Introduction
Cancer cells, in contrast to normal cells, are
rapidly proliferating, invasive and metastasizing. To facilitate
this increased malignant activity, cancer cells reprogram and
upregulate their metabolism (1,2).
This consequently generates an increased degree of utilization and
hence need for ‘metabolic building materials’, for example glucose,
amino acids etc. - any component participating in the concerned
metabolic processes. The blood circulation constitutes the
principal supply lines for these building materials, and targeting
these metabolic supply lines, practically defined as the
availability of the specific building materials in the blood, could
therefore carry treatment potential (3).
Glucose and amino acids are among the metabolic
building materials which are central to the metabolic alterations
in cancer cells (4), and studies
have indicated that the blood levels of these might have a clinical
impact. A meta-analysis including nearly 10,000 patients diagnosed
with cancer reported that hyperglycemia was associated with shorter
overall survival as well as shorter disease-free survival (5), with similar results also being
reported by other large studies (n=1770-301,948) (6–9).
Furthermore, other studies have found an association between
glucose level and the occurrence of distant metastases in different
cancers (10,11). Likewise, the levels of various
amino acids in the blood have been shown to associate with both
metastasis occurrence and survival (12–16).
A central aspect in this context is that the
processes involved in malignancy are incredibly complex. This
materializes with multiple supply lines delivering various building
materials to multiple different processes (4,17).
Furthermore, cancer cells are metabolically flexible, which may
enable them, in the event that one supply line is limited, to
compensate through upregulation of another (18). Therefore, to eventually achieve
therapeutic effect, presumably more supply lines should be
targeted, and these should constitute patterns that are most
crucial to the malignant processes and prevents compensation from
occurring (3).
Thus, the aim should be to target the metabolic
supply line patterns underlying the processes essential to
malignancy, for instance uncontrolled proliferation, metastasis
development etc., as this would in theory compromise the delivery
of the most basal components required to exert these processes. A
key aspect in this strategy is that it targets the thermodynamic
foundation which enables the activities that ‘make a cancer a
cancer’ (3).
As an initial step, this study exploratively
investigates whether such metabolic supply line patterns can be
seen to correlate with the development of distant metastases. The
examined supply lines were those of glucose, glutamine, arginine
and cystathionine. They were selected because they are involved in
central malignant processes and because they can be interpreted to
supplement each other in these processes. Therefore, they serve the
purpose of investigating the combinatorial effects of metabolic
patterns. Specifically, this study investigates whether a pattern
comprising the levels of glucose (HbA1c), glutamine, arginine and
cystathionine in the blood can be seen to correlate with the
development of distant metastases in 64 women diagnosed with breast
cancer.
Materials and methods
Patient population and blood
samples
The study was approved by the Danish Committees on
Health Research Ethics (H-19042347). 64 women diagnosed with breast
cancer between 2011 and 2015 were included in the study. Among
these, 32 had developed distant metastases and 32 had not. The
patients were age 50-80 years at the time of diagnosis. Tumors were
either estrogen-receptor-positive (ER+) ductal carcinomas or ER+
lobular carcinomas. Exclusion criteria were 1) previous or any
other current cancer, 2) neoadjuvant treatment and 3) known
diabetes mellitus or any other metabolic disease. Among the
patients who had developed distant metastases, the mean age was
63.9 years (SD: 8.34) while the histological distribution included
26 (81.3%) ER+ ductal carcinomas and 6 (18.8%) ER+ lobular
carcinomas. Among the patients who had not developed distant
metastases, the mean age was 63.5 years (SD: 7.78) while the
histological distribution was 25 (78.1%) ER+ ductal carcinomas and
7 (21.9%) ER+ lobular carcinomas.
Within 2 weeks prior to surgery, a blood sample was
drawn from the patients and subsequently administered and stored in
the Danish Cancer Biobank (Bio- and Genome Bank, Denmark,
http://rbgb.dk/cancer) as EDTA whole blood, EDTA
buffy coat, EDTA serum and plasma fractions. The samples were
stored at −80°C. To ensure optimal conditions for the metabolic
analyses, all samples were processed and placed in the freezer
within 3 h. The patients included in the study were from two
locations, Herlev Hospital, Copenhagen University Hospital,
Copenhagen and Rigshospitalet, Copenhagen University Hospital,
Copenhagen.
Metabolic analyses
The level of glucose in the blood was measured as
Hemoglobin A1c (HbA1c). EDTA whole blood samples were thawed and 20
ml of whole blood were diluted with 1,500 ml wash (HSi Hemolysis
and Wash Solution-(L)) (Tosoh Bioscience, USA) and then analyzed
using the Tosoh G8 (HLC-723G8) HPLC Analyzer (Tosoh Bioscience,
USA). HbA1c levels were expressed in mmol/mol. The analyses were
carried out at the Department of Clinical Biochemistry,
Rigshospitalet.
