Introduction
Although the development of therapeutic agents has
improved the treatment outcomes of patients with colorectal cancer
(CRC) over the years, CRC is still a leading cause of
cancer-associated mortality worldwide (1). Despite the impact of targeted agents
on the survival of patients with CRC, limitations of current
treatments have led investigators to further explore novel
treatment agents.
Phloretin, a dihydrochalcone flavonoid, is an apple
polyphenol that exerts anti-inflammatory, antioxidant and
anticancer effects (2,3). Notably, several studies have reported
the anti-neoplastic role of phloretin in various cancer cells,
including gastric cancer, prostate cancer, cervical cancer and CRC
cells (4–8). A previous study reported that its
inhibitory effect on CRC was mediated by inducing apoptosis through
elevation of BAX expression and cleavage of caspases-8, −9, −7 and
−3 (8). Another study reported
that phloretin inhibited proliferation of COLO 205 colon cancer
cells by cell cycle arrest in a p53-dependent manner, accompanied
by suppression of glucose transporter activities (7).
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL), a member of the tumor necrosis factor superfamily,
induces apoptosis in cancer cells via a TRAIL-induced signaling
pathway. The TRAIL signaling pathway is initiated by binding of
trimeric TRAIL to TRAIL-receptors (TRAIL-Rs), leading to formation
of the death-inducing signaling complex, which subsequently
activates pro-caspase 8. Activated caspase 8/10 is released and
cleaves BH3 interacting domain death agonist (Bid) and caspase 3.
Truncated Bid (tBid) then translocates to mitochondria to activate
BAX and Bcl-2 homologous antagonist/killer, releasing cytochrome
c (9). The released
cytochrome c, apoptotic protease-activating factor 1 and
pro-caspase 9 assemble to form the apoptosome, and subsequent
activation of caspase 9 leads to apoptosis by enhancing caspase-3
cleavage (9). Apoptosis is
modulated by interactions between subfamilies of the Bcl-2 family.
MCL1 apoptosis regulator BCL2 family member (Mcl-1), a member of
the pro-survival subfamily of the Bcl-2 family, is known to serve a
role in protecting cells from cell death through interaction with
tBid (10).
Several previous studies have demonstrated the
central role of TRAIL-TRAIL-R signaling in the process of
tumorigenesis. In vivo mouse models of various malignant
tumors have revealed that Trail deficiency promotes tumor
development, growth and metastasis in lymphoma, sarcoma and breast
cancer (11–13). Inhibitory effects of TRAIL
signaling on the growth of various human cancer types and efforts
to introduce TRAIL-R agonists as therapeutic agents for malignant
tumors have also been reported in several studies (14–16).
In addition to its anticancer effects, TRAIL-mediated apoptosis
causes little or no harm to normal cells (17). Therefore, attempts have been made
to investigate the mechanisms by which it suppresses malignant
tumors, including colon cancer. A previous study suggested that the
inhibitory mechanisms of TRAIL in colon cancer included promotion
of apoptosis and lymphocyte infiltration, as well as the inhibition
of invasion and migration (14).
To identify a successful cancer treatment method using TRAIL-R
agonists, a variety of chemical compounds or natural products have
been evaluated for their efficacies and mechanisms in sensitizing
cancer cells to TRAIL-induced apoptosis (18–20).
Kim et al (18) reported
that sea cucumber enhanced TRAIL-mediated apoptosis by increasing
proteasomal degradation of X-linked inhibitor of apoptosis protein
(XIAP) and activating endoplasmic reticulum stress in CRC. Another
study reported that icariin, a chemical compound classified as a
prenylated flavonol glycoside, sensitized colon cancer cells to
TRAIL-induced apoptosis through reactive oxygen species-, ERK- and
CCAAT enhancer-binding protein homologous protein-mediated
modulation of death receptor (DR)-4 and −5 expression (19).
Despite efforts to clarify the inhibitory mechanism
of phloretin in colon cancer, to the best of our knowledge, the
association between phloretin and TRAIL-induced apoptosis has not
been reported yet. Given that one anticancer mechanism of phloretin
in CRC is the induction of apoptosis (17,18),
the present study investigated possible synergistic effects of
phloretin on TRAIL-induced apoptosis in CRC. The present study
provided information on the mechanism by which phloretin exerts
inhibitory effects on CRC using human colon cancer cell lines.
Materials and methods
Cell lines
DLD-1 (ATCC® CCL-221™) and
HCT116 (ATCC® CCL-247™) human CRC cell lines
were purchased from the American Type Culture Collection. SNU283
cells (KCLB no. 00283) were obtained from the Korean Cell Line
Bank; Korean Cell Line Research Foundation. HT-29-Luc cells
(JCRB1383) were purchased from the Japanese Collection of Research
Bioresources Cell Bank. Cells were cultured as monolayers in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) or in
Eagle's minimum essential medium (American Type Culture Collection)
with 10% FBS (HyClone; Cytiva) and antibiotic-antimycotic (X100)
(cat. no. CA002-010; GenDEPOT, LLC). The CCD18-Co human normal
colon cell line (ATCC® CRL-1459™), WI-38
human lung fibroblast cell line (ATCC CCL-75™) and VERO
monkey kidney epithelial cell line (ATCC CCL-81™) were
purchased from American Type Culture Collection. All cells were
cultured at 37°C in a humidified chamber with 5%
CO2.
Reagents and antibodies
Phloretin was purchased from Merck KGaA (cat. no.
P7912). Recombinant human TRAIL protein was purchased from Merck
KGaA (cat. no. 310-04). Anti-cleaved (c-)caspase-9 (rabbit
anti-mouse polyclonal; cat. no. 9509), anti-c-caspase-8 (rabbit
anti-human monoclonal; cat. no. 9496), anti-c-caspase-3 (rabbit
anti-human monoclonal; cat. no. 9664), anti-Bid (rabbit anti-human
polyclonal; cat. no. 2002), anti-Bcl-2-like protein 11 (rabbit
anti-human polyclonal; cat. no. 2819), anti-XIAP (rabbit anti-human
polyclonal; cat. no. 2042), anti-Mcl-1 (rabbit anti-human
polyclonal; cat. no. 4572), anti-Survivin (rabbit anti-human
monoclonal; cat. no. 2808), anti-p53-upregulated modulator of
apoptosis (rabbit anti-human polyclonal; cat. no. 4976) and
anti-c-poly(ADP-ribose) polymerase (PARP) (rabbit anti-human
polyclonal; cat. no. 9541) were purchased from Cell Signaling
Technology, Inc. Anti-ubiquitin (Ub; mouse anti-bovine monoclonal;
cat. no. sc-53509), anti-Bcl-2 (mouse anti-human monoclonal; cat.
no. sc-509), anti-Bax (mouse anti-mouse monoclonal; cat. no.
sc-7480), anti-DR4 (goat anti-human polyclonal; cat. no. sc-6823),
anti-DR5 (mouse anti-human monoclonal; cat. no. sc-166624) and
anti-Bcl-xl (rabbit anti-human polyclonal; cat. no. sc-7195) and
protein-G PLUS-agarose (cat. no. sc-2002) were purchased from Santa
Cruz Biotechnology, Inc. Anti-β-actin (mouse monoclonal; dilution,
1:5,000; cat. no. A5316) was purchased from MilliporeSigma.
Anti-mouse secondary antibody (170–6516) conjugated to HRP was
purchased from Bio-Rad Laboratories, Inc. Anti-rabbit IgG-linked
HRP (cat. no. 7074S) was purchased from Cell Signaling Technology,
Inc.
