Introduction
Hodgkin lymphoma (HL) is a unique lymphoid neoplasm
characterized by malignant Reed-Sternberg cells in an inflammatory
background (1). It has a
distinctive bimodal age distribution with peaks around the second
and sixth decade of life, but the incidence varies with the
histological subtype and geography (2,3).
Average annual observed number of new HL cases in Slovenia between
2004 and 2013 was 46.8 (4).
Staging is based on the Lugano classification, which
is derived from the older Ann Arbour classification system
(5). Afterwards, patients are
assigned to one of the three categories-limited, intermediate and
advanced stages, based on which the treatment is selected (6). Combination of chemo- and radiotherapy
are the backbone of classical HL treatment, particularly in early
stages, in late stages radiotherapy is reserved to consolidate
partial remission. Early stages of HL, comprising limited and
intermediate stages, are generally treated with ABVD chemotherapy
regimen (doxorubicin, bleomycin, vinblastine, dacarbazine) with
involved-site radiation therapy. Advanced stages (stage IIB, III
and IV) are usually treated with chemotherapy, and radiation
therapy is used exclusively as consolidation for selected patients
with partial remission (7–11). Chemotherapy regimens for advanced
stage HL used extensively in Europe include escalated BEACOPP
(bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine,
procarbazine, prednisone) and ABVD, along with BV-AVD (brentuximab
vedotin, doxorubicin, vinblastine, dacarbazine) in recent years
(12–14). Slovenian guidelines for treatment
of malignant lymphomas propose treatment with ABVD for early stages
of HL and ABVD or escalated BEACOPP for advanced stages HL.
Intensified first-line chemotherapeutic regimens
(escalated BEACOPP) have been designed to overcome the risk of
early chemo-resistance development. However, the treatment-related
toxicity of intensive approaches is fairly high and is associated
with complications that could delay the administration of further
chemotherapeutic cycles or could lead to the cytostatic dose
reductions (15). Relative dose
intensity (RDI) represents the ratio of the amount of a drug
actually administered to the patient in regard to the amount
planned for a fixed time period (16,17).
The purpose of calculating the RDI is to evaluate whether or not
the planned dose intensity of a chemotherapy treatment was actually
achieved.
The aim of this retrospective study was to
investigate the association of RDI with the outcome of HL patients
with advanced stage disease therefore receiving ABVD and escalated
BEACOPP regimen, representing all possible treatments for advanced
stages according to Slovenian guidelines.
Materials and methods
Patients
We retrospectively reviewed medical records of
histologically confirmed HL patients at the Institute of Oncology
Ljubljana, Slovenia, between 2004 and 2013. We enrolled patients
with advanced stage disease that were planned to receive either 8
cycles of ABVD or 4 cycles of escalated and 4 cycles of regular
BEACOPP until 2011 and, from 2012 on, 6 cycles of escalated
BEACOPP, because of modification of national guidelines. In line
with this guidelines, ABVD was reserved for patients older than 60
years and for younger patients, who were unfit to receive escalated
BEACOPP (patients with severe arterial hypertension, chronic
obstructive pulmonary disease, and diabetes mellitus) or reluctant
for aggressive chemotherapy (childcare, work during treatment).
Before the initial treatment, physical and blood
examination, echocardiography, chest X-ray, computed tomography or
positron emission tomography-computed tomography, and bone marrow
biopsy was conducted in all patients. In addition, performance
status and the Charlson comorbidity index (CCI), which predicts the
risk of mortality associated with a range comorbid conditions, were
assessed as well (18).
ABVD regimen comprised of intravenous (IV)
doxorubicin 25 mg/m2, bleomycin 10 units/m2,
vinblastine 6 mg/m2 and dacarbazine 375 mg/m2
on days 1 and 15, every 28 days (13). Escalated BEACOPP regimen comprised
of IV bleomycin 10 units/m2 on day 8, etoposide 200
mg/m2 on days 1 through 3, doxorubicin 35
mg/m2 on day 1, cyclophosphamide 1,250 mg/m2
on day 1, vincristine 1.4 mg/m2 (maximum 2 mg) on day 8,
and oral procarbazine 100 mg/m2 on days 1 through 7 plus
prednisone 40 mg/m2 on days 1 through 14, every 21 days
(12). Regular BEACOPP was
designed in a similar manner, however the dose of etoposide was
reduced to 100 mg/m2, doxorubicin to 25 mg/m2
and cyclophosphamide to 650 mg/m2.
The study was approved by the Institutional Review
Board at the Institute of Oncology Ljubljana (approval no.