The levels of free glutamine, arginine and
cystathionine in EDTA plasma were determined using the MassTrak
Amino Acid Analysis (AAA) Solution Kit (Waters Corporation, USA),
an Acquity UHPLC system with a C18 BEH column (1.7 µm;2.1×150 mm)
and an integrated photodiode array (PDA) detector (operating at
λ=260 nm) (all from Waters Corporation, USA). 100 µl of thawed EDTA
plasma was deproteinized with an equivalent amount of 10%
sulfosalicylic acid with norvaline as internal standard. The
samples were then thoroughly mixed followed by centrifugation at
14,000 g. 20 µl of the supernatant were alkalized with a borate
buffer/NaOH solution and derivatized with
6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) and analyzed
by UHPLC-UV using a gradient of provided eluents, a column
temperature of 43°C and a flowrate of 0.45 ml/min. UHPLC conditions
were set according to the instructions provided by the manufacturer
with specific exceptions: 1) to enhance the separation of amino
acid eluting midway in the chromatogram, AccQ-Taq Ultra Eluent B
(Waters Corporation, USA) was chosen instead of the MassTrak AAA
Eluent B (19); 2) to enhance the
separation of arginine and glycine, a steeper gradient curve
between 2 and 5.5 min was applied. Concentrations were expressed in
µmol/l. The analyses were carried out at the Department of Clinical
Genetics, The Metabolic Laboratory, Rigshospitalet.
For the interpretation, it is important to note that
during the analyses, the supply lines are represented by the blood
concentrations of the metabolic building materials. The blood
glucose levels are measured as HbA1c and higher HbA1c values will
directly correspond to a higher circulatory availability of
glucose. The interpretation of the blood levels of glutamine,
arginine and cystathionine is, however, slightly more complex. The
general interpretation provided is that the higher use of these
materials in cancer cells can lead to a lower concentration in the
blood. The observation of lower levels will therefore be presumed
to be indicative of a greater consumption and thus higher
utilization of the supply lines (20). Accordingly, these observations
concerning the supply lines of the metabolic building materials
constitute the general framework for the interpretation of the
following results.
Study design and statistical
analysis
The study was conducted using a case-cohort study
design. The 32 patients who had developed distant metastases were
selected as cases, whereas the 32 patients who had not developed
distant metastases were randomly sampled as the subcohort. All 64
patients were selected/sampled from a total cohort consisting of
816 patients diagnosed with breast cancer who were potentially
eligible for inclusion. The patients were followed from the day the
blood sample was drawn between 2011 and 2015 until November 30,
2019. During that period, any pathologically verified distant
metastasis occurring was recorded.
Cox regression was used to determine the impact of
the levels of the metabolic building materials on the development
of distant metastases. To account for the non-random sampling of
the cases inherent to the case-cohort design, inverse probability
weighting was applied in the regressions using Lin-Ying
weights.
First, regressions were performed to initially
assess the impact of the supply lines for the metabolic building
materials individually while adjusting for tumor histology and age.
Next, a regression model was created containing the supply lines of
all selected metabolic building materials, i.e., HbA1c, glutamine,
arginine and cystathionine at the same time, along with tumor
histology and age as covariates. In an attempt to elucidate
potential pattern effects, this model was subsequently modified to
include an interaction term based on a selected set of supply
lines. Finally, an ‘index model’ was created to examine the
potential impact of metabolic supply line patterns comprising the
cumulated increased utilization of multiple supply lines
simultaneously. In this model, an ‘index value’ of 0 to 4 was
calculated for each of the 64 patients where 1 point is given for
displaying a higher utilization of glucose, glutamine, arginine and
cystathionine, respectively. Specifically, 1 point is given for
displaying an HbA1c value above the third quartile, 1 point is
given for displaying a glutamine value below the median, 1 point is
given for displaying an arginine value below the median and 1 point
is given for displaying a cystathionine value below the median. The
third quartile is used for the HbA1c values since the HbA1c
analysis presents an averaged value of the blood glucose
concentration. For glutamine, arginine and cystathionine, the
median is used since these values are less stable and more prone to
be presenting fluctuations. Thus, the ‘index value’ is the summed
value of these points and can therefore range from 0 to 4. The
‘index model’ then performs a Cox regression investigating the
association between this ‘index value’ and distant metastasis
development. Afterwards, a small set of variations of the model are
run to further detail this association.
P-values <0.05 were considered statistically
significant. All statistical analyses were performed using R
statistical software version 3.6.1.
Results
In the regression analyses examining the effects of
the metabolic supply lines individually, only HbA1c was
significantly associated with the development of distant
metastases, when adjusted for tumor histology and age. For
glutamine, arginine and cystathionine, no significant association
was found. Likewise, for tumor histology and age, no significant
association was found (Table
I).
 | Table I.Development of distant metastases:
Individual impact of the metabolic supply lines (adjusted for tumor
histology and age). |
Table I.
