Introduction
Immune checkpoint inhibitors (ICIs), which can block
the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or
programmed cell death 1 (PD-1) pathways, have recently played an
important role in the treatment of advanced malignancies (1,2).
Atezolizumab, an ICI that inhibits PD-L1 to promote endogenous
antitumour immunity, has been utilised in the treatment of
malignant tumours. However, as ICIs have become more commonly used,
different autoimmune side effects known as immune-related adverse
events (irAEs) have become a rising source of concern. Neurological
irAEs are seldom documented, and extensive studies show that the
prevalence of neurological AEs caused by immune checkpoint
inhibitor medication is ~1% (3).
Neurological irAEs can cause central and peripheral nervous system
damage, resulting in encephalopathy/encephalitis, central nervous
system (CNS) demyelination, aseptic meningitis, transverse
myelitis, posterior reversible leukoencephalopathy syndrome,
peripheral neuropathy, myasthenia-like syndrome, Guillain-Barré
syndrome-like illness and myopathy (4,5).
Encephalitis is estimated to occur in 0.1-0.2% of patients treated
with ICIs (6). To the best of our
knowledge, atezolizumab-associated encephalitis has been reported
in bladder cancer, cervical cancer, lung cancer, hepatocellular
carcinoma and breast cancer (7–15).
It is clinically characterized by seizures, confusion, altered
behavior, headaches and alterations of consciousness (16). Brain MRI revealed T2
hyperintensities with or without contrast enhancement. Lumbar
puncture frequently revealed lymphocytic and leucocytic
pleiocytosis, while cytopathology was negative (17). Patients with ICI-related
encephalitis generally recovered completely or partially after
corticosteroid therapy. The current study presents a case of irAEs
in the CNS. To the best of our knowledge, this is the first case of
encephalitis in China closely linked to atezolizumab.
Case report
In May 2019, a 65-year-old female was admitted to
Boai Hospital of Zhongshan (Zhongshan, China) with redness,
swelling, heat, and pain in the left breast for one month. The
patient was diagnosed with infiltrating ductal carcinoma of the
left breast via fine-needle aspiration. No metastatic signs were
present at first, and stage II disease (18) was indicated from the histological
analysis. Six sessions of chemotherapy were administered, the first
four of which consisted of epirubicin and cyclophosphamide,
followed by two treatments of docetaxel. As the patient did not
respond well to docetaxel, the chemotherapy regimen was modified to
paclitaxel and carboplatin. The breast tumour was significantly
decreased in size after a further three lines of treatment. Due to
grade IV myelosuppression, the fourth round of chemotherapy was not
finished.
In December 2019, the patient was scheduled for a
subcutaneous mastectomy with axillary and subclavian lymph node
dissection. Pathological findings revealed multiple metastases to
the left axillary lymph nodes and latissimus dorsi. A diagnosis of
triple-negative breast carcinoma (rT4N0M1) (19) was made based on the
immunohistochemistry results. The patient refused the chemotherapy
regimens recommended by the doctors and instead received
radiotherapy. After 6 months, the patient was diagnosed with a
metastatic tumour of the 9th thoracic vertebrae and left anterior
chest using integrated positron emission tomography and computed
tomography, indicating progression of the disease. At that time,
PDL-1 testing was negative. To inhibit the progression of the
tumor, doctors decided to begin treatment with atezolizumab at
1,200 mg in conjunction with paclitaxel at 200 mg per dose.
Chemotherapy had to be put on hold during treatment due to a lung
infection and a urinary tract infection.