KSOPKR/72) and National Medical Ethics Committee of Republic of
Slovenia (approval no. 0120-481/2017/5). Because of retrospective
design of the study, informed consents were waived by the National
Medical Ethics Committee of Republic of Slovenia.
Relative dose intensity
calculation
According to the chemotherapy regimen, all patients
were planned to receive the full doses of cytostatic drugs,
however, treatment delays and/or dose reductions were found in most
of them. RDI was calculated as described below.
We defined the RDI as the ratio of the drug dose
administered in the actual time, over the planned dose in the
planned time. Dose intensity (DI), which can be presented as the
amount of a drug administered per time unit, is used to assess the
intensity of chemotherapy. It is calculated as a dose of a drug per
cycle (mg/m2) divided by the number of weeks in a cycle
(17). RDI of each drug is
acquired as a fraction of actual DI and planned DI (according to
full doses of drugs and total number of cycles-8 for ABVD and 6 or
8 for BEACOPP), whereas average RDI of chemotherapy regimen as a
sum of RDI of each drug divided with the number of drugs in a
regimen (4 for ABVD and 6 for BEACOPP). The purpose of calculating
RDI of chemotherapy regimen is to evaluate if the planned DI was
actually achieved.
Statistical analysis
Categorical variables were summarized with
frequencies and percentages, numerical variables with medians,
interquartile ranges (IQR) and ranges (due to the asymmetric shape
of distributions). Patients' characteristics were compared between
treatment groups by using chi-squared tests for categorical
variables and Mann-Whitney U tests for numerical variables
(Table I).
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Characteristic | All (n=114) | ABVD (n=54) | BEACOPP (n=60) | P-value |
|---|
| Median age, years
(IQR) | 39.2 (28.8-59.2) | 59.8 (40.6-67.9) | 32.9 (25.7-40.0) |
<0.001a |
| Male gender, n
(%) | 66 (57.9) | 31 (57.4) | 35 (58.3) | 0.920 |
| Clinical stage IV, n
(%) | 68 (59.6) | 33 (61.1) | 35 (58.3) | 0.763 |
| Median CCI (IQR) | 2 (2–4) | 4 (2–6) | 2 (2–2) |
<0.001a |
| Median RDI, %
(IQR) | 91.0 (81.6-96.1) | 82.3 (68.2-89.9) | 95.9 (90.7-98.9) |
<0.001a |
| Radiation therapy, n
(%) | 34 (29.8) | 15 (27.8) | 19 (31.7) | 0.650 |
| Median PS (IQR) | 0 (0–1) | 1 (0–2) | 0 (0-0.2) |
<0.001a |
| PS ≥2, n (%) | 19 (16.7) | 15 (27.8) | 4 (6.7) | 0.003a |
Overall survival (OS) and progression-free survival
(PFS) probabilities were estimated from the end of treatment (as
our aim was to investigate association with RDI which is known at
the end of treatment) with Kaplan-Meier method (19), confidence intervals (CIs) were
reported. To make comparison with other literature possible, 5- and
10-year OS from the start of treatment were additionally reported.
The difference between OS from the start and end of treatment was
small as there were no deaths or lost to follow-up during the
treatment. Time to second malignancy was not analysed due to too
few events. The association of variables with OS and PFS was
analysed using univariate and multivariate Cox proportional hazards
(CPH) models (20). The
proportional hazards assumption was tested using Schoenfeld
residuals, and it has not been violated for any of the variables in
any of the models.
The main model for OS of ABVD patients was
multivariate CPH model with variables RDI and age, allowing only
linear effects (Table II). As a
part of sensitivity analysis, RDI was included also nonlinearly
(using restricted cubic splines with three knots). The association
of RDI and age was evaluated with Pearson correlation coefficient.
BEACOPP treatment group was not analysed due to too few events. The
low number of events per variable prevented also analysis in all
patients as groups significantly differed in age and RDI which
would require too many variables in the model. As a part of
exploratory analysis, the association of other variables with OS
was additionally tested with and without age in the model (Table III). The sole purpose of the many
models in Table III was to
demonstrate the strong effect of age on OS (see section Results),
and not to build a model (this would require more independent
variables in one model and thus a much greater number of events).
All analyses were repeated also for PFS, results were similar as
for OS (see also Fig. S1, and
Tables SI and SII).
 | Table II.Multivariate model for overall
survival in ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine)
treatment group. |
Table II.
Multivariate model for overall
survival in ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine)
treatment group.
|
| ABVD group,
multivariate model |
|---|
|
|
|
|---|
| Variable | HR | 95% CI for HR | P-value |
|---|
| RDI, % | 1.01 | [0.98, 1.04] | 0.590 |
| Age, years | 1.07 | [1.03, 1.11] | 0.001a |
 | Table III.The demonstration of the effect of
age on overall survival. |
Table III.