Development of distant metastases:
Individual impact of the metabolic supply lines (adjusted for tumor
histology and age).
| Parameter | Hazard ratio | 95% CI | P-value |
|---|
| HbA1c (glucose) |
|
|
|
| HbA1c,
mmol/mol | 1.148 | (1.001, 1.317) | 0.048 |
| Age,
years | 0.996 | (0.925, 1.073) | 0.916 |
| Tumor
histology (ER+ ILC) | 0.536 | (0.137, 2.097) | 0.370 |
| Glutamine |
|
|
|
|
Glutamine, µmol/l | 0.996 | (0.989, 1.003) | 0.267 |
| Age,
years | 1.018 | (0.949, 1.093) | 0.612 |
| Tumor
histology (ER+ ILC) | 0.913 | (0.241, 3.456) | 0.894 |
| Arginine |
|
|
|
| Arginine,
µmol/l | 0.997 | (0.973, 1.021) | 0.802 |
| Age,
years | 1.017 | (0.947, 1.092) | 0.641 |
| Tumor
histology (ER+ ILC) | 0.815 | (0.230, 2.892) | 0.752 |
| Cystathionine |
|
|
|
|
Cystathionine, µmol/l | 0.644 | (0.285, 1.454) | 0.289 |
| Age,
years | 1.019 | (0.950, 1.093) | 0.602 |
| Tumor
histology (ER+ ILC) | 0.717 | (0.197, 2.614) | 0.614 |
In the regression model including the effects of the
supply lines of glucose (HbA1c), glutamine, arginine and
cystathionine plus tumor histology and age, at the same time, HbA1c
and cystathionine appeared significantly associated with the
development of distant metastases (Table II). Hence, HbA1c remained
significant but with the hazard ratio increasing in this model and
with a noticeably smaller P-value. Cystathionine, which was
non-significant in the previous individual models, now turned
significant in this combined model.
 | Table II.Development of distant metastases:
Multivariate model including all metabolic supply lines (plus tumor
histology and age). |
Table II.
Development of distant metastases:
Multivariate model including all metabolic supply lines (plus tumor
histology and age).
| Parameter | Hazard ratio | 95% CI | P-value |
|---|
| HbA1c,
mmol/mol | 1.309 | (1.149, 1.490) | <0.001 |
| Glutamine,
µmol/l | 1.000 | (0.992, 1.009) | 0.925 |
| Arginine,
µmol/l | 0.968 | (0.927, 1.011) | 0.144 |
| Cystathionine,
µmol/l | 0.145 | (0.032, 0.665) | 0.013 |
| Age, years | 0.951 | (0.872, 1.036) | 0.249 |
| Tumor histology
(ER+ ILC) | 0.397 | (0.101, 1.567) | 0.187 |
Since lower values of cystathionine and higher
values of HbA1c are associated with metastasis development,
respectively, they may drive the hazard in opposite directions in
the regressions. This may explain why the initial individual
regressions fail to demonstrate the associations presented in the
latter model where adjustment is possible.
Subsequently, this model was further modified to
include an interaction term based on the potential interaction
between the supply lines of cystathionine and glucose (HbA1c). This
interaction term was significant (Table III) and is interpreted to
indicate that, if the value of cystathionine is low, the value of
HbA1c becomes more critical, whereas when the value of
cystathionine is high, the value of HbA1c becomes less critical.
Thus, the hazard of developing distant metastases appears to be
greatest in the presence of a supply line pattern where a high
supply of glucose (HbA1c) is accompanied by a high supply of
cystathionine.
 | Table III.Development of distant metastases:
Interaction between the supply lines of glucose and
cystathionine. |
Table III.
Development of distant metastases:
Interaction between the supply lines of glucose and
cystathionine.
| Parameter | Hazard ratio | 95% CI | P-value |
|---|
| HbA1c:
Cystathionine (high) | 0.906 | (0.738, 1.111) | 0.343 |
| HbA1c:
Cystathionine (low) | 1.371 | (1.236, 1.521) | <0.001 |
The results from the ‘index model’ showed that the
‘index value’ was significantly associated with the development of
distant metastases (Table IV). As
mentioned above, the ‘index value’ can range from 0 to 4 and is
calculated based on 1 point being given for displaying a higher
utilization of glucose, glutamine, arginine and cystathionine,
respectively. The ‘index model’ thereby approximates a significant
relationship where the hazard substantially increases for any
additional supply line that is subjected to increased utilization.
The model thus proposes that the hazard of developing distant
metastases is greatest when a high level of supplies from multiple
supply lines are present at the same time.
 | Table IV.Development of distant metastases:
Index model and variations of the index model. |
Table IV.