The patient became somnolent 10 days after taking a
fourth dose of atezolizumab and was admitted to Guangdong
Provincial Hospital of Chinese Medicine (Guangzhou, China). The
neurological examination revealed a disruption in consciousness
(Glasgow Coma Scale E1V1M3) (19)
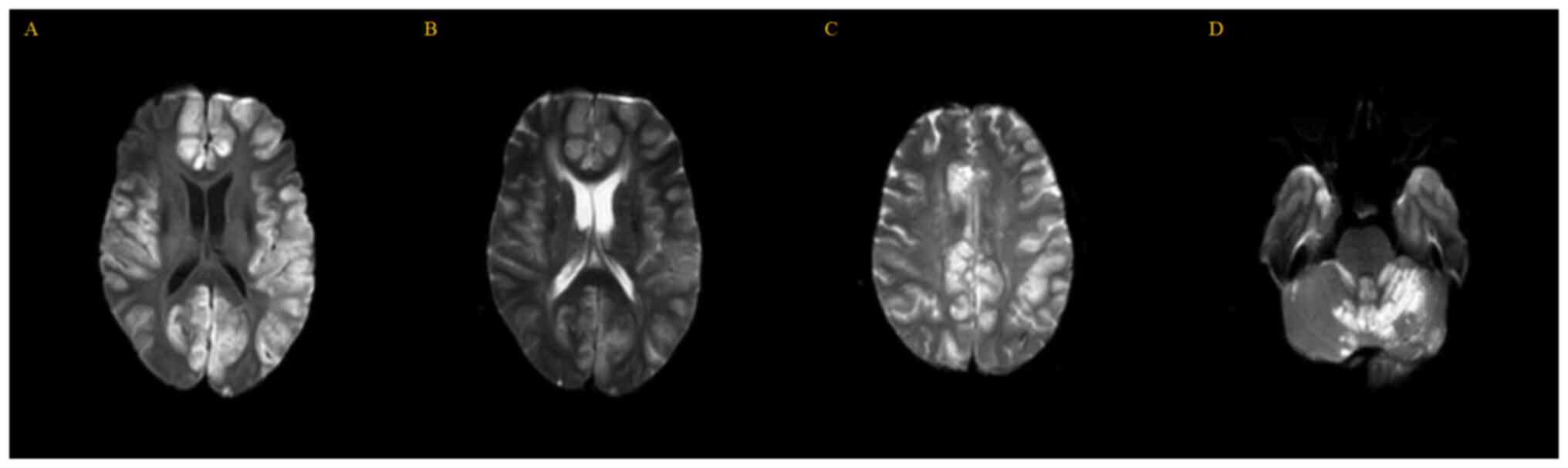
but no other abnormal findings. MRI of the brain (Fig. 1) revealed multiple patchy T2
hyperintensities in the bilateral cerebellar hemisphere, vermis of
the cerebellum, bilateral frontal lobe, temporal lobe, parietal
lobe and occipital cortex. These lesions exhibited hyperintense
signal on diffusion-weighted imaging, which was suggestive of
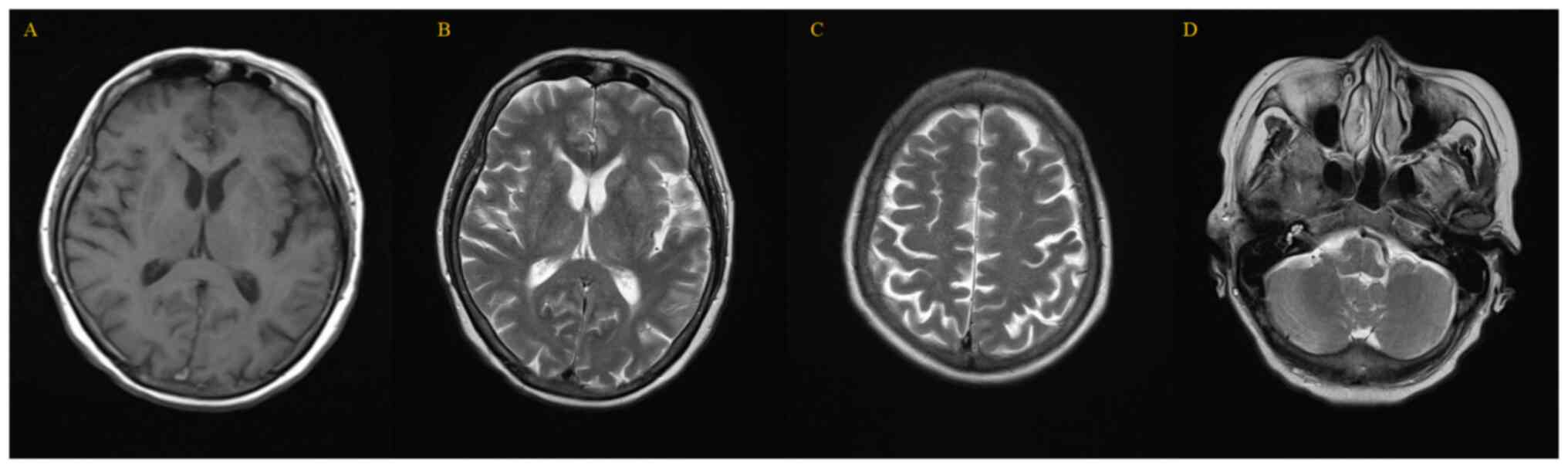
intracellular edema. However, when doctors reviewed the previous
MRI report from 23 days before, no abnormal signals were found in
the brain parenchyma, and the size and shape of each ventricle and
cistern was normal (Fig. 2). The
appearance of these new lesions confirmed the deterioration of the
patient's condition. At that time, the possibility of
immune-related encephalitis was considered first, so the patient
was administered an intravenous infusion of 10 ml dexamethasone.
However, brain metastases and paraneoplastic neurological syndrome
were not ruled out. As a result, a lumbar puncture, cerebrospinal
fluid (CSF) culture and cytology, viral serologies of the blood and
cerebrospinal fluid, and antineuronal antibody detection were all
necessary for the diagnosis. However, the patient's physical health
rapidly deteriorated and she eventually fell into a deep coma with
no response to any stimulus. The heart rate was 140 (normal ranges,
60–100) beats/min and the blood pressure was 150/110 (normal range,
90–140/60–90) mmHg. At that time, the workup revealed a white blood
cell count of 3.87 (normal range, 3.5-9.5) ×109/l, a
lymphocyte count of 0.39 (normal range, 1.1-3.2) ×109/l,
a red blood cell count of 3.63 (normal range, 3.8-5.1)
×109/l, a haemoglobin level of 88 (normal range,
115–150) g/l, a D-dimer level of 7.84 (normal range, 0–0.5) mg/l
and a fibrinogen degradation product of 25.52 (normal range, 0–5)
mg/l. Importantly, blood oxygen saturation declined to 83% (normal
