Introduction
Although the average human lifespan has been greatly
extended because of advances in medical technology, cancer has
remained the leading cause of death for the past 30 years.
Moreover, the incidence and mortality rate of cancer are expected
to continue to increase because of the aging population and
lifestyle changes (1). According
to the 2019 Breast Cancer White Paper of the Korean Breast Cancer
Society (2), breast cancer ranks
first and accounts for 24.2% of the global cancer incidence in
females and is the leading cause (15%) of cancer mortality. As of
2018, there were approximately 2 million breast cancer cases
worldwide, resulting in approximately 600,000 deaths. Metastasis is
a major cause of cancer-correlated deaths. Despite the marked
developments in current therapies, some patients experience a
relapse. Therefore, a more accurate study of metastasis is
essential for improving treatment methods for breast cancer
(3). Matrix metalloproteinases
(MMPs) are endopeptidases secreted by various cell types. The main
role of MMPs in the metastatic cascade appears to be the
interaction of tumor cells with the extracellular matrix (ECM).
Additionally, MMPs release growth factors through their degradation
process, resulting in cancer development (4,5). In
a previous study, we found that synthetic PPAR-γ ligands reduced
MMP-9 expression and cell invasion in breast cancer cells.
Therefore, revealing that a novel PPAR-γ ligand modulates MMP-9 and
helps prevent breast cancer metastasis could enhance the survival
rate of patients (6).
Triterpenoids are isopentenyl pyrophosphate oligomers that have
been successfully developed for solid cancers (7). Triterpenoids have been studied to
determine their cytotoxicity to various cancer cells and their
anticancer effects in vivo (7). Recent studies have shown that
2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), a PPAR-γ
ligand and novel synthetic triterpenoid, targets the NF-κB pathway,
which plays a pivotal role in MMP expression in cancer cells
(8). These results indicate CDDO
as a hopeful therapeutic agent for inhibiting the development of
primary tumors and metastases in solid cancers, such as breast
cancer. However, the effect of CDDO on
12-O-tetradecanoylphorbol13-acetate (TPA)-induced breast cancer
metastasis has not yet been addressed as a mechanism contributing
to MMP suppression.
Materials and methods
Reagents
TPA, T0070709 (a PPAR-γ antagonist), and
anti-β-actin (A5441) were obtained from Sigma-Aldrich. CDDO was
obtained from Cayman Chemical Co. Antibodies against MMP-9
(ab76003) and p-c-Jun (ab32385) were obtained from Abcam. p-ERK
(#4370), ERK (#4695), p-p38 (#4511), p38 (#8690), p-JNK (#4668),
JNK (#9258), c-Jun (#9165), p-c-Fos (#5348), c-Fos (#3250), p-IκBa
(#2859), and p-IKKa/β (#2697) were obtained from Cell Signaling
Technology. PPARγ (sc-7273), PCNA (sc-56), p50 (sc-7178), p65
(sc-372), IκBa (sc-371), and IKKa (sc-71333) and IKKβ (sc-56918)
were obtained from Santa Cruz Biotechnology. Secondary
HRP-conjugated anti-mouse (ADI-SAB-100-J) and anti-rabbit
(ADI-SAB-300-J) were obtained from Enzo Life Sciences.
Cell culture
The MCF-7 human breast cancer cell line was
purchased from American Type Culture Collection. The cells were
cultured in DMEM supplemented with 1% antibiotic and 10% FBS
(Thermo Fisher Scientific) in CO2 at 37°C.
Cytotoxicity assay
Cells were either treated with 0.1, 0.3, 0.5, 1, or
2 µM CDDO or left untreated at 37°C for 24 h and then washed with
PBS. The assay was performed using MTT (0.5 mg/ml; Merck KGaA).
After addition of the agent, the cells were incubated at 37°C for
40 min. DMSO was added to dissolve formazan crystals, and
absorbance was determined using a microplate reader at 570 nm
(Bio-Rad Laboratories).
Cell proliferation assay
Cell proliferation was measured using a BrdU Cell
Proliferation ELISA kit (ab126556, Abcam) following the
manufacturer's instructions. Briefly, cells (2×106) were
incubated in 96-well plates with CDDO at 37°C for 24 h, and then
BrdU was added to the cells for 2 h. Cells were fixed and incubated
with anti-BrdU monoclonal Detector Antibody for 1 h at room
temperature. Peroxidase goat anti-mouse IgG conjugate was added as
secondary antibody and incubated for 30 min at room temperature.
Color was developed using TMB peroxidase substrate, and BrdU
incorporation was measured at 450 nm using a microplate reader
(Bio-Rad).
Nuclear and cytoplasmic extracts
Cells were treated with 1 µM CDDO, treated with TPA
at 37°C for 2 h, and washed with PBS. Nuclear and cytoplasmic
extracts were obtained using NE-PER® extraction reagents
(Thermo Fisher Scientific).
siRNA transfection
Cells were transfected with PPARγ siRNA (Bioneer)
using Opti-MEM (Gibco) and Lipofectamine RNAiMAX (Invitrogen).
Negative control siRNA was purchased from Genotech. Experiments
were performed according to the Lipofectamine RNAiMAX transfection
protocol at an siRNA concentration of 10 nM. The Lipofectamine
RNAiMAX-siRNA complex was added to the cells, and after 6 h of
transfection, the growth medium was replaced. Cells were treated
with TPA and CDDO 24 h after transfection and then used in the
experiments.