Development of distant metastases:
Index model and variations of the index model.
| Parameter | Hazard ratio | 95% CI | P-value |
|---|
| Index model |
|
|
|
| Index
value | 1.935 | (1.028, 3.640) | 0.041 |
| Variations of the
index model |
|
|
|
| Index
value >2 points | 3.785 | (0.964,
14.864) | 0.056 |
| Index
value >3 points | 44.150 | (10.440,
186.708) | <0.001 |
To further detail this association, including
investigating if the effect extends beyond the two already
demonstrated significant supply lines, two ‘variations of the index
model’ were created. A model investigating the effect of increased
utilization of at least three of the four studied supply lines
(‘index value >2 points’) simultaneously was created, and a
model investigating the effect of increased utilization of all four
of the studied supply lines (‘index value >3 points’)
simultaneously was created (Table
IV). The first variation model presented a quite noticeable
hazard ratio but was nevertheless just non-significant. In the
second model, a significant rather drastic hazard ratio did occur.
This drastic value seems to result from the fact that simultaneous
utilization of all four supply lines appeared to be present
exclusively among patients who had developed distant metastases.
This may, in principle, potentially convey an important
observation, but it must also be maintained that ensuing
reproduction would be valuable.
In addition, it is important to note that these
results also seem to imply an effect of glutamine and arginine on
the development of distant metastasis.
The results from these variations of the ‘index
model’, along with the abovementioned results, are interpreted as
indicative of a general tendency where a combination of a higher
number of supply lines subjected to increased utilization may be
associated with the development of distant metastases. However, at
the same time, the results also open the possibility of potential
nuances to this association.
Discussion
HbA1c appeared significantly associated with the
development of distant metastases both individually and in the
model including all the investigated metabolites. Furthermore, it
figures as a component of the significant ‘index value’ in the
‘index model’. An augmented uptake and use of glucose is a central
and presumably the most well-known alteration in the metabolic
reprogramming occurring in cancer cells. The Warburg effect
describes this change in glucose metabolism and comprises that
cancer cells direct glucose through glycolysis despite oxygen being
adequately available. The resulting amplified glycolytic flux
provides an increased amount of intermediates available to
branching pathways, which are able to support and underlie
malignant processes (4,21). For example, this may subsequently
allow for an upregulation of the branching pentose phosphate
pathway, which will then facilitate an increased production of
NADPH to ensure sufficient antioxidant capacity. This NADPH
production may function as a central mechanism enabling the
development of distant metastases, since cancer cells undergoing
metastatic progression need to counter and balance substantially
elevated levels of reactive oxygen species (ROS) associated with
this progression. This is critical because failure to mitigate
these ROS levels will result in cell damage and cell death
(2,22). This example illustrates how a
higher availability of glucose in the blood may potentially fuel
processes contributing to the development of distant
metastases.
Cystathionine was significant in the regression
model containing all the supply lines and is, similarly to HbA1c
and the other metabolites, a part of the calculated ‘index value’.
Cystathionine is a sulfur-containing metabolite and functions in
the transsulfuration pathway through which it is involved in the
production of glutathione, which is important in balancing ROS
(23). Studies have indicated that
cystathionine can be imported into cells through the system
xc− transporter via xCT to fuel glutathione
synthesis (24), and, generally,
that the exogenous supply can be used to counteract ROS-induced
apoptosis (25). Considering the
importance of ROS-mitigation in cancer cells, a higher
cystathionine supply may therefore play a role in metastasis
development. Moreover, the reactions related to the
transsulfuration pathway can generate hydrogen sulfide
(H2S), which has been proposed to support metastatic
progression by inducing epithelial-mesenchymal transition (EMT) and
cell migration and VEGFA-mediated angiogenesis (26,27).
Accordingly, a higher cystathionine supply may potentially fuel
these reactions leading to a greater production of H2S
with the ensuing metastatic consequences. As with glucose, these
are examples of how a higher availability of cystathionine in the
blood may be interpreted to potentially support the processes of
metastasis.
In addition to the individual effects of glucose
(HbA1c) and cystathionine, respectively, the interaction term based
on the interaction between the supply lines of these building
materials appeared significant, thereby indicating that the risk of
developing metastases is greater with a supply line pattern where a
high supply of both glucose and cystathionine is present. Hence,
glucose and cystathionine may act synergistically in promoting
metastatic progression. Specifically, the interaction term
indicated that the value of HbA1c becomes more critical, if, at the
same time, the value of cystathionine is low.
One possible theoretical interpretation of this
interaction is based on the contemplation that cystathionine may
potentially fuel the production of glutathione, which, in order to
exert its antioxidant effect, needs NADPH that can be derived from
glucose. This interplay may explain that, in order for cancer cells
to counter ROS levels to allow for metastatic progression, adequate
supply lines for both building (cystathionine) and subsequently
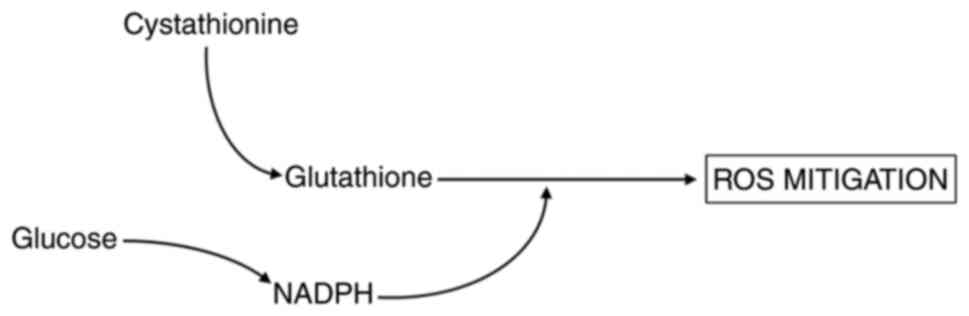
running (glucose) the antioxidant machinery are needed (Fig. 1).
