Introduction
Lung cancer is one of the leading causes of cancer
morbidity and mortality in the world. According to GLOBOCAN
estimates for 2020, lung cancer is the most common type of cancer
in men and the third most common type of cancer in women (1). Furthermore, lung cancer has the
highest cancer-associated death rate in men and the second highest
cancer-associated death rate in women (1). The World Health Organization predicts
that by 2025, the number of individuals with lung cancer in China
will reach 1 million (2). Thus,
lung cancer is a considerable public health concern.
The development of lung cancer is affected by
several factors, such as environmental and genetic factors
(3). Environmental factors include
smoking, drinking, infection and exposure to ionizing radiation,
amongst others (4). As
environmental factors play such a strong role in lung cancer, less
attention is paid to genetic factors. The ABO blood types are a
very stable genetic trait. Reports have linked it to cancer risk
(5–7); however, the molecular mechanisms
involved are less clear. Blood group antigens may influence
systemic inflammatory responses associated with malignancy
(8–11). In addition, blood group antigens
are expressed in several tissues, including certain malignant
cells. However, there are some differences between ABO antigens
expressed on the surface of malignant cells and those on normal
tissues (12,13). This may influence the behaviors of
the tumor cells, thereby promoting or inhibiting the proliferation
of tumor cells (14).
The association between gastric cancer and blood
type A was first noted by Aird et al (5) in 1953. Since this, a study by Hems
(6) reported a correlation between
breast cancer and type A blood, and a study by Vioque and Walker
(7) also reported that type A
blood was associated with an increased risk of pancreatic cancer in
1991. There have been several reports on the association between
lung cancer and blood type. However, consistent conclusions have
not been drawn. Urun et al (15) showed that non-O blood types were
associated with an increased risk of lung cancer. However, Peng
et al (16) reported that
the occurrence of lung cancer was independent of blood type.
Association studies with small sample sizes lack statistical power
and may result in contradictory results. Based on the
aforementioned points, a meta-analysis was conducted on the
association between the ABO blood classification types and the
occurrence of lung cancer.
Materials and methods
Search strategy
A comprehensive search of PubMed
(pubmed.ncbi.nlm.nih.gov/), Embase
(embase.com/landing?status=grey), Web of Science
(webofscience.com), Medline (https://www.nlm.nih.gov/medline/index.html), China
National Knowledge Infrastructure (CNKI, http://www.cnki.net/), Google Scholar
(scholar.google.com), Science Direct (https://www.sciencedirect.com) and Wanfang databases
(https://www. wanfangdata.com.cn/) was
performed for studies published before February 1, 2022. The
following English search strategy was used: (‘lung carcinoma’ OR
‘lung cancer’) AND ‘ABO’. A manual search was performed by
reviewing a list of references in the retrieved studies. The
studies were included if they were in English or Chinese only.
Eligibility criteria
The literature inclusion criteria were: i) Clear
pathological diagnosis and ABO blood group typing; ii)
case-controlled study or a cohort study; iii) the source and the
raw data for the cases and controls were present; and iv) data on
ethnicity, geographical distribution and publication year of the
study were available.
The exclusion criteria were: i) Review articles and
meta-analyses; ii) irrelevant or repetitive literature; iii)
studies without a control group; and iv) studies with no useful
data.
Data extraction
Information was extracted from all eligible studies
by two reviewers independently. The information was then
cross-checked to ensure no required data were missing. The
following variables were extracted from each study: The year of
publication, the name of the first author, the country of origin,
the source of the control group (social means that the control
group used routine patients attending health checkups or healthy
blood donors from the area. Hospital means that the control group
used non-cancer patients or patients attending health checkups from
the same hospital as the experimental group), the study design, and
the number of cases and controls with different ABO blood group
types. If there was a disagreement in the extraction of
information, it was discussed and reviewed with a third author. All
the data presented in the study were agreed upon.
Study quality assessment
The Newcastle-Ottawa Scale (NOS) was used to
evaluate the quality of the included articles. Articles with a NOS
score of ≥6 were considered high quality (17). The evaluation of case-controlled
studies included selection (4 points), comparability (2 points) and
exposure (3 points). The evaluation content of the cohort study
included selection (4 points), comparability (2 points) and outcome
(3 points).
Trial sequential analysis (TSA)
TSA was performed using TSA v0.9.5.10 Beta software
developed by The Clinical Trial Center in Copenhagen, Denmark
(18). In a case-controlled study,
the OR was set to be reduced to 20% with a probability of type I
error of A=0.05 and b=0.2 to estimate the required information size
(RIS). If the cumulative Z value exceeded the RIS threshold, the
result was considered statistically significant and the sample size
was sufficient. If it did not exceed the RIS, the sample size was
considered insufficient, suggesting that additional data were
needed to draw the conclusion.
Statistical analysis
Case-controlled studies and cohort studies used odds
ratios (ORs) and relative risks (RRs) with 95% confidence intervals
(CIs) to assess the association between different blood types and
lung cancer risk, respectively. Heterogeneity was assessed using
I2 statistics and a χ2 test.
I2>50% or P<0.10 was considered statistically
significant heterogeneity. In cases where significant heterogeneity
was detected, the random-effects model was used. Otherwise, the
fixed-effect model was used. In this paper, funnel plots were used
to identify publication bias. Each article was sequentially removed
for sensitivity analysis to determine the impact and stability of
merging OR or RR from individual studies. In addition, subgroup
analysis was conducted for publication year, ethnicity, study type
and source of control. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using Software Review Manager 5.4 (RevMan 5.4; Cochrane).
Results
Study selection and
characteristics
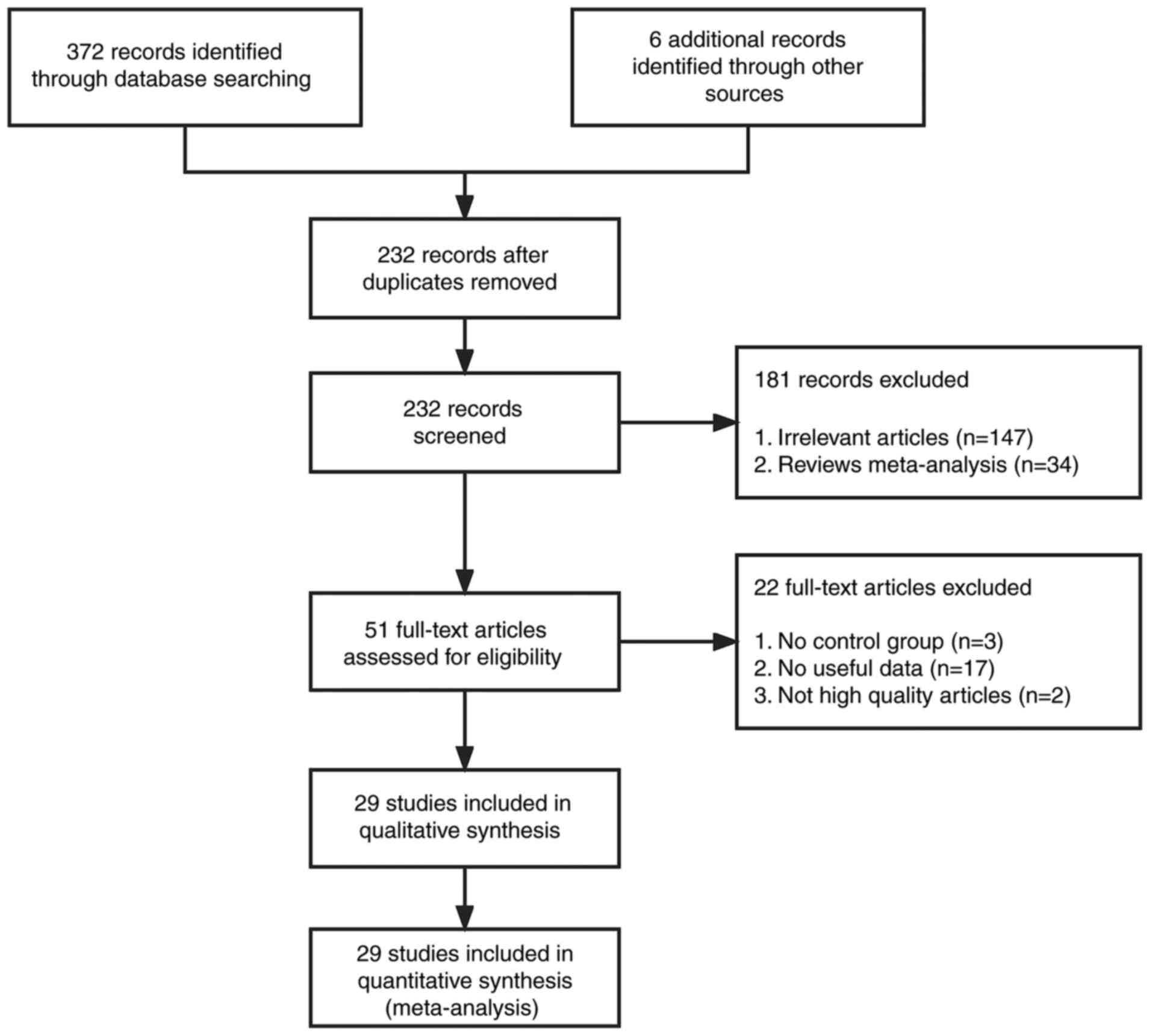
According to the search strategy, 372 articles were
identified from the PubMed, Embase, Web of Science, Medline, CNKI,
Google Scholar, Science Direct and Wanfang databases. A total of 6
articles were identified through citation searching. After removal
of duplications, the search returned 232 records. Finally, after
further screening using the aforementioned inclusion and exclusion
criteria, 29 studies (15,16,19–45)
were eligible for evaluation of ABO blood types and lung cancer
risk (Fig. 1). There were 26
case-controlled studies involving 12,598 patients with lung cancer
and 3,299,927 healthy controls. The characteristics of the included
studies are shown in Table I. Of
these, 22 experiments were based on individuals of Chinese descent
and 4 were based on individuals of Caucasian descent. In terms of
selection of the control group, 20 studies were from the general
populace and 6 studies were from healthy individuals in hospitals.