Survival assay
Cells from four colon cancer cell lines and one
normal colon cell line were seeded (1×104/well) into
96-well plates (cat. no. 31020; SPL Life Sciences) and treated with
phloretin (5 µM), TRAIL (10 ng/ml), or a combination of phloretin
and TRAIL at 37°C for 24 h. Survival was examined using MTT (cat.
no. M5655; MilliporeSigma). The treated cells were incubated with
50 µl MTT solution (1 mg/ml) at 37°C for 4 h. The purple formazan
formed was dissolved with 200 µl dimethyl sulfoxide. Absorbance at
595 nm was measured using ELISA spectroscopy.
Colony formation assay
HT-29-Luc cells were seeded into a 6-well plate at a
density of 500 cells per well and treated with phloretin (5 µM),
TRAIL (10 ng/ml), or both phloretin and TRAIL. Cells were then
incubated at 37°C for 1 week. The medium was changed every 3 days.
After 1 week, cells were washed with PBS and fixed with 4%
paraformaldehyde at 25°C for 30 min, followed by staining with
crystal violet at 25°C for 30 min. Colonies (>0.1 mm) were then
counted and visualized (Image J version 1.5.2; National Institutes
of Health).
In vitro bioluminescent assay
HT-29-Luc cells were seeded in triplicate into a
6-well plate (1 ml/well) at a concentration of 1×105
cells/well. Cells were then incubated for 6 h under standard
conditions before the addition of 150 µg/ml D-luciferin (cat. no.
#7903; BioVision, Inc.) The luciferase signal of live tumor cells
was detected by an immunofluorometer In Vivo Imaging System
(NightOWL II LB983; Titertek-Berthold).
Apoptosis assay
The induction of apoptosis was detected through
binding of FITC-conjugated annexin V. Briefly, Cells treated with
phloretin (5 µM), TRAIL (10 ng/ml) or both at 37°C for 24 h were
resuspended for 24 h in the binding buffer provided in the annexin
V-FITC Apoptosis Detection Kit (ApoScan kit; cat. no. LS-02-100;
BioBird). Cells were then mixed with 1.25 µl annexin V-FITC and a
5-µl solution of propidium iodide reagent. The mixture was then
incubated for 30 min at room temperature (RT) in the dark. Flow
cytometry (Navios EX; Beckman Coulter, Inc.) was performed within 1
h after staining. For apoptosis analysis, the Navios EX software
(version 2.0) provided by the manufacturer was used. For
statistical analysis, the percentage of cells in a specific gate,
which were Annexin V-FITC+ cells, was examined.
Immunoblotting assay
To prepare cell lysates, lysis buffer [containing
phosphatase inhibitor (cat no. P5726; MilliporeSigma), protease
inhibitor (cat no. P8340; MilliporeSigma) and RIPA buffer (cat no.
R0278; MilliporeSigma)] was added, and the cells were lysed by
sonication for 3 sec seven times. The suspension was then
centrifuged at 18,000 × g for 5 min at 4°C. The protein content of
the supernatant was quantified using a bicinchoninic acid assay kit
(Pierce™ BCA Protein Assay Kit; Thermo Fisher
Scientific, Inc.). Proteins (50 µg per lane) were separated on
8–12% gels using SDS-PAGE and then electroblotted onto
nitrocellulose membranes (cat. no. 10600002; Cytiva) for western
blot analysis. Skimmed milk powder (5%; cat. no. SM2010; BioPrince)
was used as a blocking buffer at 4°C for 2 h. The membranes were
then probed with primary antibodies diluted in a primary antibody
dilution buffer [0.5% BSA (cat. no. 160069; MP Biomedicals, LLC)]
with 0.1% sodium azide (cat no. S2002; MilliporeSigma) in PBS at
4°C overnight. After washing with 1X Tris-buffered saline
containing 0.1% Tween 20 (cat. no. 0777; VWR International, LLC),
the membranes were probed with specific secondary antibodies at 4°C
for 2 h and then detected with chemiluminescence kit (EZ-Western
Lumi Pico; DG-WP250; DoGenBio). The protein bands were quantified
using Image J software (version 1.5.2; National Institutes of
Health).
Immunofluorescence staining
HT-29 Luc cells were grown on glass coverslips and
fixed with 3.7% formaldehyde at RT for 15 min, followed by
permeabilization with 0.5% Triton X-100 for 15 min at RT. Cells
were then blocked at RT for 1 h with 3% BSA and probed with primary
antibodies (Mcl-1; anti-mouse monoclonal; cat. no. sc-377487;
1:200; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The cells
were washed and then incubated with FITC-conjugated secondary
antibody at 4°C (1:200; cat. no. F0257; MilliporeSigma). The nuclei
were stained with DAPI at 37°C for 15 min (cat. no. P36935;
Invitrogen; Thermo Fisher Scientific, Inc.). Cells were mounted
with VECTASHIELD mounting medium (cat. no. 101098-042; Vector
Laboratories, Inc.) and visualized using confocal microscopy
(Olympus CKX53; Olympus Corporation). The immunofluorescence
density was quantified using Image J software (version 1.5.2;
National Institutes of Health).
Reverse transcription-quantitative
PCR
Total RNA was isolated from treated cells using
TRIzol® reagent (cat. no. 15596; Thermo Fisher
Scientific, Inc.). The transcript was converted into cDNA using a
reverse transcription PCR kit according to the manufacturer's
protocol (High-Capacity cDNA Reverse Transcription kit; cat. no.
368814; Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR
was performed with an Applied Biosystems 9700 instrument using
TaqMan™ Gene Expression Master mix (cat. no. 4369016;
Applied Biosystems; Thermo Fisher Scientific, Inc.). Taqman probes
and gene-specific oligonucleotide primers (Applied Biosystems;
Thermo Fisher Scientific, Inc.) were used with the following
cycling conditions: 5 min at 95°C, followed by 35 cycles of 95°C
for 15 sec, 60°C for 30 sec and 72°C for 40 sec, and 5 min at 72°C
for a final extension. The probes used were as follows: Mcl-1
(Hs01050896_m1; Applied Biosystems; Thermo Fisher Scientific, Inc.)
and GAPDH (Hs99999905_m1; Applied Biosystems; Thermo Fisher
Scientific, Inc.). For mRNA quantification, gene expression was
normalized to GAPDH. The relative gene expression ratios were
analyzed using the 2−ΔΔCq method (21).
Transfection
HT-29-Luc cells were transfected with 2 µg human
pcDNA3.1-h Mcl-1 His-tagged plasmid (cat. no. #25375; Addgene,
Inc.) and their corresponding negative control empty plasmids using
Lipofectamine® 2000 (cat. no. 11668027; Invitrogen;
Thermo Fisher Scientific, Inc.). For transfection, the cells were
incubated at 37°C with 5% CO2 for 8 h. At 24 h
post-transfection, the cells were treated with TRAIL (10 ng/ml) and
phloretin (5 µM) at 37°C for 24 h.
Analysis of Mcl-1 protein
stability
HT-29-Luc cells were treated with phloretin (5 µM)
at 37°C for 24 h and then treated with 10 µg/ml cycloheximide (CHX;
cat no. 01810; Merck KGaA). Cells were harvested at 0, 15, 30 and
60 min after CHX treatment, and the levels of Mcl-1 and β-actin
were determined by western blotting as aforementioned.
Proteasome degradation assay
HT-29-Luc cells were treated with phloretin (5 µM)
at 37°C for 18 h and then treated with 5 µM MG132 (cat no. M8699;
Merck KGaA). The cells were harvested at 6 h after MG132 treatment,
and the levels of Mcl-1 and β-actin were determined by western
blotting as aforementioned.
