Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare form
of non-small cell lung cancer (NSCLC), accounting for 0.1-0.4% of
all lung cancers (1). According to
the standards established by the World Health Organization (WHO),
PSCs may be subclassified into pleomorphic carcinoma, spindle-cell
carcinoma, giant-cell carcinoma, carcinosarcoma and pneumoblastoma
(2). PSC is commonly observed in
older males with a history of smoking and the average age at
diagnosis is 60 years (3).
PSCs are insensitive to conventional radiotherapy
and chemotherapy and patients with PSC tend to have a poorer
prognosis than other patients with NSCLC due to rapid tumor growth,
early metastasis and limited treatment options (4,5). In
the past decade, targeted therapy and immunotherapy have made
tremendous progress in treating lung cancer and improving patient
quality of life. The discovery of therapeutic targets, including
the epidermal growth factor receptor (EGFR), anaplastic lymphoma
kinase (ALK) and receptor tyrosine kinase ROS, programmed death-1
(PD-1) and programmed death ligand 1 (PD-L1) in NSCLC has had a
significant impact on treatment outcomes; however, their PSC
remains genetically unexplored. There have been no large
prospective studies of PSC due to its rarity and limited targeted
therapeutic options, and therefore, the clinical effectiveness of
these agents against PSC has remained elusive (6,7). The
present study reported a unique case of a young patient with lung
adenocarcinoma and sarcomatoid carcinoma, both of which exhibited
echinoderm microtubule-associated protein-like 4-anaplastic
lymphoma kinase (EML4-ALK) gene fusion. After surgery, the disease
progressed rapidly despite treatment with the ALK inhibitor