Blood types were recorded for both the case and control groups in
all studies. There were 3 cohort studies with 363,805 participants,
and ultimately, 2,198 patients with lung cancer. The
characteristics of the included studies are shown in Table II.
 | Table I.Main characteristics of case-control
studies included in the present meta-analysis. |
Table I.
Main characteristics of case-control
studies included in the present meta-analysis.
|
|
|
|
| Lung cancer group,
n | Control group,
n |
|
|---|
| First author/s | Publication
year | Area | Source of
controla |
|
|
|
|---|
| A | B | AB | O | A | B | AB | O | (Refs.) |
|---|
| Xu et
al | 2006 | China | Social | 10 | 14 | 3 | 17 | 1952 | 1211 | 434 | 1822 | (19) |
| Oguz et
al | 2013 | Turkey | Social | 97 | 30 | 20 | 74 | 7756 | 2819 | 1316 | 5423 | (20) |
| Li et
al | 2014 | China | Hospital | 357 | 279 | 83 | 373 | 648 | 492 | 168 | 670 | (21) |
| Sun and Zheng | 2001 | China | Social | 76 | 24 | 29 | 53 | 92 | 66 | 31 | 115 | (22) |
| Yang et
al | 2000 | China | Social | 47 | 56 | 45 | 41 | 984 | 1060 | 344 | 909 | (23) |
| Li et
al | 1995 | China | Social | 35 | 49 | 23 | 44 | 5979 | 7184 | 2189 | 5899 | (24) |
| Wang and Liang | 2000 | China | Social | 30 | 24 | 9 | 55 | 238 | 281 | 79 | 265 | (25) |
| Gao et
al | 1998 | China | Social | 128 | 114 | 42 | 98 | 312 | 252 | 96 | 340 | (26) |
| Xiao et
al | 2021 | China | Hospital | 297 | 276 | 74 | 256 | 342 | 259 | 81 | 379 | (27) |
| Feng and Ying | 2013 | China | Social | 164 | 122 | 37 | 140 | 9274 | 7986 | 2717 | 10542 | (29) |
| Chen et
al | 2004 | China | Social | 230 | 270 | 50 | 346 | 11958 | 13979 | 2634 | 14848 | (30) |
| Tang et
al | 2001 | China | Social | 29 | 58 | 11 | 45 | 23 | 36 | 13 | 49 | (32) |
| McConnell et
al | 1954 | UK | Social | 312 | 55 | 31 | 379 | 406 | 81 | 32 | 481 | (33) |
| Peng et
al | 2014 | China | Hospital | 306 | 265 | 69 | 367 | 4101 | 3308 | 975 | 4819 | (16) |
| Zhao et
al | 1993 | China | Social | 45 | 51 | 11 | 69 | 1664 | 1712 | 406 | 2714 | (34) |
| Rennie and
Haber | 1961 | Australia | Social | 90 | 18 | 3 | 107 | 11520 | 2910 | 900 | 14670 | (35) |
| Jiang and Wang | 1989 | China | Social | 92 | 62 | 22 | 112 | 6262 | 4672 | 1463 | 6781 | (36) |
| Pan et
al | 2006 | China | Social | 382 | 268 | 93 | 399 | 771 | 727 | 251 | 714 | (37) |
| Liu et
al | 2017 | China | Hospital | 41 | 30 | 15 | 29 | 24 | 33 | 7 | 34 | (39) |
| Zhang | 1990 | China | Social | 139 | 81 | 8 | 113 | 6382 | 4491 | 1581 | 7207 | (40) |
| Jin et
al | 2000 | China | Hospital | 43 | 45 | 19 | 51 | 331 | 402 | 123 | 403 | (41) |
| Urun et
al | 2013 | Turkey | Social | 896 | 354 | 167 | 627 | 1276032 | 493769 | 229554 | 1023528 | (15) |
| Liu et
al | 2006 | China | Social | 97 | 46 | 9 | 67 | 3576 | 1870 | 824 | 3820 | (43) |
| Guo | 2001 | China | Social | 99 | 43 | 13 | 66 | 9270 | 6060 | 2463 | 10055 | (42) |
| Cai et
al | 2006 | China | Hospital | 187 | 152 | 41 | 228 | 998 | 1087 | 297 | 1312 | (44) |
| Wang | 1993 | China | Social | 178 | 163 | 26 | 119 | 1484 | 1922 | 650 | 1597 | (45) |
 | Table II.Main characteristics of cohort
studies included in this meta-analysis. |
Table II.
Main characteristics of cohort
studies included in this meta-analysis.
|
|
|
| All participants,
n | Lung cancer group,
n |
|
|---|
| First author | Publication
year | Area |
|
|
|
|---|
| A | B | AB | O | A | B | AB | O | (Refs.) |
|---|
| Huang et
al | 2017 | China | 5586 | 4891 | 1890 | 5702 | 302 | 256 | 104 | 302 | (28) |
| Hsiao et
al | 2015 | China | 1716 | 1388 | 335 | 2865 | 54 | 35 | 13 | 67 | (31) |
| Sun et
al | 2015 | China | 90972 | 82631 | 20279 | 145550 | 294 | 281 | 61 | 429 | (38) |
Study quality
The quality of the included literature was evaluated
according to the NOS. Finally, 29 high-quality studies were
included. The 26 case-controlled studies included were of high
quality (Table III). The 3
cohort studies were all of high quality as well (Table IV).
 | Table III.Newcastle-Ottawa Scale scores for
case-control studies. |
Table III.
Newcastle-Ottawa Scale scores for
case-control studies.
|
| Selection |
| Exposure |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | Adequacy of case
definition | Representativeness
of the cases | Selection of
controls | Definition of
controls | Comparability
cases/controls | Ascertainment of
exposure | Same method of
ascertainment | Non-response
rate | Total scores | (Refs.) |
|---|
| Li et al,
2014 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 | (21) |
| Urun et al,
2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (15) |
| Liu et al,
2017 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 | (39) |
| Oguz et al,
2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (20) |
| Rennie and Haber,
1961 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (35) |
| McConnell et
al, 1954 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (33) |
| Xiao et al,
2021 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 | (27) |
| Peng et al,
2014 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | (16) |
| Cai et al,
2006 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | (44) |
| Xu et al,
2006 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (19) |
| Feng and Ying,
2013 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (29) |
| Liu et al,
2006 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (43) |
| Pan et al,
2006 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (37) |
| Guo, 2001 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (42) |
| Tang et al,
2001 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (32) |
| Sun and Zheng,
2001 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (22) |
| Chen et al,
2004 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (30) |
| Gao et al,
1998 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (26) |
| Yang et al,
2000 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (23) |
| Jin et al,
2000 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | (41) |
| Wang and Liang,
2000 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (25) |
| Jiang and Wang,
1989 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (36) |
| Zhang, 1990 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (40) |
| Zhao et al,
1993 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (34) |
| Wang, 1993 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (45) |
| Li et al,
1995 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (24) |
 | Table IV.Newcastle-Ottawa Scale scores for
cohort studies. |
Table IV.
Newcastle-Ottawa Scale scores for
cohort studies.
|
| Selection |
| Outcome |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposed | Demonstration that
outcome of interest was not present at start of study | Comparability of
cohorts on the basis of the design or analysis | Assessment of
outcome | Was follow-up long
enough for outcomes to occur | Adequacy of
follow-up of cohorts | Total scores | (Refs.) |
|---|
| Huang et al,
2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (28) |
| Hsiao et al,
2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (31) |
| Sun et al,
2015 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (38) |
Meta-analyses of the case-controlled
studies
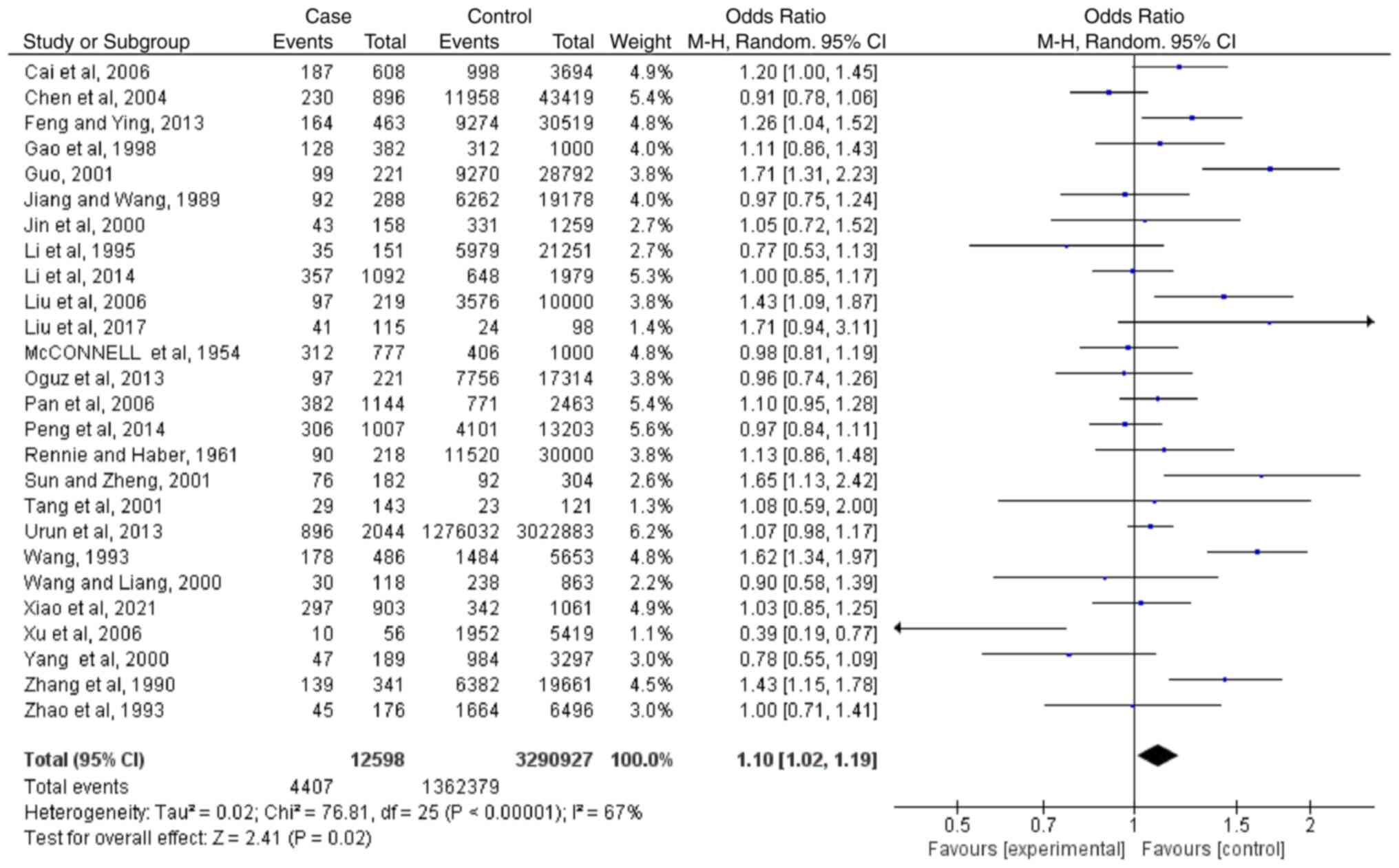
Meta-analysis regarding blood type A
Based on the results of 26 case-controlled studies,
the OR (CI; P-value) of type A blood and the risk of lung cancer
was 1.10 (1.02-1.19; P=0.02). This showed that there was a
difference in the distribution of type A blood between healthy
individuals and patients with lung cancer (Fig. 2). The heterogeneity in the study
was statistically significant (I2=67%; P<0.00001),
and the random-effects model was used.