This would be consistent with the observation that
the highest risk of developing metastases arises when the supply of
both glucose and cystathionine is increased. Furthermore, it would
be consistent with the specific observation that the HbA1c value is
more important when the cystathionine value is low. If the
cystathionine value is high (indicating low utilization), adequate
amounts of NADPH would be less important, as glutathione would be
present in only suboptimal measures.
Another possible theoretical interpretation of the
interaction is related to the potential involvement of
cystathionine in generating H2S. It has been suggested
that H2S-mediated S-sulfhydration of lactate
dehydrogenase A (LDHA) can upregulate its enzymatic activity and
thereby serve to accelerate glycolysis and consequently the Warburg
effect (26). The accelerated
Warburg effect may then promote metastatic progression by
generating NADPH for ROS-mitigation, or, for instance, through the
resulting lactate production which can induce cell migration,
angiogenesis and reduced immune responses (28). The most effective manifestation of
this effect would again be in the presence of an adequate supply of
both cystathionine and glucose, since this pattern would allow for
the cystathionine derived H2S to accelerate the Warburg
effect and glucose to fuel and run through it. Concordantly, the
HbA1c value becomes more important when the cystathionine value is
low, as glucose would be needed to ensure adequate flux through the
elevated glycolysis.
The above examples are hypothesized possible
interpretations of the basis for the observed interaction effect
and more interpretations could be made. What remains central in
this study, is that the observed interaction effect points towards
the effect of patterns as a central aspect. Furthermore, this is in
line with the results of the ‘index model’.
The ‘index model’ proposes a general relationship
where the greatest risk of developing distant metastases occurs
during a supply line pattern where high supplies from multiple
supply lines are present at the same time. This reflects the
complexity of cancer metabolism with multiple metabolic processes
being involved in malignant progression. Moreover, it is in
accordance with an emphasis on the importance of pattern
effects.
High supplies from multiple supply lines may provide
a set of favorable conditions for cancer to develop. In general,
such conditions may ensure the fueling of the various processes
driving different aspects of malignancy. For example, while glucose
and cystathionine may facilitate ROS-mitigation, other metabolites
may facilitate different functions likewise contributing to the
malignant progression. Arginine may potentially fuel processes
involved in immune evasion (29).
Likewise, glutamine is a building material central to the metabolic
alterations in cancer cells, and it has been demonstrated that
highly invasive cancer cells can be characterized by upregulated
glutamine metabolism (30). This
observation can be extrapolated to include more potential effects
derived from any additionally utilized supply of building
materials. In this way, a supply line pattern comprising high
supplies from multiple supply lines may potentially facilitate the
employment of different malignant processes at the same time, for
instance ROS-mitigation, immune evasion, invasiveness etc., which
together and combined may propel disease progression. Moreover,
utilization of multiple supply lines is a prerequisite for the
interacting and synergistically working supply lines patterns, as
described with regard to glucose and cystathionine. More of such
interacting patterns obviously exist, and the simultaneous
involvement of multiple supply lines serves as the foundation for
the manifestation of these patterns.
Thus, a metabolic supply line pattern comprising
multiple supply lines may hypothetically be able to simultaneously
underlie multiple malignant processes, thereby enabling the
manifestation of a cancer with more comprehensive malignant
capacity.
It is important to note that the results appeared to
allow for nuances in the relationship concerning the simultaneous
utilization of multiple supply lines. While the general association
is that increased utilization of high supplies of multiple building
materials may support metastatic development, it is possible that
in some cases, a low circulatory supply of a given building
material might be beneficial to the cancer. For example, arginine
is, while potentially being involved in immune evasion processes,
coincidently also required by immune cells to maintain their
optimal function (29,31). Based on this, it is possible that
the supply line pattern driving malignancy most effectively might
potentially also include a restricted supply of given metabolites.
Nevertheless, an emphasis on the effect of patterns is maintained
in both cases.
Together, the results from this study collectively
seem to demonstrate that the condition of metabolic supply lines
may impact outcome in patients with cancer, and furthermore, the
results place a significant emphasis on the effects of patterns
with regard to these supply lines.
When evaluating the results of this study, there are
certain aspects and limitations that must be taken into
consideration. When interpreting the results for glutamine,
arginine and cystathionine, it is important to acknowledge that the
observed blood concentrations are presumed to be indicative of the
utilization of these materials rather than their actual
availability in the blood; it does therefore not directly follow
from this that intervening this availability would carry an effect.