Immunoprecipitation
HT-29-Luc cells were seeded into 100-mm plates and
treated with or without phloretin (5 µM) at 37°C for 24 h. The
100-mm dishes were then washed with ice-cold PBS and incubated on
ice for 5 min with 500 µl cell lysis buffer (cat no. #9803; Cell
Signaling Technology, Inc.) containing protease, phosphatase
inhibitors and 1 mM PMSF (cat. no. 10837091001; Merck KGaA). Cells
were scrape-harvested and cellular debris was removed by
centrifugation at 18,000 × g for 5 min at 4°C. The protein
concentration was determined using a BCA Protein Assay Kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Supernatants of HT-29-Luc cells (200 µg cell lysate) were incubated
with IgG and an anti-Ub antibody (anti-mouse monoclonal; cat. no.
sc-53509; Santa Cruz Biotechnology, Inc.) overnight at 4°C,
followed by addition of 50 µl protein G agarose beads (cat no.
sc-2002; Santa Cruz Biotechnology, Inc.) and incubation for 1 h at
4°C. Immunoprecipitates were washed five times with cold lysis
solution (Cell lysis buffer), separated by centrifugation at 4°C
for 30 sec at 12,000 × g, and then heated with 2X sample buffer for
SDS-PAGE and western blot analysis as aforementioned.
Animal experiments
In vivo experiments were conducted in
accordance with the guidelines approved by the Korea University
Institutional Animal Care and Use Committee (Seoul, Republic of
Korea). A total of 20 female BALB/c nude mice (weight, 14–16 g; 4
weeks old) were purchased from Orient Bio, Inc. and kept in a
specific pathogen-free environment. Mice were fed standard bottled
water and pelleted food. The temperature was maintained at 20–24°C,
with a relative humidity of 45–65% and a 12/12-h light/dark cycle.
A total of 1×107 HT-29-Luc cells resuspended in PBS were
subcutaneously injected into the right thigh of 5-week-old mice.
After 1 week, tumor bearing mice were divided into 4 groups.
Phloretin (dissolved in saline, 10 mg/kg), TRAIL (dissolved in
saline, 4 ng/ml), or a combination of phloretin and TRAIL was then
injected intraperitoneally every 2–3 days. Αt 19 days post-cell
injection, mice were sacrificed by inhalation of 30% CO2
(4.5 l/min) for 2 min in a CO2 gas chamber to measure
the weights of tumors. Tumor size was calculated at the same time
using the following formula: Length × width. Volume was calculated
as 0.5 × length × (width)2. A total of 5 mice were
examined in each treatment group. During the experiment, 1 of the 5
mice treated with phloretin and TRAIL died from an unidentified
cause. This mouse was not included in the calculation of the mean
values of weight and volume.
TUNEL staining
The tumor tissue was fixed in 4% paraformaldehyde
solution (cat. no. PC2031-100-00; Biosesang) overnight at 4°C. The
entire tumor tissue was then paraffin-embedded. TUNEL staining was
performed using the In Situ Cell Death Detection Kit, TMR
red (cat. no. 12156792910; Merck KGaA) according to the
manufacturer's instructions. Paraffin sections of tumor samples
were deparaffinized in xylene and rehydrated in a series of graded
ethanol. After the microwave antigen retrieval process for the
dehydrated sections, TUNEL reaction mixture was added and incubated
at 37°C for 1 h. The tissue sections were mounted with
ProLong™ Gold antifade reagent with DAPI (cat no. 36935;
Invitrogen; Thermo Fisher Scientific, Inc.). Stained tissue was
visualized using confocal microscopy (Olympus CKX53; Olympus
Corporation). The TUNEL-positive cells were quantified in three
random fields using Image J software (version 1.5.2; National
Institutes of Health).
Statistical analysis
GraphPad InStat 6 software (GraphPad Software, Inc.)
was used for all statistical analyses. For comparisons among
groups, one-way ANOVA followed by Tukey's multiple comparisons test
was used. Values are presented as the mean ± SD. All experiments
were performed three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Phloretin treatment is associated with
decreased survival of colon cancer cells
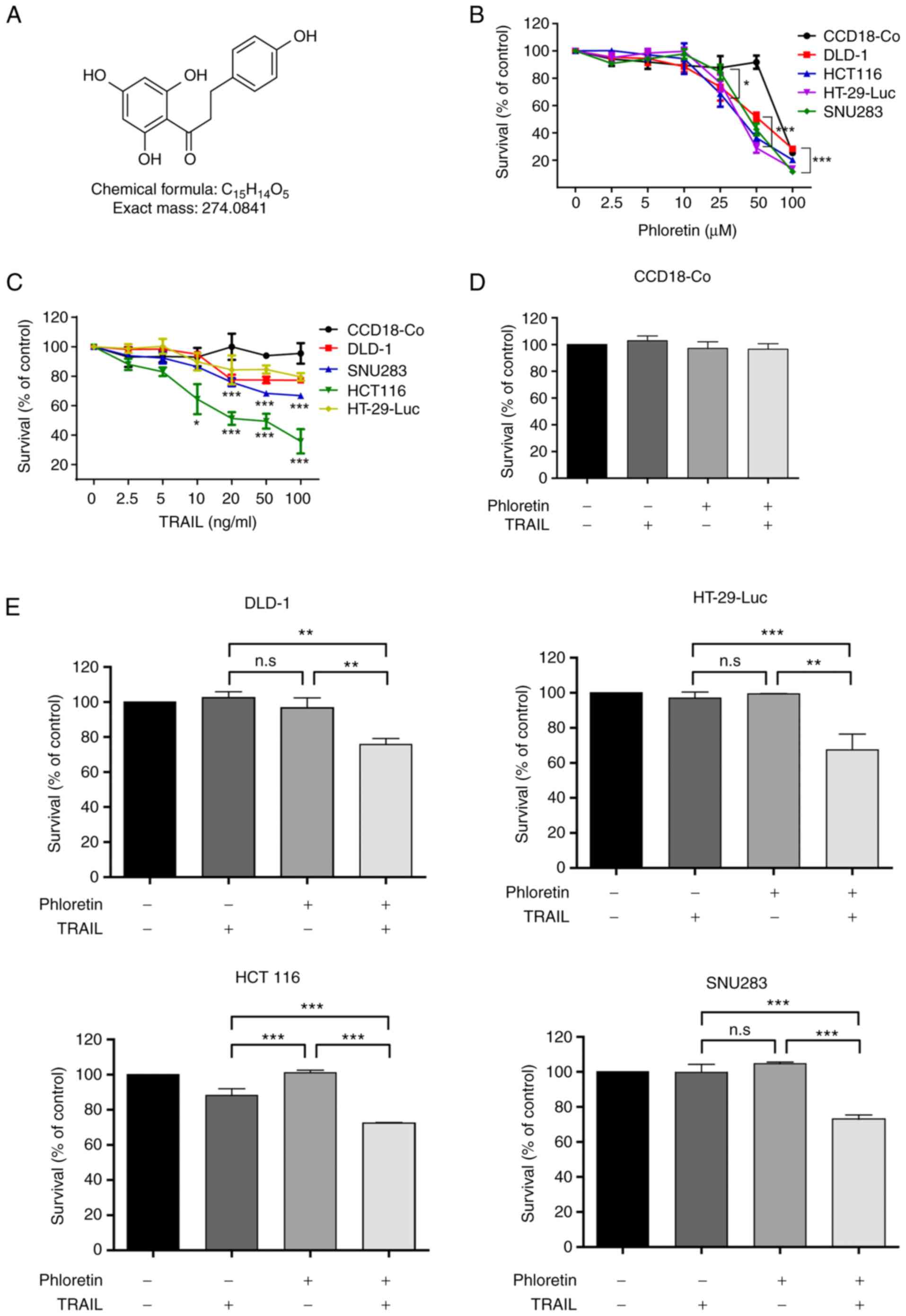
Phloretin (C15H14O5; Fig. 1A) is a plant-derived natural
product known to exert antitumor activities in several cancer cells
(22). The viability of several
colon cancer cell lines (DLD-1, HT-29-Luc, HCT116 and SNU283) was
examined after treatment with TRAIL, phloretin, or TRAIL and
phloretin using an MTT assay. Increasing concentrations of
treatment of colon cancer cells with either phloretin or TRAIL
decreased the viability compared with that of control colon cells
(CCD18-Co; Fig. 1B and C). In
order to avoid the effect of toxicity from DMSO, 5 µM was selected
as the phloretin concentration for treatment of normal colon and
colon cancer cells. As expected, survival of normal colon cells
(CCD18-Co) was not affected by treatment with phloretin (5 µM) or
TRAIL (10 ng/ml) (Fig. 1D). Other
normal cells, including the VERO (monkey kidney epithelial cells)
and WI-38 (human lung fibroblasts) cell lines, were also examined
for their sensitivities to phloretin or TRAIL. Survival of these
normal cells was not affected by treatment with phloretin or TRAIL
(Fig. S1A-C). Survival of colon
cancer cells treated with a combination of phloretin (5 µM) and
TRAIL (10 ng/ml) was suppressed compared with that of cells treated
with either phloretin or TRAIL alone (Fig. 1E).