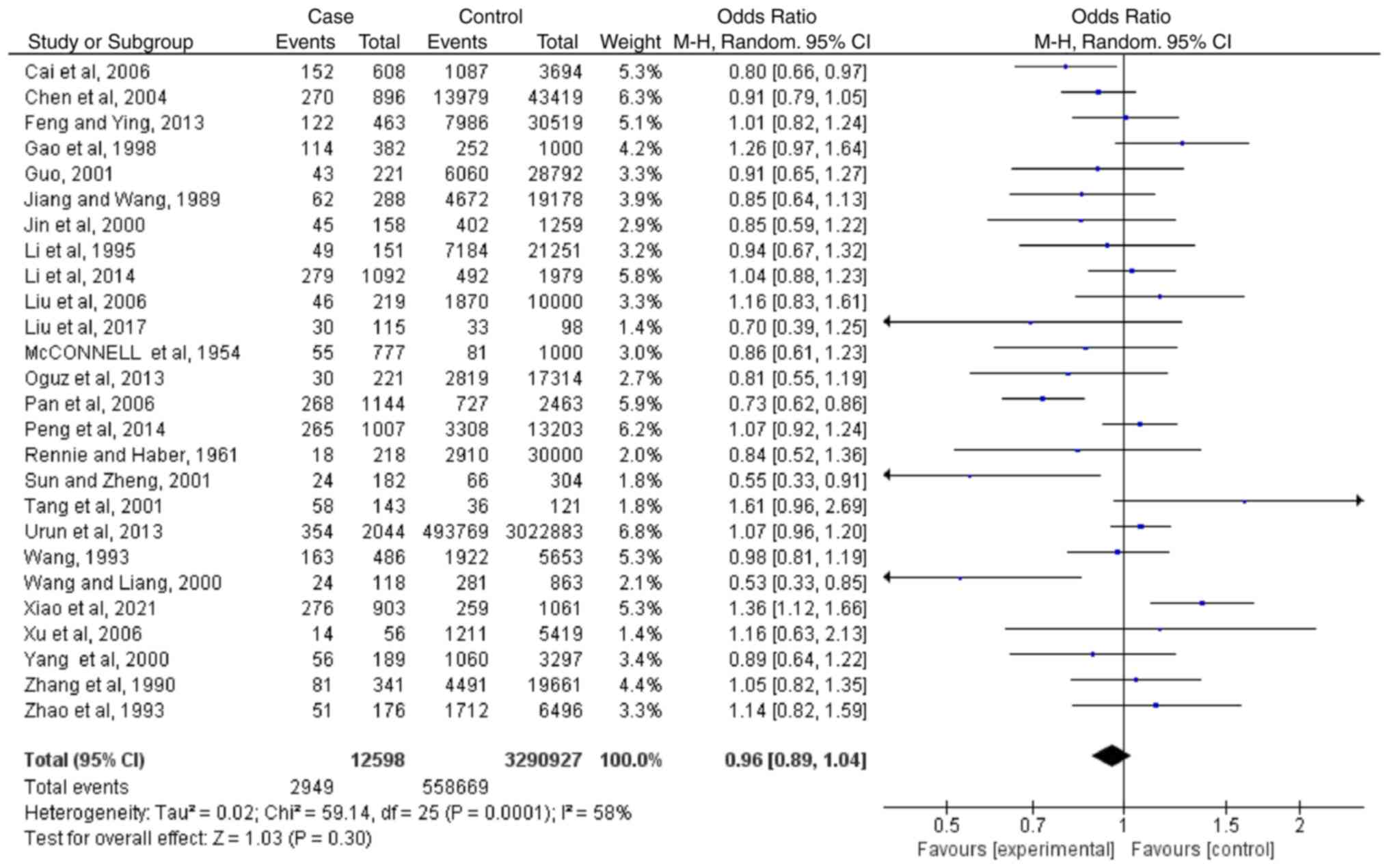
Meta-analyses regarding blood type
B
Based on the results of 26 case-controlled studies,
the OR of type B blood and the risk of lung cancer was 0.96
(0.89-1.04; P=0.30). This showed that there was no significant
difference in the proportion of type B blood between healthy
individuals and patients with lung cancer (Fig. 3). The heterogeneity in the study
was statistically significant (I2=58%; P=0.0001), and
the random-effects model was used.
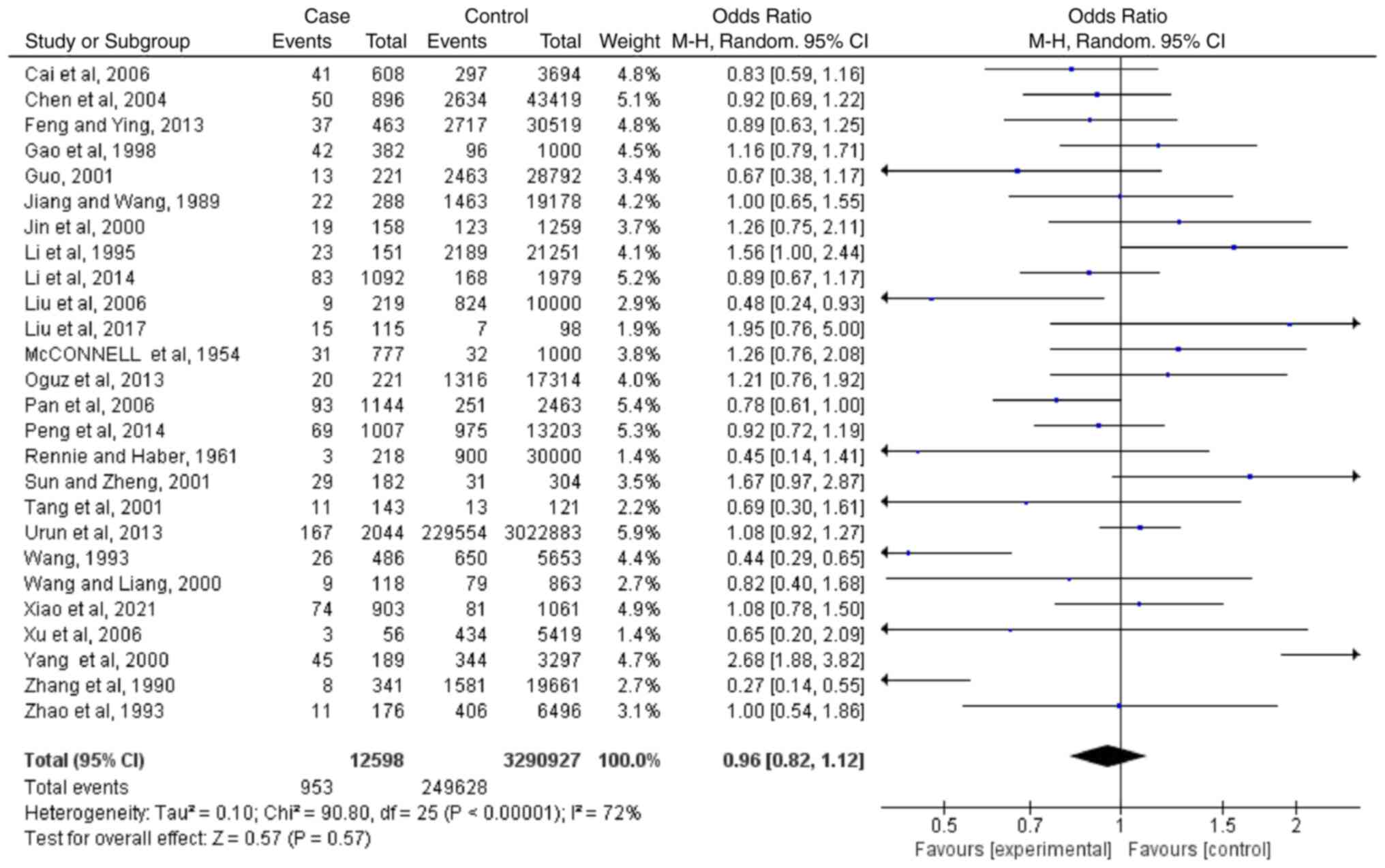
Meta-analyses regarding blood type
AB
Based on the results of 26 case-controlled studies,
the OR of type AB blood and the risk of lung cancer was 0.96
(0.82-1.12; P=0.57). This showed that there was no significant
difference in the proportion of type AB blood between healthy
individuals and patients with lung cancer (Fig. 4). The heterogeneity in the study
was statistically significant (I2=72%; P<0.00001),
and the random-effects model was used.
