Introduction
An association between malignancies and venous
thromboembolism (VTE) was first reported by the French physician
Armand Trousseau in 1865 (1).
Since its identification, the combination of cancer and conditions
of hypercoagulation is often termed Trousseau syndrome
(cancer-associated thrombosis). Cancer-associated thrombosis,
including deep vein thrombosis (DVT), pulmonary embolism and
visceral thrombosis, are classified as VTE (2). VTE is a frequent and burdensome
complication in patients with active cancer, and its estimated
overall 12-month incidence rate is 6–8% (3,4).
Risk factors for VTE in these patients include patient-related
factors such as obesity, lying down for too long, primary cancer
site, staging, comorbidities and histopathological subclass, as
well as treatment-related factors such as surgery, chemotherapy,
molecular targeted agents and central venous catheter placement
(5–7). Trousseau syndrome-related cerebral
infarction is a relatively rare complication of malignant disease
compared with those related to DVT or pulmonary embolism; however,
its prognosis is markedly poor (median survival, 4.5 months from
diagnosis) (2,3,8). The
mechanisms underlying stroke due to Trousseau syndrome include
hypercoagulopathy, disseminated intravascular coagulation (DIC),
nonbacterial thrombotic endocarditis and production of tissue
factors (9,10).
In a previous study of 3,426 autopsy cases of
malignant tumors conducted in 1985, 14.6% of cases had combined
cerebrovascular accidents, of which 51% had cerebral infarction and
49% had cerebral hemorrhage (11).
Malignant tumors and thromboembolism have been reported to be
strongly associated with mortality, and thromboembolism has been
reported as the second leading cause of mortality in patients with
malignant tumors (5,12,13).
It is known that, in patients with Trousseau syndrome, those with
cerebral infarction have a higher mortality rate than those with
only VTE, and treatment and prevention of cerebral infarction
reduce mortality and improve patient quality of life (6,14,15).
However, effective treatment methods and predictors of its onset
have not been established. It is important to understand these
clinical features, since thrombosis associated with cancer is not
just an accidental complication but also a major cause of morbidity
and mortality directly associated with malignancy (15).
A large-scale epidemiological study demonstrated
that patients with brain tumors, hematological malignancies and
adenocarcinomas of the lung, pancreas, stomach and ovary are at a
markedly high risk of developing VTE; however, the actual
prevalence of Trousseau syndrome has not been clarified thus far
(16). Few reports have examined
the clinical features of Trousseau syndrome-related cerebral
infarction developed during chemotherapy in patients with ovarian,
pancreatic and colorectal cancers (17–21);
however, the information on patients with gastrointestinal (GI)
cancer is limited.
The present study aimed to investigate the
prevalence and detailed clinical features of patients with
Trousseau syndrome who developed cerebral infarction during
chemotherapy for GI cancer. In addition, the current study
investigated the factors associated with the occurrence of
Trousseau syndrome-related cerebral infarction under
chemotherapy.
Materials and methods
Patients
The present retrospective study consecutively
enrolled 878 patients with unresectable pathologically-diagnosed GI
cancer who received chemotherapy at the Multidisciplinary Treatment
Cancer Center of Kurume University Hospital (Kurume, Japan) between
April 2014 and March 2020. Patients (aged ≥18 years) were eligible
if they had histologically confirmed advanced gastrointestinal
cancer (colorectal cancer, pancreatic cancer, gastric cancer,
biliary tract cancer, neuroendocrine tumor, duodenal cancer) and a
performance status of 0–2. Patients were excluded if they had a
perfromance status of 3 and multiple cancers. The following data
were collected from medical charts and reviewed: Age, sex,
performance status, type of comorbid malignancies, cancer stage
[according to the 8th edition of the Union for International Cancer
Control (UICC) TNM classification] (22), risk factors for cerebrovascular
accident, number of days from onset of cerebral infarction to
mortality, serum C-reactive protein (CRP), carcinoembryonic antigen
(CEA), carbohydrate antigen (CA)19-9, treatment method and outcome.
Risk factors for cerebrovascular accidents, including diabetes
mellitus, hypertension, hyperlipidemia, chronic kidney disease
(CKD), Khorana VTE risk score and DIC score, were evaluated. Serum
markers (CRP, CEA, CA19-9) and risk factors (diabetes,
hypertension, hyperlipidemia, CKD, performance status, Khorana VTE
risk score) at the time of initiating chemotherapy were evaluated.
DIC score were evaluated at the time of Trousseau syndrome-related
cerebral infarction.
Definition of diabetes mellitus,
hypertension, hyperlipidemia, CKD, Khorana VTE risk score and DIC
score
Subjects were confirmed to have diabetes mellitus if
they had fasting plasma glucose ≥126 mg/dl and hemoglobin A1c ≥6.5%
(23). Hypertension was regarded
as present if a patient had a systolic blood pressure ≥140 mmHg
and/or a diastolic blood pressure ≥90 mmHg (24). Hyperlipidemia was defined as
present if the serum triglyceride levels were ≥240 mg/dl and/or the
serum low-density lipoprotein cholesterol level was ≥160 mg/dl
(25). Subjects were defined as
patients with CKD if they had CKD stage ≥3 (26). The serum D-dimer level measured
within 48 h from the diagnosis of cerebral infarction was used to
evaluate blood coagulability. The risk of VTE was evaluated using
the Khorana VTE risk score (0 points, low risk; 1–2 points,
moderate risk; and 3 points, high risk) (12). The diagnosis for DIC was based on
the Japanese Association for Acute Medicine DIC diagnostic criteria
(0–3 points, not DIC; 4–8 points, DIC) (27).