The demonstration of the effect of
age on overall survival.
|
| All patients | ABVD group |
|---|
|
|
|
|
|---|
| Variable | P-value in
univariate model | P-value in model
controlled for age | P-value in
univariate model | P-value in model
controlled for age |
|---|
| RDI |
<0.001a | 0.898 | 0.242 | 0.590 |
| All cycles of
CTb | 0.064 | 0.979 | 0.418 | 0.868 |
| Treatment |
<0.001a | 0.146 | NA | NA |
| CCI |
<0.001a | 0.506 |
<0.001a | 0.726 |
| Clinical stage
IV | 0.649 | 0.364 | 0.617 | 0.190 |
| Gender | 0.733 | 0.105 | 0.957 | 0.275 |
| PS ≥2 | 0.014a | 0.340 | 0.224 | 0.420 |
| Age |
<0.001a | NA |
<0.001a | NA |
P<0.05 was considered as statistically
significant. All analyses were performed using R statistical
software, version 3.6.3 (21).
Results
Patients' characteristics
Between May 2004 and December 2013, 114 patients
received treatment for advanced HL and were enrolled for
evaluation. Patients' and their disease characteristics are
presented in Table I. The median
age of patients was 39 years, majority of patients were males and
had stage IV disease. Patients in the BEACOPP group were
significantly younger, with less comorbidities, better performance
status and they received a higher RDI (all *P<0.05, Table I).
There were 28 deaths (24.6%), 24 (44.4%) in ABVD and
4 (6.7%) in BEACOPP group. Relapse occurred in 15 (13.2%) patients,
12 (22.2%) in ABVD and 3 (5%) in BEACOPP group. Median follow-up
time was 8.0 years. OS and PFS from the end of treatment are
presented on the left panels of Figs.
1 and S1. Five-year OS was
84.2% [95% CI: (77.8, 91.2%)], 10-year OS 74.1% [95% CI: (66.0,
83.2%)], 5-year PFS 78.9% [95% CI: (71.8, 86.8%)], and 10-year PFS
was 68.9% [95% CI: (60.3, 78.9%)]. Additionally, 5- and 10-year OS
from the start of treatment was 87.7% [95% CI: (81.9, 94.0%)] and
73.7% [95% CI: (65.5, 83.0%)], respectively. There were only 7
(6.1%) secondary malignancies, 4 (7.4%) in ABVD and 3 (5%) in
BEACOPP group, which prevented further analysis of this event.
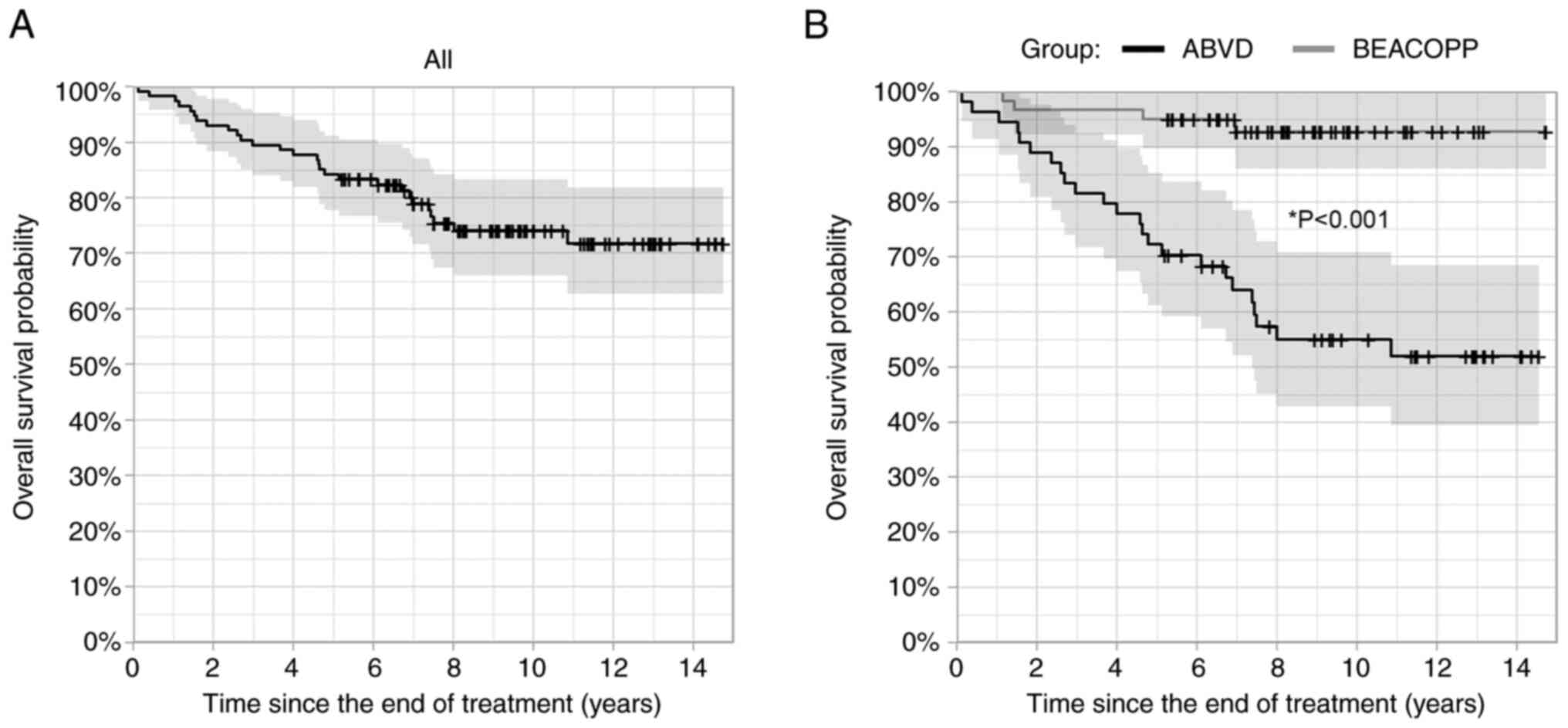 | Figure 1.Overall survival of (A) all patients
with Hodgkin lymphoma, and (B) for ABVD (doxorubicin, bleomycin,