Meta-analyses regarding blood type
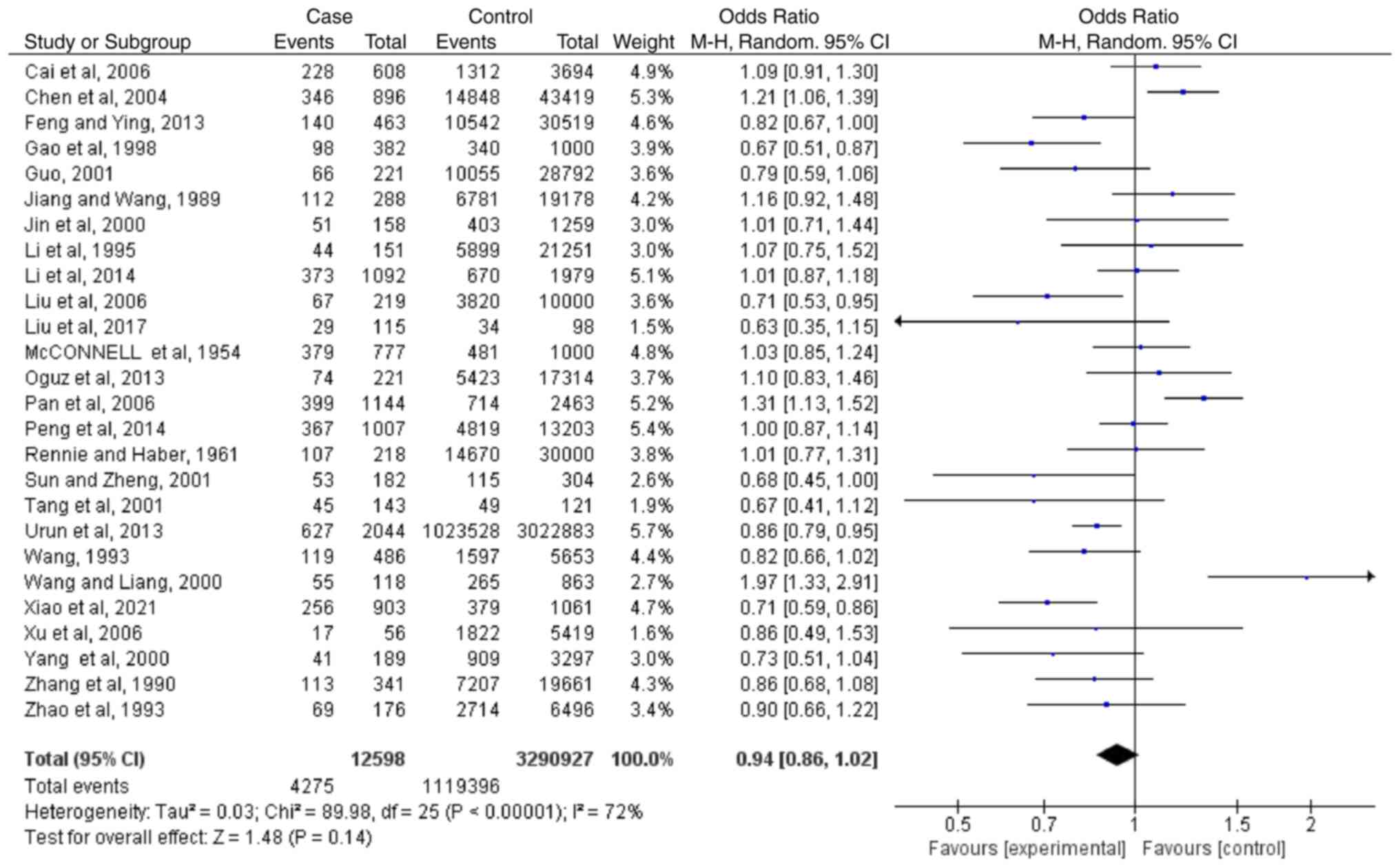
O
Based on the results of 26 case-controlled studies,
the OR of type O blood and the risk of lung cancer was 0.94
(0.86-1.02; P=0.14). This shows that there was no significant
difference in the proportion of type AB blood between healthy
individuals and patients with lung cancer (Fig. 5). The heterogeneity in the study
was statistically significant (I2=72%; P<0.00001),
and the random-effects model was used.
Sensitivity analyses
Sensitivity analysis was performed by removing each
individual study in turn. The results showed that the combined
results were not significantly affected by any specific individual,
indicating that the combined results of the meta-analysis were
reliable (Table V).
 | Table V.Sensitivity analysis of the
association between blood type A and lung cancer risk in the
case-controlled studies. |
Table V.
Sensitivity analysis of the
association between blood type A and lung cancer risk in the
case-controlled studies.
| First author | Publication
year | OR | (95%CI) | P-value | I2 % | (Refs.) |
|---|
| Cai et
al | 2006 | 1.10 | 1.01-1.19 | 0.030 | 68 | (44) |
| Chen et
al | 2004 | 1.11 | 1.03-1.21 | 0.008 | 66 | (30) |
| Feng and Ying | 2013 | 1.10 | 1.01-1.19 | 0.030 | 68 | (29) |
| Gao et
al | 1998 | 1.10 | 1.02-1.20 | 0.020 | 69 | (26) |
| Guo | 2001 | 1.08 | 1.00-1.17 | 0.040 | 63 | (42) |
| Jiang and Wang | 1989 | 1.11 | 1.02-1.20 | 0.010 | 68 | (36) |
| Jin et
al | 2000 | 1.10 | 1.02-1.20 | 0.020 | 69 | (41) |
| Li et
al | 2014 | 1.11 | 1.02-1.20 | 0.020 | 68 | (21) |
| Li et
al | 1995 | 1.11 | 1.03-1.21 | 0.008 | 67 | (24) |
| Liu et
al | 2017 | 1.10 | 1.01-1.19 | 0.020 | 68 | (39) |
| Liu et
al | 2006 | 1.09 | 1.01-1.18 | 0.030 | 67 | (43) |
| McConnell et
al | 1954 | 1.11 | 1.02-1.20 | 0.010 | 68 | (33) |
| Oguz et
al | 2013 | 1.11 | 1.02-1.20 | 0.010 | 68 | (20) |
| Pan et
al | 2006 | 1.10 | 1.01-1.20 | 0.020 | 69 | (37) |
| Peng et
al | 2014 | 1.11 | 1.02-1.21 | 0.010 | 67 | (16) |
| Rennie and
Haber | 1961 | 1.10 | 1.01-1.20 | 0.020 | 69 | (35) |
| Sun and Zheng | 2001 | 1.09 | 1.01-1.18 | 0.030 | 67 | (22) |
| Tang et
al | 2001 | 1.10 | 1.02-1.19 | 0.020 | 69 | (32) |
| Urun et
al | 2013 | 1.10 | 1.01-1.21 | 0.030 | 69 | (15) |
| Wang | 1993 | 1.08 | 1.00-1.16 | 0.040 | 60 | (45) |
| Wang and Liang | 2000 | 1.11 | 1.02-1.20 | 0.010 | 68 | (25) |
| Xiao et
al | 2021 | 1.11 | 1.02-1.20 | 0.020 | 69 | (27) |
| Xu et
al | 2006 | 1.12 | 1.03-1.20 | 0.005 | 65 | (19) |
| Yang et
al | 2000 | 1.11 | 1.03-1.21 | 0.007 | 67 | (23) |
| Zhang | 1990 | 1.09 | 1.01-1.18 | 0.030 | 66 | (40) |
| Zhao et
al | 1993 | 1.11 | 1.02-1.20 | 0.020 | 69 | (34) |
Publication bias regarding blood
type
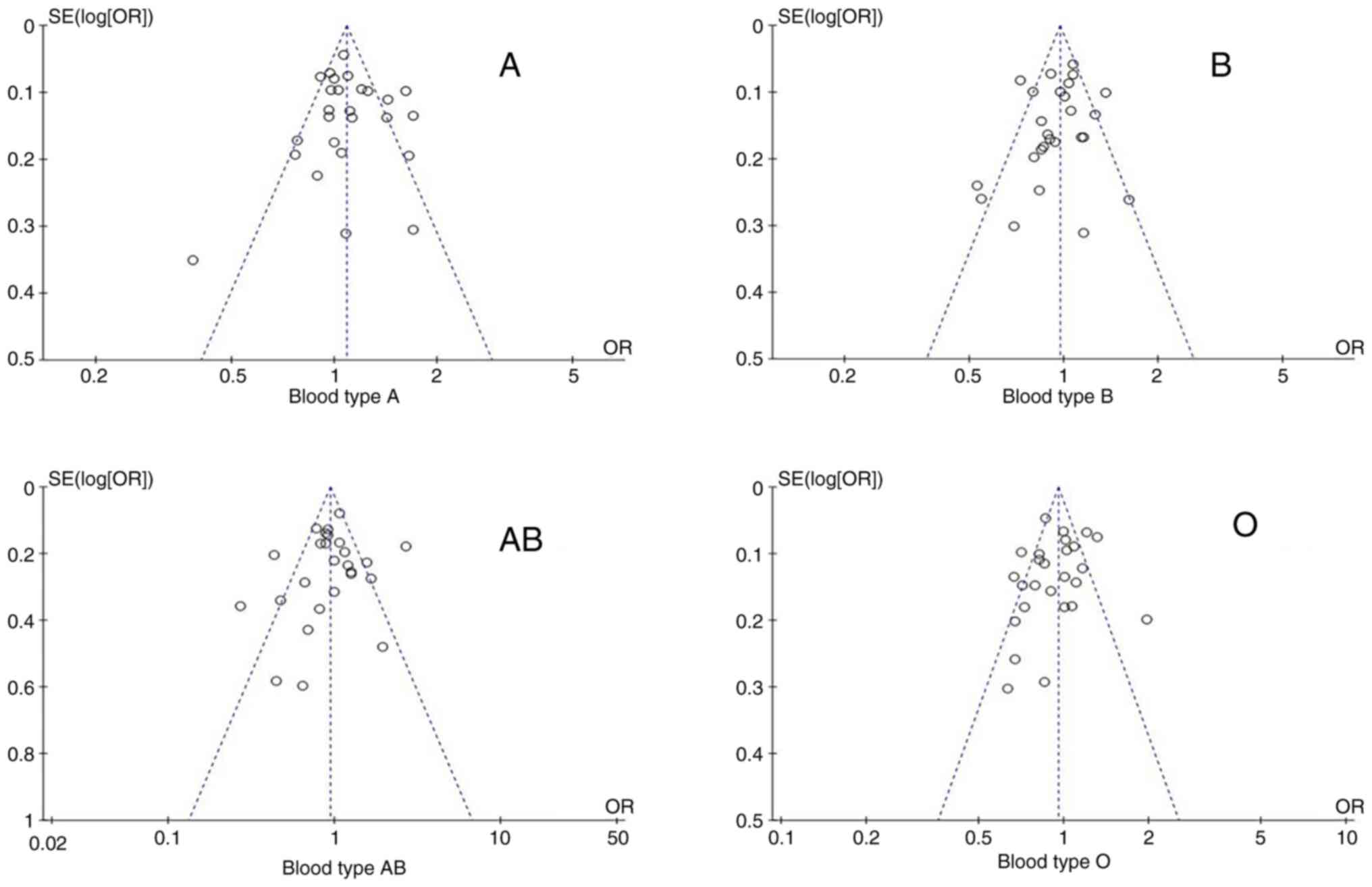
Publication bias was assessed using funnel plots.