range, 91.9–99%). The partial pressures of oxygen and carbon
dioxide were 54.1 (normal range, 80–100) and 53.8 (normal range,
35–45) mmHg, respectively, indicating type 2 respiratory failure.
Ventilation with a non-invasive ventilator was provided; however,
the patient's condition worsened and cardiac and respiratory arrest
occurred. Doctors performed chest compressions, airbag
mask-assisted ventilation, medication boosting, stimulated
breathing and other symptomatic treatments. The patient and family
refused endotracheal intubation or being transferred to the
intensive care unit. After a temporary stabilization of viatal
signs as a result of a series of rescue measures, the patient was
discharged. A telephone follow-up conversation later revealed that
the patient had died a few days after being discharged from the
hospital.
Discussion
Immune checkpoint inhibitors have a well-documented
history of causing neurological adverse results, with an incidence
ranging from 3.8-6.1% (16,20).
The beginning of neurological irAEs ranges from 6–13 weeks after
the initiation of ICI, but they can occur at any time during
treatment and even after termination (10,21).
It is possible that the central and/or peripheral nervous systems
are affected. According to the European Society for Medical
Oncology clinical practice guidelines recommended, symptoms of
irAEs may be divided into four grades (22). Asthenia, headaches, dizziness,
paresthesia or dysgeusia are common symptoms of grades 1 and 2
(22,23). Myasthenia gravis, Guillain-Barré
syndrome, chronic inflammatory polyneuropathy, myelitis, aseptic
meningitis, encephalitis and posterior-reversible encephalopathy
are the more common authentic neurological syndromes in grades 3
and 4 (22,23). Although neurological irAEs are
uncommon, they often manifest as serious diseases with a high
fatality rate (24). Encephalitis
caused by atezolizumab has only been recorded in a few cases as an
irAE. To the best of our knowledge, only 9 cases have been reported
(7–15). The main information for these cases
is shown in Table I.
 | Table I.Main information on cases of
atezolizumab-associated encephalitis. |
Table I.
