
|
thymidylate synthase
|
|
UMP
|
uridine monophosphate
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toi M, Imoto S, Ishida T, Ito Y, Iwata H,
Masuda N, Mukai H, Saji S, Shimizu A, Ikeda T, et al: Adjuvant S-1
plus endocrine therapy for oestrogen receptor-positive,
HER2-negative, primary breast cancer: A multicentre, open-label,
randomised, controlled, phase 3 trial. Lancet Oncol. 22:74–84.
2021. View Article : Google Scholar
|
|
4
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takashima T, Mukai H, Hara F, Matsubara N,
Saito T, Takano T, Park Y, Toyama T, Hozumi Y, Tsurutani J, et al:
Taxanes versus S-1 as the first-line chemotherapy for metastatic
breast cancer (SELECT BC): An open-label, non-inferiority,
randomised phase 3 trial. Lancet Oncol. 17:90–98. 2016. View Article : Google Scholar
|
|
6
|
Kufe DW and Major PP: 5-Fluorouracil
incorporation into human breast carcinoma RNA correlates with
cytotoxicity. J Biol Chem. 256:9802–9805. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito K, Nagashima H, Noguchi K, Yoshisue
K, Yokogawa T, Matsushima E, Tahara T and Takagi S: First-in-human,
phase I dose-escalation study of single and multiple doses of a
first-in-class enhancer of fluoropyrimidines, a dUTPase inhibitor
(TAS-114) in healthy male volunteers. Cancer Chemother Pharmacol.
73:577–83. 2014. View Article : Google Scholar
|
|
8
|
Francini G, Petrioli R, Lorenzini L,
Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G,
Civitelli S, et al: Folinic acid and 5-fluorouracil as adjuvant
chemotherapy in colon cancer. Gastroenterology. 106:899–906. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goodsell DS: The molecular perspective:
methotrexate. Oncologist. 4:340–341. 1999. View Article : Google Scholar
|
|
10
|
Rose MG, Farrell MP and Schmitz JC:
Thymidylate synthase: A critical target for cancer chemotherapy.
Clin Colorectal Cancer. 1:220–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolmark N, Rockette H, Fisher B, Wickerham
DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ,
et al: The benefit of leucovorin-modulated fluorouracil as
postoperative adjuvant therapy for primary colon cancer: Results
from national surgical adjuvant breast and bowel project protocol
C-03. J Clin Oncol. 11:1879–1887. 1993. View Article : Google Scholar
|
|
12
|
Hironaka S, Sugimoto N, Yamaguchi K,
Moriwaki T, Komatsu Y, Nishina T, Tsuji A, Nakajima TE, Gotoh M,
Machida N, et al: S-1 plus leucovorin versus S-1 plus leucovorin
and oxaliplatin versus S-1 plus cisplatin in patients with advanced
gastric cancer: A randomised, multicentre, open-label, phase 2
trial. Lancet Oncol. 17:99–108. 2016. View Article : Google Scholar
|
|
13
|
Drake JC, Allegra CJ and Johnston PG:
Immunological quantitation of thymidylate
synthase-FdUMP-5,10-methylenetetrahydrofolate ternary complex with
the monoclonal antibody TS 106. Anticancer Drugs. 4:431–435. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacFarlane AJ, Anderson DD, Flodby P,
Perry CA, Allen RH, Stabler SP and Stover PJ: Nuclear localization
of de novo thymidylate biosynthesis pathway is required to prevent
uracil accumulation in DNA. J Biol Chem. 286:44015–44022. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kodera Y, Ito S, Fujiwara M, Mochizuki Y,
Nakayama G, Ohashi N, Koike M, Yamamura Y and Nakao A: Gene
expression of 5-fluorouracil metabolic enzymes in primary gastric
cancer: Correlation with drug sensitivity against 5-fluorouracil.
Cancer Lett. 252:307–313. 2007. View Article : Google Scholar
|
|
16
|
Tsutani Y, Yoshida K, Sanada Y, Wada Y,
Konishi K, Fukushima M and Okada M: Decreased orotate
phosphoribosyltransferase activity produces 5-fluorouracil
resistance in a human gastric cancer cell line. Oncol Rep.
20:1545–1551. 2008.PubMed/NCBI
|
|
17
|
Isshi K, Sakuyama T, Gen T, Nakamura Y,
Kuroda T, Katuyama T and Maekawa Y: Predicting 5-FU sensitivity
using human colorectal cancer specimens: comparison of tumor
dihydropyrimidine dehydrogenase and orotate phosphoribosyl
transferase activities with in vitro chemosensitivity to 5-FU. Int
J Clin Oncol. 7:335–342. 2002. View Article : Google Scholar
|
|
18
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar
|
|
19
|
Shitara K, Doi T, Dvorkin M, Mansoor W,
Arkenau HT, Prokharau A, Alsina M, Ghidini M, Faustino C, Gorbunova
V, et al: Trifluridine/tipiracil versus placebo in patients with
heavily pretreated metastatic gastric cancer (TAGS): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
19:1437–1448. 2018. View Article : Google Scholar
|
|
20
|
Mori R, Futamura M, Tanahashi T, Tanaka Y,
Matsuhashi N, Yamaguchi K and Yoshida K: 5FU resistance caused by
reduced fluoro-deoxyuridine monophosphate and its reversal using
deoxyuridine. Oncol Lett. 14:3162–3168. 2017. View Article : Google Scholar
|
|
21
|
Mori R, Yoshida K, Futamura M, Suetsugu T,
Shizu K, Tanahashi T, Tanaka Y, Matsuhashi N and Yamaguchi K: The
inhibition of thymidine phosphorylase can reverse acquired
5FU-resistance in gastric cancer cells. Gastric Cancer. 22:497–505.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suetsugu T, Mori R, Futamura M, Fukada M,
Tanaka H, Yasufuku I, Sato Y, Iwata Y, Imai T, Imai H, et al:
Mechanism of acquired 5FU resistance and strategy for overcoming
5FU resistance focusing on 5FU metabolism in colon cancer cell
lines. Oncol Rep. 45:272021. View Article : Google Scholar : PubMed/NCBI
|