The funnel diagram of the association between the ABO blood group
and the risk of lung cancer is shown in Fig. 6. Funnel plots were mostly
symmetric, and the corresponding points of the majority of data
were within the 95% CI, indicating that publication bias had been
adequately controlled.
Subgroup analysis
To assess the effect of each parameter on outcomes,
subgroup analyses were performed based on ethnicity and the source
of the control group (Table VI).
In the subgroup analysis of ethnicity, blood type A was associated
with the risk of lung cancer in patients from China (P=0.03), but
was not associated with lung cancer risk in Caucasians (P=0.18).
Blood type O was not associated with lung cancer risk in patients
from China (P=0.14), but was associated with lung cancer risk in
Caucasian patients (P=0.03). The other blood types did not show
heterogeneity regarding ethnicity. In the subgroup analyses of the
control source, type A blood was associated with the risk of lung
cancer in the control groups that were from the general populace
(P=0.04). In the control groups from healthy individuals in the
hospital, there was no association with the risk of lung cancer
(P=0.34). The other blood types did not show heterogeneity
regarding the source of the control group.
 | Table VI.Subgroup analysis of the association
between ABO blood group and lung cancer risk in case-control
studies. |
Table VI.
Subgroup analysis of the association
between ABO blood group and lung cancer risk in case-control
studies.
|
|
|
|
|
| Test for
heterogeneity |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | n | Blood type | OR (95% CI) | P-value | I2, % | P-value | Analysis model |
|---|
| Ethnicity |
|
|
|
|
|
|
|
|
Chinese | 22 | A | 1.12
(1.01-1.23) | 0.03 | 72 | <0.0001 | R |
|
| 22 | B | 0.96
(0.88-1.05) | 0.40 | 62 | <0.0001 | R |
|
| 22 | AB | 0.93