Main information on cases of
atezolizumab-associated encephalitis.
| First author,
year | Patient |
|
|
|
|
|
|
|
|---|
| Manifestation of
encephalitis |
|
|
|
|---|
| Age, years | Sex | Type of malignant
tumour |
|
|
|
|
|---|
| Time of onset | Main symptoms | MRI | CSF | Interventions | Outcome | (Refs.) |
|---|
| Levine et al,
2017 | 59 | Female | Metastatic bladderr
cance | 12 days after first
dose | Confusion, fatigue,
spastic tremors and vomiting | Left frontal lobe
mildly enhancing lesion | Glucose: 80 mg/dl;
protein: 100 mg/dl; negative paraneoplastic tests | Steroids | Symptoms improved,
with upper extremity weakness left | (7) |
| Laserna et al,
2018 | 53 | Female | Cervical squamous
cell carcinoma | 13 days after first
dose | Altered mental
statusand headache | Diffuse
leptomeningeal enhancement | Protein: >600
mg/dl; glucose: 92 mg/dl; negative CSF cultures | Steroids | Symptoms improved,
with muscle weakness left | (8) |
| Arakawa et al,
2018 | 78 | Male | Metastatic lung
cancer | 13 days after first
dose | Confusion and
fever | Unmentioned | Cell count: 139/µl;
protein 132 mg/dl | Steroids | Recovered | (9) |
| Robert et al,
2020 | 48/F | Female | Metastatic lung
adenocarcinoma | 13 days after first
dose | Fever, temporospatial
disorientation, memory impairment and aphasia | Pachytomeningitis and
leptomeningitis. | Elevated protein | Steroids | Recovered | (10) |
| Yamaguchi et
al, 2020 | 56 | Female | Metastatic lung
adenocarcinoma | 17 days after first
dose | Fever, consciousness
disorder and motor aphasia | Unmentioned | Cell count: 20/µl;
protein: 166 mg/glucose: 73 mg/dl; interleukin: 682.9 pg/ml | Steroids | Recovered | (11) |
| Tatsumi et al,
2020 | 76 | Male | Small cell lung
cancer | 5 months after first
dose | Irritability and
forgetfulness | T2-hyper-intensity in
the bilateral striatum. | A high titer of
anti-CRMP5 antibody | Steroids | Recovered | (12) |
| Nader et al,
2021 | 38 | Female | Metastatic
triple-negative breast cancer | 10 days after first
dose | Fever, seizures and
somnolence | Moderate diffuse
leptomeningeal enhancement bilaterally | No malignant cells
and negative CSF cultures | Steroids | Remained stable for
~1 year, passed away after 5 years due to an infection | (13) |
| Özdirik et
al, 2021 | 70 | Female | Multifocal
hepatocellular carcinoma | 10 days after first
dose | Impaired cognition
and language, somnolence, emesis and dyspnea | Unmentioned | Leucocyte count:
179/ml; protein: 5,494 mg/dl; negative CSF cultures | Steroids and
plasma-pheresis | Died due to
multi-organ failure | (14) |
| Nishijima et
al, 2022 | 72 | Female | Non-small cell lung
cancer | Uncertain | Gait disturbance
and mild disturbance of consciousness | Symmetrical high
signal in the thalamus bilaterally | Unmentioned | Steroids and
IVIG | Died due to
aspiration pneumonia | (15) |
| Chen et al,
2022 | 65 | Female | Metastatic
triple-negative breast cancer | 10 days after
fourth dose | Coma and
respiratory failure | T2 and DWI
hyperintense signals in the bilateral cerebellar hemisphere, vermis
of the cerebellum, bilateral frontal lobe, temporal lobe, parietal
lobe and occipital cortex | None | Steroids | Dead | Present case |
Table I reveals
that atezolizumab-associated encephalitis has been described in
bladder cancer, cervical cancer, lung cancer, hepatocellular
carcinoma and breast cancer. Furthermore, 3 cases were noted in an
atezolizumab clinical study in triple-negative breast cancer
(25). The current case is the
fifth case associated with breast cancer.
