Introduction
Cancer is a major public health problem and one of
the main causes of mortality worldwide (1). Although there are numerous treatment
options available, including chemotherapy, surgery, radiotherapy,
hormone therapy and immunotherapy, according to the World Health
Organization, 19.3 million patients were diagnosed with cancer and
10 million succumbed to the disease in 2020 (1). There are multiple reasons for this,
including lack of specific and effective tumor markers, late
detection due to atypical early symptoms, rapid growth and
metastasis of tumors, and drug resistance caused by tumor
chemotherapy, which results in poor therapeutic effect (2–4).
Circular RNAs (circRNAs) participate in the regulation of all
principal hallmarks of malignancy, and are considered promising
markers for predicting cancer diagnosis and prognosis (5).
Formation and function of circRNAs
circRNAs are endogenous RNA molecules that are
widely present in mammalian transcriptomes and are involved in the
regulation of gene expression, belonging to the family of
non-coding RNAs (ncRNAs) (6,7).
circRNAs are generated by a precursor mRNA through back-splicing or
non-canonical splicing with no free end or polyadenylated tail.
Compared with linear ncRNAs, circRNAs are more stable, since their
circular structure protects them from degradation by the majority
of RNA decay mechanisms (8,9).
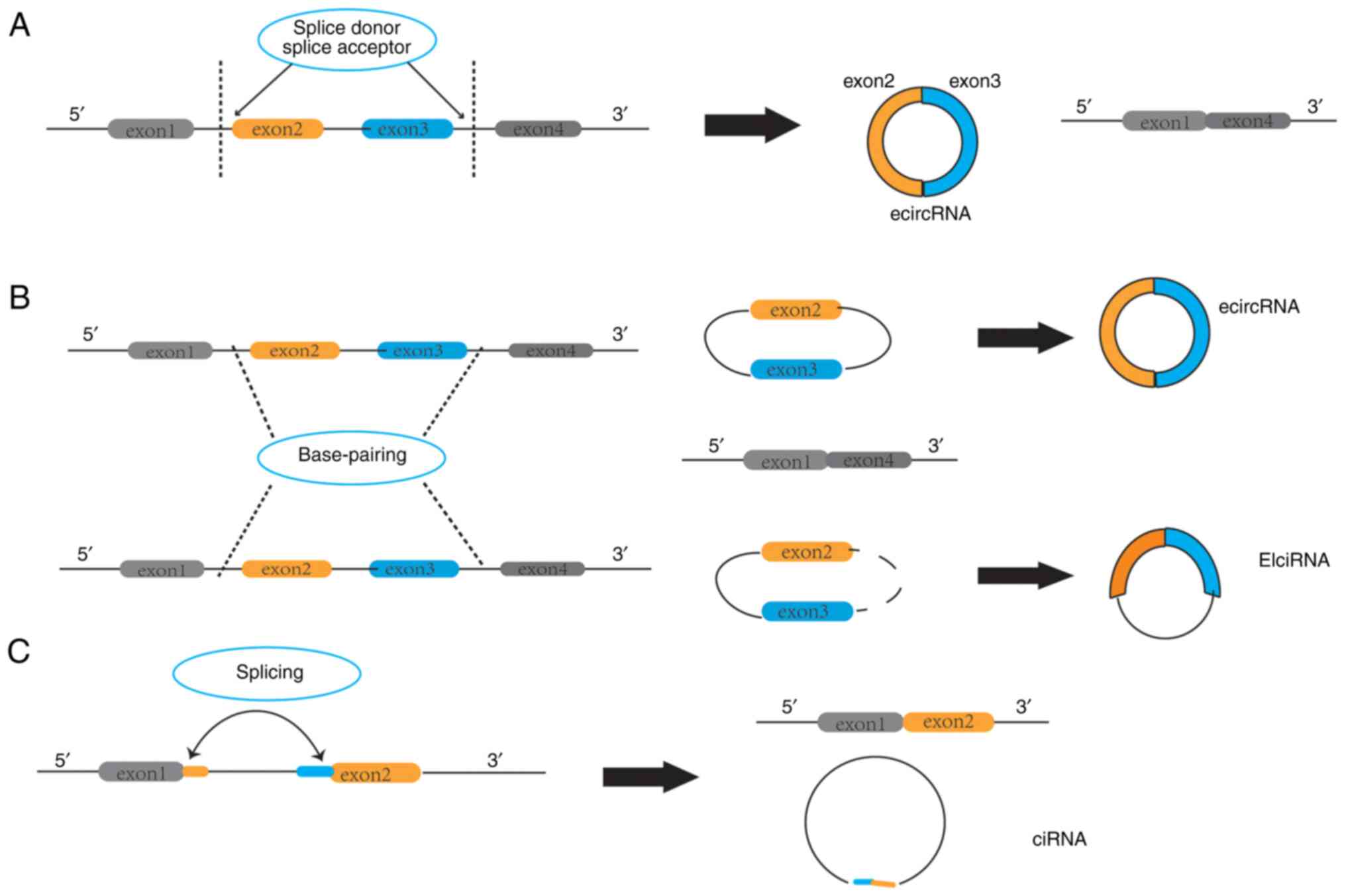
Based on the exons and introns that they contain from the parental
genes, circRNAs can be divided into three categories: i) Exonic
circRNAs (ecircRNA), which only contain back-spliced exons; ii)
circular intronic RNAs (ciRNA) that come from introns; and iii)
exon-intron circRNAs (EIciRNAs), which are circularized with both
exons and introns (10). A
schematic diagram of their structure is shown in Fig. 1.