(0.78-1.12) | 0.47 | 75 | <0.0001 | R |
|
| 22 | O | 0.93
(0.83-1.03) | 0.14 | 75 | <0.0001 | R |
|
Caucasian | 4 | A | 1.05
(0.98-1.13) | 0.18 | 0 | 0.7300 | F |
|
| 4 | B | 1.02
(0.92-1.13) | 0.73 | 17 | 0.31 | F |
|
| 4 | AB | 1.08
(0.94-1.25) | 0.27 | 0 | 0.42 | F |
|
| 4 | O | 0.92
(0.85-0.99) | 0.03 | 41 | 0.17 | F |
| Source of
control |
|
|
|
|
|
|
|
|
Social | 20 | A | 1.11
(1.00-1.23) | 0.04 | 72 | <0.0001 | R |
|
| 20 | B | 0.94
(0.86-1.03) | 0.21 | 52 | 0.0030 | R |
|
| 20 | AB | 0.92
(0.75-1.13) | 0.45 | 78 | <0.0001 | R |
|
| 20 | O | 0.94
(0.84-1.04) | 0.24 | 75 | <0.0001 | R |
|
Hospital | 6 | A | 1.04
(0.96-1.12) | 0.34 | 19 | 0.29 | F |
|
| 6 | B | 1.00
(0.84-1.18) | 0.97 | 71 | 0.004 | R |
|
| 6 | AB | 0.96
(0.83-1.10) | 0.53 | 0 | 0.43 | F |
|
| 6 | O | 0.94
(0.81-1.08) | 0.36 | 64 | 0.02 | R |
TSA
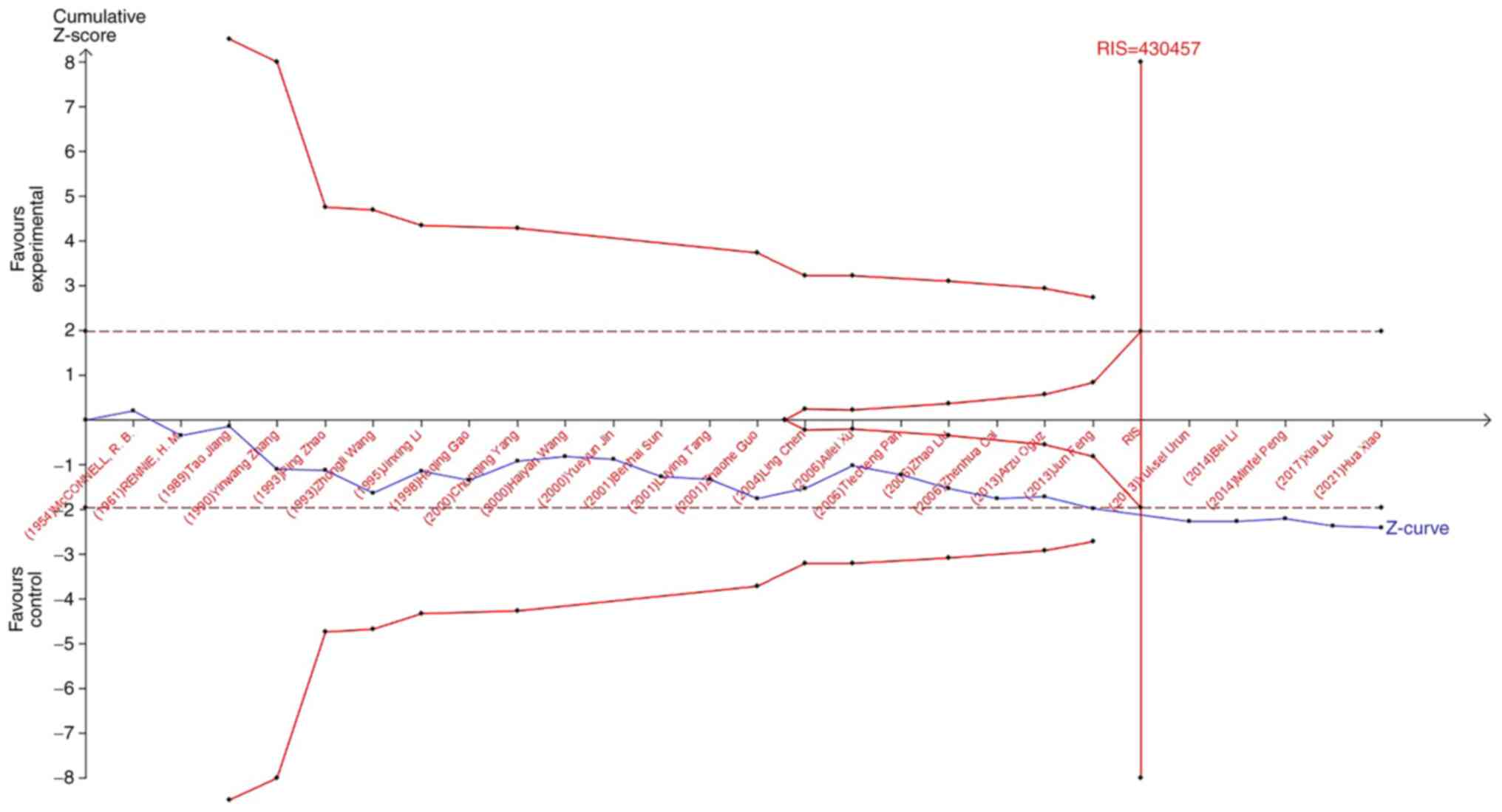
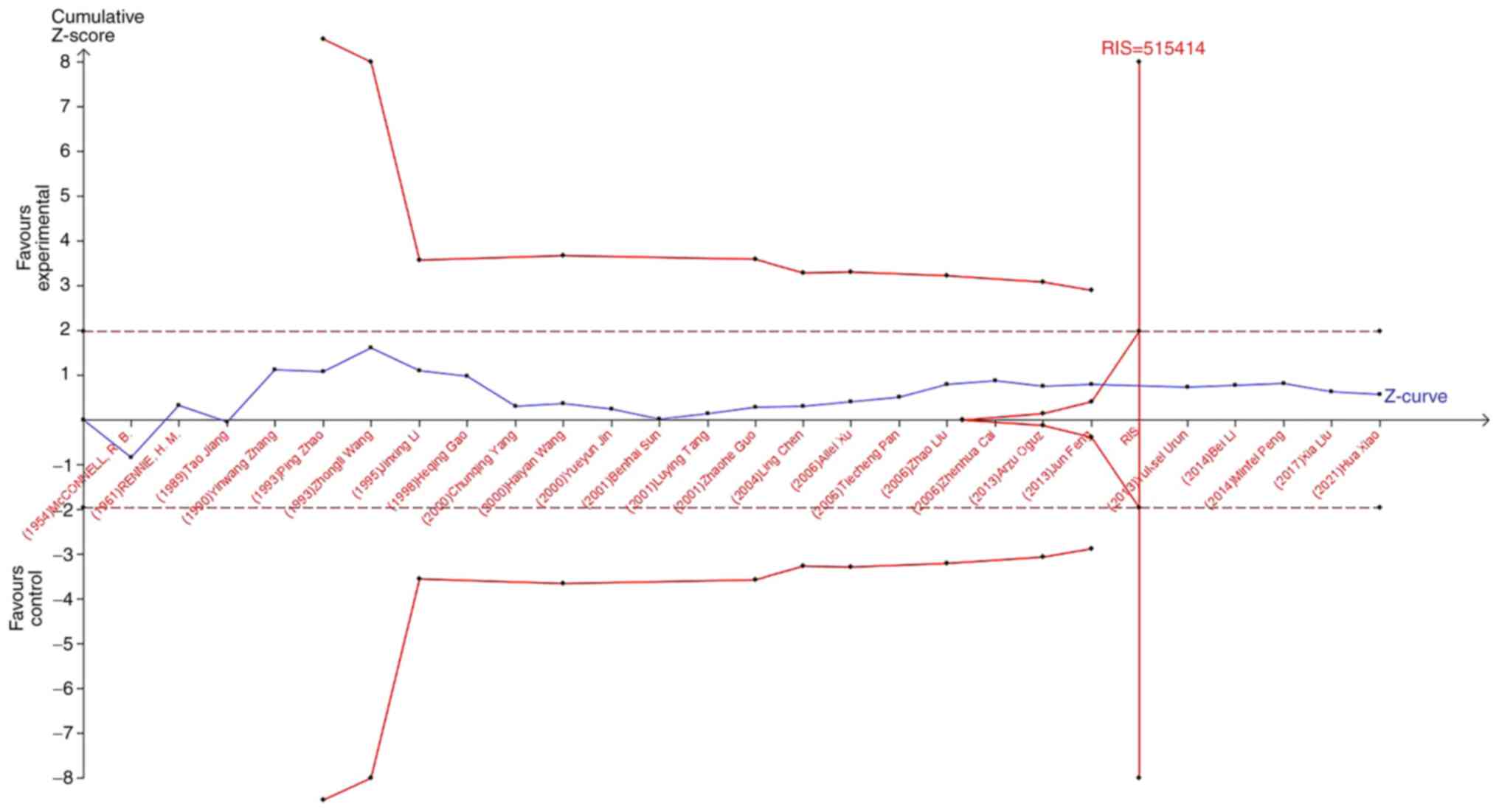
TSA was used to reduce the risk of type 1 error, and
the RIS was evaluated by maintaining a 5% risk of type 1 error and
a 20% relative risk reduction (80% power). As shown in Fig. 7, when studying the effects of blood
type A on the occurrence of lung cancer, the sample size of study
21 (Jun Feng, 2013) crossed the TSA boundary and reached a positive
conclusion in advance. This is consistent with previous
meta-analysis results, suggesting that blood type A increases the
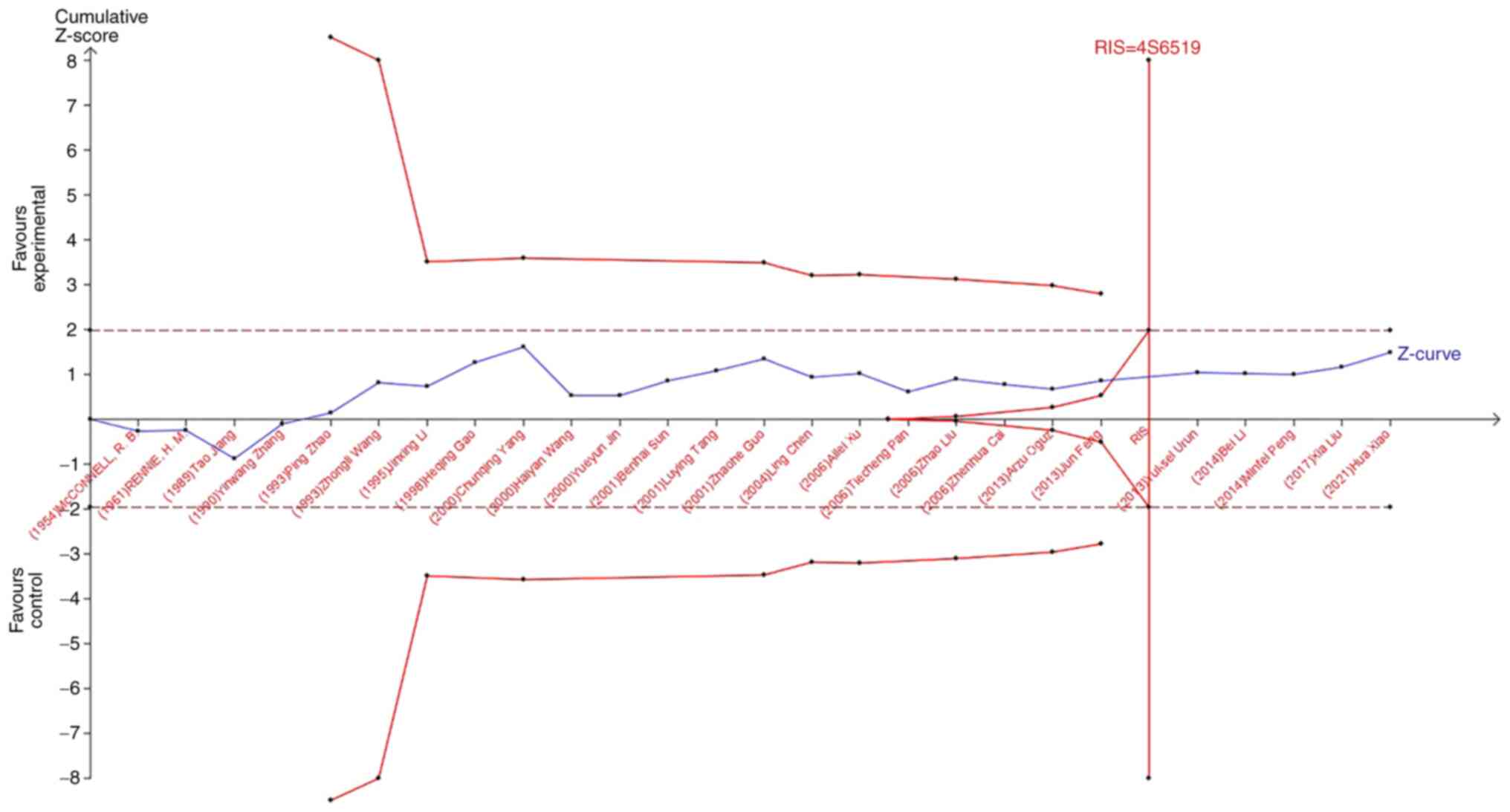
risk of lung cancer. In the study of the influence of blood types
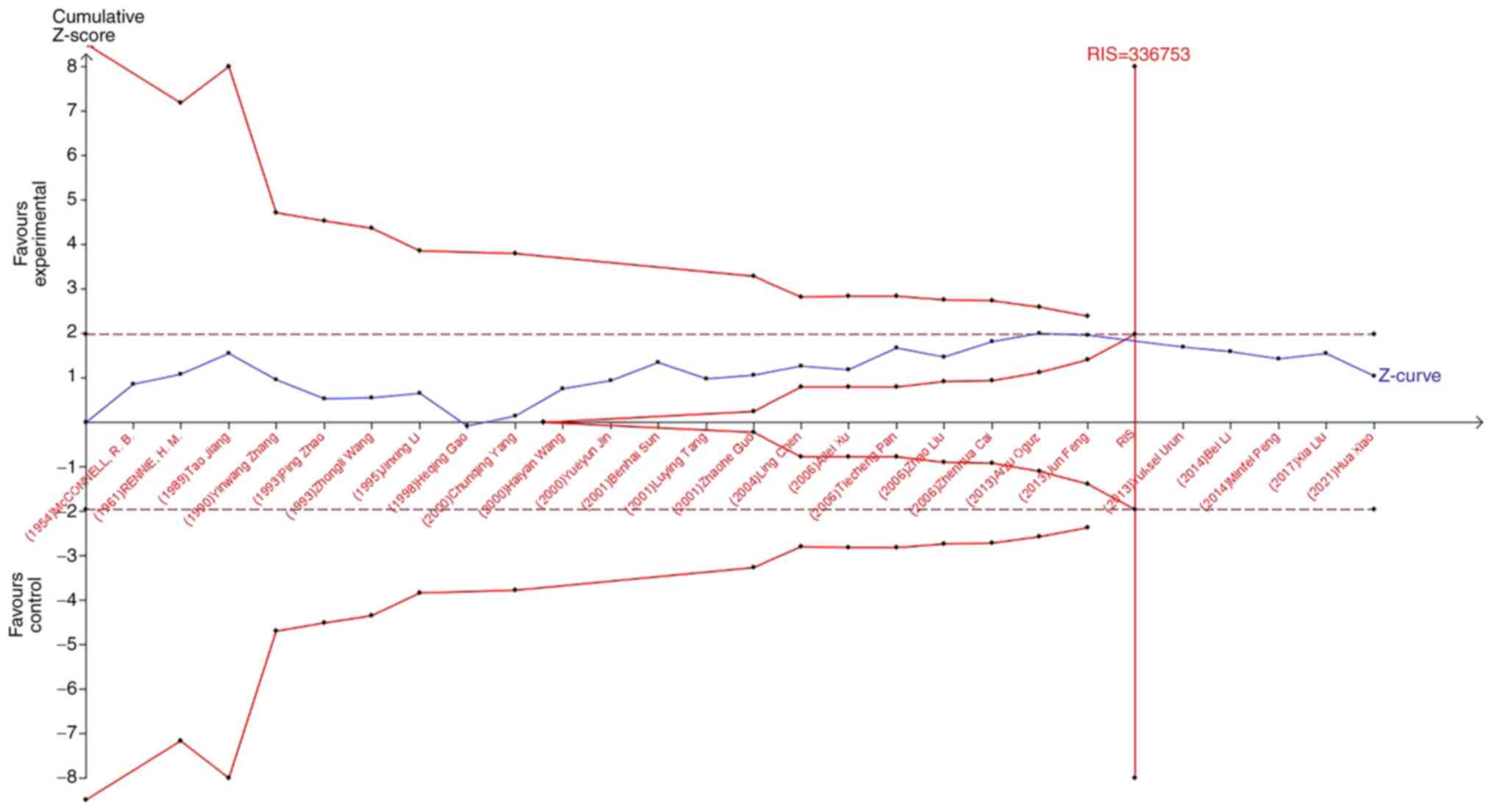
B, O, and AB blood on the occurrence of lung cancer, the Z-curve
did not cross the TSA boundary, but crossed the RIS line (Fig. 8, Fig.
9, Fig. 10). The results
showed that blood types B, AB, and O had no effect on the
occurrence of lung cancer. Moreover, the sample size was sufficient
and no more case-controlled trials are required.