Definition of Trousseau
syndrome-related cerebral infarction
Patients who presented with acute cerebral
infarction on brain magnetic resonance imaging (MRI) with
neurological symptoms were further diagnosed with cerebral
infarction by neurology specialists, and were diagnosed with
Trousseau syndrome-related cerebral infarction.
Statistical analysis
Data are presented as the median (range) or n (%).
Each parameter was compared between patients without Trousseau
syndrome-related cerebral infarction groups and patients with
Trousseau syndrome-related cerebral infarction groups using the
Fisher's exact test or categorical variables and Mann-Whitney
U-test for continuous variables. Firth's logistic regression was
performed to assess the association between Trousseau
syndrome-related cerebral infarction and possible risk factors, and
to reduce biases of ordinal logistic regression in the analysis of
rare events such as Trousseau syndrome-related cerebral infarction
(28). P<0.05 was considered to
indicate a statistically significant difference. Data analysis was
performed using JMP Pro version 15.0 and SAS9.4 (both from SAS
Institute, Inc.).
Results
Baseline patient characteristics
Of the 878 patients, 39% were female (341/878). The
median age was 67 years (range, 23–86 years). Colorectal cancer
(35%; 308/878) was the most common malignancy, followed by
pancreatic (28%; 242/878), gastric (25%; 222/878) and biliary tract
(7%; 61/878) cancer, as well as neuroendocrine tumors (4%; 34/878)
and duodenal cancer (1.3%; 11/878) (Table I). The majority of patients in the
study had a performance status of 0 and 89 patients had a
performance status of 1 (Table I).
Only 0.9% (8/878) of the patients developed Trousseau
syndrome-related cerebral infarction during chemotherapy. There
were no significant differences in age or sex between patients with
and without Trousseau syndrome-related cerebral infarction. In all
patients who developed Trousseau syndrome-related cerebral
infarction, the tumor stage was IV (8th edition of the UICC TNM
classification). According to their cancer type, Trousseau
syndrome-related cerebral infarction occurred in 3.28% (2/61) of
patients with biliary tract cancer, 1.35% (3/222) of patients with
gastric cancer and 1.24% (3/242) of patients with pancreatic
cancer. By contrast, no patients with colorectal cancer or
neuroendocrine tumors were diagnosed with Trousseau
syndrome-related cerebral infarction (Table I).
 | Table I.Baseline clinical characteristics of
the patients (n=878). |
Table I.
Baseline clinical characteristics of
the patients (n=878).
| Characteristic | Total | Patients without
Trousseau syndrome-related cerebral infarction (n=870) | Patients with
Trousseau syndrome-related cerebral infarction (n=8) | P-value |
|---|
| Age, years | 67 (23–86) | 67 (23–86) | 70.5 (58–75) | 0.64 |
| Sex
female/male | 341 (39)/537 (61) | 337 (39)/533 (61) | 4 (50)/4 (50) | 0.72 |
| Performance status
0/1/2 | 789 (90)/89 (10)/0