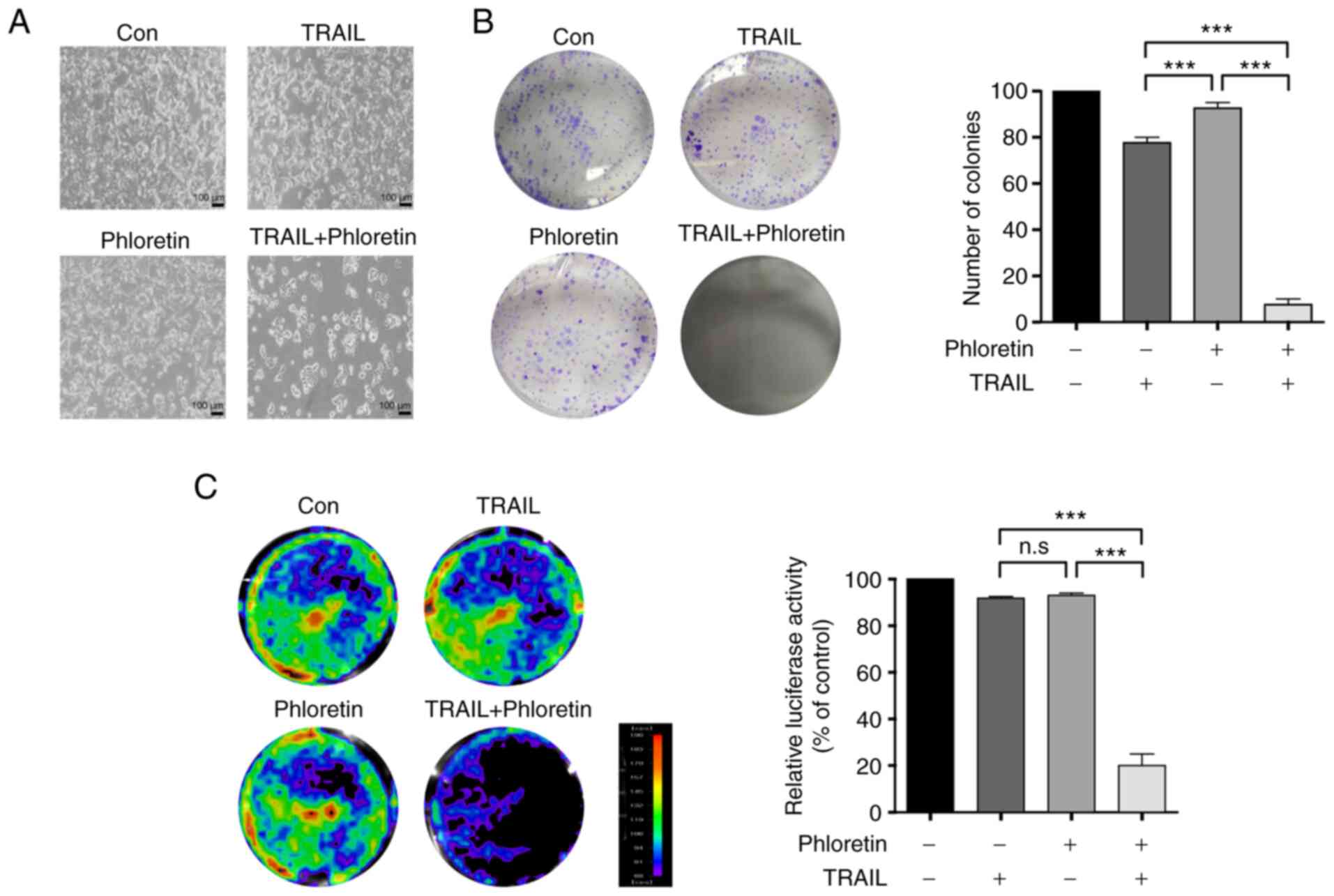
The number of colonies of HT-29-Luc cells treated
with phloretin (5 µM), TRAIL (10 ng/ml), or both phloretin and
TRAIL was examined using a colony formation assay. Treatment of
HT-29-Luc cells with phloretin and TRAIL combined decreased colony
counts compared with treatment with either phloretin (P<0.0001)
or TRAIL alone (P<0.0001) (Fig. 2A
and B).
The viability of HT-29-Luc colon cancer cells
expressing luciferase constitutively was also examined. Treatment
of HT-29-Luc cells with a combination of phloretin (5 µM) and TRAIL
(10 ng/ml) resulted in a decrease in luciferase activity compared
with treatment with either phloretin (P<0.0001) or TRAIL alone
(P<0.0001) (Fig. 2C),
suggesting a potential synergistic effect of phloretin on
TRAIL-induced apoptosis.
Enhanced TRAIL-induced apoptosis by
phloretin via downregulation of Mcl-1 expression
Subsequently, the mechanism of decreased viability
of colon cancer cells treated with phloretin and TRAIL was
evaluated. HT-29-Luc cells treated with phloretin and TRAIL
exhibited enhanced apoptosis on Annexin-V staining compared with
HT-29-Luc cells treated with either phloretin (P<0.0001) or
TRAIL (P<0.0001) (Fig. 3A).
There was no statistically significant difference between the
non-treated control cells and cells treated with either phloretin
or TRAIL. Enhanced apoptosis in combined phloretin- and
TRAIL-treated HT-29-Luc cells was accompanied by increased levels
of c-PARP as well as c-caspase-3, −8 and −9, as examined by western
blotting (Fig. 3B; Table SI). Combined treatment with
phloretin and TRAIL also induced increased levels of c-PARP and
c-caspase-3, −8 and −9 in SNU283 (Fig. S2A) and DLD-1 (Fig. S2B) colon cancer cells.