Alectinib and chemotherapy; after initial treatment failure, the
patient received combined Alectinib with Tirelizumab (anti-PD-1
antibody) and Anlotinib [anti-vascular endothelial growth factor
receptor (VEGFR), anti-angiogenic], which yielded positive
results.
Case report
A 29-year-old male patient with no history of
smoking presented at The Affiliated Hospital of Panzhihua
University (Panzhihua, China) in March 2021 with a cough and chest
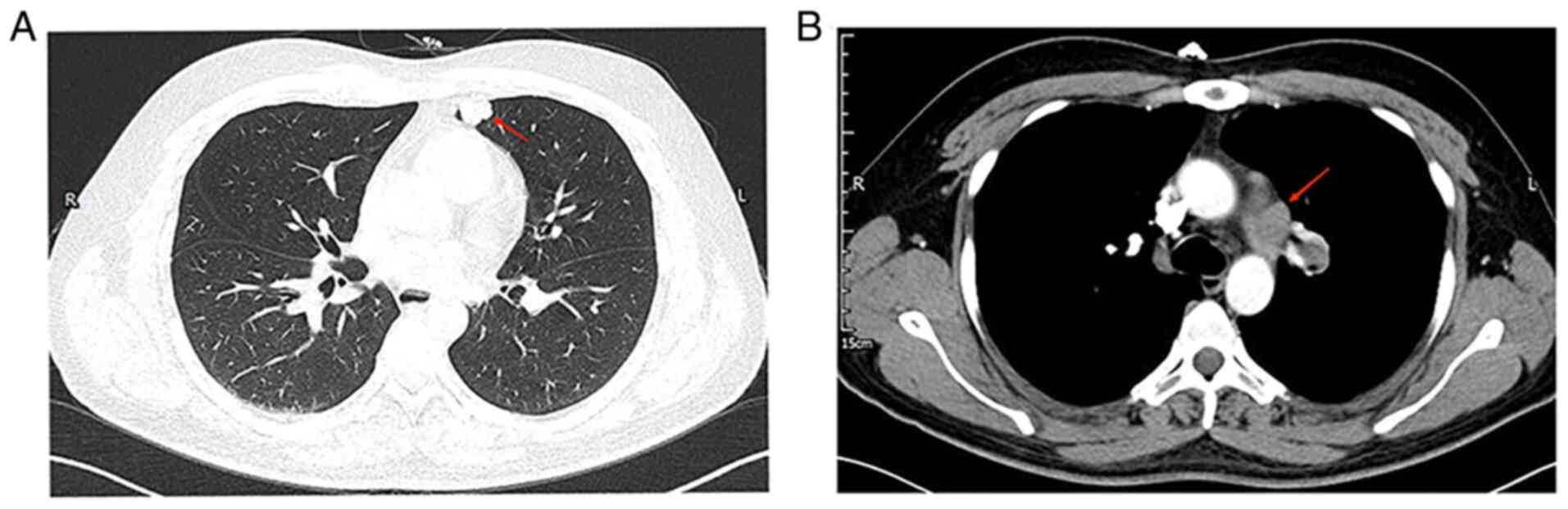
pain. An enhanced chest CT scan revealed a nodular shadow in the
left upper lung (~13×21 mm), a mass-like hyper-density shadow in
the left upper lung hilum and enlarged lymph nodes in the left
hilum and mediastinum (Fig. 1); no
distant organ metastases were observed during the systemic
examination. After five days, the patient underwent left upper lung
lobectomy, left upper lobe pulmonary artery sleeve resection and
systemic lymph node dissection. Post-operatively, the pathological
report identified two tumor nodules, one small and one large, in
the upper lobe of the left lung. The small nodule was close to the
lung pleura (measuring ~2.5×2.0×1.1 cm) and diagnosed as lung
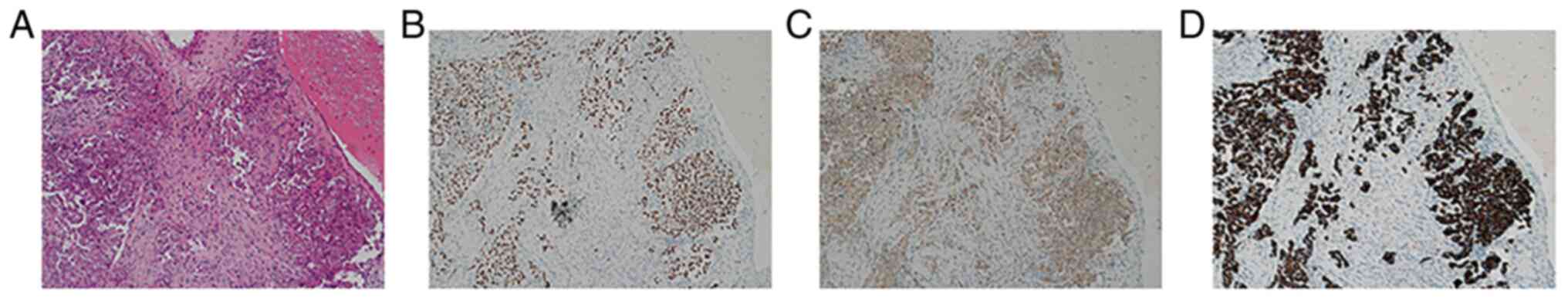
adenocarcinoma by immunohistochemistry (IHC) (Fig. 2). The large nodule was located in
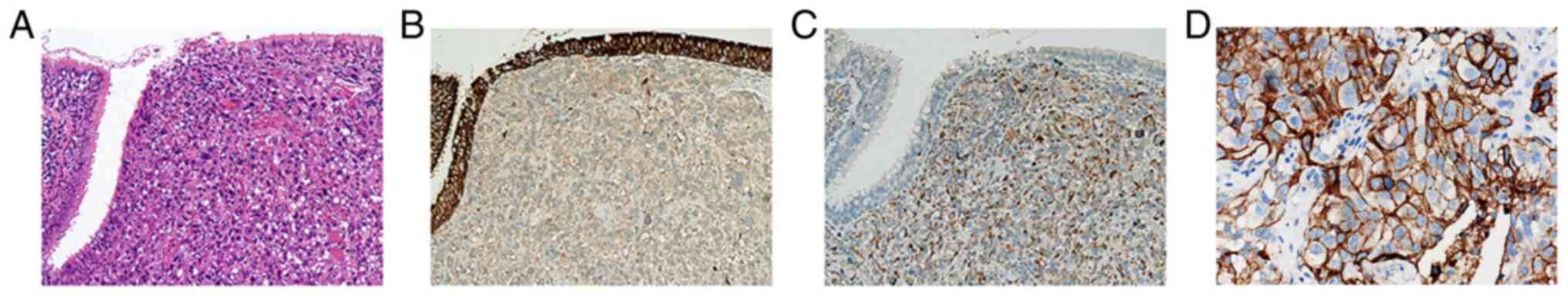
the bronchus (measuring ~3.5×3.3×2.3 cm) and IHC supported
pulmonary sarcomatoid carcinoma that was positive for
pancytokeratin (partially) and vimentin (Fig. 3). The IHC protocols are provided in
the supplemental data. These findings led to the diagnosis of
adenocarcinoma of the upper lobe of the left lung combined with
sarcomatoid carcinoma (T4N2M0 stage IIIB).