Zymography assay
Supernatant medium was added with loading buffer and
separated using PAGE (0.1% gelatin). The gel was washed for 40 min
with Triton X-100 solution (2.5%) at 15~20°C and then incubated for
24 h in a digestion buffer at 37°C. The gel was stained for 40 min
with Coomassie brilliant blue (0.25%) and measured with an image
analyzer (FUJIFILM Corporation). Densitometric analysis was
determined using the Multi-Gauge image analysis software
(Multi-Gauge v3.0; FUJIFILM Corporation).
Western blot analysis
Cells were treated with CDDO 1 µM at 37°C for 1 h
and then treated with TPA at 37°C for the time required by each
experiment. Proteins were lysed using the M-PER Mammalian Protein
Extraction Reagent (Thermo Fisher Scientific) and a proteinase
inhibitor. Protein concentration was analyzed using a Protein Assay
Dye Reagent Concentrate (Bio-Rad Laboratories). Lysates (10 µg
protein) were separated using SDS-PAGE (10%) and transferred to
Hybond™ polyvinylidene fluoride membranes (GE Healthcare
Life Sciences). Each membrane was blocked at 4°C for 3 h with 5%
skim milk or 5% bovine serum albumin (MP Biomedicals) and then
incubated at 4°C for 16 h with primary antibodies (diluted
1:1,000). Secondary antibody of HRP-conjugated anti-IgG (diluted
1:1,000) was applied and incubated at 4°C for 1 h. Immunoreactive
signals were visualized using an HRP substrate peroxide solution
and luminol reagent (Merck Millipore). Protein density were
determined using an imaging system (Las-4000; FUJIFILM Corporation)
and image analyzer software (Multi-Gauge v3.0; FUJIFILM
Corporation).
Real-time polymerase chain
reaction
Cells were treated with CDDO (1 µM) at 37°C for 1 h
and then treated with TPA at 37°C for 24 h. RNA was isolated using
the RNAiso Plus Reagent. cDNA was synthesized using a PrimeScript
RT Reagent Kit by heating based on the kit protocol. The mRNA
levels were analyzed by qPCR using an ABI PRISM™ 7900
Sequence Detection and Power SYBR® Green PCR Master Mix
(Thermo Fisher Scientific). The primers used were as follows: GAPDH
(NM002046) sense, 5′-ATGGAAATCCCATCACCATCTT-3′ and antisense,
5′-CGCCCCACTTGATTTTGG-3′; MMP-9 (NM 004994) sense,
5′-CCTGGAGACCTGAGAACCAATCT-3′ and antisense,
5′-CCACCCGAGTGTAACCATAGC-3′. The qPCR cycle was as follows: 40
cycles at 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec, and
60°C for 1 min. Target genes were normalized to GAPDH. Relative
quantitation was performed using the comparative Cq
(2−ΔΔCq) method, following the manufacturer's
instructions (9).
Luciferase assay
Luciferase reporter assays was performed using a
Dual-Luciferase® Reporter Assay System (E1910, Promega)
following the manufacturer's instructions. Cells were transfected
with AP-1 or NF-κB reporter plasmids (provided by Professor Kim
Chul Ho, SungKyunKwan University) using the Lipofectamine 3000
Reagent (Thermo Fisher Scientific). Transfected cells were treated
with 1 µM CDDO at 37°C for 1 h and then treated with TPA at 37°C
for 2 h. Renilla luciferase activity was measured using a
luminometer (Lumat LB 9507; Berthold Technologies GmbH &
Co.).
Migration assay
The assay was performed using well chambers (8 µm
pore size) without Matrigel. Cells (3×105) were added to
the upper chamber in DMEM (FBS-free media), and the lower chamber
contained supernatant medium (1% antibiotic and 10% FBS). TPA with
or without 1 µM CDDO was added to the upper chamber. After
incubation at 37°C for 24 h, upper chamber cells were removed. The
migrated cells at the bottom were fixed with formalin (3.5%) for 10
min and stained with crystal violet (0.25%) for 1 h. Migrated cells
were counted in four random parts of the layer using a light
microscope (40× magnification).
Invasion assay
The assays were conducted using well chambers (8 µm
pore size) in which the upper area of the Transwell insert was
coated with Matrigel at 37°C for 40 min (BD Biosciences). Cells
(3×105) were added to the upper chamber in DMEM
(FBS-free media), and the lower chamber contained supernatant
medium (1% antibiotic and 10% FBS). TPA with or without 1 µM CDDO
was added to the upper chamber. After incubation at 37°C for 24 h,
cells in the upper chamber were removed. The invading cells on the
bottom were fixed with formalin (3.5%) for 10 min and stained with
crystal violet (0.25%) for 1 h. Invading cells were counted in four
random parts of the layer using light microscopy (40×
magnification).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of three independent experiments. Statistical analyses
were conducted using one-way ANOVA and Tukey test. Differences were
statistically significant at P<0.05.