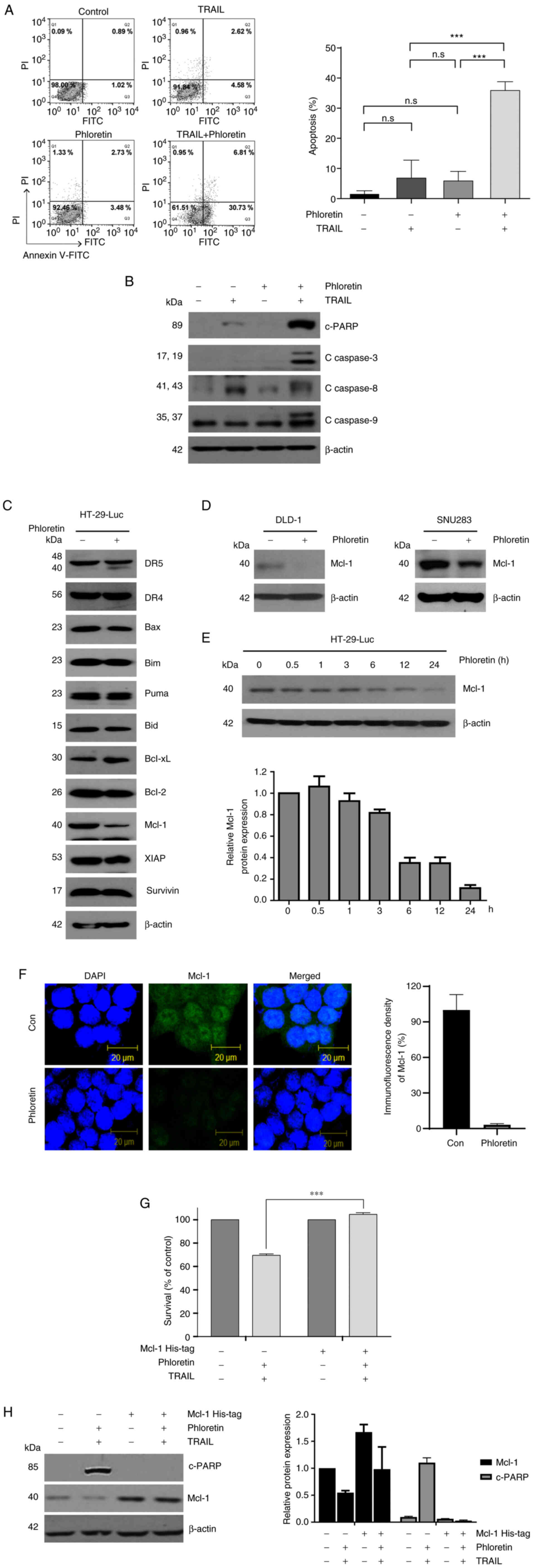 | Figure 3.Mechanism for the synergistic effect
of phloretin on TRAIL-induced apoptosis. (A) Annexin V assay
showing the induction of apoptosis was greater in phloretin- and
TRAIL-treated HT-29-Luc cells compared with either treatment alone
(***P<0.0001). (B) Increased levels of c-PARP, and c-caspase-3,
−8 and −9 in HT-29-Luc cells treated with a combination of
phloretin and TRAIL. (C) Decreased expression levels of Mcl-1 in
phloretin-treated HT-29-Luc cells. (D) Decreased expression levels
of Mcl-1 under treatment with phloretin in DLD-1 and SNU283 colon
cancer cells. (E) Time-dependent decreased expression levels of
Mcl-1 in HT-29-Luc cells after treatment with phloretin. (F)
Immunofluorescence staining showing decreased expression levels of
Mcl-1 in phloretin-treated HT-29-Luc cells. (G) Overexpression of
Mcl-1 reversed decreased survival in phloretin- and TRAIL-treated
HT-29-Luc cells (***P<0.0001). (H) Increased c-PARP expression
after treatment with both phloretin and TRAIL was reversed by
overexpression of Mcl-1 in HT-29-Luc cells. Data are presented as
the mean ± SD (n=3). Bid, BH3 interacting domain death agonist;
Bim, Bcl-2-like protein 11; c-, cleaved; Con, control; DR, death
receptor; Mcl-1, MCL1 apoptosis regulator BCL2 family member; n.s.,
non-significant; PARP, poly (ADP-ribose) polymerase; Puma,
p53-upregulated modulator of apoptosis; TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; XIAP, X-linked inhibitor
of apoptosis protein. |
The signaling cascade of the activation of caspase-3
by TRAIL was explored to examine how phloretin exerted its
synergistic effect on TRAIL (Fig.
3C). Phloretin (5 µM) decreased the expression levels of Mcl-1
in HT-29-Luc colon cancer cells leaving expression levels of other
proteins involved in apoptosis unchanged (Fig. 3C). Phloretin also suppressed
expression levels of Mcl-1 in colon cancer DLD-1 and SNU 283 cells
(Fig. 3D). A time-dependent
decrease in Mcl-1 expression due to phloretin was verified again in
colon cancer HT-29-Luc cells (Fig.
3E). Consistent with the results of western blotting, Mcl-1
expression was decreased but still faintly visible in
phloretin-treated HT-29-Luc cells in immunofluorescence staining,
although it was hard to distinguish Mcl-1 expression from the black
background (Fig. 3F). Decreased
Mcl-1 expression was also verified by semi-quantifying the results
of the immunoblotting assays (Tables
SII and SIII). Decreased
viability of HT-29-Luc cells treated with phloretin (5 µM) and
TRAIL (10 ng/ml) was reversed by overexpression of Mcl-1
(P<0.0001; Fig. 3G). The levels
of c-PARP in HT-29-Luc cells treated with phloretin (5 µM) and
TRAIL (10 ng/ml) were decreased following overexpression of Mcl-1
(Fig. 3H).