(0) | 781 (90)/89 (10)/0
(0) | 8 (100)/0(0)/0
(0) | >0.99 |
| Type of cancer and
staginga |
|
|
|
|
|
Colorectal cancer | 308 (35) | 308 (35) | 0 (0) | 0.06 |
|
Stage
IIA/IIIA/IIIB/IVA/IVB | 1/11/8/204/73 | 1/11/8/204/73 | 0/0/0/0/0 |
|
|
Pancreatic cancer | 242 (28) | 239 (27) | 3 (38) | 0.69 |
|
Stage III/IV | 52/190 | 52/187 | 0/3 |
|
| Gastric
cancer | 222 (25) | 219 (25) | 3 (38) | 0.42 |
|
Stage
IIIA/IIIB/IIIC/IV | 2/1/5/214 | 2/1/5/211 | 0/0/0/3 |
|
| Biliary
tract cancer | 61 (7) | 59 (7) | 2 (25) | 0.10 |
|
Stage
IV/other | 61/0 | 59/0 | 2/0 |
|
|
Neuroendocrine tumor | 34 (4) | 34 (4) | 0 (0) | >0.99 |
|
GIST/NET/NEC | 7/17/10 | 7/17/10 | 0/0/0 |
|
|
Duodenal cancer | 11 (1.3) | 11 (1.3) | 0 (0) | >0.99 |
|
Stage
IV/other | 11/0 | 11/0 | 0/0 |
|
Symptoms and radiographic findings of
cerebral infarction in patients with Trousseau syndrome-related
cerebral infarction
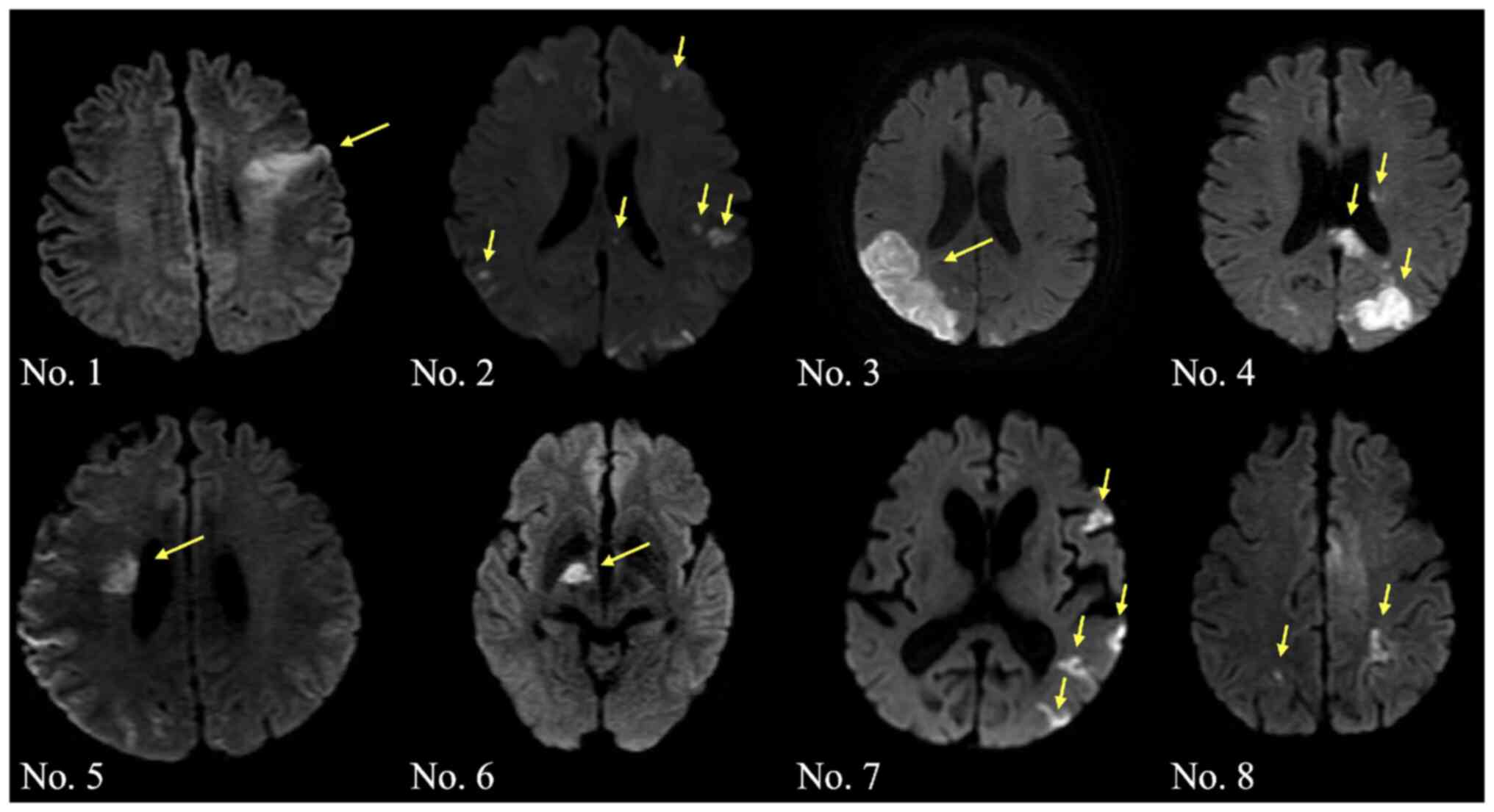
The cerebral infarction lesions detected by MRI of
the brain (diffusion-weighted image) were as follows: Single lesion
in one vascular territory infarction in 4 cases (cases 1, 3, 5 and
6) and multiple infarctions in 4 cases (cases 2, 4, 7 and 8)
(Fig. 1). Neurological symptoms at
onset were as follows: Disorientation in 5 cases, hemiparesis in 3
cases, dysarthria in 3 cases and loss of consciousness in 1
case.
Clinical characteristics of patients
with Trousseau syndrome-related cerebral infarction
The clinical characteristics of the patients with
Trousseau syndrome-related cerebral infarction are summarized in
Table II. Among the 8 patients
who developed Trousseau syndrome-related cerebral infarction, the
median age was 70.5 years (range, 58–75 years), and 50% were
females. Although the histological differentiation of the different
malignancies was not the same, all cases were adenocarcinoma. In
almost all patients (87.5%; 7/8), elevated serum D-dimer levels
were observed (median serum D-dimer level, 8.925 µg/ml; normal
range, <1.0 µg/ml) at disease onset. Metastatic lesions were
found in the liver in 6 cases and in the peritoneum in 2 cases
(Table II). There were no
patients with lung and/or brain metastases. In total, 3 cases
(37.5%) were diagnosed with DIC at onset (Table II). Notably, all these
characteristics were observed in patients with pancreatic cancer
(Table II). The line of therapy
at the time of onset was as follows: A total of 6 cases (75.0%)
were on first-line chemotherapy, 1 case (12.5%) was on second-line
chemotherapy and 1 case (12.5%) was on third-line chemotherapy
(Table II). In total, 5 patients
underwent a chemotherapy regimen using gemcitabine, while 4
patients underwent chemotherapy using cisplatin. As acute-phase
treatment for cerebral infarction, 3 cases were treated with
heparin, 2 with anti-platelet drugs, 2 with edaravone (a free
radical scavenger) and 1 with thrombectomy (Table II).
 | Table II.Clinical characteristics of patients
with Trousseau syndrome-related cerebral infarction. |
Table II.