Results
Effects of CDDO on MMP-9
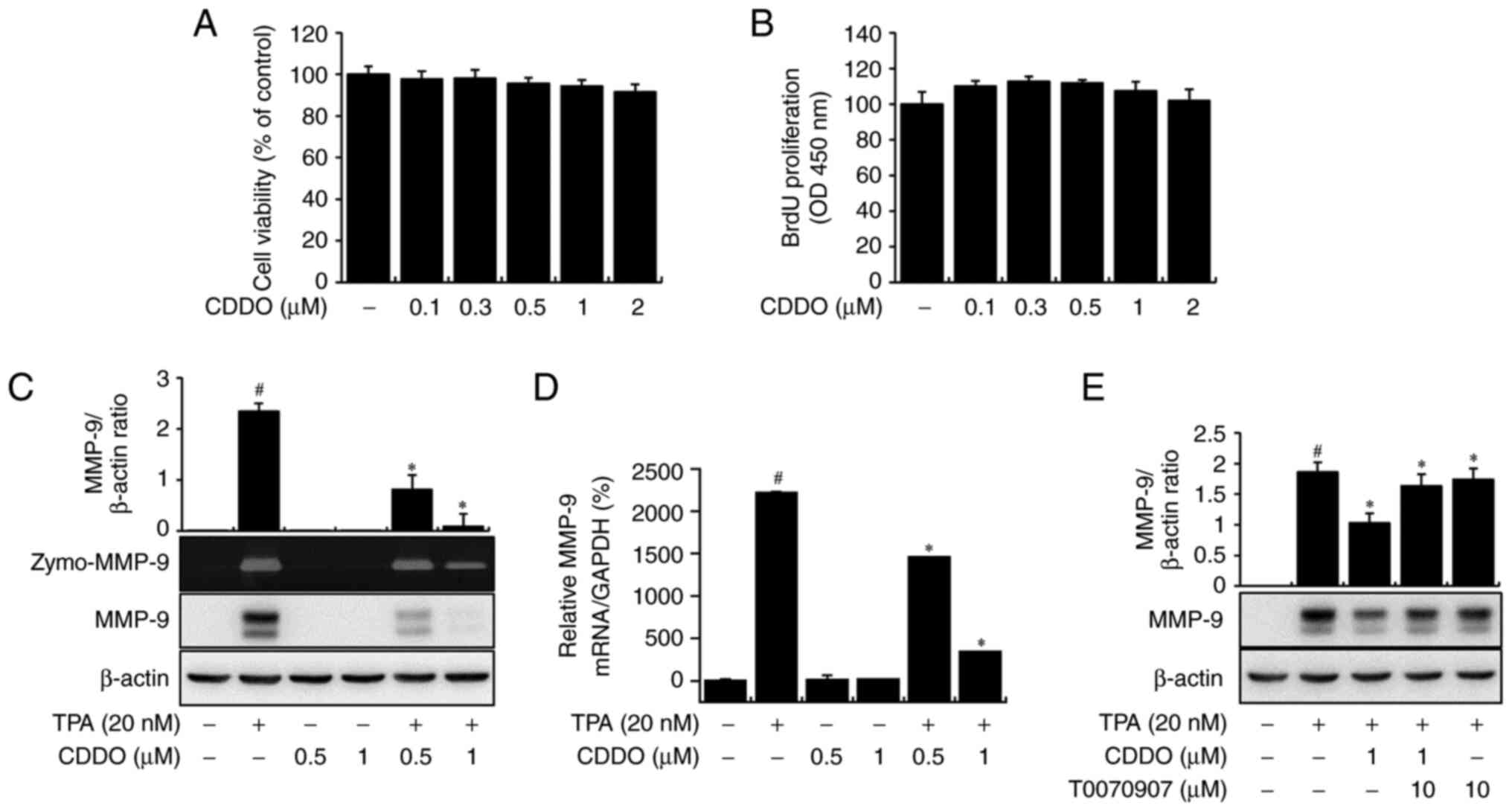
MCF-7 cells were treated with CDDO (0–2 µM) for 24
h, and cytotoxicity and cell proliferation were confirmed (Fig. 1A and B). Non-toxic concentrations
of CDDO were used in subsequent experiments. TPA-mediated MMP-9
secretion was observed, and CDDO suppressed this secretion in a
concentration-dependent manner, as determined using zymography
(Zymo-MMP-9). Western blot analysis showed that CDDO reduced
TPA-mediated MMP-9 protein expression in a concentration-dependent
manner (Fig. 1C). Additionally,
RT-qPCR indicated that CDDO treatment inhibited TPA-mediated MMP-9
mRNA level (Fig. 1D). Together,
these data indicate that CDDO downregulates MMP-9 expression. We
next determined whether CDDO inhibited MMP-9 in a PPAR-γ-dependent
manner and confirmed that the inhibition of MMP-9 expression after
treatment with CDDO was reversed by the PPAR-γ antagonist T0070907
(Fig. 1E). These data showed that
CDDO downregulated MMP-9 expression.
CDDO blocks TPA-mediated MAPK and AP-1
activation through a PPAR-γ-independent pathway
The downregulation of MMP-9 by CDDO could be
PPAR-γ-dependent or independent. To investigate an associative
relationship between downregulation of MMP-9 by CDDO and PPAR-γ, we
investigated the change of the expression level of MMP-9 by CDDO in
TPA-treated breast cancer cells after knock-out of PPAR-γ by siRNA.
In a previous study, we confirmed the expression of PPARγ in the
nuclear fraction of MCF-7 cells (10). In the present study, the
transfection efficiency of si-PPARγ was verified at the protein
level in the nuclear fraction of MCF-7 cells (Fig. 2A). As shown in Fig. 2B, CDDO suppressed TPA-mediated
expression of MMP-9 regardless of PPAR-γ presence, meaning that
CDDO downregulates MMP-9 through a PPAR-γ-independent pathway.