After one month, a tumor specimen from the patient
was examined for a pathological consultation at a different
hospital, which corroborated our results. Genetic testing using
next-generation sequencing revealed no mutations in commonly
implicated genes, such as EGFR, BRAF, MET or RAS; however, the
sequencing identified an EML4-ALK fusion and further IHC analysis
revealed a high degree of PD-L1 expression [tumor proportion score
(TPS) >70%] (Fig. 3).
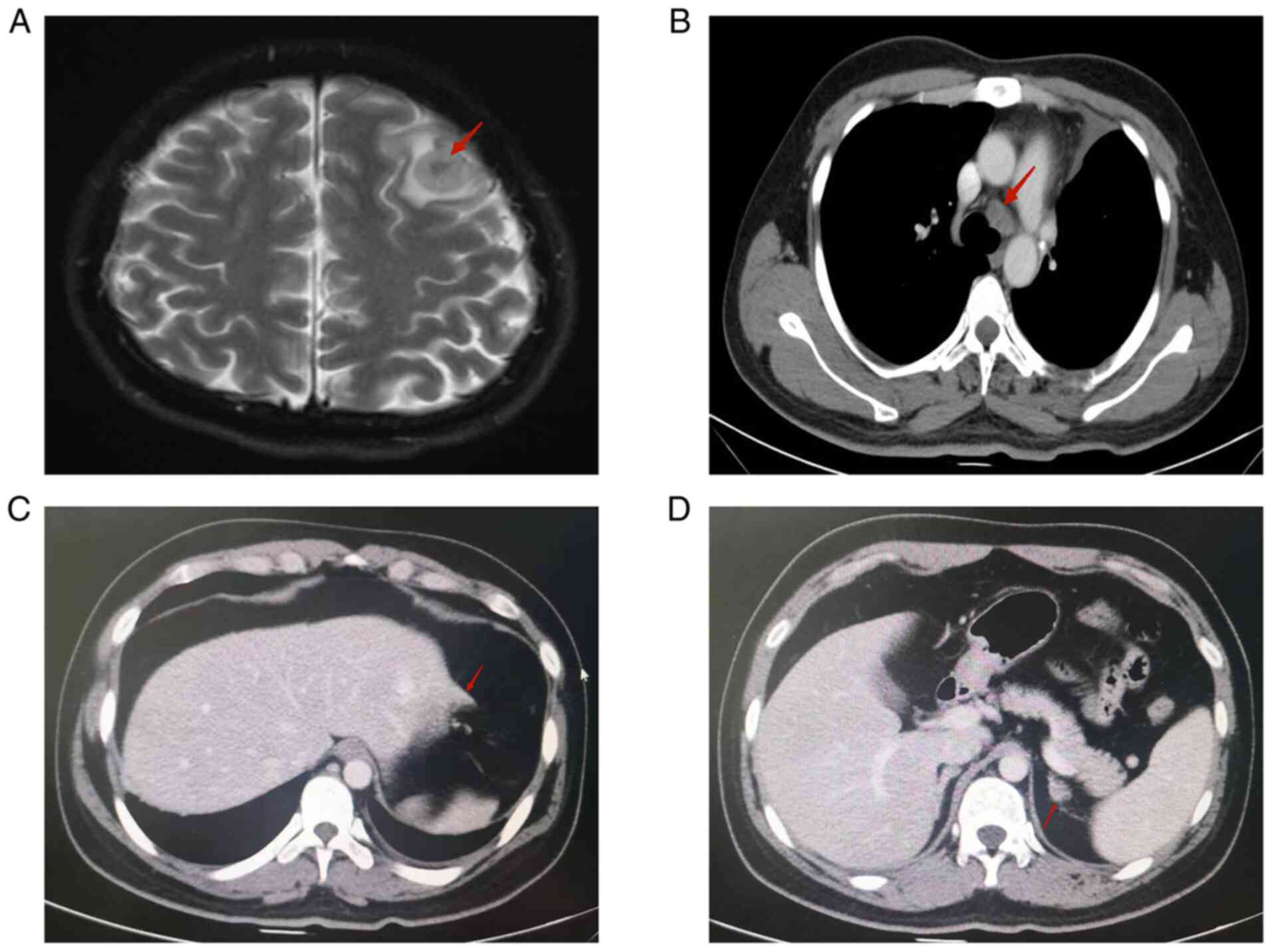
Unfortunately, the patient's cancer progressed rapidly and a
comprehensive evaluation revealed enlargement of the bilateral
hilar and mediastinal intramural lymph nodes. There were also
multiple distant metastatic lesions involving the abdominal
mesenteric region, the omental region (the largest measuring
~3.4×2.4 cm), the lateral left lobe of the liver and the left
adrenal gland (measuring ~1.3×1.0 cm). Furthermore, multiple
bilateral metastases, with edema, were discovered in the brain
(Fig. 4). These findings led to
the diagnosis of postoperative mediastinal lymph node, abdominal,
adrenal and brain metastases from the adenocarcinoma of the upper
lobe of the left lung combined with sarcomatoid carcinoma (T4N2M1c
stage IVB, EML4/ALK fusion, PD-L1 TPS >70%).
Considering the patient's young age, rapid disease
progression and the combination of two tumor components, the
adenocarcinoma and sarcomatoid carcinoma, a combination treatment
strategy was implemented using the ALK inhibitor Alectinib (600 mg
po bid) + Bevacizumab (600 mg dl) + Paclitaxel liposome (270 mg dl)
+ Carboplatin (500 mg dl); the therapy was administered in 21-day