Suppression of Mcl-1 expression via
modulation of protein degradation
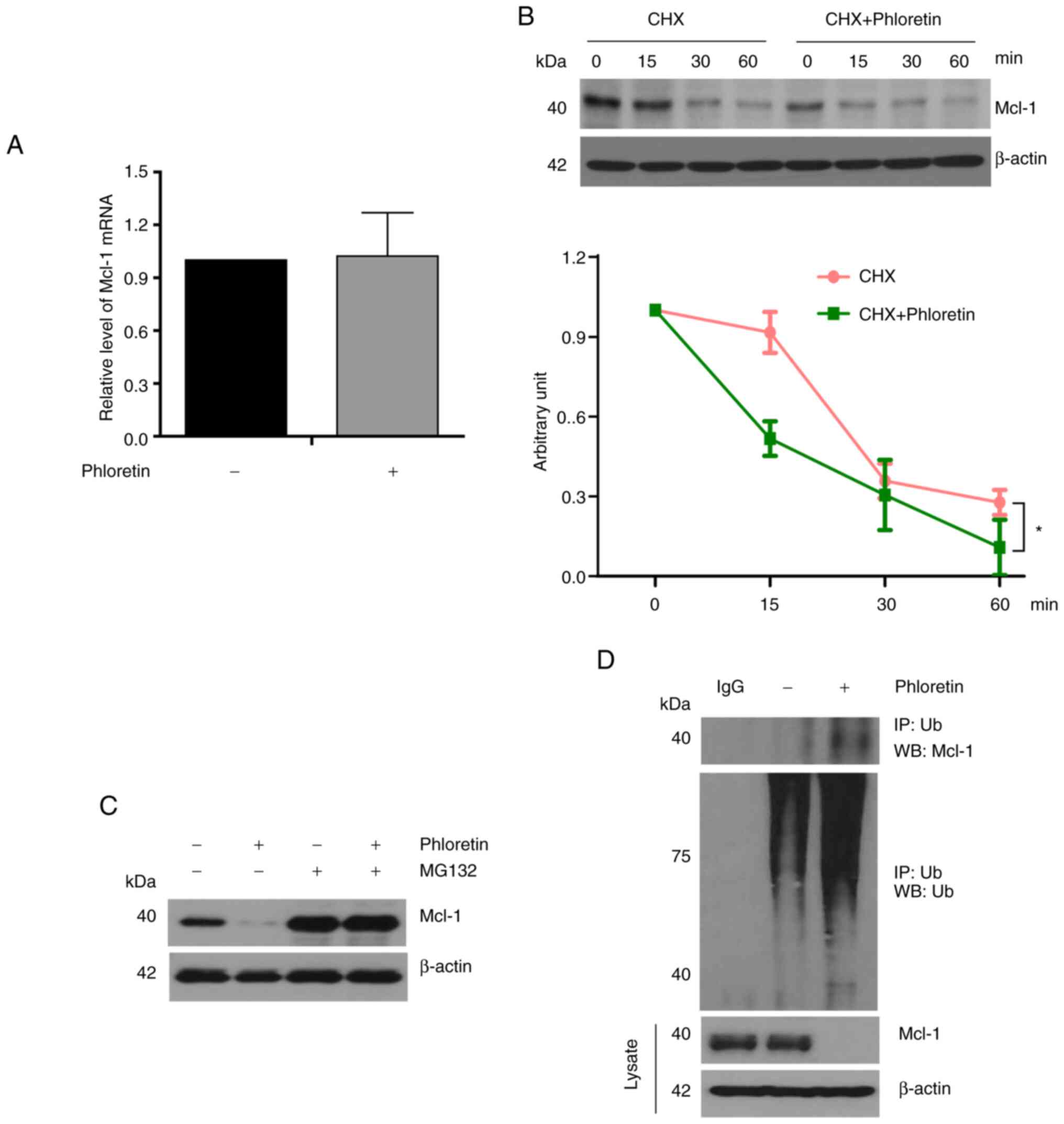
To further examine the mechanism by which phloretin
suppressed Mcl-1 expression, quantitative PCR was performed for
HT-29-Luc cells treated with or without phloretin. Notably, the
mRNA expression levels of Mcl-1 were not altered after
addition of phloretin (5 µM) in HT-29-Luc cells (Fig. 4A). Next, Mcl-1 expression was
compared between HT-29-Luc cells treated with cycloheximide (CHX)
alone and those treated with both CHX (10 µg/ml) and phloretin (5
µM). Combined treatment with CHX and phloretin suppressed Mcl-1
expression compared with treatment with CHX alone in HT-29-Luc
cells at 60 min (P<0.01; Fig.
4B). Suppression of Mcl-1 expression by phloretin was reversed
after the addition of MG132 in phloretin-treated (5 µM) HT-29-Luc
cells (Fig. 4C; Table SIV). In addition, modulation of
Mcl-1 expression by protein degradation in phloretin-treated
HT-29-Luc cells was verified again by immunoprecipitation, which
indicated binding of Mcl-1 with Ub (Fig. 4D; Table SV).
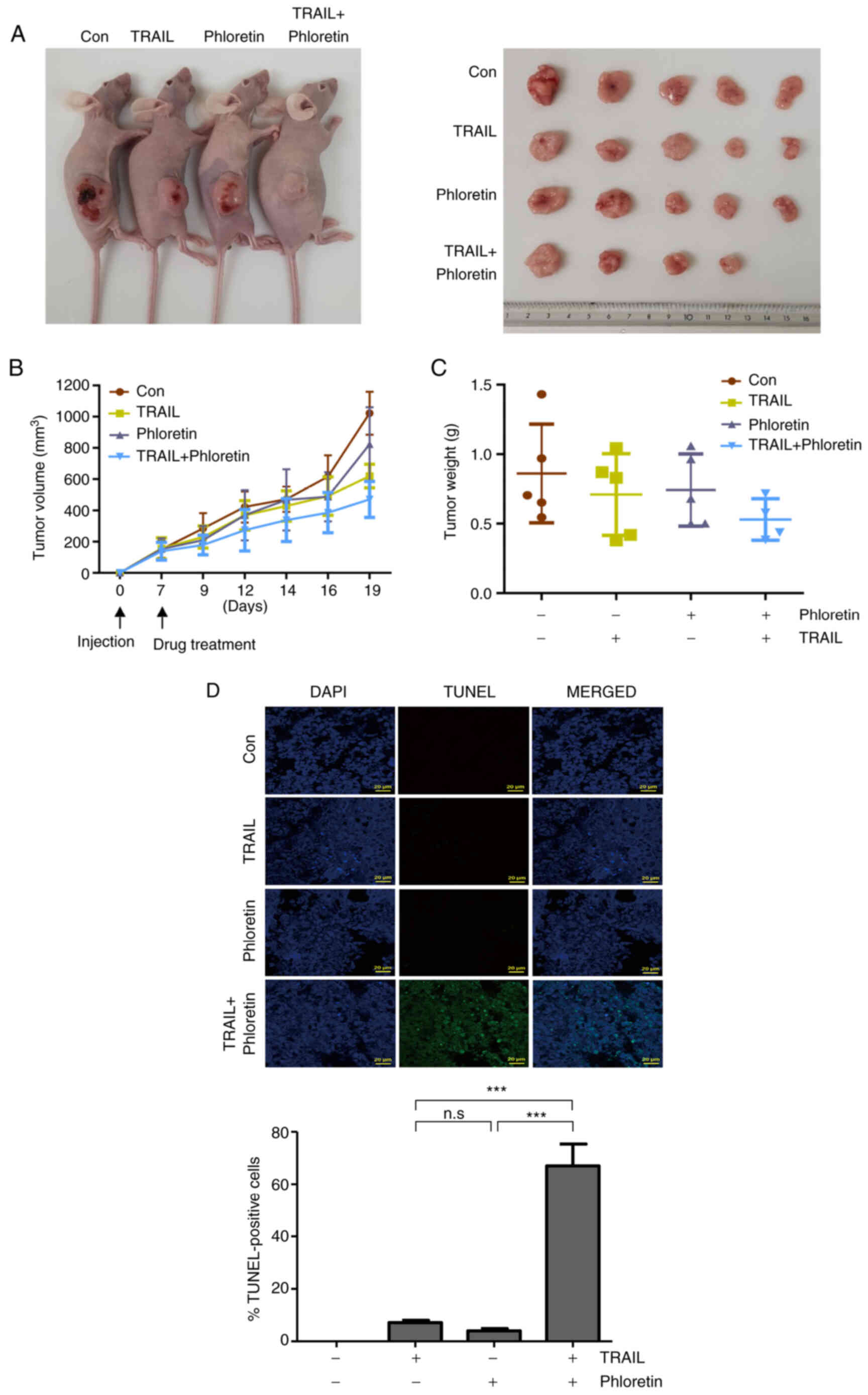
Shrinkage of tumor mass in xenografts
treated with phloretin and TRAIL
HT-29-Luc xenograft-bearing rats were
intraperitoneally injected with TRAIL (4 ng/kg), phloretin (10
mg/kg), or a combination of TRAIL and phloretin. The tumors from
mice injected with both TRAIL and phloretin were observed to shrink
more than the tumors from the mice injected with either TRAIL or
phloretin alone, confirming the synergistic effect of phloretin in
an in vivo colon cancer model (Fig. 5A). Both the volumes and weights of
the tumors were decreased most prominently in xenografts injected
with a combination of TRAIL and phloretin, although the results
were not significant (Fig. 5B and
C). The measured mean tumor volume was 470.8±115.1
mm3 in xenografts treated with both TRAIL and phloretin,
and 823.8±236.5 and 619.9±84.7 mm3 in xenografts treated
with either phloretin or TRAIL, respectively, on day 19
post-injection (Fig. 5B). The mean
tumor weight was 0.53±0.15 g in mice injected with both TRAIL and
phloretin, and 0.74±0.26 and 0.71±0.29 g in xenografts injected
with phloretin or TRAIL, respectively (Fig. 5C). The percentages of tumor weight
to mouse weight when mice were sacrificed are presented in Table SVI. The most prominent increase in
the level of apoptosis was found in tumors from mice injected with
a combination of TRAIL and phloretin, as confirmed by a TUNEL assay
(Fig. 5D).