A potential effect of such an intervention could, however, for
example be considered with reference to the current clinical use of
asparaginase, which is used to lower circulatory asparagine in the
treatment of acute lymphoblastic leukemia (ALL). Furthermore, it is
important to consider that plasma generally contains cystathionine
at very low concentrations, which are around the limit of
quantification for the method, and the measurement of cystathionine
is therefore subjected to a degree of uncertainty. However, it
seems improbable that this measurement uncertainty would apply
differently based on following metastasis status, and that this
would manifest as significant associations. Such non-differential
measurement error would be presumed to attenuate the associations
(32). The inclusion of
cystathionine as a binary variable in the interaction analysis and
index model would also reduce this uncertainty. Moreover, common
difficulties when examining amino acid concentrations in relation
to cancer exist. While the general interpretation provided is that,
for the amino acids, a higher use will lead to a lower
concentration in the blood, it is important to note that higher
concentrations can sometimes also be found to correlate with
malignancy, and that generally multiple conditions may influence
the association (20). Studies
have demonstrated the observation that lower concentrations can
reflect an increased use. For instance, this has been performed
during colon cancer surgery by determining the arterio-venous
difference of the amino acid levels in the tumor region and in the
adjacent non-tumor region. The study generally observed a
significantly greater arterio-venous difference for multiple amino
acids in the tumor region, which, accordingly, was considered to
result from an increased uptake by the tumor (33). However, the aforementioned
difficulties should be maintained. Furthermore, it is important to
note that the scale of this study is relatively small in terms of
the number of included patients, the focus on one type of cancer
and the fact that few metabolic building materials are examined.
Furthermore, corresponding tissue enzyme activity for the
metabolites measured in the blood was not investigated. In general,
the explorative nature of this work must be considered. This study
should be seen as an initial step aiming to investigate any
potential effect of patterns occurring between the metabolic supply
lines underlying malignant processes in relation to the clinical
outcome in patients with cancer. The results from this study seem
to indicate that such pattern effects may in fact correlate with
clinical outcome, and ensuing studies are therefore warranted.
Concerning the reflections upon a certain level of
the supply lines compromising malignant capacity, a purely
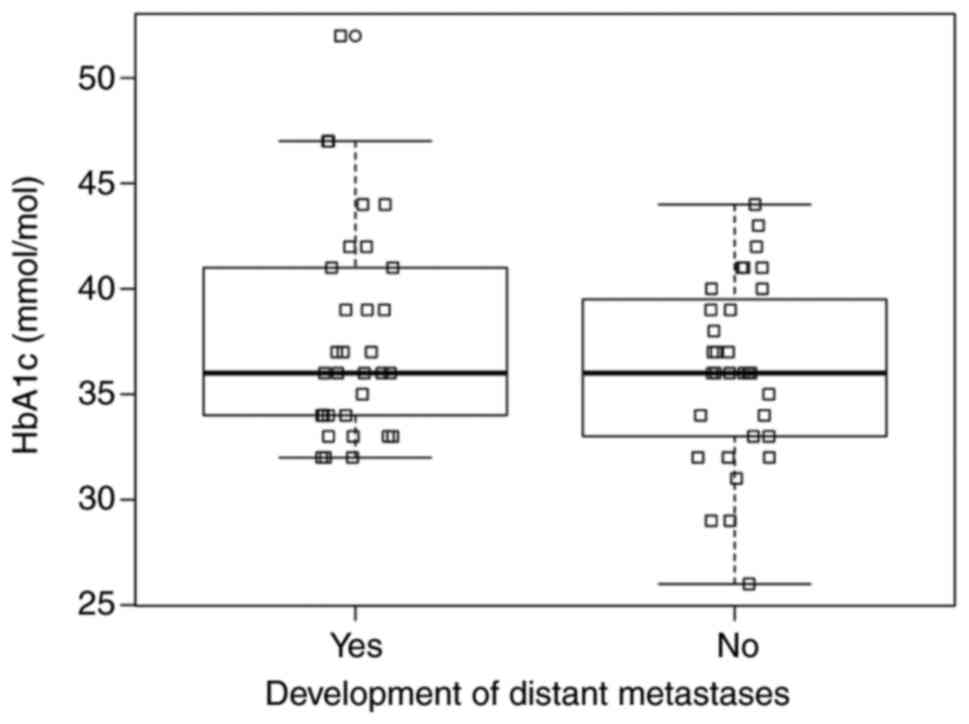
descriptive observation of the HbA1c values seems relevant. When
the HbA1c values are divided according to whether the patients had
developed distant metastases or not, it is noteworthy that, among
the patients who had developed distant metastases, no HbA1c value
below ~32 mmol/mol was displayed, while among the patients who had
not developed distant metastases, multiple patients did present
values below this point (Fig.
2).