cycles for a total of four cycles. Stereotactic body radiation
therapy targeting cranial metastases was initiated simultaneously
for 10 irradiation sessions. Following radiotherapy, the
intracranial lesions and edema were significantly reduced. After
the fourth treatment cycle, enhanced CT revealed that certain
metastases in the peritoneal, mesenteric and omental areas were
larger than before (the larger one was ~4.5×2.4 cm and had invaded
the left lobe of the liver). The number of remaining lesions did
not significantly change.
After four months, the patient returned, presenting
with cough and back pain. Hematologic examination revealed that the
number of white blood cells (WBC) was increased to
14.68×109/l [normal range (NR), 4.0-10.0
×109/l], accompanied with a platelet count of
517×109/l (NR, 100–300×109/l), without any
chills or fever. After five months, the patient returned to the
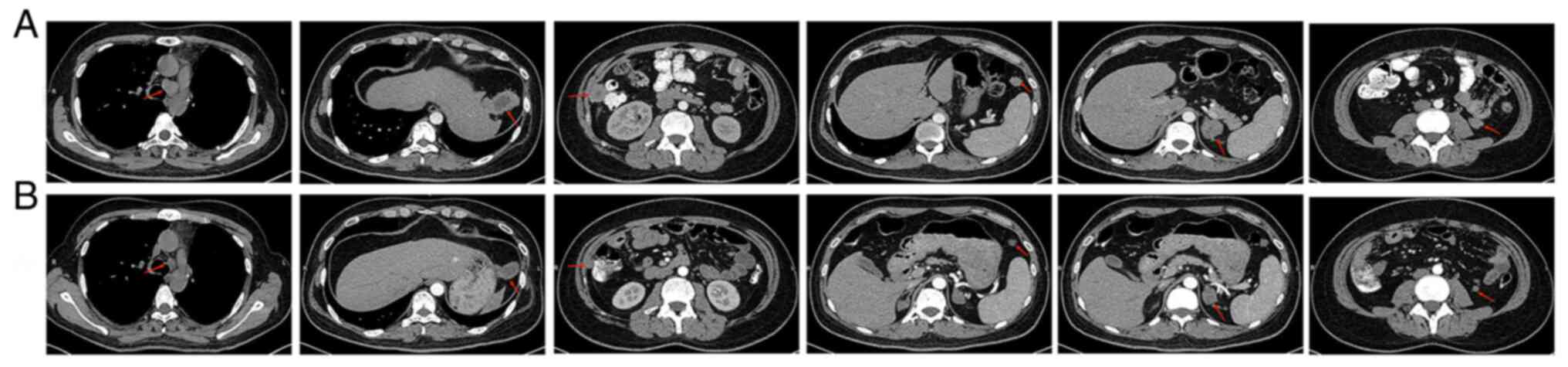
hospital and CT examination revealed enlarged lymph nodes in the
mediastinum and a small amount of fluid in the right pleural
cavity. Multiple metastases were found in the abdominal cavity, the
largest of which was located in the lateral left lobe of the liver
(~5.7×3.0×4.0 cm in size), markedly enlarged adrenal glands
bilaterally (~2.7 cm) and soft tissue lesions below the left
kidney, anterior to the spleen and in the right paracolic sulcus,
~1.8, 1.5 and 2.9 cm in diameter, respectively (Fig. 5). A CT-guided percutaneous biopsy
of the lesion on the right paracolonic sulcus was performed and
subsequent IHC indicated a malignancy of mesenchymal origin; it was
considered a possible metastasis of the sarcomatoid component.