It is possible to draw conclusions based on the
clinical symptoms of those cases mentioned in the Table I. First, symptoms of neurological
irAEs appeared ~2 weeks after the patients received their first
dose of atezolizumab in all cases. Second, the predominant symptoms
were a high fever and a disturbance of consciousness. Third, MRI
revealed encephalitis symptoms. CSF investigation revealed
encephalitis due to an increase in the number of leucocytes,
lymphocytes and protein. Furthermore, CSF was negative for
bacterial and fungal cultures, as were other associated
autoantibodies. Consequently, atezolizumab-associated encephalitis
was discovered after ruling out other possible causes of
encephalitis using MRI, CSF analysis and autoantibody assays.
Fourth, in these cases, steroid pulse therapy has been shown to be
helpful. Since atezolizumab-associated encephalitis is rare, it may
be seen from the existing case reports and clinical guidelines that
the most direct objective index of this rare encephalitis for
diagnosis is still uncertain (17). Clinicians rely on an exclusive
diagnosis, i.e., a diagnosis of atezolizumab-associated
encephalitis after excluding other causes of encephalitis,
including infectious, toxic and metabolic causes. Therefore, in the
diagnosis, the results of CSF-related examinations are necessary
(10). In terms of treatment,
timely administration of steroids may prevent rapid deterioration
of patients (16).
In contrast to those previously described cases of
atezolizumab-associated encephalitis (Table I), the patient in the present case
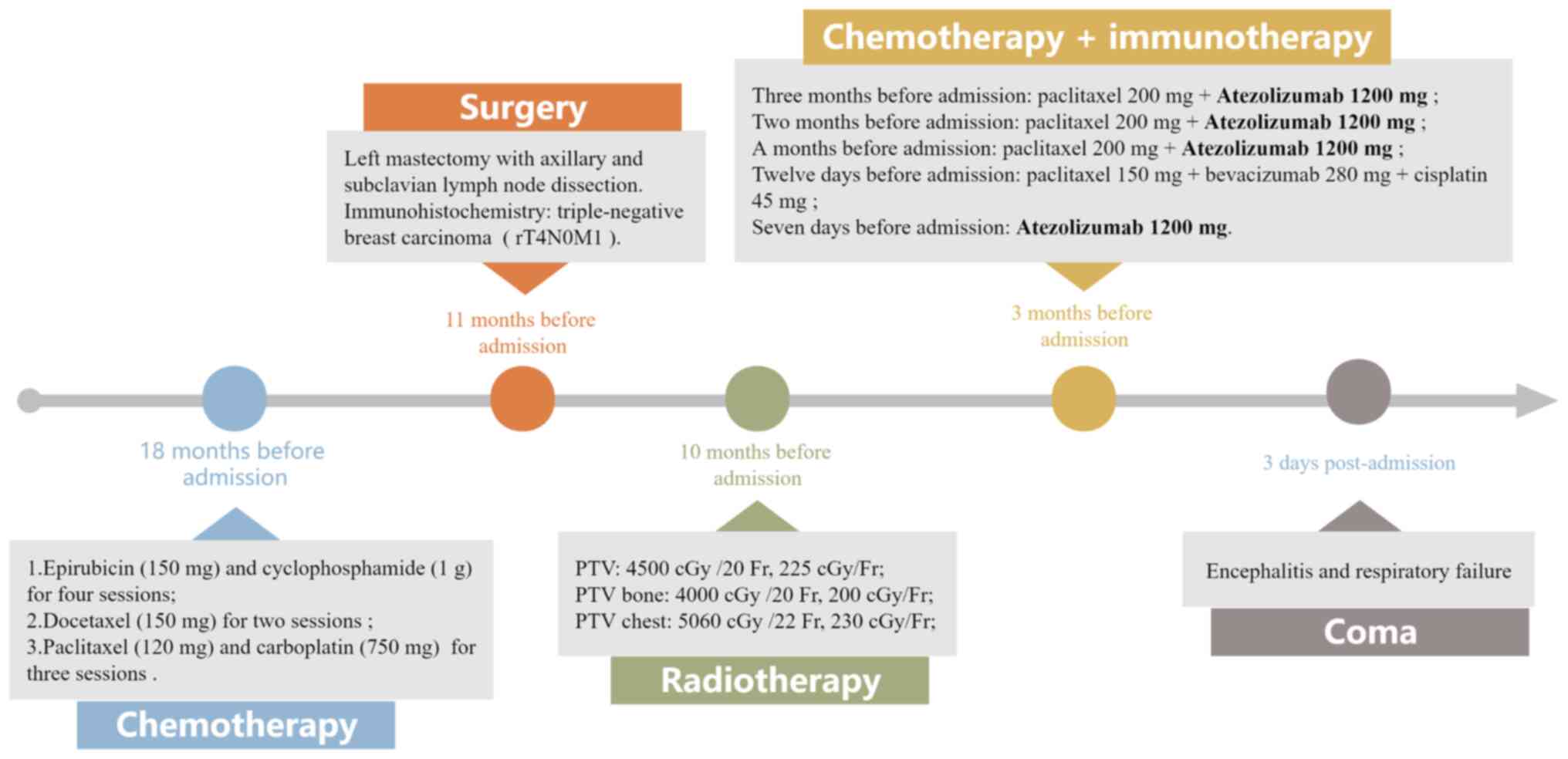
displayed distinct clinical manifestations. Fig. 3 shows the patient's overall course
of treatment, particularly the duration of atezolizumab treatment.
No discomfort was noted after the first dose of atezolizumab and