vinblastine, dacarbazine) and BEACOPP (bleomycin, etoposide,
doxorubicin, cyclophosphamide, vincristine, procarbazine,
prednisone) treatment groups separately (Kaplan-Meier method).
Shaded areas represent 95% confidence intervals, censoring times
are marked with crosses. *P<0.05 BEACOPP vs. ABVD group (Cox
proportional hazards model). ABVD, doxorubicin, bleomycin,
vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide,
doxorubicin, cyclophosphamide, vincristine, procarbazine,
prednisone. |
Fifty-four patients received ABVD and 60 received
BEACOPP chemotherapy. Median RDI in ABVD and BEACOPP group was 82.3
and 95.9%, respectively, in addition the interquartile range was
narrower for the BEACOPP group (Table
I). Eighty-one % of patients in ABVD group and 93% in BEACOPP
group received all planned cycles of chemotherapy (Table IV). Approximately one third of
patients received radiation therapy as consolidation.
 | Table IV.Average RDI in ABVD and BEACOPP
groups. |
Table IV.
Average RDI in ABVD and BEACOPP
groups.
| Planned treatment
(number of cycles) | Actual treatment,
number of cycles | Number of
patients | Average RDI, % |
|---|
| 8× ABVD | 8× ABVD | 44 | 81.2 |
|
| 7.5× ABVD | 3 | 78.7 |
|
| 7× ABVD | 3 | 73.6 |
|
| 6× ABVD | 3 | 55.6 |
|
| 5.5× ABVD | 1 | 52.7 |
| 4× eBEACOPP + 4×
rBEACOPP | 4× eBEACOPP + 4×
rBEACOPP | 42 | 94.3 |
|
| 4× eBEACOPP + 3×
rBEACOPP | 1 | 87.5 |
| 6× eBEACOPP | 6× eBEACOPP | 14 | 96.8 |
|
| 5× eBEACOPP + 1×
rBEACOPP | 1 | 85.4 |
|
| 4× eBEACOPP + 2×
rBEACOPP | 1 | 83.9 |
|
| 3× eBEACOPP + 3×
rBEACOPP | 1 | 76.5 |
Dose de-escalation for the escalated BEACOPP
chemotherapy follows a predefined scheme, which is determined by
the occurrence of toxic events in the previous cycles, such as
leukopenia, thrombocytopenia and other toxicities (22). Treatment always begins at dose
level 4, which is later reduced as necessary to level 1, before
regular BEACOPP is used. In BEACOPP group, the majority of RDI
reductions was a consequence of reduced cytostatic doses according
to de-escalation protocol, whereas in ABVD it was mostly caused by
non-protocol dose reductions and treatment delays.