After a full discussion, the therapeutic plan was changed to a
combination of targeted therapy with immunotherapy (Alectinib +
Anlotinib + Tirelizumab). Before the treatment started, informed
consent was obtained from the patient and the patient's family. The
patient began treatment with Anlotinib (12 mg, qd, d1-14, q21d) and
with Tirelizumab (200 mg, q21d). After the first treatment cycle,
the patient experienced a significant improvement in his cough and
complaints of pain. Furthermore, the patient's WBC and platelet
counts decreased to normal and the right retroauricular mass had
disappeared. A repeat CT after the second treatment cycle revealed
a significant reduction in the pleural effusion of the right lung
and in the size of the mediastinal lymph nodes, and the lesions in
the abdominal cavity (largest lesion was ~3.2×2.0×2.3 cm);
furthermore, the lesions in the lower left kidney, the anterior
spleen and the right paracolic sulcus were reduced to ~1.0, 1.1 and
1.2 cm in diameter, respectively (Fig.
5). The tumor response to treatments was evaluated according to
the New Response Evaluation Criteria In Solid Tumours: Revised
RECIST Guideline (version 1.1) (8). The sum of the diameters of the target
lesions was reduced by >30%, taking as reference the baseline
sum diameter. These observations resulted in the rating as partial
response (PR) for the disease. The patient tolerated the combined
treatment (Alectinib + Anlotinib + Tirelizumab) well, only
experiencing mild diarrhea over 7 months on the regimen; currently,
the patient remains on this treatment plan.
Discussion
PSC is essentially a carcinoma of epithelial origin
with a sarcomatoid component, a spindle-cell variant of cancer
cells (9). It is more common in
older males who smoke than in younger males (3). The present case was unique, as the
patient was 29 years old and denied any history of smoking, but
still developed PSC concurrently with an adenocarcinoma component
in the lung. Due to its rarity, there is no standardized protocol
for treating metastatic PSC and the currently used treatment
regimens are derived from those for NSCLC. Current case reports and
studies of PSC demonstrate that patients with PSC have worse
survival rates and prognosis than other histological subtypes of
NSCLC (10). PSC exhibits
significant resistance against conventional first-line
chemotherapy, with a median progression-free survival (PFS) of only
2.0 months and a median overall survival (OS) of 6.3 months
(3). Therefore, the development of
new treatment options is necessary to improve the prognosis of
patients with PSC.
Over the past decade, there have been significant
advances in targeted therapy and immunotherapy for NSCLC, such as
EGFR and ALK inhibitors, and more recently, PD-1 inhibitors.
Patients with NSCLC with specific driver mutations (e.g., EGFR and
ALK) may benefit significantly from targeted therapy (11). The high mutation rate of EGFR in
NSCLC makes tyrosine kinase inhibitors (TKI) the first-line
therapies for affected patients; however, the mutation rate of EGFR
in PSC is significantly lower than that in other types of NSCLC,
although the true rate remains controversial (0–28%) (12,13).