there was no sign of prodromal infection based on the results of an
MRI and a chest X-ray prior to admission. However, encephalitis
developed 10 days after the fourth dose of atezolizumab therapy. An
immediate loss of consciousness, with multiple T2 hyperintensities
in the initial brain MRI, indicated encephalitis. This outbreak of
encephalitis affected a wide area, including bilateral cerebellar
hemispheres, vermis, bilateral frontal lobe, temporal lobe,
parietal lobe, occipital cortex and medulla oblonata, resulting in
loss of consciousness and respiratory failure. Although physicians
fully explained the patient's condition and treatment plans to the
family, they refused to allow further treatments or a full
inspection, including the CSF and autoantibody examinations, which
accounted for the difficulty in verifying the diagnosis. After
ruling out other possible causes, the physicians highly suspected
that the encephalitis was caused by atezolizumab.
The precise mechanism of neurological irAEs has not
yet been established. In ICI therapies, monoclonal antibodies are
employed to block the expression of proteins [CTLA4, PD-1 and
programmed death-ligand 1 (PD-L1)] and thereby increase T-cell
activation against tumours (5).
The onset of immune-associated AEs may be more closely associated
with anti-PD-1 and anti-PD-L1 antibody responses than with
anti-CTLA-4 antibody responses (26). A post-hoc analysis of a phase II
trial assessing the efficacy of ipilimumab as a combination with
chemotherapy in the treatment of metastatic small-cell lung cancer
indicated that the presence of antineuronal antibodies was
associated with more irAEs and particularly neurological toxicity
(27).
ICI-mediated encephalitis is a medical emergency and
a diagnosis by exclusion; it can be present in a variety of
clinical manifestations, making diagnosis and therapy problematic.