Overall survival, PFS and their
association with RDI
Overall survival and PFS were significantly better
in the BEACOPP group compared to ABVD group (both *P<0.05,
Figs. 1 and S1). However, direct comparison between
the two groups is not reasonable, because the groups differed
markedly, especially in terms of age and CCI. Median age in the
ABVD group was 59.8 years, whereas it was only 32.9 years in the
BEACOPP group. Furthermore, 27 patients in ABVD group were older
than 60 years while none was in the BEACOPP group. Likewise, the
median CCI was 4 in ABVD and 2 in BEACOPP group, respectively.
Twenty-eight % of patients in ABVD group and only 2% in BEACOPP
group had a CCI of more than 5, for which the estimated 10-year
survival is 2% or lower (18).
The different patients' characteristics in treatment
groups led to a separate analysis of the ABVD group. The low number
of events prevented further analysis in the BEACOPP group. In ABVD
group, RDI was not significantly associated with OS (P=0.590) or
PFS (P=0.354) in a multivariate model where age was controlled (see
Table II for OS and Table SI for PFS). As a part of
sensitivity analysis, we included RDI in models for OS and PFS also
nonlinearly, the effect of RDI remained non-significant (P=0.436
for OS, P=0.434 for PFS). This could be explained with a strong
negative correlation between the RDI and age (Fig. 2). Pearson correlation coefficient
was-0.61 for all patients and −0.45 for ABVD treatment group,
indicating that patients with higher age received a lower RDI.
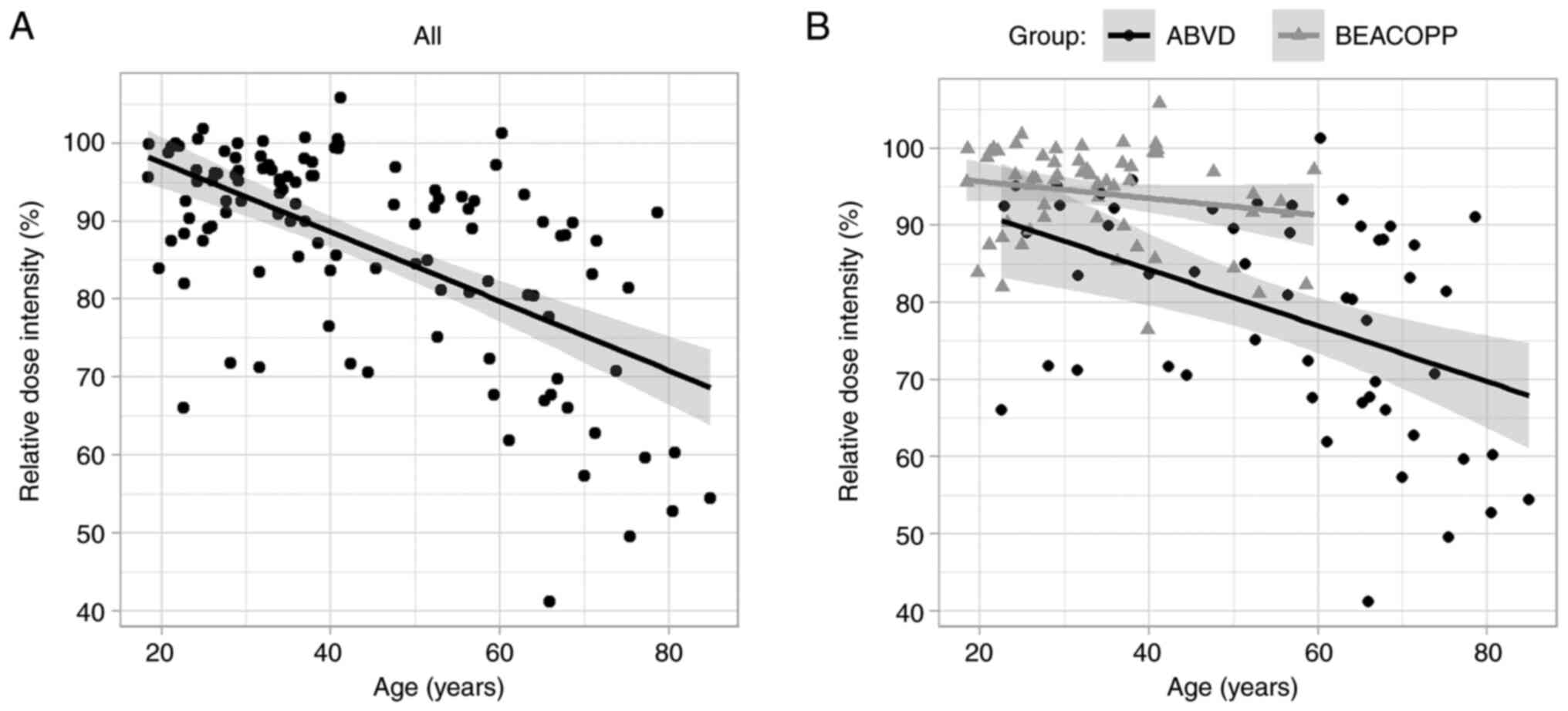 | Figure 2.Correlation between relative dose
intensity and age (A) for all patients with Hodgkin lymphoma, and
(B) for ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) and
BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide,
vincristine, procarbazine, prednisone) treatment groups separately.