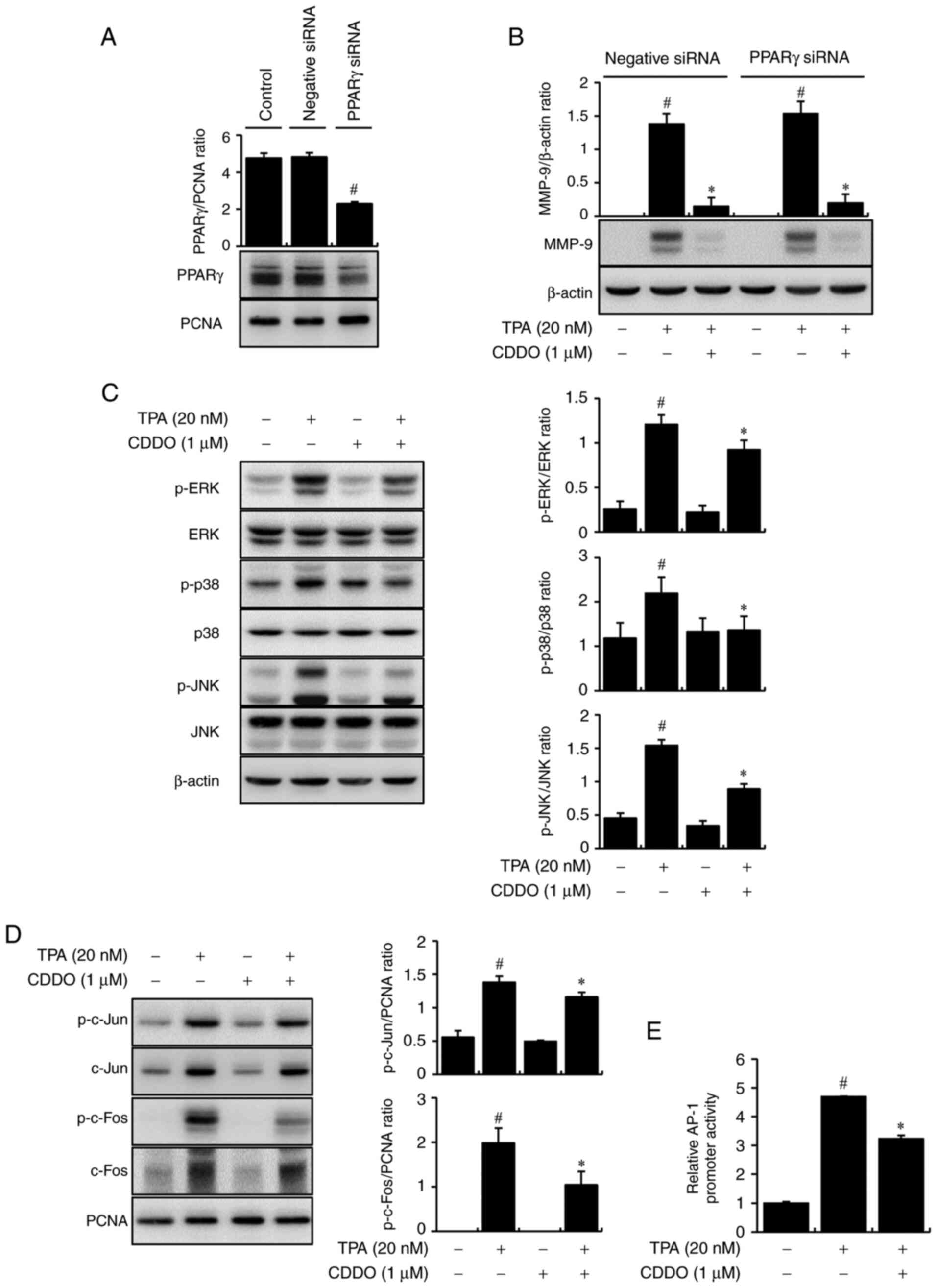 | Figure 2.Effects of CDDO on TPA-mediated MAPK
and AP-1 activation through a PPARγ-independent pathway. (A) The
transfection efficiency of si-PPARγ was confirmed in the nuclear
fraction of cells. PCNA was used as the internal control. (B) Cells
were transfected with PPARγ siRNA and then treated with TPA and
CDDO for 24 h. MMP-9 protein expression was analyzed using western
blotting. (C) Cells were treated with CDDO and/or TPA for 30 min.
The phosphorylation of ERK, p38, and JNK was determined by western
blotting using β-actin as a loading control. (D) Cells were treated
with CDDO and/or TPA. After a 2 h incubation, prepare the extracted
cytoplasmic and nuclear. The nuclei of p-c-Jun, p-c-Fos, total
c-Jun, and c-Fos were quantitated by western blotting. PCNA was the
internal control for the nucleus. (E) AP-1-luc reporters were
co-transfected with a Renilla luciferase reporter. Cells
were treated with CDDO, TPA was added for 2 h, and the AP-1
promoter activities were analyzed using a luciferase reporter
assay. The ratio of western blots was quantified with ImageJ.