Clinical characteristics of patients
with Trousseau syndrome-related cerebral infarction.
| Case no. | Age, years | Sex | Performance
status | Primary cancer
site | Metastatic
lesions | Histopathological
diagnosis | D-dimer, µg/ml | Duration from onset
to diagnosis, days | DIC score | Acute-phase
treatment for cerebral infarction | Chemotherapy
regimen |
|---|
| 1 | 64 | F | 0 | Gastric cancer | Peritoneum | Poorly
differentiated adenocarcinoma | 19.4 | 1 | 1 | Edaravone | Nivolumab (3rd
line) |
| 2 | 58 | M | 0 | Gastric cancer | Liver | Tubular
adenocarcinoma | 10.5 | 2 | 1 | Heparin | S1 + CDDP (1st
line) |
| 3 | 75 | M | 0 | Gastric cancer | Liver | Poorly
differentiated adenocarcinoma | 1.3 | 3 | 0 | Heparin | S1 + CDDP (1st
line) |
| 4 | 71 | F | 0 | Pancreatic
cancer | Peritoneum | Adenocarcinoma | 0.1 | 3 | 4 | Edaravone | GEM + Nab-PTX (1st
line) |
| 5 | 72 | F | 0 | Pancreatic
cancer | Liver | Adenocarcinoma | 28.4 | 1 | 6 | Thrombectomy | GEM (2nd line) |
| 6 | 70 | F | 0 | Pancreatic
cancer | Liver | Adenocarcinoma | 5.5 | 7 | 4 | Anti-platelet
drug | GEM + Nab-PTX (1st
line) |
| 7 | 74 | M | 0 | Biliary tract
cancer | Liver | Adenocarcinoma | 4.3 | 4 | 1 | Anti-platelet
drug | GEM + CDDP (1st
line) |
| 8 | 63 | M | 0 | Biliary tract
cancer | Liver | Adenocarcinoma | 1.3 | 7 | 3 | Heparin | GEM + CDDP (1st
line) |
Clinical course of each patient with
Trousseau syndrome-related cerebral infarction
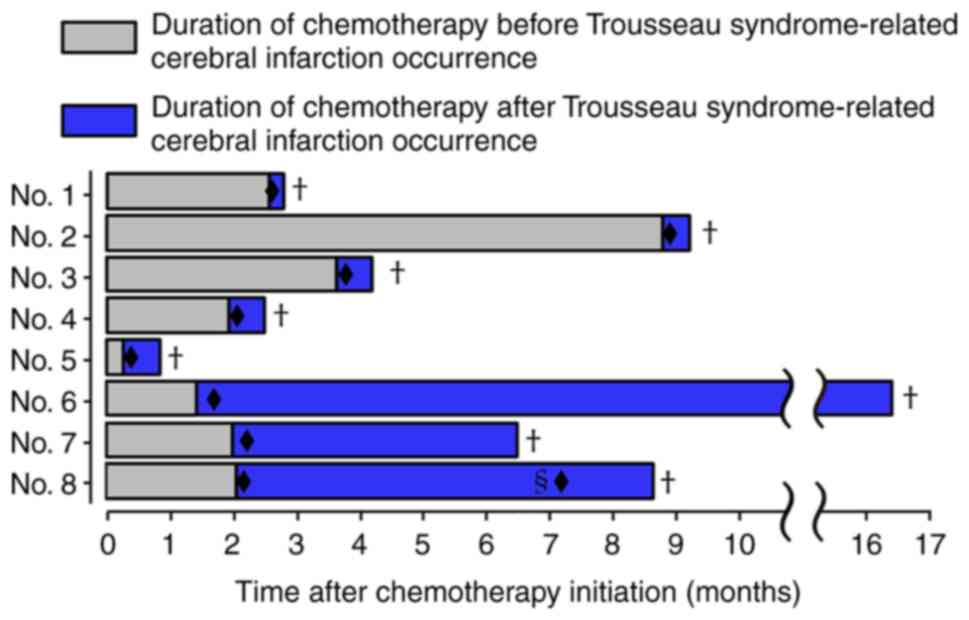
In the 8 patients who developed Trousseau
syndrome-related cerebral infarction, the median time from the
initiation of chemotherapy to onset of Trousseau syndrome-related
cerebral infarction diagnosis was 59.5 days (range, 7–263 days),
and 62.5% (5/8) of these patients were diagnosed within 60 days
(Fig. 2). In addition, the median
survival time after Trousseau syndrome-related cerebral infarction
occurrence was 17.5 days (range, 5–455 days), and 62.5% (5/8) of
these patients succumbed to disease within 20 days (Fig. 2). By contrast, long-term survival,
defined as >120 days after Trousseau syndrome-related cerebral
infarction diagnosis, was observed in 3 cases (cases 6, 7 and 8).
The median time from onset to diagnosis, which was the same as the
initiation of treatment, was 3 days (range, 1–7 days). Cases 6 and
7 were able to continue chemotherapy without sequelae, and survived
for 455 and 136 days, respectively. Case 8 resumed chemotherapy
upon recovering from a stroke; however, the patient had a
recurrence of a stroke associated with Trousseau syndrome 5 months
later (Fig. 2).