Discussion
Although substantial numbers of clinically
applicable targeted agents have been developed along with extensive
investigation on the molecular mechanisms of tumorigenesis in
metastatic CRC (23), there still
are unmet needs for therapeutic agents in this disease. TRAIL-R
agonists may be one solution, due to their selective tumoricidal
activities that do not harm normal cells (24,25);
however, the results of clinical trials using TRAIL-R agonists have
been disappointing (9,26). Several factors might have
contributed to this failure. First, TRAIL-R agonists with
suboptimal activity were selected for clinical trials due to
concerns regarding possible toxicity. In addition, a number of
cancer types exhibit primary resistance against TRAIL-R agonists,
and there was a lack of selection of patients who are likely to
benefit from treatment with TRAIL-R agonists based on biomarker
investigation (24).
Development of TRAIL-sensitizing agents has been
suggested as a solution to overcome TRAIL resistance in cancer
cells, and several natural products to sensitize cancer to
TRAIL-induced apoptosis signaling have been explored. A recent
study reported that curcumin exerts inhibitory effects on leukemic
cells by modulating the expression of TRAIL-Rs and anti-apoptotic
proteins, leading to enhancement of TRAIL-induced apoptosis
(27). Another study reported the
antitumorigenic role of sea cucumber in CRC. Kim et al
(18) demonstrated that sea
cucumber sensitizes colon cancer cells to TRAIL-induced apoptosis
signaling by enhancing proteasomal degradation of XIAP and
activating ER stress.
The anticancer activities of phloretin, one of the
apple polyphenols, have been constantly studied in various
malignancies (4–8). Due to the various suggested
anticancer mechanisms, including enhancement of apoptosis by
phloretin, the present study examined its contribution to
TRAIL-induced apoptosis signaling as a TRAIL sensitizer. The
present results revealed a synergistic effect of phloretin on
TRAIL-induced apoptosis signaling in colon cancer cells. This
synergistic effect was exerted via activation of extrinsic and
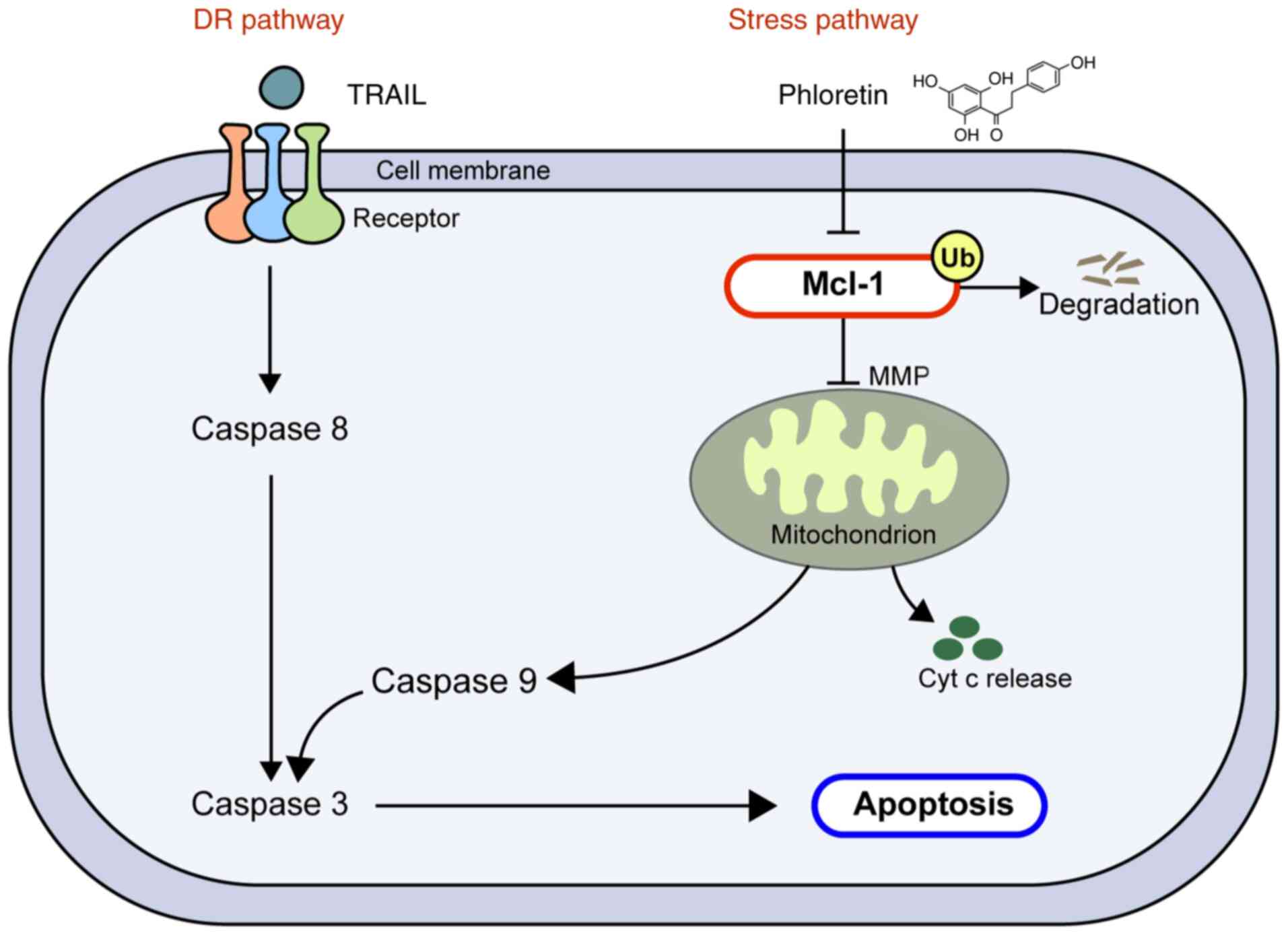
intrinsic pathways by TRAIL and phloretin, respectively. In the
present study, phloretin served a role in activating the stress
pathway by regulating the expression of Mcl-1, a key modulator to
switch the apoptosis signal on or off, without interfering with
expression of other apoptotic proteins (Fig. 6). Notably, Mcl-1 expression was
demonstrated to be modulated by Ub-mediated degradation, not by
suppression of transcription. Contrary to a previous study,
phloretin did not appear to upregulate the expression levels of
TRAIL-Rs, such as DR4 or DR5 (19), since the expression levels of these
receptors were not altered following treatment with phloretin.
Additionally, it was noted that expression levels of Bax were not
altered following addition of phloretin, suggesting that
degradation of Mcl-1 did not lead to modulation of Bax protein
expression. Notably, cells from normal colon, lung and kidney cell
lines were not affected by treatment with phloretin, suggesting
phloretin had the least impact on normal cells in inducing
apoptosis regardless of the origin of the organ. The role of
phloretin as a sensitizer of colon cancer cells to TRAIL-induced
apoptosis was also demonstrated in an in vivo colon cancer
xenograft model. A tendency for decreased volumes and weights in
tumors from mice injected with phloretin, TRAIL, or phloretin and
TRAIL was observed. The non-significance might be due to the small
number of mice examined or a more complicated in vivo tumor
environment. However, the results of the in vivo experiments
in the present study may support the potential application of
phloretin in human studies in the future.