Values are the mean ± standard error of the mean of three
independent experiments. #P<0.05 vs. untreated cells,
*P<0.05 vs. TPA alone. PPARγ, peroxisome proliferator-activated
receptor γ; siRNA, small interfering RNA; CDDO,
2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-oic acid; TPA,
12-O-tetradecanoylphorbol-13-acetate; p-, phosphorylated; AP-1,
activator protein-1. |
By applying CDDO and TPA to the MCF-7 cells, we
further determined the mechanism by which CDDO inhibited MMP-9
expression using western blot analysis. TPA increased the
phosphorylation of ERK, p38, and JNK. CDDO suppressed TPA-mediated
phosphorylation of MAPKs. However, the total protein levels of ERK,
p38, and JNK were unchanged (Fig.
2C). Additionally, the nuclear target of MAPK signaling was the
transcription factor AP-1. The results showed that phosphorylation
of c-Jun and c-Fos (AP-1 subunit) in the nucleus of TPA-mediated
cells was reduced subsequent to treatment with CDDO (Fig. 2D). Luciferase assays were used to
analyze the DNA-binding activities of AP-1. TPA treatment
significantly reduced these interactions in CDDO-treated cells
(Fig. 2E). These data indicate
that CDDO blocks MMP-9 by inhibiting the MAPK and AP-1 signaling
pathways.
CDDO blocks TPA-mediated NF-κB
activation
We further elucidated the mechanism underlying MMP-9
transcriptional activation by CDDO. The effects of CDDO on NF-κB
regulation were assessed using western blot analysis. MCF-7 cells
were treated with CDDO and TPA. CDDO suppressed TPA-mediated
nuclear translocation of p50/p65 (NF-κB subunit) and
phosphorylation of cytoplasmic IκBα and IKKα/β. Levels of total
IκBα, IKKα, and IKKβ in the cytosol were unchanged (Fig. 3A). Luciferase assays were used to
analyze the DNA-binding activities of NF-κB. TPA treatment
significantly reduced these interactions in CDDO-treated cells
(Fig. 3B). These data indicate
that CDDO inhibits MMP-9 expression by regulating NF-κB
transcription activity.
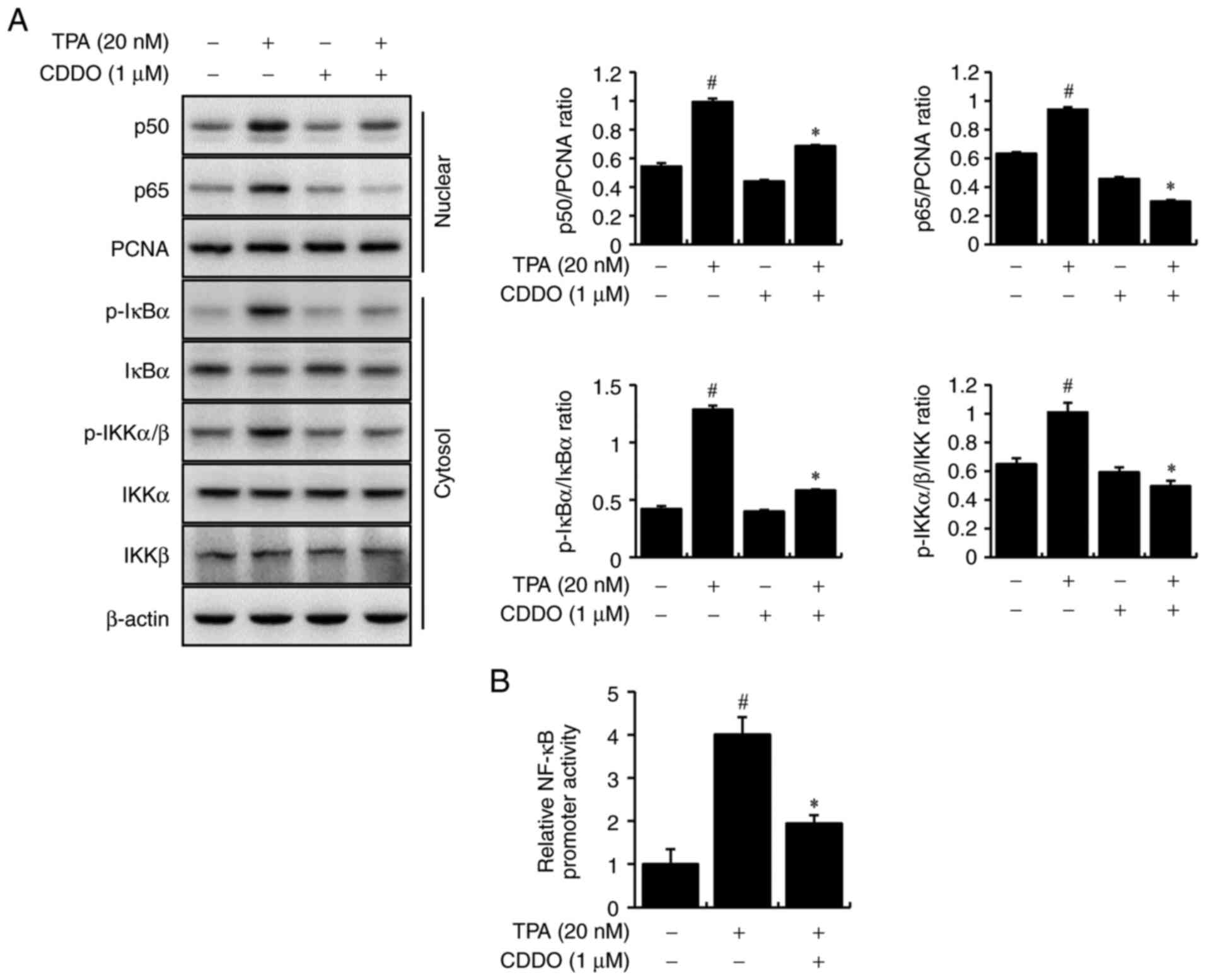 | Figure 3.NF-κB activation of CDDO in MCF-7
cells. (A) Cells were treated with CDDO and/or TPA. After
incubation for 2 h, prepare the extracted cytoplasmic and nuclear.