It must be stressed that it is possible that the
observation, to some extent, occurs randomly due to a relatively
small number of included patients. On the contrary, the daring
supposition would be to interpret this as to some degree consistent
with the contemplation that an adequate supply from the metabolic
supply lines may potentially be required to facilitate the
processes of malignancy, and likewise, more importantly, that an
intervention of the supply lines could potentially compromise these
processes.
The potential of the approach presented in this
study is based on the observation that the metabolic supply lines
and the supply patterns between them serve as the most basic
foundation for the different malignant processes of a cancer. They
deliver the building blocks that are needed to perform rapid
proliferation, metastatic development etc.; in other words, the
different processes that intrinsically constitute and define the
disease. Therefore, intervening the circulatory availability of the
patterns most crucial to these processes would, in theory, target
the thermodynamic foundation that facilitates the activities that
‘make a cancer a cancer’ (3).
In conclusion, the results from this study indicate
that the levels of metabolic supply lines and the patterns between
them may impact clinical outcome in patients with cancer. The
results particularly place a significant emphasis on the effect of
supply line patterns. Ensuing attempts to conduct investigations
identifying the patterns most crucial to malignancy should
therefore be made. This aim is based on the contemplation that
intervening the circulatory availability of these patterns may
compromise the most basic foundation for exerting these processes
intrinsic to the disease.
Acknowledgements
Not applicable.
Funding
This study was funded by The Medical Society of Copenhagen
(DMSK) and The Research Council of Herlev and Gentofte
Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
OA conceived the idea for the study and drafted the
manuscript. EH took part in all planning. OA, EB, MC, FW, EBJ and
EH designed the study and provided critical evaluation of the work.
All authors provided scientific expertise within their respective
fields. MC carried out the amino acid analysis. OA and EBJ
performed the statistical analysis. OA and EH confirm the
authenticity of all the raw data. All authors edited the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Danish Committees on
Health Research Ethics (approval no. H-19042347). Informed consent
was obtained from all participants. The study was performed in
accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
2
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv. 2:e16002002016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abrahamsen O, Balslev E and Høgdall E:
Targeting the supply lines of cancer-A possible strategy for
combating the disease? Anticancer Res. 41:2737–2744. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barua R, Templeton AJ, Seruga B, Ocana A,
Amir E and Ethier JL: Hyperglycaemia and survival in solid Tumours:
A systematic review and Meta-analysis. Clin Oncol (R Coll Radiol).
30:215–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon JM, Thomas F, Czernichow S, Hanon O,
Lemogne C, Simon T, Pannier B and Danchin N: Hyperglycaemia is
associated with cancer-related but not non-cancer-related deaths:
Evidence from the IPC cohort. Diabetologia. 61:1089–1097. 2018.
View Article : Google Scholar
|
|
7
|
Murtola TJ, Sälli SM, Talala K, Taari K,
Tammela TLJ and Auvinen A: Blood glucose, glucose balance, and
disease-specific survival after prostate cancer diagnosis in the
Finnish randomized study of screening for prostate cancer. Prostate
Cancer Prostatic Dis. 22:453–460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Tao M, Zhao L and Zhang X: The
association between diabetes/hyperglycemia and the prognosis of
cervical cancer patients: A systematic review and meta-analysis.
Medicine (Baltimore). 96:e79812017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu D, Peng F, Lin X, Chen G, Liang B, Li
C, Zhang H, Liao X, Lin J, Zheng X and Niu W: The elevated
preoperative fasting blood glucose predicts a poor prognosis in
patients with esophageal squamous cell carcinoma: The Fujian
prospective investigation of cancer (FIESTA) study. Oncotarget.
7:65247–65256. 2016. View Article : Google Scholar
|
|
10
|
Contiero P, Berrino F, Tagliabue G,
Mastroianni A, Di Mauro MG, Fabiano S, Annulli M and Muti P:
Fasting blood glucose and long-term prognosis of non-metastatic
breast cancer: A cohort study. Breast Cancer Res Treat.
138:951–959. 2013. View Article : Google Scholar
|
|
11
|
Nik-Ahd F, Howard LE, Eisenberg AT,
Aronson WJ, Terris MK, Cooperberg MR, Amling CL, Kane CJ and
Freedland SJ: Poorly controlled diabetes increases the risk of
metastases and castration-resistant prostate cancer in men
undergoing radical prostatectomy: Results from the SEARCH database.
Cancer. 125:2861–2867. 2019.PubMed/NCBI
|
|
12
|
Berker Y, Vandergrift LA, Wagner I, Su L,
Kurth J, Schuler A, Dinges SS, Habbel P, Nowak J, Mark E, et al:
Magnetic resonance spectroscopy-based metabolomic biomarkers for
typing, staging, and survival estimation of early-stage human lung
cancer. Sci Rep. 9:103192019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling HH, Pan YP, Fan CW, Tseng WK, Huang
JS, Wu TH, Chou WC, Wang CH, Yeh KY, Chang PH, et al: Clinical
significance of serum glutamine level in patients with colorectal
cancer. Nutrients. 11:8982019. View Article : Google Scholar
|
|
14
|
Ma H, Hasim A, Mamtimin B, Kong B, Zhang
HP and Sheyhidin I: Plasma free amino acid profiling of esophageal
cancer using high-performance liquid chromatography spectroscopy.