Association between risk factors and
Trousseau syndrome-related cerebral infarction
The associations between risk factors and their
occurrence were assessed to explore the predictive factors for
Trousseau syndrome-related cerebral infarction development. The
prevalence of hyperlipidemia in patients with Trousseau
syndrome-related cerebral infarction (38%; 3/8) was significantly
higher than that in patients without Trousseau syndrome-related
cerebral infarction (8.2%; 72/870; P=0.005) (Table III). Hyperlipidemia was found to
be a risk factor for occurrence of Trousseau syndrome-related
cerebral infarction with an odds ratio of 7.009 [95% confidence
interval (CI), 1.785-27.513] (Table
III). By contrast, there were no significant differences
between the groups for other factors, including prevalence of
hypertension, CKD, diabetes mellitus, performance status, Khorana
VTE risk score, serum CRP, and serum tumor markers such as CEA and
CA19-9 (Table III).
 | Table III.Risk factors for Trousseau
syndrome-related cerebral infarction. |
Table III.
Risk factors for Trousseau
syndrome-related cerebral infarction.
| Risk factor | Patients without
Trousseau syndrome-related cerebral infarction (n=870) | Patients with
Trousseau syndrome-related cerebral infarction (n=8) | Odds ratio | 95% CI | P-value |
|---|
| Hypertension | 214 (24) | 1 (13) | 0.612 | 0.105-3.570 | 0.585 |
| Hyperlipidemia | 72 (8.2) | 3 (38) | 7.009 | 1.785-27.513 | 0.005 |
| Chronic kidney
disease | 31 (3.6) | 0 (0) | 1.568 | 0.085-29.005 | 0.763 |
| Diabetes | 158 (18) | 2 (25) | 1.726 | 0.396-7.527 | 0.467 |
| Performance status
>1 | 89 (10) | 0 (0) | 0.514 | 0.029-9.112 | 0.650 |
| Khorana VTE risk
score |
|
|
|
|
|
|
Low | 300 (34) | 2 (25) | 1.000 | Ref. | Ref. |
|
Intermediate | 433 (50) | 4 (50) | 1.248 | 0.263-5.910 | 0.796 |
|
High | 137 (16) | 2 (25) | 2.185 | 0.373-12.821 | 0.373 |
| CRP >0.3
mg/dl | 440 (51) | 4 (50) | 0.977 | 0.262-3.639 | 0.973 |
| CEA >5
ng/ml | 473 (56) | 4 (50) | 0.795 | 0.213-2.962 | 0.733 |
| CA19-9 >37.0
ng/ml | 416 (49) | 5 (63) | 1.620 | 0.421-6.241 | 0.483 |
Discussion
A recent population-based study of patients with
different cancer types showed that the standardized mortality ratio
of fatal stroke, including cerebral infarction, was 2.17 (95% CI,
2.15-3.21). The highest ratio was observed in patients with brain
or GI cancer (29). In addition,
it has also been reported that cancer treatment options, including
chemotherapy and molecular-targeted therapy, aggravate the risk of
stroke (29). In the present
study, which analyzed 878 patients with unresectable GI cancer on
chemotherapy, only 0.9% of patients developed Trousseau
syndrome-related cerebral infarction. Notably, of the 308 cases of
colorectal cancer, none of the patients were diagnosed with
Trousseau syndrome-related cerebral infarction. However, its
occurrence rate has been reported to be 10–12% in patients with
this malignancy (21). In the
present study, the occurrence rate of Trousseau syndrome-related
cerebral infarction developed during chemotherapy was 1.24, 1.35
and 3.28% in patients with pancreatic, gastric and biliary tract
cancer, respectively. Previous studies have reported an occurrence
rate of 6–8% in patients with pancreatic cancer and of 4–6% in
patients with gastric cancer (19,20).
The majority of patients examined in the present study had a good
general condition, with a performance status of 0 and a low Khorana
VTE score, suggesting that the risk of thrombosis may have been
lower than previously reported (19,20).
Furthermore, the current study did not include cases with Trousseau
syndrome-related cerebral infarction before diagnosis of cancer,
which likely led to the observed differences in prevalence rates.
Therefore, including various cancer stages in the evaluation of
prospective trials is desirable.
In the present study, common risk factors for
cerebral infarction, including hypertension, CKD and diabetes
mellitus, were not associated with the development of Trousseau
syndrome-related cerebral infarction. Consistent with a previous
study in which hyperlipidemia was an independent predictor of
cerebrovascular events such as stroke (30), only hyperlipidemia was detected as
a predictive factor for Trousseau syndrome-related cerebral
infarction in the present study. It has been demonstrated that
hyperlipidemia induces endothelial dysfunction and oxidative
stress, leading to endothelial damage, resulting in an increased
risk of thromboembolism (31).
Furthermore, lowering cholesterol is important to prevent cerebral
infarction in patients with hyperlipidemia (31). Lowering cholesterol in patients
with cancer may also be important for preventing the development of
Trousseau syndrome-related cerebral infarction. Adenocarcinomas
that secrete mucus are one of the risk factors for Trousseau
syndrome (32). Since
mucin-related tumor markers such as CA19-9, CA125 and CA15-3 are
considered to trigger thrombotic events, particularly in ovarian
cancer (31), the serum levels of
CA19-9 and CEA were assessed in the current study. However, these
tumor markers were not found to be predictive factors for the
occurrence of Trousseau syndrome-related cerebral infarction.