Meta-analyses of cohort studies
Forest plot for meta-analysis
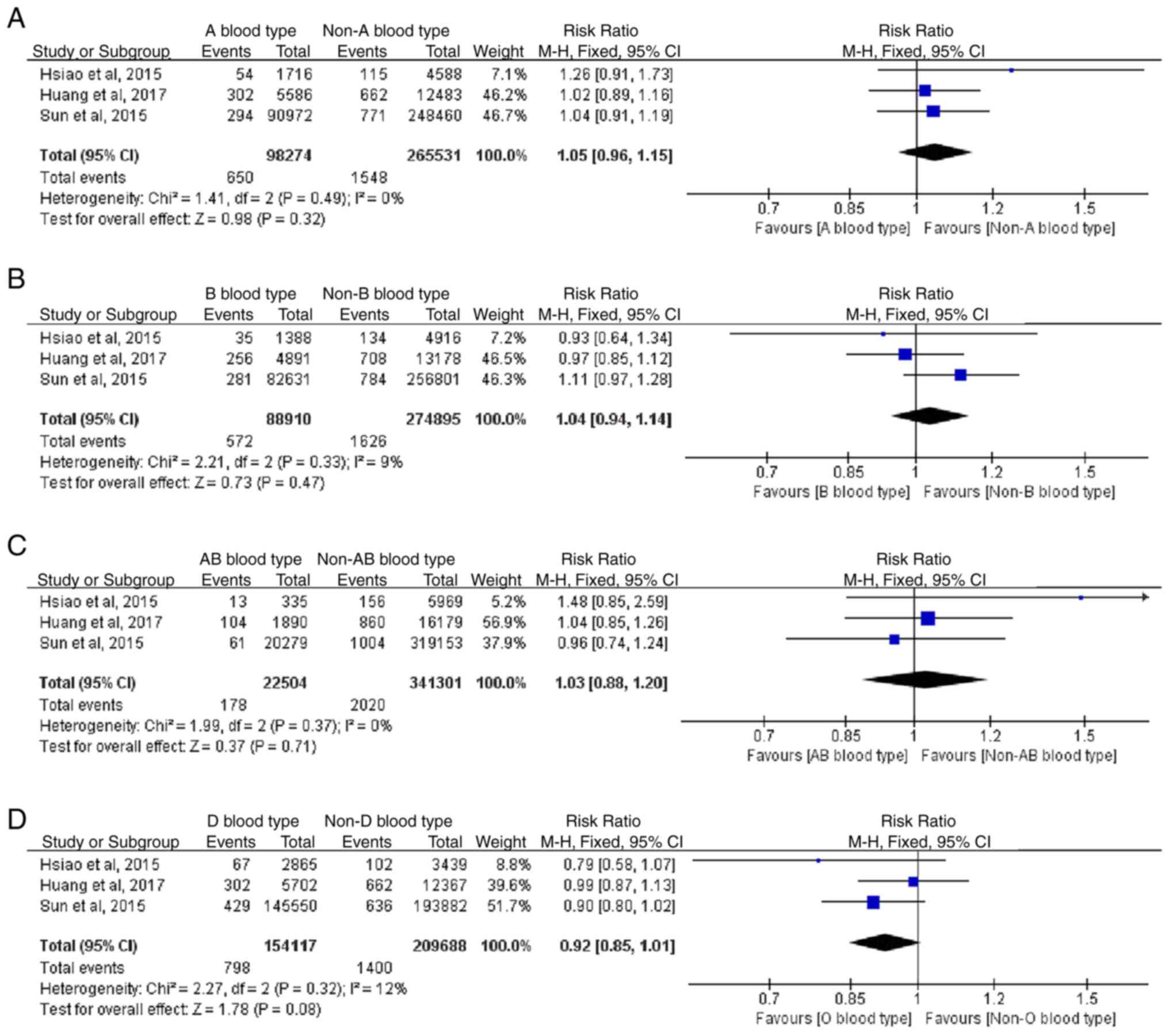
Based on the results of 3 cohort studies, the RR of
blood type A and lung cancer was 1.05 (0.96-1.15; P=0.32), the RR
of blood type B and lung cancer was 1.04 (0.94-1.14; P=0.47) the RR
of blood type AB and lung cancer was 1.03 (0.88-1.20; P=0.71), and
the RR of blood type O and lung cancer was 0.92 (0.85-1.01;
P=0.08). This indicated that there was no statistically significant
difference in blood type regarding the risk of lung cancer
(Fig. 11). Heterogeneity was not
statistically significant in the study, and a fixed-effect model
was adopted.
Publication bias regarding the cohort
studies
Due to the small number of included cohort studies,
funnel plots were not used to assess publication bias.
Discussion
Lung cancer seriously affects the quality of life of
patients. Thus, identifying similarities in the occurrence and
development of lung cancer is key to identifying methods to reduce
the incidence and mortality of affected patients. Since the
discovery of the ABO blood group system by Landsteiner (46,47),
>20 independent systems have been developed for human
erythrocyte surface antigens. Due to its stable heritability, an
increasing number of medical researchers are paying attention to
its role in the occurrence and development of diseases (5–7).
Multiple researchers have performed studies on the ABO blood group
and the risk of lung cancer (15,16).
The present study comprehensively analyzed the
influence of the ABO blood classification on the risk of lung
cancer. By reviewing all eligible case-controlled studies, it was
determined that blood type A was associated with the occurrence of
lung cancer, and that this blood type may be a risk factor for lung
cancer. The other blood types were not associated with the overall
risk of lung cancer. In addition, to further explore the impact of
ethnicity and source of control, subgroup analyses were performed.
The results showed that type A blood was heterogeneous regarding
ethnicity and source of control. These results were obtained when
the study ethnicity was Chinese or the control group was from the
social population. In addition, type O blood was determined to be a
protective factor for lung cancer in Caucasian individuals. In
Chinese individuals, type O blood had no effect on the prevalence
of lung cancer. TSA results suggested that the sample size of the
case-controlled study was sufficient; thus, additional
case-controlled studies are not needed. Furthermore, the results
from the cohort studies suggested that blood type was not
associated with the risk of lung cancer.
The ABO blood group system consists of A and B
antibodies and their corresponding antigens. The ABO blood type of
can be determined by simply testing for the presence of antigens A
or B in the blood. Individuals with type A blood have only A
antigens on their red blood cells, and individuals with type B
blood have only B antigens on their red blood cells. Individuals
with type O blood have neither A nor B antigens in their red blood
cells. Conversely, individuals with type AB have both A and B
antigens. These antigens are present on the surface of red blood
cells and also in several other tissues in the human body. The
genes that determine ABO blood groups are located in the long arm
of chromosome 9, region 3 and band 4 (9q34) (48). It was found that 9q34 contains the
human DNA repair gene XPA, and proto-oncogene C-abl. If these genes
are mutated or defective, they may cause tumor cell proliferation
(49). Additionally, blood group
antigen-associated glycosyltransferases encoded by the 9q34 gene
can regulate intercellular adhesion and signal transduction
(50). This may play an important
role in immune monitoring of tumor cells and their sensitivity to
apoptosis (51). On the other
hand, the underlying mechanism associated with the ABO blood group
and tumorigenesis also includes the inflammatory state of the body.
Studies have identified an association between the ABO blood group
and the circulating levels of TNF-α, soluble ICAM-1, e-selectin and
p-selectin. The association was precisely found to be associated
with the genotype of the A allele (8–10).
This suggests that blood type A may influence inflammation
throughout the body, leading to the development of cancer.
Experimental study has also found that antigen A may improve immune
escape capacity and prevent apoptosis (52). The aforementioned conclusions may
underlie the increased incidence of patients with lung cancer with
type A blood. The effect of ethnicity on the results may be due to
the fact that lung cancer is caused by several factors. The
incidence of lung cancer differs in different regions due to the
different lifestyles of individuals. Furthermore, the ABO blood
group affects several diseases. Therefore, the proportion of blood
types in the control group from the hospital may differ from that
of the total population, resulting in different results in the
control groups from the different sources in this study.
The present study covered a wide range of subjects
over a relatively large span of time. ABO blood group is a very
stable genetic factor, which has not changed over decades.
Therefore, the data from early studies are still valuable and can
be included in this study. This meta-analysis provides a more
accurate assessment of the association of the ABO blood type with
lung cancer risk than previous studies. Additionally, the cohort
study was added based on the inclusion of case-controlled studies.
However, this analysis also has some limitations, as follows: i)
Most of the studies included in the paper included patients of
Chinese descent, thus there is a notable selection bias; ii) lung
cancer has several different types of pathology, and different
pathological types have different paths of pathogenesis (53); therefore, the study results may
change when studying a specific pathological type of lung cancer;
iii) case-controlled studies are observational studies that may
have a selection bias due to incomplete randomization; iv) only a
portion of the case-controlled studies retrieved in this paper
corrected for traditional risk factors; therefore, the confounding
effect of other risk factors cannot be completely controlled; and
v) only the Chinese and English literature were included in this
study, and the results may be affected by the inclusion of
incomplete data.
In conclusion, the meta-analysis of the
case-controlled studies analyzed in the present study suggest that
patients with blood type A are at a higher risk of lung cancer.
However, this result does not apply to Caucasians. In addition,
this study also confirmed that Caucasians with type O blood have a
lower risk of lung cancer. No association was found between other
blood types and the prevalence of lung cancer. Differing study
designs have a considerable impact on the research outcomes. The
results of only three cohort studies showed that blood type was not
associated with the risk of lung cancer. Larger and higher quality
prospective studies recruiting patients from several international
hospitals are required to better explore a more precise association
between ABO blood group and the risk of lung cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PH, HY and ZT contributed to the conception and
design of the study. HY, ZT, YZ and JS prepared the materials,
collected the data and performed the analysis. HY drafted the
manuscript. HY and ZT confirm the authenticity of all the raw data.
All authors revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu X, Zhao H, Suk R and Christiani DC:
Genetic susceptibility to tobacco-related cancer. Oncogene.
23:6500–6523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vachani A, Sequist LV and Spira A: AJRCCM:
100-Year anniversary. The shifting landscape for lung cancer: Past,
present, and future. Am J Respir Crit Care Med. 195:1150–1160.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anand P, Kunnumakkara AB, Sundaram C,
Harikumar KB, Tharakan ST, Lai OS, Sung B and Aggarwal BB: Cancer
is a preventable disease that requires major lifestyle changes.