The levels of p50 and p65 in the nucleus, of p-IκBα and p-IKKα/β in
the cytoplasm, and of total IκBα, IKKα, and IKKβ were analyzed by
western blotting. PCNA was the internal control for the nucleus,
and β-actin was the loading control for the cytoplasm. (B)
NF-κB-luc reporters were co-transfected with a Renilla
luciferase reporter. Cells were treated with CDDO, TPA was added
for 2 h, and the NF-κB promoter activities were analyzed using a
luciferase reporter assay. The ratio of western blots was
quantified with ImageJ. Values are the mean ± standard error of the
mean of three independent experiments. #P<0.05 vs.
untreated cells, *P<0.05 vs. TPA alone. CDDO,
2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-oic acid; TPA,
12-O-tetradecanoylphorbol-13-acetate; NF-κB, nuclear factor κB. |
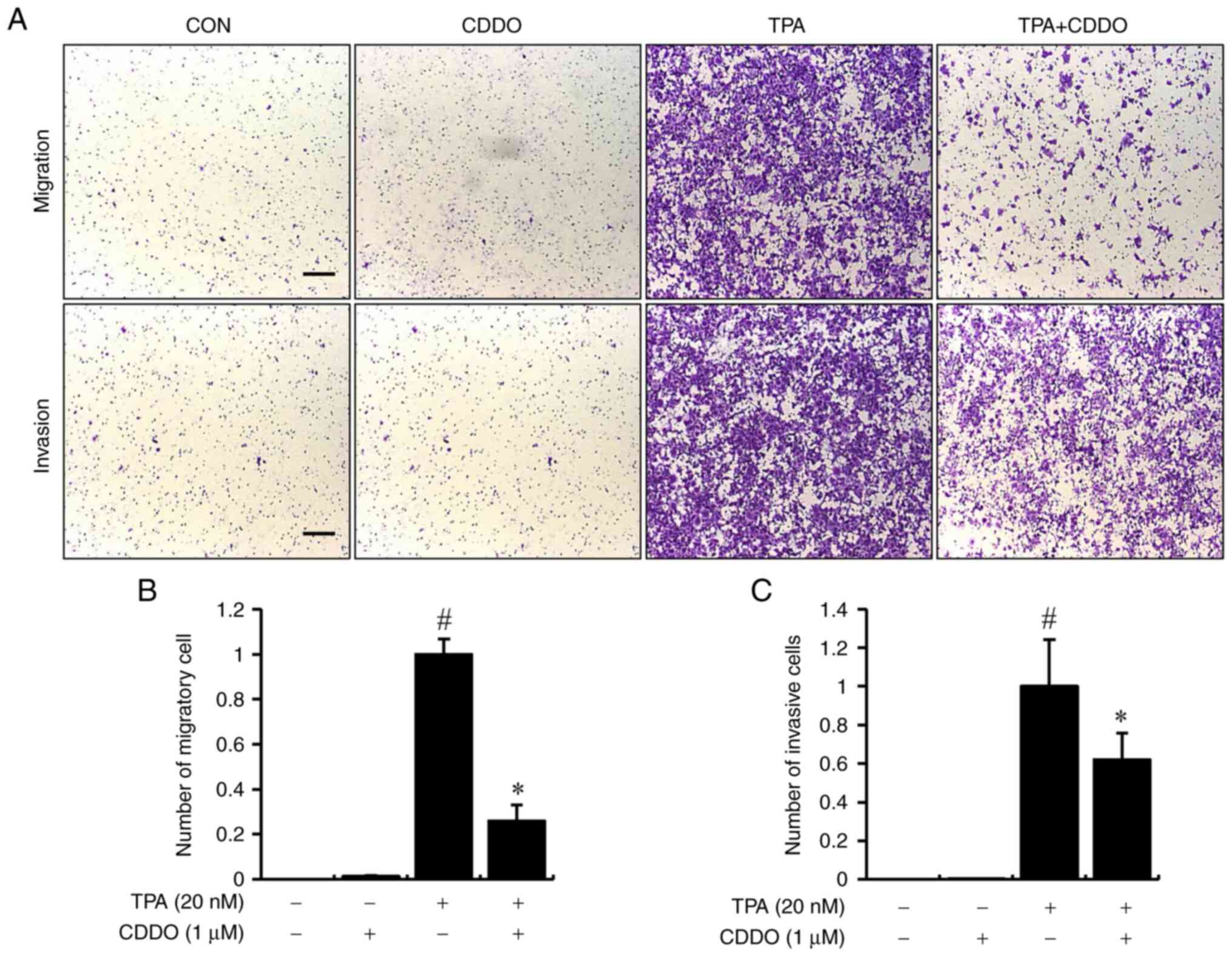
CDDO inhibits TPA-induced chamber
migration and Matrigel invasion in vitro
CDDO inhibited TPA-induced migration of cells, as
determined by chamber migration without Matrigel, compared with the
untreated control cells. (Fig. 4A,
upper panel). We investigated the effect of CDDO on the
invasiveness of MCF-7 breast cancer cells using a Matrigel invasion
assay. The results indicated that CDDO effectively inhibited the
TPA-mediated invasion of cells relative to non-treated control
cells (Fig. 4A). Fig. 4B and C show the relative numbers of
migrating cells and Matrigel-invading cells. These data indicate
that CDDO inhibited the migratory and invasive capabilities of
breast cancer cells.