Cancer may mediate the pathophysiology of stroke
either directly or via coagulation disorders and infections
(33). D-dimer, a degradation
product of fibrin thrombus produced by the action of thrombin,
activated factor XIII and plasmin, is known to be a highly
sensitive index for thrombosis (34). D-dimer is also nonspecifically
elevated in various conditions, including DVT, pregnancy, DIC and
aortic dissection (35). Trousseau
syndrome, which is caused by cancer-related hypercoagulability, has
been reported to present with higher D-dimer values than accidental
cerebral infarction not associated with hypercoagulability, and is
often accompanied by DIC (12,36).
Consistent with a previous study reporting that solid tumors,
particularly hepatocellular carcinoma, lung cancer and pancreatic
cancer, are more likely to cause DIC, all cases diagnosed with DIC
at the onset of Trousseau syndrome in the present study were cases
of pancreatic cancer (37).
Recently, it has been reported that D-dimer and CRP are potential
biomarkers for diagnosing Trousseau syndrome in patients with
cerebral embolism (38).
Consistent with the previous report, the serum D-dimer levels in
almost all patients (87.5%; 7/8) with Trousseau syndrome-related
cerebral infarction in the present study were elevated. However,
not all patients were evaluated for serum D-dimer levels, and
D-dimer levels could not be evaluated as predictors in the current
study. In addition, serum CRP levels were not significantly
different between the group with Trousseau syndrome-related
cerebral infarction and the group without Trousseau
syndrome-related cerebral infarction. Although it depends on the
histopathological subclass and primary cancer site, the incidence
rate of cancer-related VTE has been reported to be 6–8% (3,4).
According to the Khorana VTE score, which was introduced in 2008 to
stratify the risk of VTE and select cases requiring therapeutic
intervention (12,39), the incidence of VTE was 5.7% in the
low-risk group, 8.6% in the medium-risk group and 14% in the
high-risk group, showing a positive association between the Khorana
VTE score and the incidence of VTE (40). By contrast, the Khorana VTE score
could not be used as a predictor in the present study. It is known
that malignant cells activate coagulation through multiple
mechanisms such as tissue factor production and inflammatory
cytokines (41). Tissue factor is
one of the principal initiators of the extrinsic coagulation
cascade and directly converts factor VII to factor VIIa (42). Furthermore, inflammatory cytokines
can also promote the coagulation cascade by inducing tissue factors
from vascular cells, monocytes and macrophages (41).
In the current retrospective study, almost all
patients who developed Trousseau syndrome-related cerebral
infarction underwent chemotherapy with gemcitabine and/or
cisplatin. Gemcitabine is a widely used anticancer drug for
patients with different types of cancer, including pancreatic,
biliary tract and lung cancer (43). Several case reports have
demonstrated the occurrence of Trousseau syndrome under
chemotherapy using gemcitabine (44,45).
A previous study revealed that gemcitabine kills proliferating
endothelial cells by activating acid sphingomyelinase (46). Cisplatin is also a widely approved
anticancer drug for patients with different types of cancer,
including gastric, lung and ovarian cancer (47). In vitro examination using
human endothelial cells revealed that exposure to cisplatin
upregulated the production of inflammatory proteins, which were
assumed to initiate vascular inflammation and endothelial
dysfunction (48). It was
suggested that these drugs may damage endothelial cells, resulting
in an increased risk of thromboembolism.
It has been reported that infarcts involving
multiple vascular areas detected by diffusion-weighted imaging are
highly sensitive to Trousseau syndrome-related cerebral infarction
(18). A previous study that
assessed the radiological features of 31 patients with Trousseau
syndrome-related cerebral infarction showed that multiple lesions
in multiple vascular territories were the most frequent pattern
(87.1%; 27/31) (36). By contrast,
only 50% of the patients in the present study exhibited this
feature. Due to the small number of cases, further research on
patterns of cerebral infarction in patients with GI cancer in a
large number of patients is needed.
In patients with Trousseau syndrome-related cerebral
infarction, the median survival time has been reported to be 4.5
months, with 25% of patients succumbing to the disease within 30
days (8). Consistent with this
report, the prognoses of the patients in the present study were
remarkably poor, and 62.5% (5/8) of them succumbed to the disease
within 20 days. Only 2 patients were able to continue chemotherapy
without sequelae. Notably, 62.5% (5/8) and 87.5% (7/8) of these
patients developed cerebral infarction within 60 and 110 days,
respectively. These results indicated that Trousseau
syndrome-related cerebral infarction may develop soon after the
initiation of chemotherapy. Heparin, a standard treatment for
Trousseau syndrome-related cerebral infarction, is preferred over
oral anticoagulants (4). In the
physiology of Trousseau syndrome, warfarin potassium is considered
ineffective due to the suggested presence of vitamin K-independent
coagulation abnormalities (49).
In a study regarding the efficacy of direct oral anticoagulants, it
was reported that dabigatran was not effective in suppressing the
recurrence of Trousseau syndrome-related cerebral infarction
(50). Case accumulation is
desired for optimal management and long-term survival of patients
with Trousseau syndrome-related cerebral infarction.
To the best of our knowledge, the present study was
the first to reveal the prevalence and clinical features of
Trousseau syndrome-related cerebral infarction in patients with
unresectable GI cancer. However, the current study has several
limitations. In total, >800 patients with unresectable GI cancer
who received chemotherapy were assessed. The prevalence of
Trousseau syndrome-related cerebral infarction was calculated, and
the risk factors associated with its occurrence were evaluated.