Studies reported that the efficacy of TKI is variable among
patients with PSC who present with EGFR mutations (14,15),
suggesting that there are other, more significant, oncogenic
drivers for PSC. TP53 and KRAS mutations are also prevalent among
patients with PSC; however, the presence of KRAS mutations is
frequently predictive of a poor prognosis (16,17).
The incidence of MET exon 14 jump mutations is reported to be
higher (~20-30%) than lung adenocarcinoma (18,19);
furthermore, the incidence of ALK fusion-positive NSCLC is known to
be 3–7%. In lung adenocarcinoma, the first-generation ALK inhibitor
Crizotinib improved PFS (up to 11.1 months); its second-generation
counterpart, Alectinib, further prolonged patient PFS to 34.8
months. ALK fusion is rarely present in PSC and only a small number
of cases have been reported. However, a study by Chen et al
(20) suggested that the incidence
of ALK rearrangement in SC (5/141, 3.5%) was similar to that in
other NSCLC subtypes and, of note, EML4-ALK-positive lung cancer is
associated with a young age of onset and a history of non-smoking
or light smoking. In the present study, the patient was a young
non-smoker, who presented with an EML4-ALK gene fusion; therefore,
the patient was given the second-generation ALK inhibitor
Alectinib. Considering the presence of two tumor components
(sarcomatoid and adenocarcinoma) and rapid disease progression, it
is understandable that the initial chemotherapeutic approach proved
ineffective and the sarcomatoid component progressed despite the
robust treatment regimen.
In the era of immunotherapy, immune checkpoint
inhibitors (ICIs) targeting PD-1/PD-L1 have become the standard
treatment strategy for NSCLC. PD-L1 expression in tumors has become
a common and optimal biomarker for predicting the effect of
immunotherapy. Naito et al (21) reported that among 35 patients with
PSC, ~91% (32/35) were positive for PD-L1 expression (TPS ≥1%) and
60% (21/35) had high PD-L1 expression (TPS ≥50%). By contrast, Yang
et al (22) reported that
36.5% of 148 patients with PSC were PD-L1-positive. The differences
in PD-L1 expression among studies may be related to differences in
antibody clones, positive detection thresholds in testing and tumor
heterogeneity; in the present case, the patient had high PD-L1
expression (TPS >70%). Therefore, ICIs are a potential treatment
option for patients with PSC concurrent with high PD-L1
expression.
There are a limited number of case reports that
demonstrate successful treatment with ICIs and long-term efficacy
(23,24), likely due to the rarity of PSC. In
a study of 37 patients with PSC treated with a second-generation or
newer immunotherapy, the overall response rate was 40.5% and the
median OS was 12.7 months (25).
However, one-third of the patients in the high PD-L1 expression or
KRAS mutation subgroup experienced early disease progression within
2 months.
Tirelizumab was approved by the National Medical
Products Administration (NMPA) in December 2019 for the treatment
of Hodgkin lymphoma in China. It causes tumor-cell death by
blocking PD-L1/PD-L2-related cell signaling used to avoid an immune
response, promoting cytokine production and restoring T-cell
clearance (26). Previous studies
have demonstrated high tolerability, safety and efficacy of
Tirelizumab in patients with advanced NSCLC, regardless of the
PD-L1 status (27). In 2020,
Tirelizumab has gained NMPA approval for the treatment of NSCLC
(both advanced squamous cell carcinoma and non-squamous cell
carcinoma) (28). However, the use
of Tirelizumab in PSC has rarely been reported. In the present
case, Tirelizumab immunotherapy was administered along with
Anlotinib; Anlotinib is an orally administered anti-angiogenic
agent that potently inhibits multiple targets, including VEGFR,
platelet-derived growth factor receptor, fibroblast growth factor
receptor and c-Ki) and has been approved by the NMPA for
soft-tissue sarcomas, and third-line treatment of advanced NSCLC.
VEGFR signaling may be inhibited by reducing tumor T-cell
infiltration and increasing suppressive immune cells, such as
regulatory cells and myeloid-derived suppressor cells, to modulate
the immune response and ultimately create an immunosuppressive
tumor microenvironment (29).
Thus, Anlotinib combined with immunotherapy may synergistically
inhibit tumors to improve patient outcomes. The patient in the
present case report had a positive response to this combination
therapy and continues to demonstrate a controlled disease
state.