Pharm Res. 25:2097–2116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aird I, Bentall HH and Roberts JA: A
relationship between cancer of stomach and the ABO blood groups. Br
Med J. 1:799–801. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hems G: Epidemiological characteristics of
breast cancer in middle and late age. Br J Cancer. 24:226–234.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vioque J and Walker AM: Pancreatic cancer
and ABO blood types: A study of cases and controls. Med Clin
(Barc). 96:761–764. 1991.(In Spanish). PubMed/NCBI
|
|
8
|
Melzer D, Perry JR, Hernandez D, Corsi AM,
Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR, Paolisso G,
et al: A genome-wide association study identifies protein
quantitative trait loci (pQTLs). PLoS Genet. 4:e10000722008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbalic M, Dupuis J, Dehghan A, Bis JC,
Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A,
et al: Large-scale genomic studies reveal central role of ABO in
sP-selectin and sICAM-1 levels. Hum Mol Genet. 19:1863–1872. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paterson AD, Lopes-Virella MF, Waggott D,
Boright AP, Hosseini SM, Carter RE, Shen E, Mirea L, Bharaj B, Sun
L, et al: Genome-wide association identifies the ABO blood group as
a major locus associated with serum levels of soluble E-selectin.
Arterioscler Thromb Vasc Biol. 29:1958–1967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi L, Cornelis MC, Kraft P, Jensen M, van
Dam RM, Sun Q, Girman CJ, Laurie CC, Mirel DB, Hunter DJ, et al:
Genetic variants in ABO blood group region, plasma soluble
E-selectin levels and risk of type 2 diabetes. Hum Mol Genet.
19:1856–1862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vowden P, Lowe AD, Lennox ES and Bleehen
NM: The expression of ABH and Y blood group antigens in benign and
malignant breast tissue: The preservation of the H and Y antigens
in malignant epithelium. Br J Cancer. 53:313–319. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strauchen JA, Bergman SM and Hanson TA:
Expression of A and B tissue isoantigens in benign and malignant
lesions of the breast. Cancer. 45:2149–2155. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Pendu J, Marionneau S, Cailleau-Thomas
A, Rocher J, Le Moullac-Vaidye B and Clément M: ABH and Lewis
histo-blood group antigens in cancer. APMIS. 109:9–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Urun Y, Utkan G, Cangir AK, Oksuzoglu OB,
Ozdemir N, Oztuna DG, Kocaman G, Coşkun HŞ, Kaplan MA, Yuksel C, et
al: Association of ABO blood group and risk of lung cancer in a
multicenter study in Turkey. Asian Pac J Cancer Prev. 14:2801–2803.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng M, Yu S, Wang J and Wang D:
Relationship between ABO blood group and risk of 8 kinds of
malignant tumors. Chin J Health Inspection. 24:811–813+823.
2014.(In Chinese).
|
|
17
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa scale (NOS)
for assessing the quality of non-randomized studies in
meta-analysis. Appl Eng Agric. 18:727–734. 2014.https://www.ohri.ca/programs/clinical_epidemiology/oxford.aspMarch
2–2022
|
|
18
|
Thorlund K, Engstrøm J, Wetterslev J, Brok
J, Imberger G and Gluud C: User Manual for Trial Sequential
Analysis (TSA). 2nd edition. Copenhagen: Copenhagen Trial Unit; pp.
1–119. 2017
|
|
19
|
Xu A, He X, Wang W, Tang Y and Ping W:
Relationship between ABO blood group and gastric cancer, liver
cancer and lung cancer. J Clin Mil Med. 722–723. 2006.(In
Chinese).
|
|
20
|
Oguz A, Unal D, Tasdemir A, Karahan S,
Aykas F, Mutlu H, Cihan YB and Kanbay M: Lack of any association
between blood groups and lung cancer, independent of histology.
Asian Pac J Cancer Prev. 14:453–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li B, Tan B, Chen C, Zhao L and Qin L:
Association between the ABO blood group and risk of common cancers.
J Evid Based Med. 7:79–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun B and Zheng Y: ABO blood group typing
in lung cancer patients. J Clin Pulmonology. 2:302001.(In
Chinese).
|
|
23
|
Yang C, Zhang R, Zhou S and Wang Q:
Discussion on the relationship between ABO blood group and lung
cancer, liver cancer and gastric cancer. Clin Transfus Lab.
1:11–12. 2000.(In Chinese).
|
|
24
|
Li J, Hu J and Wang M: The correlation
between esophageal cancer, lung cancer and ABO blood group. Chin J
Blood Transfus. 1:45–46. 1995.(In Chinese).
|
|
25
|
Wang H and Liang X: Relationship between
ABO blood group and 10 kinds of malignant tumors of Han nationality
in Guangxi. Med Lit. 5:643–644. 2000.(In Chinese).
|
|
26
|
Gao H, Zhao L, Wu S, Wang D, Li Y and
Heyun S: Relationship between malignant tumor and ABO blood group.
Chin J Prim Med. 4:41–42. 1998.(In Chinese).
|
|
27
|
Xiao H, Xiang S, Shan Z and Bin X:
Relationship between ABO blood group and different pathological
types of lung cancer in southern Sichuan. Chin J Mod Med.
31:98–102. 2021.(In Chinese).
|
|
28
|
Huang JY, Wang R, Gao YT and Yuan JM: ABO
blood type and the risk of cancer-findings from the Shanghai cohort
study. PLoS One. 12:e01842952017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng J and Ying X: Correlation analysis
between ABO blood group and lung cancer in Zhejiang Han people.
Harbin Med. 33:96–97. 2013.(In Chinese).
|
|
30
|
Chen L, Heng C, Shen W and Xiao X:
Correlation analysis between ABO blood group and malignant tumor.
Chin J Oncol. 3:131–133. 2004.(In Chinese).
|
|
31
|
Hsiao LT, Liu NJ, You SL and Hwang LC: ABO
blood group and the risk of cancer among middle-aged people in
Taiwan. Asia Pac J Clin Oncol. 11:e31–e36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang L, Ren Z, Zhou X, Su Z and Zhuang Z:
ABO blood group and its interaction with smoking and lung cancer
susceptibility. Chronic Dis Prev Control China. 2:70–71. 2001.(In
Chinese).
|
|
33
|
McConnell RB, Clarke CA and Downton F:
Blood groups in carcinoma of the lung. Br Med J. 2:323–325. 1954.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao P, Wu Q and Wang S: Lung cancer and
ABO blood group relationship. Chin J Eugenics Genet. 59–60.
1993.(In Chinese).
|
|
35
|
Rennie HM and Haber RW: Blood groups and
carcinoma of the lung. Med J Aust. 48:61–62. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang T and Wang Z: ABO blood group
distribution in 288 patients with lung cancer. J Chongqing Med
Univ. 220–221. 1989.(In Chinese).
|
|
37
|
Pan T, Zheng Z, Li J, Chen T, Wei X, Hu M
and Liu L: ABO blood group and lung cancer in Hubei province and
literature review. Sixth National Thoracic and Cardiovascular
Surgery Conference; Beijing, China: pp. 22006, (In Chinese).
PubMed/NCBI
|
|
38
|
Sun W, Wen CP, Lin J, Wen C, Pu X, Huang
M, Tsai MK, Tsao CK, Wu X and Chow WH: ABO blood types and cancer
risk-a cohort study of 339,432 subjects in Taiwan. Cancer
Epidemiol. 39:150–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Chen X, Yang J and Guo R:
Association of ABO blood groups with von Willebrand factor, factor
VIII and ADAMTS-13 in patients with lung cancer. Oncol Lett.
14:3787–3794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang YW: Preliminary analysis of ABO
blood group distribution in cancer patients. J Hubei Med Coll.
369–371. 1990.(In Chinese).
|
|
41
|
Jin YY, Wang ZY and Cheng CH: Relationship
between ABO blood group and 3 common cancers. J Luoyang Med Coll.
3:2482000.(In Chinese).
|
|
42
|
Guo ZH: Lung cancer and ABO blood group.
Chin J Mod Med. 1:61–63. 2001.(In Chinese).
|
|
43
|
Liu Z, Qiao Z and Huang P: Association
between ABO blood group and lung cancer. Mod Med Health.
14:2134–2135. 2006.(In Chinese).
|
|
44
|
Cai Z, Fang W, Qin J, Chen S and Lai K:
Correlation between ABO blood group and malignant tumors in
Guangzhou area. J Appl Med Technol. 21:3727–3729. 2006.(In
Chinese).
|
|
45
|
Wang Z: ABO blood group distribution
analysis of lung cancer patients. Shaanxi Med Lab. 242–243.
1993.(In Chinese).
|
|
46
|
Landsteiner K: ZTo know the
antifermentative, lytic and agglutinative effects of blood serum
and lymph. Centr Bakt Orig. 27:357–362. 1900.(In German).
|
|
47
|
Landsteiner K: About agglutination
symptoms of normal human blood. Wien Klin Wochschr. 14:1132–1134.
1901.(In German).
|
|
48
|
Wagner FF, Flegel WA, Bittner R and
Döscher A: Molecular typing for blood group antigens within 40 min
by direct polymerase chain reaction from plasma or serum. Br J
Haematol. 176:814–821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tang Z: Introduction to modern oncology.
Med Res Lett. 242001.
|
|
50
|
Hakomori S: Antigen structure and genetic
basis of histo-blood groups A, B and O: Their changes associated
with human cancer. Biochim Biophys Acta. 1473:247–266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang S, Zhang HS, Cordon-Cardo C, Reuter
VE, Singhal AK, Lloyd KO and Livingston PO: Selection of tumor
antigens as targets for immune attack using immunohistochemistry:
II. Blood group-related antigens. Int J Cancer. 73:50–56. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Marionneau S, Le Moullac-Vaidye B and Le
Pendu J: Expression of histo-blood group A antigen increases
resistance to apoptosis and facilitates escape from immune control
of rat colon carcinoma cells. Glycobiology. 12:851–856. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sequist LV and Lynch TJ: EGFR tyrosine
kinase inhibitors in lung cancer: An evolving story. Annu Rev Med.
59:429–442. 2008. View Article : Google Scholar : PubMed/NCBI
|