However, the number of cases in the Trousseau syndrome-related
cerebral infarction group was small, which may have led to bias in
the results. Therefore, a future study with a large number of cases
is required. In the present retrospective study, DVT was not
confirmed on transesophageal echocardiography prior to
chemotherapy. Furthermore, the smoking status (which is known to be
strongly associated with stroke), such as current or never smokers,
could not be evaluated. In addition, D-dimer was not determined in
all of the patients. Therefore, clinical trials that address these
issues require to be performed in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ToT and HS conceived the study and wrote the
manuscript. KM, HK, YA and TaT made substantial contributions to
the conception and design of the study. TU, SN, MF, KI, TN, HI, AM
and TS collected the clinical data and were involved in the raw
data analysis. KM was involved in the raw data statistical analysis
and revised the manuscript. All authors discussed the results and
contributed to the final manuscript. ToT, HS and KM confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and was reviewed and approved by the
Ethics Committee of Kurume University School of Medicine (Kurume,
Japan; approval no. 21166). Written informed consent was obtained
from all patients regarding treatment; however, the requirement for
patient consent for participation in the study was waived due to
the retrospective design of the current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trousseau A: Phlegmasia alba dolens. Clin
Med Hotel-dieu Paris. 3:654–712. 1865.
|
|
2
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW,
et al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J Clin Oncol.
38:496–520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Timp JF, Braekkan SK, Versteeg HH and
Cannegieter SC: Epidemiology of cancer-associated venous
thrombosis. Blood. 122:1712–1723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen AT, Katholing A, Rietbrock S, Bamber
L and Martinez C: Epidemiology of first and recurrent venous
thromboembolism in patients with active cancer. A population-based
cohort study. Thromb Haemost. 117:57–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prandoni P, Lensing AW, Piccioli A,
Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins
MH, Noventa F and Girolami A: Recurrent venous thromboembolism and
bleeding complications during anticoagulant treatment in patients
with cancer and venous thrombosis. Blood. 100:3484–3488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AY: Epidemiology and management of
venous thromboembolism in patients with cancer. Thromb Res.
110:167–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prandoni P, Falanga A and Piccioli A:
Cancer and venous thromboembolism. Lancet Oncol. 6:401–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cestari DM, Weine DM, Panageas KS, Segal
AZ and DeAngelis LM: Stroke in patients with cancer: Incidence and
etiology. Neurology. 62:2025–2030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rickles FR: Mechanisms of cancer-induced
thrombosis in cancer. Pathophysiol Haemost Thromb. 35:103–110.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grisold W, Oberndorfer S and Struhal W:
Stroke and cancer: A review. Acta Neurol Scand. 119:1–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graus F, Rogers LR and Posner JB:
Cerebrovascular complications in patients with cancer. Medicine
(Baltimore). 64:16–35. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwarzbach CJ, Schaefer A, Ebert A, Held
V, Bolognese M, Kablau M, Hennerici MG and Fatar M: Stroke and
cancer: The importance of cancer-associated hypercoagulation as a
possible stroke etiology. Stroke. 43:3029–3034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varki A: Trousseau's Syndrome: Multiple
definitions and multiple mechanisms. Blood. 110:1723–1729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noble S and Pasi J: Epidemiology and
pathophysiology of cancer-associated thrombosis. Br J Cancer. 102
(Suppl 1):S2–S9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wun T and White RH: Epidemiology of
cancer-related venous thromboembolism. Best Pract Res Clin
Haematol. 22:9–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takano H, Nakajima K, Nagayoshi Y,
Komazaki H, Suzuki J, Tanabe H, Niimi S, Isonishi S and Okamoto A:
Clinical associations of Trousseau's syndrome associated with
cerebral infarction and ovarian cancer. J Gynecol Oncol.
29:e672018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, He Y and Su Y: Multifocal cerebral
infarction as the first manifestation of occult malignancy: Case
series of Trousseau's syndrome and literature review. Brain Circ.
4:65–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SG, Hong JM, Kim HY, Lee J, Chung PW,
Park KY, Kim GM, Lee KH, Chung CS and Bang OY: Ischemic stroke in
cancer patients with and without conventional mechanisms: A
multicenter study in Korea. Stroke. 41:798–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaturvedi S, Ansell J and Recht L: Should
cerebral ischemic events in cancer patients be considered a
manifestation of hypercoagulability? Stroke. 25:1215–1218. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pinzon R, Drewinko B, Trujillo JM, Guinee
V and Giacco G: Pancreatic carcinoma and Trousseau's syndrome:
Experience at a large cancer center. J Clin Oncol. 4:509–514. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brierley JD, Gospodarowicz MK and
Christian Wittekind C: TNM Classification of Malignant Tumours. 8th
edition. Wiley Publications; Hoboken, NJ: 2016
|
|
23
|
Araki E, Goto A, Kondo T, Noda M, Noto H,
Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K and Yoshioka N:
Japanese clinical practice guideline for diabetes 2019. Diabetol
Int. 11:165–223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umemura S, Arima H, Arima S, Asayama K,
Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al:
The Japanese society of hypertension guidelines for the management
of hypertension (JSH 2019). Hypertens Res. 42:1235–1481. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lipsy RJ: The National cholesterol
education program adult treatment panel III guidelines. J Manag
Care Pharm. 9 (Suppl 1):S2–S5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
KDIGO CKD Work Group, : KDIGO 2012
Clinical Practice Guideline for the Evaluation and Management of
Chronic Kidney Disease. Kidney Int. (Suppl 3):1–150. 2013.
|
|
27
|
Gando S, Iba T, Eguchi Y, Ohtomo Y,
Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, et
al: A multicenter, prospective validation of disseminated
intravascular coagulation diagnostic criteria for critically ill
patients: Comparing current criteria. Crit Care Med. 34:625–631.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Puhr R, Heinze G, Nold M, Lusa L and
Geroldinger A: Firth's logistic regression with rare events:
Accurate effect estimates and predictions? Stat Med. 36:2302–2317.