Shaded areas represent 95% confidence intervals around regression
lines. ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine;
BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide,
vincristine, procarbazine, prednisone. |
Exploratory analysis showed that patients' age was
strongly associated with both OS and PFS. To illustrate this, the
association of other variables with OS (Table III) and PFS (Table SII, similar results) was
additionally tested with and without age in the model. As a part of
univariate model, the chemotherapy regimen, as well as CCI, RDI and
performance status at least 2 were statistically significant
predictors of OS (*P<0.05) in all patients combined. Noteworthy,
none of these characteristics remained a statistically significant
predictor in the multivariate model where age was controlled. In
the ABVD group, only the CCI was a statistically significant
predictor of OS (*P<0.05) in the univariate model. Similarly,
CCI lost its effect in the multivariate analysis in the ABVD group,
after age was controlled. Based on our observations, we can
conclude that RDI is not associated with the OS or PFS after the
age is controlled, neither in all patients combined nor in ABVD
group.
Discussion
The ABVD and the BEACOPP represent the standard of
care for patients with advanced HL (13,23).
The aim of this study was to assess the association of RDI with the
outcome of advanced HL patients, thus receiving either ABVD or
BEACOPP regimen. Multiple works have reported an association
between the RDI and survival prognosis, especially in breast cancer
and aggressive lymphoma (17,24–26).
However, only a few studies have addressed this issue in HL.
The results of present analysis indicate that there
is no evidence that a higher RDI results in better prognosis of HL
patients. These findings are in concordance with the key study
published on this topic on 380 patients by Owadally et al,
who also found no clear evidence that DI influences the outcome
(27). However, in the study of
Owadally et al the DI was measured only in first two cycles
of ABVD chemotherapy, whereas in our study the RDI was measured
throughout the whole treatment with either ABVD or BEACOPP, the
treatments lasting from 6 to 8 cycles. It is worth noting that it
is especially hard to maintain high DI in the last cycles of
treatment, particularly on account of accumulated toxicities and
complications from previous cycles. The median RDI in the study of
Owadally et al was 89% with the lower and upper quartiles of
79 and 97%, respectively, whereas we found a median RDI of 82.3%
and the lower and upper quartiles of 68.2 and 89.9% for the ABVD
group, respectively. Patients included by Owadally et al
were younger, with a median age of 36 years, having both early and
advanced stages of HL, while our patients in the ABVD group were
characterized with advanced stage disease and a median age of 59.8
years. Therefore, the calculation of RDI for all 8 cycles of ABVD,
the older age of patients and the advanced stage HL in all patients
might explain the difference in lower RDI achieved in our
study.
Similar conclusions were also drawn by Raida et
al (28). Likewise, they found
no influence of primary chemotherapy DI on the probability of
complete remission, disease relapse, event-free survival and OS.
They included 194 heterogeneous patients with predominantly early
HL (63.4%), who had the median age of 28 years and have received
diverse chemotherapeutic regimes, including ABVD, BEACOPP, and
Stanford V among others. In the study of Raida et al, the
median RDI was not reported, however 76.3% of patients received a
RDI of 90% or more, which is considerably higher than 51.8% of
patients with the RDI of 90% or more achieved in our study for both
chemotherapy groups combined. Again, the reason for the difference
in the attained RDI is most likely due to the significantly older
patient population and more intensive chemotherapy regimens in our
study. As shown in Results, because of a strong negative
correlation between the RDI and age, we can assume that patients
with higher age achieve a lower RDI.
Landgren et al evaluated the effect of RDI on
prognosis of 88 elderly (>60 years) HL patients, though the
final study cohort consisted of 59 patients only (29). Unlike our study and previous two
studies, Landgren et al reported a significantly better OS
in patients with the RDI >65% compared to those with the RDI
≤65%, despite the relatively low number of enrolled patients. It is
worth noting that RDI was not controlled for age in a multivariate
model, which might explain the association with OS. Similar to the
report of Owadally et al the calculated RDI values were
based on the initial two cycles of chemotherapy only. Patients
included by Landgren et al had various stages of HL, the
majority (69.3%) of them being advanced stage, they also received
five different chemotherapy protocols. We agree with Raida et
al that the RDI of ≤65% suggested by Landgren et al may
be considered as a significant violation of primary chemotherapy
protocol, and is as such not appropriate to arbitrary divide the
patients with good or bad prognosis.