In conclusion, to the best of our knowledge, the
present study was the first report of a young patient exhibiting
both PSC and lung adenocarcinoma with an EML4/ALK fusion, who
achieved a good response to the combined treatment with Tirelizumab
and Anlotinib after failure of initial ALK inhibitor combination
with chemotherapy. Immunotherapy in combination with the
anti-angiogenic drug Anlotinib may be a promising treatment
strategy for patients with PSC. Further study of this strategy in
patients with PSC is necessary to corroborate these findings and
improve patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW was responsible for collecting clinical, imaging
and pathological data of the patient and responsible for the
conception, design, content and writing of the manuscript. XL
analyzed the data and revised the manuscript. YG and YC
participated in making the pathological diagnosis, and made
substantial contributions to design, acquisition of data, analysis
and interpretation of data. WW was involved in the clinical and
therapeutic management of the patient and was responsible for the
acquisition, analysis and interpretation of the images. LY made
substantial contributions to the conception and design of the
study, revising and proofreading the manuscript. LY and MW
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Affiliated Hospital of Panzhihua University
(Panzhihua, China; reference no. 202207001).
Patient consent for publication
Written informed consent was provided by the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yendamuri S, Caty L, Pine M, Adem S,
Bogner P, Miller A, Demmy TL, Groman A and Reid M: Outcomes of
sarcomatoid carcinoma of the lung: A surveillance, epidemiology,
and end results database analysis. Surgery. 152:397–402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pelosi G, Sonzogni A, De Pas T, Galetta D,
Veronesi G, Spaggiari L, Manzotti M, Fumagalli C, Bresaola E, Nappi
O, et al: Review article: Pulmonary sarcomatoid carcinomas: A
practical overview. Int J Surg Pathol. 18:103–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vieira T, Girard N, Ung M, Monnet I, Cazes
A, Bonnette P, Duruisseaux M, Mazieres J, Antoine M, Cadranel J and
Wislez M: Efficacy of first-line chemotherapy in patients with
advanced lung sarcomatoid carcinoma. J Thorac Oncol. 8:1574–1577.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen M, Yang Q, Xu Z, Luo B, Li F, Yu Y
and Sun J: Survival analysis and prediction model for pulmonary
sarcomatoid carcinoma based on SEER database. Front Oncol.
11:6308852021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsao AS, Scagliotti GV, Bunn PA Jr,
Carbone DP, Warren GW, Bai C, de Koning HJ, Yousaf-Khan AU,
McWilliams A, Tsao MS, et al: Scientific advances in lung cancer
2015. J Thorac Oncol. 11:613–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terra SB, Jang JS, Bi L, Kipp BR, Jen J,
Yi ES and Boland JM: Molecular characterization of pulmonary
sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol.
29:824–831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin LW, Correa AM, Ordonez NG, Roth JA,
Swisher SG, Vaporciyan AA, Walsh GL and Rice DC: Sarcomatoid
carcinoma of the lung: A predictor of poor prognosis. Ann Thorac
Surg. 84:973–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouziane I, Boutayeb S, Mrabti H, Lalya I,
Rimani M and Errihani H: Sarcomatoid carcinoma of the lung: A model
of resistance of chemotherapy. N Am J Med Sci. 6:342–345. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fallet V, Saffroy R, Girard N, Mazieres J,
Lantuejoul S, Vieira T, Rouquette I, Thivolet-Bejui F, Ung M,
Poulot V, et al: High-throughput somatic mutation profiling in
pulmonary sarcomatoid carcinomas using the lungcarta panel:
Exploring therapeutic targets. Ann Oncol. 26:1748–1753. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schrock AB, Li SD, Frampton GM, Suh J,
Braun E, Mehra R, Buck SC, Bufill JA, Peled N, Karim NA, et al:
Pulmonary sarcomatoid carcinomas commonly harbor either potentially
targetable genomic alterations or high tumor mutational burden as
observed by comprehensive genomic profiling. J Thorac Oncol.