2017.PubMed/NCBI
|
|
29
|
Zaorsky NG, Zhang Y, Tchelebi LT, Mackley
HB, Chinchilli VM and Zacharia BE: Stroke among cancer patients.
Nat Commun. 10:51722019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alloubani A, Nimer R and Samara R:
Relationship between hyperlipidemia, cardiovascular disease and
stroke: A systematic review. Curr Cardiol Rev.
17:e0511211890152021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dardiotis E, Aloizou AM, Markoula S,
Siokas V, Tsarouhas K, Tzanakakis G, Libra M, Kyritsis AP, Brotis
AG, Aschner M, et al: Cancer-associated stroke: Pathophysiology,
detection and management (Review). Int J Oncol. 54:779–796.
2019.PubMed/NCBI
|
|
32
|
Lv J, Yang L, Guo R, Shi Y, Zhang Z and Ye
J: Ox-LDL-induced microRNA-155 promotes autophagy in human
endothelial cells via repressing the Rheb/mTOR pathway. Cell
Physiol Biochem. 43:1436–1448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Awkar N, Amireh S, Rai S, Shaaban H, Guron
G and Maroules M: Association between level of tumor markers and
development of VTE in patients with pancreatic, colorectal and
ovarian Ca: Retrospective case-control study in two community
hospitals. Pathol Oncol Res. 24:283–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adam SS, Key NS and Greenberg CS: D-dimer
antigen: Current concepts and future prospects. Blood.
113:2878–2887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tripodi A: D-Dimer testing in laboratory
practice. Clin Chem. 57:1256–1262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao L, Zhang S, Gong X and Cui G:
Trousseau syndrome related cerebral infarction: Clinical
manifestations, laboratory findings and radiological features. J
Stroke Cerebrovasc Dis. 29:1048912020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taniguchi T, Kakkar AK, Tuddenham EG,
Williamson RC and Lemoine NR: Enhanced expression of urokinase
receptor induced through the tissue factor-factor VIIa pathway in
human pancreatic cancer. Cancer Res. 58:4461–4467. 1998.PubMed/NCBI
|
|
38
|
Tsushima M, Metoki N, Hagii J, Saito S,
Shiroto H, Yasujima M, Kato T, Kudo N, Toyama Y, Yokono Y, et al:
D-Dimer and C-reactive protein as potential biomarkers for
diagnosis of trousseau's syndrome in patients with cerebral
embolism. J Stroke Cerebrovasc Dis. 29:1045342020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mulder FI, Candeloro M, Kamphuisen PW, Di
Nisio M, Bossuyt PM, Guman N, Smit K, Büller HR and van Es N;
CAT-prediction collaborators, : The khorana score for prediction of
venous thromboembolism in cancer patients: A systematic review and
meta-analysis. Haematologica. 104:1277–1287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Donati MB and Lorenzet R: Thrombosis and
cancer: 40 years of research. Thromb Res. 129:348–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ikushima S, Ono R, Fukuda K, Sakayori M,
Awano N and Kondo K: Trousseau's Syndrome: Cancer-associated
thrombosis. Jpn J Clin Oncol. 46:204–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noble S and Goa KL: Gemcitabine. A review
of its pharmacology and clinical potential in non-small cell lung
cancer and pancreatic cancer. Drugs. 54:447–472. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
van Hell AJ, Haimovitz-Friedman A, Fuks Z,
Tap WD and Kolesnick R: Gemcitabine kills proliferating endothelial
cells exclusively via acid sphingomyelinase activation. Cell
Signal. 34:86–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sasaki R, Ohya Y, Hayashida S, Maeda Y,
Murahashi S, Kumamoto S, Tsuji A, Shibata H, Kuramoto K, Hayashi H,
et al: A case of Trousseau's syndrome due to intrahepatic
cholangiocarcinoma with an extremely high level of CA19-9. Surg
Case Rep. 6:752020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamamoto R, Kato S, Takahashi M, Ono S,
Sakurada T, Nagoshi S, Nisikawa K and Yakabi K: Trousseau syndrome
accompanied by cholangiocellular carcinoma: Report of two. J
Saitama Medical University. 40:131–134. 2014.
|
|
47
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nuver J, De Haas EC, Van Zweeden M,
Gietema JA and Meijer C: Vascular damage in testicular cancer
patients: A study on endothelial activation by bleomycin and
cisplatin in vitro. Oncol Rep. 23:247–253. 2010.PubMed/NCBI
|
|
49
|
Walsh-McMonagle D and Green D:
Low-molecular-weight heparin in the management of Trousseau's
syndrome. Cancer. 80:649–655. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoshida K, Kimura T, Aburakawa Y, Suzuki
Y, Kuroda K and Yahara O: Recurrent ischemic stroke in a patient
with the trousseau syndrome treated with dabigatran. J Stroke
Cerebrovasc Dis. 23:1724–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|