Discussion
The target of this study was to determine the effect
of CDDO, a novel synthetic triterpenoid and PPAR-γ ligand, on MMP-9
expression and invasiveness of breast cancer cells. Our findings
indicate that CDDO inhibits MMP-9 and cancer cell invasion.
Furthermore, we described the mechanism of CDDO action using the
transcriptional effects on AP-1 and NF-κB and determined that the
CDDO mechanism uses MAPK signaling to repress MMP-9. We showed that
the major signaling pathways in the migration and invasion of
cancer cells, such as MAPKs, AP-1, and NF-κB, are targets of CDDO
in a PPAR-γ-independent manner, suggesting CDDO as a potential
therapeutic phytochemical agent.
MMP-9 is an important molecule that plays a major
role in cancer growth and metastasis primarily via degradation of
the ECM, which could result in cancer cell migration (11,12).
Elevated MMP-9 expression is key in the invasive process of
numerous cancers, especially breast cancers (13). The induction of MMP-9 is affected
by many factors, such as cytokines, growth factors, and certain
oncogenes. TPA, a phorbol ester, activates protein kinase C (PKC),
which excites MMP-9 secretion and synthesis during cancer cell
invasion (14). MMP is considered
a marker of cancer invasion and could be used as a prognostic
indicator for various cancers (15).
TPA mediates MMP-9 via regulation of transcription
factors, such as AP-1 and NF-κB, which have binding sites on the
MMP-9 promoter (16).
Additionally, the MAPK signaling pathway is important for AP-1 and
NF-κB regulation, which are known modulators of the MMP-9 promoter
(17). AP-1 and NF-κB are nuclear
transcription factors involved in several important diseases,
including cancer (18). NF-κB is
dominant in breast cancer cells and might play a key role in
tumorigenesis, such as cancer cell migration. Studies have shown
that NF-κB activation induces the growth and expansion of breast
cancer cells (19). Clinical data
have revealed that NF-κB inhibitors are beneficial in some patients
with breast cancer (20).
It has been well known that troglitazone, a PPAR-γ
ligand, shows anticancer activity in breast cancer cells in
vitro (21). Furthermore, some
studies have indicated that the PPAR-γ ligand is effective in
inhibiting breast cancer in vitro, but this drug has a
marginal effect in vivo (22), suggesting that the role of the
PPAR-γ ligand in breast cancer cells should be decided for the
development of effective and safe drugs for patients with breast
cancer (23).
Recent studies have shown that CDDO, a PPAR-γ ligand
and novel synthetic triterpenoid, plays a pivotal role in MMP
expression in several cancer lines (24). These results indicate CDDO as a
hopeful therapeutic agent for inhibiting the development of primary
tumors and metastases in cancers (25). However, the effect of CDDO on
TPA-induced breast cancer metastasis has not yet been addressed as
a mechanism contributing to MMP repression. Consequently, the role
of the PPAR-γ ligand, including CDDO, in cancer growth inhibition
has been studied (26), but its
dependency on PPAR-γ remain uncertain.
In conclusion, we observed the effects of CDDO on
regulation of PPAR-γ signaling and MMP-9 expression of breast
cancer cell. CDDO reduced MMP-9 expression, cell migration, and
invasion of breast cancer by inhibiting TPA-induced phosphorylation
of MAPKs and downregulating the activities of AP-1 and NF-κB. In
addition, knock-out of PPAR-γ by siRNA in MCF-7 cells showed that
TPA induced MMP-9 expression by a PPAR-γ-independent pathway. These
data showed that the downregulation effect of CDDO on MMP-9
expression occurred through a mechanism independent of PPAR-γ. Our
findings support those of several other reports demonstrating that
CDDO can regulate cell movement independent of PPAR-γ. These
results suggest that the function of CDDO is to act as a key agent
in the regulation of breast cancer metastasis in a
PPAR-γ-independently.
Acknowledgements
Not applicable.
Funding
This paper was supported by the Biomedical Research Institute,
Jeonbuk National University Hospital, and the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant nos.