Phloretin has also been reported to sensitize SW 620
colon cancer cells to a chemotherapeutic agent, daunorubicin, in
terms of anticancer activity and apoptosis, by inhibiting glucose
uptake under hypoxia (28). Since
numerous targeted agents exert their effects when used in
combination with conventional chemotherapeutic agents, previous
results (28) provide a basis for
planning clinical trials combining conventional chemotherapeutic
agents with TRAIL and phloretin.
In summary, the present study clarified the role of
phloretin as a sensitizer of TRAIL-induced apoptosis signaling in
colon cancer. Co-treatment with TRAIL and phloretin exhibited
synergistic effects in suppressing growth of colon cancer cells
through apoptosis induction. The synergistic effect was exerted by
activating the intrinsic pathway through phloretin, in addition to
activation of the extrinsic pathway of apoptosis by TRAIL.
Proteasomal degradation of Mcl-1 was the major mechanism leading to
activation of the stress pathway. The findings of the present study
provide useful information for overcoming the primary resistance of
colon cancer cells to TRAIL-R agonists by suggesting a potential
sensitizing candidate to TRAIL-induced apoptosis. This may
represent the potential to develop a novel therapeutic agent in the
treatment of CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Korea University Guro
Hospital ‘KOREA RESEARCH-DRIVEN HOSPITALS’ Grant (no.
O1801471).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYL, SCO and DHL conceived and designed the present
study. JLK and DHL performed the experiments. DHL, CHP, SJP, SYL
and SCO assisted in the data analysis of the present study. SYL
drafted the manuscript. SYL and JLK wrote the manuscript. SYL, CHP,
SJP, and SCO edited and revised the manuscript. JLK, DHL, SYL and
SCO confirm the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the guidelines approved by the Korea University Institutional
Animal Care and Use Committee (Seoul, Republic of Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Q, Han L, Li J, Xu H, Liu X, Wang X,
Pan C, Lei C, Chen H and Lan X: Activation of Nrf2 by phloretin
attenuates palmitic acid-induced endothelial cell oxidative stress
via AMPK-dependent signaling. J Agric Food Chem. 67:120–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang G, Gao Y, Wang H, Wang J and Niu X:
Phloretin reduces cell injury and inflammation mediated by
staphylococcus aureus via targeting sortase B and the molecular
mechanism. Appl Microbiol Biotechnol. 102:10665–10674. 2018.
View Article : Google Scholar
|
|
4
|
Xu M, Gu W, Shen Z and Wang F: Anticancer
activity of phloretin against human gastric cancer cell lines
involves apoptosis, cell cycle arrest, and inhibition of cell
invasion and JNK signalling pathway. Med Sci Monit. 24:6551–6558.
2018. View Article : Google Scholar
|
|
5
|
Kim U, Kim CY, Lee JM, Oh H, Ryu B, Kim J
and Park JH: Phloretin inhibits the human prostate cancer cells
through the generation of reactive oxygen species. Pathol Oncol
Res. 26:977–984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsiao YH, Hsieh MJ, Yang SF, Chen SP, Tsai
WC and Chen PN: Phloretin suppresses metastasis by targeting
protease and inhibits cancer stemness and angiogenesis in human
cervical cancer cells. Phytomedicine. 62:1529642019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin ST, Tu SH, Yang PS, Hsu SP, Lee WH, Ho
CT, Wu CH, Lai YH, Chen MY and Chen LC: Apple polyphenol phloretin
inhibits colorectal cancer cell growth via inhibition of the type 2
glucose transporter and activation of p53-mediated signaling. J
Agric Food Chem. 64:6826–6837. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SY, Kim EJ, Shin HK, Kwon DY, Kim MS,
Surh YJ and Park JH: Induction of apoptosis in HT-29 colon cancer
cells by phloretin. J Med Food. 10:581–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nat Rev Cancer. 17:352–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sedger LM, Glaccum MB, Schuh JC, Kanaly
ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R and
Gliniak B: Characterization of the in vivo function of
TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using
TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 32:2246–2254. 2002.
View Article : Google Scholar
|
|
12
|
Cretney E, Takeda K, Yagita H, Glaccum M,
Peschon JJ and Smyth MJ: Increased susceptibility to tumor
initiation and metastasis in TNF-related apoptosis-inducing
ligand-deficient mice. J Immunol. 168:1356–1361. 2002. View Article : Google Scholar
|
|
13
|
Takeda K, Smyth MJ, Cretney E, Hayakawa Y,
Kayagaki N, Yagita H and Okumura K: Critical role for tumor
necrosis factor-related apoptosis-inducing ligand in immune
surveillance against tumor development. J Exp Med. 195:161–169.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong H, Cheng W and Wang Y: Tumor necrosis
factor-related apoptosis-inducing ligand inhibits the growth and
aggressiveness of colon carcinoma via the exogenous apoptosis
signaling pathway. Exp Ther Med. 17:41–50. 2019.PubMed/NCBI
|
|
15
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XD, Franco A, Myers K, Gray C,
Nguyen T and Hersey P: Relation of TNF-related apoptosis-inducing
ligand (TRAIL) receptor and FLICE-inhibitory protein expression to
TRAIL-induced apoptosis of melanoma. Cancer Res. 59:2747–2753.
1999.PubMed/NCBI
|
|
17
|
Abe K, Kurakin A, Mohseni-Maybodi M, Kay B
and Khosravi-Far R: The complexity of TNF-related
apoptosis-inducing ligand. Ann N Y Acad Sci. 926:52–63. 2000.
View Article : Google Scholar
|
|
18
|
Kim JL, Park SH, Jeong S, Kim BR, Na YJ,
Jo MJ, Jeong YA, Yun HK, Kim DY, Kim BG, et al: Sea cucumber
(Stichopus japonicas) F2 enhanced TRAIL-induced apoptosis via XIAP
ubiquitination and ER stress in colorectal cancer cells. Nutrients.
11:10612019. View Article : Google Scholar
|
|
19
|
Kim B, Seo JH, Lee KY and Park B: Icariin
sensitizes human colon cancer cells to TRAIL-induced apoptosis via
ERK-mediated upregulation of death receptors. Int J Oncol.
56:821–834. 2020.
|
|
20
|
Mahalingam D, Carew JS, Espitia CM, Cool
RH, Giles FJ, de Jong S and Nawrocki ST: Heightened JNK activation
and reduced XIAP levels promote TRAIL and sunitinib-mediated
apoptosis in colon cancer models. Cancers (Basel). 11:8952019.
View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi BY: Biochemical basis of
anti-cancer-effects of phloretin-a natural dihydrochalcone.
Molecules. 24:2782019. View Article : Google Scholar
|
|
23
|
Wu C: Systemic therapy for colon cancer.
Surg Oncol Clin N Am. 27:235–242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar
|
|
26
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Surapally S, Jayaprakasam M and Verma RS:
Curcumin augments therapeutic efficacy of TRAIL-based immunotoxins
in leukemia. Pharmacol Rep. 72:1032–1046. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao X, Fang L, Gibbs S, Huang Y, Dai Z,
Wen P, Zheng X, Sadee W and Sun D: Glucose uptake inhibitor
sensitizes cancer cells to daunorubicin and overcomes drug
resistance in hypoxia. Cancer Chemother Pharmacol. 59:495–505.
2007. View Article : Google Scholar
|