Our study has several limitations that merit
consideration. The major limitation is the modest sample size and
the difference in patients' characteristics between the ABVD and
BEACOPP groups. The ABVD group was considerably older and had more
comorbidities, therefore we cannot compare the two groups directly.
Moreover, too few events prevented the analysis of RDI in BEACOPP
group. Additionally, the RDI analyses performed in our study were
retrospective in design, therefore only hypotheses about possible
association can be formulated.
The available evidence suggests that small dose
reductions or short delays between chemotherapy cycles, which still
result in a decreased RDI, may not affect overall outcomes of HL
patients, most likely due to a relatively good prognosis and
chemosensitivity of this disease. To our knowledge, this is the
first study to evaluate the impact of RDI throughout whole
treatment in patients with advanced HL treated exclusively with
ABVD or BEACOPP chemotherapy. The lack of association between the
RDI and response to treatment is in concordance with the current
literature. However, in order to fully elucidate the relationship
between the RDI and response, a prospective trial with a larger
number of patients would be required.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research work was partially supported by a grant from the
Slovenian Research Agency (Program P3-0321).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SR contributed to the conception of the study. SR
and BJN confirm the authenticity of all the raw data. SR, BJN and
SN designed the study and analysed the clinical data. NRG performed
statistical analysis. All authors contributed to the acquisition,
analysis or interpretation of data for this article and drafts of
the article. All authors participated in writing the manuscript and
approved the final version of it. All authors were involved in
revising the paper critically for intellectual content and gave
final approval for the submission of the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board at the Institute of Oncology Ljubljana (approval number
KSOPKR/72) and National Medical Ethics Committee of Republic of
Slovenia (approval number 0120-481/2017/5). Because of
retrospective design of the study, informed consents were waived by
the National Medical Ethics Committee of Republic of Slovenia.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shanbhag S and Ambinder RF: Hodgkin
lymphoma: A review and update on recent progress. CA Cancer J Clin.
68:116–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al:
SEER cancer statistics review, 1975–2017, https://seer.cancer.gov/csr/1975_2017/simplehttps://seer.cancer.gov/csr/1975_2017/,
based on November 2019 SEER data submission, posted to the SEER web
site. National Cancer Institute; Bethesda, MD: 2020
|
|
3
|
Evens AM, Antillón M, Aschebrook-Kilfoy B
and Chiu BC: Racial disparities in Hodgkin's lymphoma: A
comprehensive population-based analysis. Ann Oncol. 23:2128–2137.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zadnik V, Primic Zakelj M, Lokar K, Jarm
K, Ivanus U and Zagar T: Cancer burden in Slovenia with the time
trends analysis. Radiol Oncol. 51:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia and Lymphoma Group and Eastern Cooperative Oncology
Group, ; et al: Recommendations for initial evaluation, staging,
and response assessment of Hodgkin and non-Hodgkin lymphoma: The
Lugano classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eichenauer DA, Aleman BMP, André M,
Federico M, Hutchings M, Illidge T, Engert A and Ladeto M; ESMO
Guidelines Committee, : Hodgkin lymphoma: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl 4):iv19–iv29. 2018. View Article : Google Scholar
|
|
7
|
Engert A, Plütschow A, Eich HT, Lohri A,
Dörken B, Borchmann P, Berger B, Greil R, Willborn KC, Wilhelm M,
et al: Reduced treatment intensity in patients with early-stage
Hodgkin's lymphoma. N Engl J Med. 363:640–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Specht L, Yahalom J, Illidge T, Berthelsen
AK, Constine LS, Eich HT, Girinsky T, Hoppe RT, Mauch P, Mikhaeel
NG, et al: Modern radiation therapy for Hodgkin lymphoma: Field and
dose guidelines from the international lymphoma radiation oncology
group (ILROG). Int J Radiat Oncol Biol Phys. 89:854–862. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fermé C, Eghbali H, Meerwaldt JH, Rieux C,
Bosq J, Berger F, Girinsky T, Brice P, van't Veer MB, Walewski JA,
et al: Chemotherapy plus involved-field radiation in early-stage
Hodgkin's disease. N Engl J Med. 357:1916–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Canellos GP, Niedzwiecki D and Johnson JL:
Long-term follow-up of survival in Hodgkin's lymphoma. N Engl J
Med. 361:2390–2391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engert A, Haverkamp H, Kobe C, Markova J,
Renner C, Ho A, Zijlstra J, Král Z, Fuchs M, Hallek M, et al:
Reduced-intensity chemotherapy and PET-guided radiotherapy in
patients with advanced stage Hodgkin's lymphoma (HD15 trial): A
randomised, open-label, phase 3 non-inferiority trial. Lancet.