World J Gastroenterol. 20:8653–8659. 2014. View Article : Google Scholar
|
|
15
|
Zheng H, Dong B, Ning J, Shao X, Zhao L,
Jiang Q, Ji H, Cai A, Xue W and Gao H: NMR-based metabolomics
analysis identifies discriminatory metabolic disturbances in tissue
and biofluid samples for progressive prostate cancer. Clin Chim
Acta. 501:241–251. 2020. View Article : Google Scholar
|
|
16
|
Jobard E, Pontoizeau C, Blaise BJ,
Bachelot T, Elena-Herrmann B and Tredan O: A serum nuclear magnetic
resonance-based metabolomic signature of advanced metastatic human
breast cancer. Cancer Lett. 343:33–41. 2014. View Article : Google Scholar
|
|
17
|
Cantor JR and Sabatini DM: Cancer cell
metabolism: One hallmark, many faces. Cancer Discov. 2:881–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boroughs LK and Deberardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View
Article : Google Scholar
|
|
19
|
Peake RWA, Law T, Hoover PN, Gaewsky L,
Shkreta A and Kellogg MD: Improved separation and analysis of
plasma amino acids by modification of the MassTrakTM AAA
Solution Ultraperformance® liquid chromatography method.
Clin Chim Acta. 423:75–82. 2013. View Article : Google Scholar
|
|
20
|
Bi X and Henry CJ: Plasma-free amino acid
profiles are predictors of cancer and diabetes development. Nutr
Diabetes. 7:e2492017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teoh ST and Lunt SY: Metabolism in cancer
metastasis: Bioenergetics, biosynthesis, and beyond. Wiley
Interdiscip Rev Syst Biol Med. 102018.doi: 10.1002/wsbm.1406.
|
|
23
|
Mosharov E, Cranford MR and Banerjee R:
The quantitatively important relationship between homocysteine
metabolism and glutathione synthesis by the transsulfuration
pathway and its regulation by redox changes. Biochemistry.
39:13005–13011. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi S, Sato M, Kasakoshi T, Tsutsui
T, Sugimoto M, Osaki M, Okada F, Igarashi K, Hiratake J, Homma T,
et al: Cystathionine is a novel substrate of cystine/glutamate
transporter: Implications for immune function implications for
immune function. J Biol Chem. 290:8778–8788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu M, Du J, Chen S, Liu AD, Holmberg L,
Chen Y, Zhang C, Tang C and Jin H: L-Cystathionine inhibits the
mitochondria-mediated macrophage apoptosis induced by oxidized low
density lipoprotein. Int J Mol Sci. 15:23059–23073. 2014.
View Article : Google Scholar
|
|
26
|
Wang RH, Chu YH and Lin KT: The hidden
role of hydrogen sulfide metabolism in cancer. Int J Mol Sci.
22:65622021. View Article : Google Scholar
|
|
27
|
Wang M, Yan J, Cao X, Hua P and Li Z:
Hydrogen sulfide modulates epithelial-mesenchymal transition and
angiogenesis in non-small cell lung cancer via HIF-1α activation.
Biochem Pharmacol. 172:1137752020. View Article : Google Scholar
|
|
28
|
Payen VL, Porporato PE, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 1: Tumor pH, glycolysis and the pentose phosphate pathway.
Cell Mol Life Sci. 73:1333–1348. 2016. View Article : Google Scholar
|
|
29
|
Kim SH, Roszik J, Grimm EA and Ekmekcioglu
S: Impact of l-arginine metabolism on immune response and
anticancer immunotherapy. Front Oncol. 8:672018. View Article : Google Scholar
|
|
30
|
Simpson NE, Tryndyak VP, Beland FA and
Pogribny IP: An in vitro investigation of metabolically sensitive
biomarkers in breast cancer progression. Breast Cancer Res Treat.
133:959–968. 2012. View Article : Google Scholar
|
|
31
|
Albaugh VL, Pinzon-Guzman C and Barbul A:
Arginine-Dual roles as an onconutrient and immunonutrient. J Surg
Oncol. 115:273–280. 2017. View Article : Google Scholar
|
|
32
|
Carroll RJ, Ruppert D, Stefanski LA and
Crainiceanu CM: Measurement error in nonlinear models: A modern
perspective. Chapman and Hall/CRC; 2006
|
|
33
|
Wang LB, Shen JG, Zhang SZ, Ding KF and
Zheng S: Amino acid uptake in arterio-venous serum of normal and
cancerous colon tissues. World J Gastroenterol. 10:1297–1300. 2004.
View Article : Google Scholar
|