2019R1A2C1003454 and 2020R1I1A1A01054100).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSK and OYH conceived and designed the study and
wrote the manuscript. HYJ performed the experiments and analyzed
the data. JSK and SHJ contributed to the design and acquisition of
funding. HYJ, HJY, JJ and EYC were involved in additional
experiments and the revision processes. SHJ and OYH evaluated and
revised the manuscript. JSK and SHJ confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Glossary
Abbreviations
Abbreviations:
|
PPAR-γ
|
peroxisome proliferator-activated
receptor-γ
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide
assay
|
|
PCR
|
polymerase chain reaction
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
p38
|
mitogen-activated protein kinase
|
|
AP-1
|
activator protein-1
|
|
NF-κB
|
nuclear factor κB
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DY and Park HL: Breast cancer risk
prediction in Korean women: Review and perspectives on personalized
breast cancer screening. J Breast Cancer. 23:331–342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin X and Mu P: Targeting breast cancer
metastasis. Breast Cancer (Auckl). 9 (Suppl 1):S23–S34. 2015.
|
|
4
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Zang C, Fenner MH, Possinger K and
Elstner E: PPARgamma ligands and ATRA inhibit the invasion of human
breast cancer cells in vitro. Breast Cancer Res Treat. 79:63–74.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong OY, Youn HJ, Jang HY, Jung SH, Noh
EM, Chae HS, Jeong YJ, Kim W, Kim CH and Kim JS: Troglitazone
inhibits matrix metalloproteinase-9 expression and invasion of
breast cancer cell through a peroxisome proliferator-activated
receptor γ-dependent mechanism. J Breast Cancer. 21:28–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YY, Zhe H and Zhao R: Preclinical
evidences toward the use of triterpenoid CDDO-Me for solid cancer
prevention and treatment. Mol Cancer. 13:302014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lapillonne H, Konopleva M, Tsao T, Gold D,
McQueen T, Sutherland RL, Madden T and Andreeff M: Activation of
peroxisome proliferator-activated receptor gamma by a novel
synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic
acid induces growth arrest and apoptosis in breast cancer cells.
Cancer Res. 63:5926–5939. 2003.PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang HY, Hong OY, Youn HJ, Kim MG, Kim CH,
Jung SH and Kim JS: 15d-PGJ2 inhibits NF-κB and AP-1-mediated MMP-9
expression and invasion of breast cancer cell by means of a heme
oxygenase-1-dependent mechanism. BMB Rep. 53:212–217. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
12
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong OY, Jang HY, Park KH, Jeong YJ, Kim
JS and Chae HS: Triptolide inhibits matrix metalloproteinase-9
expression and invasion of breast cancer cells through the
inhibition of NF-κB and AP-1 signaling pathways. Oncol Lett.
22:5622021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH,
Rho JY and Kim JH: PMA-induced up-regulation of MMP-9 is regulated
by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp
Mol Med. 39:97–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JM, Noh EM, Kim HR, Kim MS, Song HK,
Lee M, Yang SH, Lee GS, Moon HC, Kwon KB and Lee YR: Suppression of
TPA-induced cancer cell invasion by Peucedanum japonicum Thunb.
extract through the inhibition of PKCα/NF-κB-dependent MMP-9
expression in MCF-7 cells. Int J Mol Med. 37:108–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YR, Noh EM, Han JH, Kim JM, Hwang BM,
Kim BS, Lee SH, Jung SH, Youn HJ, Chung EY and Kim JS: Sulforaphane
controls TPA-induced MMP-9 expression through the NF-κB signaling
pathway, but not AP-1, in MCF-7 breast cancer cells. BMB Rep.
46:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uzzo RG, Crispen PL, Golovine K, Makhov P,
Horwitz EM and Kolenko VM: Diverse effects of zinc on NF-kappaB and
AP-1 transcription factors: Implications for prostate cancer
progression. Carcinogenesis. 27:1980–1990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Nakshatri H: NF-kappaB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lichtor T, Spagnolo A, Glick RP and
Feinstein DL: PPAR-gamma thiazolidinedione agonists and
immunotherapy in the treatment of brain tumors. PPAR Res.
2008:5474702008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arif IS, Hooper CL, Greco F, Williams AC
and Boateng SY: Increasing doxorubicin activity against breast
cancer cells using PPARγ-ligands and by exploiting circadian
rhythms. Br J Pharmacol. 169:1178–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krieger-Hinck N, Schumacher U, Müller A
and Valentiner U: The effect of the PPAR-gamma agonist
rosiglitazone on neuroblastoma SK-N-SH cells in a metastatic
xenograft mouse model. Oncol Res. 8:387–393. 2010.PubMed/NCBI
|
|
23
|
Wang YY, Yang YX, Zhe H, He ZX and Zhou
SF: Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update
on its pharmacokinetic and pharmacodynamic properties. Drug Des
Devel Ther. 8:2075–2088. 2014.PubMed/NCBI
|
|
24
|
Marx N, Sukhova G, Murphy C, Libby P and
Plutzky J: Macrophages in human atheroma contain PPARgamma:
Differentiation-dependent peroxisomal proliferator-activated
receptor gamma(PPARgamma) expression and reduction of MMP-9
activity through PPARgamma activation in mononuclear phagocytes in
vitro. Am J Pathol. 153:17–23. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shishodia S, Sethi G, Konopleva M,
Andreeff M and Aggarwal BB: A synthetic triterpenoid, CDDO-Me,
inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF
and chemotherapeutic agents through down-regulation of expression
of nuclear factor kappaB-regulated gene products in human leukemic
cells. Clin Cancer Res. 12:1828–1838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo X, Wu J and Wu G: PPARγ activation
suppresses the expression of MMP9 by downregulating NF-κB post
intracerebral hemorrhage. Neurosci Lett. 752:1357702021. View Article : Google Scholar : PubMed/NCBI
|