379:1791–1799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eich HT, Diehl V, Görgen H, Pabst T,
Markova J, Debus J, Ho A, Dörken B, Rank A, Grosu AL, et al:
Intensified chemotherapy and dose-reduced involved-field
radiotherapy in patients with early unfavorable Hodgkin's lymphoma:
Final analysis of the German Hodgkin study group HD11 trial. J Clin
Oncol. 28:4199–4206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Canellos GP, Anderson JR, Propert KJ,
Nissen N, Cooper MR, Henderson ES, Green MR, Gottlieb A and
Peterson BA: Chemotherapy of advanced Hodgkin's disease with MOPP,
ABVD, or MOPP alternating with ABVD. N Engl J Med. 327:1478–1484.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Connors JM, Jurczak W, Straus DJ, Ansell
SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illés A, Picardi M,
et al: Brentuximab vedotin with chemotherapy for stage III or IV
Hodgkin's lymphoma. N Engl J Med. 378:331–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skoetz N, Will A, Monsef I, Brillant C,
Engert A and von Tresckow B: Comparison of first-line chemotherapy
including escalated BEACOPP versus chemotherapy including ABVD for
people with early unfavourable or advanced stage Hodgkin lymphoma.
Cochrane Database Syst Rev. 5:CD0079412017.PubMed/NCBI
|
|
16
|
Hryniuk WM and Goodyear M: The calculation
of received dose intensity. J Clin Oncol. 8:1935–1937. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wildiers H and Reiser M: Relative dose
intensity of chemotherapy and its impact on outcomes in patients
with early breast cancer or aggressive lymphoma. Crit Rev Oncol
Hematol. 77:221–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
20
|
Cox DR: Regression models and life tables.
J R Stat Soc Ser B (Methodological). 34:187–220. 1972.
|
|
21
|
R Core Team R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing. (Vienna, Austria). 2021.Available from. https://www.R-project.org/
|
|
22
|
Borchmann P, Goergen H, Kobe C, Lohri A,
Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J,
Feuring-Buske M, et al: PET-guided treatment in patients with
advanced-stage Hodgkin's lymphoma (HD18): Final results of an
open-label, international, randomised phase 3 trial by the German
Hodgkin study group. Lancet. 390:2790–2802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dann EJ, Bar-Shalom R, Tamir A, Haim N,
Ben-Shachar M, Avivi I, Zuckerman T, Kirschbaum M, Goor O, Libster
D, et al: Risk-adapted BEACOPP regimen can reduce the cumulative
dose of chemotherapy for standard and high-risk Hodgkin lymphoma
with no impairment of outcome. Blood. 109:905–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gregory SA and Trümper L: Chemotherapy
dose intensity in non-Hodgkin's lymphoma: Is dose intensity an
emerging paradigm for better outcomes? Ann Oncol. 16:1413–1424.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi H, Hirakawa T and Inokuchi K:
Importance of relative dose intensity in chemotherapy for diffuse
large B-cell lymphoma. J Clin Exp Hematop. 51:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutiérrez A, Bento L, Bautista-Gili AM,
Garcia F, Martinez-Serra J, Sanchez B, Martorell C, Gines J, Garcia
L, Gimeno E, et al: Differential impact of relative dose-intensity
reductions in diffuse large B-cell lymphoma treated with R-CHOP21
or R-CHOP14. PLoS One. 10:e01239782015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Owadally WS, Sydes MR, Radford JA, Hancock
BW, Cullen MH, Stenning SP and Johnson PW: Initial dose intensity
has limited impact on the outcome of ABVD chemotherapy for advanced
Hodgkin lymphoma (HL): Data from UKLG LY09 (ISRCTN97144519). Ann
Oncol. 21:568–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raida L, Papajik T, Rusinakova Z,
Prochazka V, Faber E, Cahova D, Tucek P and Indrak K: Reduced
relative dose intensity of primary chemotherapy does not influence
prognosis of patients with Hodgkin lymphoma. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 158:428–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Landgren O, Algernon C, Axdorph U, Nilsson
B, Wedelin C, Porwit-MacDonald A, Grimfors G and Björkholm M:
Hodgkin's lymphoma in the elderly with special reference to type
and intensity of chemotherapy in relation to prognosis.
Haematologica. 88:438–444. 2003.PubMed/NCBI
|
