12:932–942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaira K, Horie Y, Ayabe E, Murakami H,
Takahashi T, Tsuya A, Nakamura Y, Naito T, Endo M, Kondo H, et al:
Pulmonary pleomorphic carcinoma: A clinicopathological study
including EGFR mutation analysis. J Thorac Oncol. 5:460–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakagomi T, Goto T, Hirotsu Y, Shikata D,
Yokoyama Y, Higuchi R, Amemiya K, Okimoto K, Oyama T, Mochizuki H
and Omata M: New therapeutic targets for pulmonary sarcomatoid
carcinomas based on their genomic and phylogenetic profiles.
Oncotarget. 9:10635–10649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lococo F, Gandolfi G, Rossi G, Pinto C,
Rapicetta C, Cavazza A, Cesario A, Galeone C, Paci M and Ciarrocchi
A: Deep sequencing analysis reveals that KRAS mutation is a marker
of poor prognosis in patients with pulmonary sarcomatoid carcinoma.
J Thorac Oncol. 11:1282–1292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Wang D, Zhao Q, Ren D, Ren F, Chen
G, Liu H and Chen J: Clinical significance and next-generation
sequencing of chinese pulmonary sarcomatoid carcinoma. Sci Rep.
7:39472017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong JH, Yeung SF, Chan AW, Chung LY, Chau
SL, Lung RWM, Tong CY, Chow C, Tin EKY, Yu YH, et al: MET
Amplification and exon 14 splice site mutation define unique
molecular subgroups of non-small cell lung carcinoma with poor
prognosis. Clin Cancer Res. 22:3048–3056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Jia Y, Stoopler MB, Shen Y, Cheng
H, Chen J, Mansukhani M, Koul S, Halmos B and Borczuk AC:
Next-generation sequencing of pulmonary sarcomatoid carcinoma
reveals high frequency of actionable MET gene mutations. J Clin
Oncol. 34:794–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Zhang Y, Lu J, Xu C, Liang J, Wang
F, Sun W, Fang S, Yuan J, Wang H, et al: Pulmonary sarcomatoid
carcinoma with ALK rearrangement: Frequency, clinical-pathologic
characteristics, and response to ALK inhibitor. Transl Oncol.
10:115–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naito M, Tamiya A, Takeda M, Taniguchi Y,
Saijo N, Naoki Y, Okishio K, Yoon H, Kasai T, Matsumura A and Atagi
S: A High PD-L1 expression in pulmonary pleomorphic carcinoma
correlates with parietal-pleural invasion and might predict a poor
prognosis. Intern Med. 58:921–927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Xu J, Li R, Gao Y and He J: PD-L1
and CD47 co-expression in pulmonary sarcomatoid carcinoma: A
predictor of poor prognosis and potential targets of future
combined immunotherapy. J Cancer Res Clin Oncol. 145:3055–3065.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Tao NN, Liang B, Li DW, Li HC and Su
LL: Use of PD-1 inhibitor tislelizumab in the treatment of advanced
pulmonary sarcomatoid carcinoma: A case report. Thorac Cancer.
13:502–505. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto Y, Miura T, Horiuchi H and Usui
K: The successful treatment of pulmonary pleomorphic carcinoma with
pembrolizumab: A case report. Case Rep Oncol. 10:752–757. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Domblides C, Leroy K, Monnet I, Mazières
J, Barlesi F, Gounant V, Baldacci S, Mennecier B, Toffart AC,
Audigier-Valette C, et al: Efficacy of immune checkpoint inhibitors
in lung sarcomatoid carcinoma. J Thorac Oncol. 15:860–866. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong
W, Zhang Y, Zhou X, Wang Z, Wang Y, et al: The binding of an
anti-PD-1 antibody to FcgammaRIota has a profound impact on its
biological functions. Cancer Immunol Immunother. 67:1079–1090.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai
Y, Liu T, Zhou Q, Zhao J, Shu Y, et al: Tislelizumab in chinese
patients with advanced solid tumors: An open-label,
non-comparative, phase 1/2 study. J Immunother Cancer.
8:e0004372020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee A and Keam SJ: Tislelizumab: First
approval. Drugs. 80:617–624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang H and Wang M: Prospect of
immunotherapy combined with anti-angiogenic agents in patients with
advanced non-small cell lung cancer. Cancer Manag Res.
11:7707–7719. 2019. View Article : Google Scholar : PubMed/NCBI
|