The diagnosis of ICI-mediated neurotoxicities is difficult due to
the rich variety of differential diagnoses, which includes tumour
progression, paraneoplastic neurologic disorders, metabolic
derangements, infections and complications associated with
concurrent treatment modalities (21). The present study emphasizes the
important relevance of MRI in the early detection of ICI-related
encephalitis. MRI is considered to be the one of the accurate and
non-invasive tests available to evaluate the changes of lesions in
the brain. MRI is also a powerful basis for clinicians to diagnose
and make medical decisions in such cases. Complementary
examinations are also essential, including a full biological
assessment, viral serologies in the blood and cerebrospinal fluid
and antineuronal antibody determination (10). Although they were invasive tests
and could not give immediate results, the lack of additional
examinations to support the diagnosis was considered as a
limitation in the present case.
When atezolizumab-associated encephalitis occurs,
steroid pulse treatment is used to alleviate brain inflammation,
according to current irAE care guidelines (16). After visiting a neurologist,
serious cases should be treated with intravenous immunoglobulin and
plasmapheresis (28). If the
patient and family members in the present study had agreed to
active treatment, the patient's results would likely have been
completely different. The doctors might have been able to confirm
the diagnosis and steroid pulse treatment could have been
administered to the patient. Therefore, the manner in which rare
ICI-mediated encephalitis can be accurately identified in clinical
practice and how to make the most beneficial medical decisions for
patients are directions for our future efforts.
In conclusion, as the application of ICIs for
treating diverse types of malignancies expands, the occurrence of
clinical irAEs will surely increase. Despite an uncertain cause and
the lack of focused treatment for atezolizumab-associated
encephalitis, doctors are obliged to constantly update their
working knowledge in order to appropriately diagnose and manage
these cases. Prompt identification and treatment are essential for
successful management. Therefore, it is very important to consult
experts in order to clarify a diagnosis in a timely manner.
Physicians are obliged to decide the optimal measures after
carefully examining the severity of the irAEs and the condition of
the patient. Since irAEs can affect different organs, a
collaborative approach connecting with experts from multiple fields
is useful.
Acknowledgements
Not applicable.
Funding
This case report was supported by the Special project of
Guangdong Provincial Key Laboratory of Traditional Chinese Medicine
Emergency Research (grant no. 2019KT1340).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC was responsible for the study conception,
reviewed the literature and drafted the manuscript. CZ and JL
confirmed the authenticity of all the raw data analyzed and
interpreted the data and edited the manuscript. ZL obtained medical
images (MRI scans) and analyzed patient data. YZ and HZ contributed
to the study conception, overall design and quality control. All
authors reviewed the manuscript critically and approved the
submission.
Ethics approval and consent to
participate
This case report was approved by the Ethics
Committee of Guangdong Provincial Hospital of Chinese Medicine
(Guangzhou, China).
Patient consent for publication
The patient's family provided oral consent for the
article to be published. The Ethics Committee of Guangdong
Provincial Hospital of Chinese Medicine (Guangzhou, China) approved
that oral consent was sufficient in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367:eaax01822020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Möhn N, Beutel G, Gutzmer R, Ivanyi P,
Satzger I and Skripuletz T: Neurological immune related adverse
events associated with nivolumab, ipilimumab, and pembrolizumab
therapy-review of the literature and future outlook. J Clin Med.
8:17772019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan PC and Haggiagi A: Neurologic
immune-related adverse events associated with immune checkpoint
inhibition. Curr Oncol Rep. 21:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22:392020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams TJ, Benavides DR, Patrice KA,
Dalmau JO, de Ávila AL, Le DT, Lipson EJ, Probasco JC and Mowry EM:
Association of autoimmune encephalitis with combined immune
checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol.
73:928–933. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine JJ, Somer RA, Hosoya H and
Squillante C: Atezolizumab-induced encephalitis in metastatic
bladder cancer: A case report and review of the literature. Clin
Genitourin Cancer. 15:e847–e849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laserna A, Tummala S, Patel N, El Hamouda
DEM and Gutiérrez C: Atezolizumab-related encephalitis in the
intensive care unit: Case report and review of the literature. SAGE
Open Med Case Rep. 6:2050313X187924222018.PubMed/NCBI
|
|
9
|
Arakawa M, Yamazaki M, Toda Y, Saito R,
Ozawa A, Kosaihira S and Kimura K: Atezolizumab-induced
encephalitis in metastatic lung cancer: A case report and
literature review. eNeurologicalSci. 14:49–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robert L, Langner-Lemercier S, Angibaud A,
Sale A, Thepault F, Corre R, Lena H and Ricordel C: Immune-related
encephalitis in two patients treated with immune checkpoint
inhibitor. Clin Lung Cancer. 21:e474–e477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi Y, Nagasawa H, Katagiri Y and
Wada M: Atezolizumab-associated encephalitis in metastatic lung
adenocarcinoma: A case report. J Med Case Rep. 14:882020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tatsumi S, Uryu K, Iwasaki S and Harada H:
A case of anti-CRMP5 paraneoplastic neurological syndrome induced
by atezolizumab for small cell lung cancer. Intern Med. Aug
12–2020.(Epub ahead of print).
|
|
13
|
Nader R, Tannoury E, Rizk T and Ghanem H:
Atezolizumab-induced encephalitis in a patient with metastatic
breast cancer: A case report and review of neurological adverse
events associated with checkpoint inhibitors. Autops Case Rep.
11:e20212612021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Özdirik B, Jost-Brinkmann F, Savic LJ,
Mohr R, Tacke F, Ploner CJ, Roderburg C and Müller T: Atezolizumab
and bevacizumab-induced encephalitis in advanced hepatocellular
carcinoma: Case report and literature review. Medicine (Baltimore).
100:e263772021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishijima H, Kon T, Seino Y, Yagihashi N,
Suzuki C, Nakamura T, Tanaka H, Sakamoto Y, Wakabayashi K and
Tomiyama M: Bilateral thalamic lesions associated with
atezolizumab-induced encephalitis: A follow-up report with autopsy
findings. Neurology. 98:204–205. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstof MS, Gardner JM,
Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Velasco R, Villagrán M, Jové M, Simó M,
Vilariño N, Alemany M, Palmero R, Martínez-Villacampa MM, Nadal E
and Bruna J: Encephalitis induced by immune checkpoint inhibitors:
A systematic review. JAMA Neurol. 78:864–873. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta R GP trainee and Chinthapalli K
consultant neurologist: Glasgow coma scale explained. BMJ.
365:l12962019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trinh S, Le A, Gowani S and La-Beck N:
Management of immune-related adverse events associated with immune
checkpoint inhibitor therapy: A minireview of current clinical
guidelines. Asia Pac J Oncol Nurs. 6:154–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duong SL, Barbiero FJ, Nowak RJ and
Baehring JM: Neurotoxicities associated with immune checkpoint
inhibitor therapy. J Neurooncol. 152:265–277. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haanen JB, Carbonnel F, Robert C, Kerr KM,
Peters S, Larkin J and Jordan K; ESMO Guidelines Committee, :
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(Suppl 4):iv119–iv142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Touat M, Talmasov D, Ricard D and Psimaras
D: Neurological toxicities associated with immune-checkpoint
inhibitors. Curr Opin Neurol. 30:659–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogrig A, Muñiz-Castrillo S, Joubert B,
Picard G, Rogemond V, Marchal C, Chiappa AM, Chanson E, Skowron F,
Leblanc A, et al: Central nervous system complications associated
with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry.
91:772–778. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arriola E, Wheater M, Galea I, Cross N,
Maishman T, Hamid D, Stanton L, Cave J, Geldart T, Mulatero C, et
al: Outcome and biomarker analysis from a multicenter phase 2 study
of ipilimumab in combination with carboplatin and etoposide as
first-line therapy for extensive-stage SCLC. J Thorac Oncol.
11:1511–1521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar V, Chaudhary N, Garg M, Floudas CS,
Soni P and Chandra AB: Current diagnosis and management of immune
related adverse events (irAEs) induced by immune checkpoint
inhibitor therapy. Front Pharmacol. 8:492017. View Article : Google Scholar : PubMed/NCBI
|