circRNAs used to be considered to be a byproduct of
splicing errors, with no biological function, and were considered
junk RNAs in the past (11). With
the widespread application of RNA sequencing and the rapid
expansion of bioinformatics, the functions of additional circRNAs
have been identified (12).
circRNAs perform four main functions. First, they act as competing
endogenous RNA (ceRNA) or microRNA (miRNA or miR) sponges (8). circRNAs can competitively bind to
miRNAs, leading to a decrease in functional miRNA molecules and a
subsequent upregulation of target miRNAs (8). Cerebellar degeneration-associated
protein 1 antisense transcript (CDR1as) is the most representative
miRNA sponge circRNA, and contains >70 miR-7-binding sites.
CDR1as is a circular inhibitor of miR-7. When CDR1as is highly
expressed, miR-7 activity is decreased, leading to increased
expression of miR-7′s target genes (8,13,14).
Second, circRNAs interact with RNA binding proteins and mRNAs
(15,16). For example, circ-Foxo3 can repress
cell cycle progression by binding to the G1 to S phase
transition-related proteins CDK2 and p21 (15). circRNAs can interact with mRNAs,
and circRNAs that contain a translation start site can act as mRNA
traps and regulate protein translation of mRNA (16). Third, circRNAs regulate the
expression of their parent genes, and circRNAs involved in gene
regulation are enriched in the nucleus (17). EIciRNAs, such as EIciEIF3J and
EIciPAIP2, can bind to nuclear ribonucleoprotein with U1 small
nuclear RNA and RNA polymerase II in a cis-acting form to enhance
the transcription of their parental genes (10). Fourth, circRNAs are involved in
protein translation. Although circRNAs belong to ncRNAs, a number
of these molecules have been reported to be translatable (18,19).
Legnini et al (18)
confirmed that circ-ZNF609 can be translated into a protein that
participates in myogenesis (18).
A previous study reported that upregulation of FBXW7-185aa, a novel
protein encoded by circ-FBXW7, inhibits cell proliferation and cell
cycle progression in glioblastoma, since it reduces the half-life
of c-Myc (19).
Accumulating evidence shows that circRNAs play
important roles in the development and progression of non-cancerous
and cancerous diseases, such as neurological (20) and cardiovascular (21) diseases, as well as insulin
secretion (22) and tumors
[(including lung, breast and gastric cancer (GC)] (23–25).
circRNAs regulate the apoptosis, proliferation, migration, invasion
and angiogenesis of cancer cells, and lead to tumor drug resistance
through the hypoxia-inducible factor (HIF) regulatory pathway under
hypoxia (23–26). For example, circAGFG1 promotes the
proliferation and invasion of non-small cell lung cancer (NSCLC)
cells, and inhibits apoptosis by accelerating glycolysis via the
miR-28-5p/HIF-1α axis (23).
Formation and function of HIFs
Accumulating evidence has shown that the hypoxic
microenvironment, which is critical during cancer development,
plays a key role in regulating cancer progression and metastasis.
The effects of HIF, a master regulator of the hypoxic response,
have been extensively studied during these processes (23–25).
HIFs are a family of three members, and they are
heterodimers composed of an O2-sensitive α subunit (HIF-1α, HIF-2α
or HIF-3α) and an O2-insensitive HIF-1β subunit (27). HIF-α is the most characteristic
subtype of HIF and is a conditionally regulated transcription
factor (28). HIF-1α promotes an
acute response to hypoxia, while HIF-2α promotes a chronic
response. The function of HIF-3α and its numerous splice variants
is not known in detail (29,30).
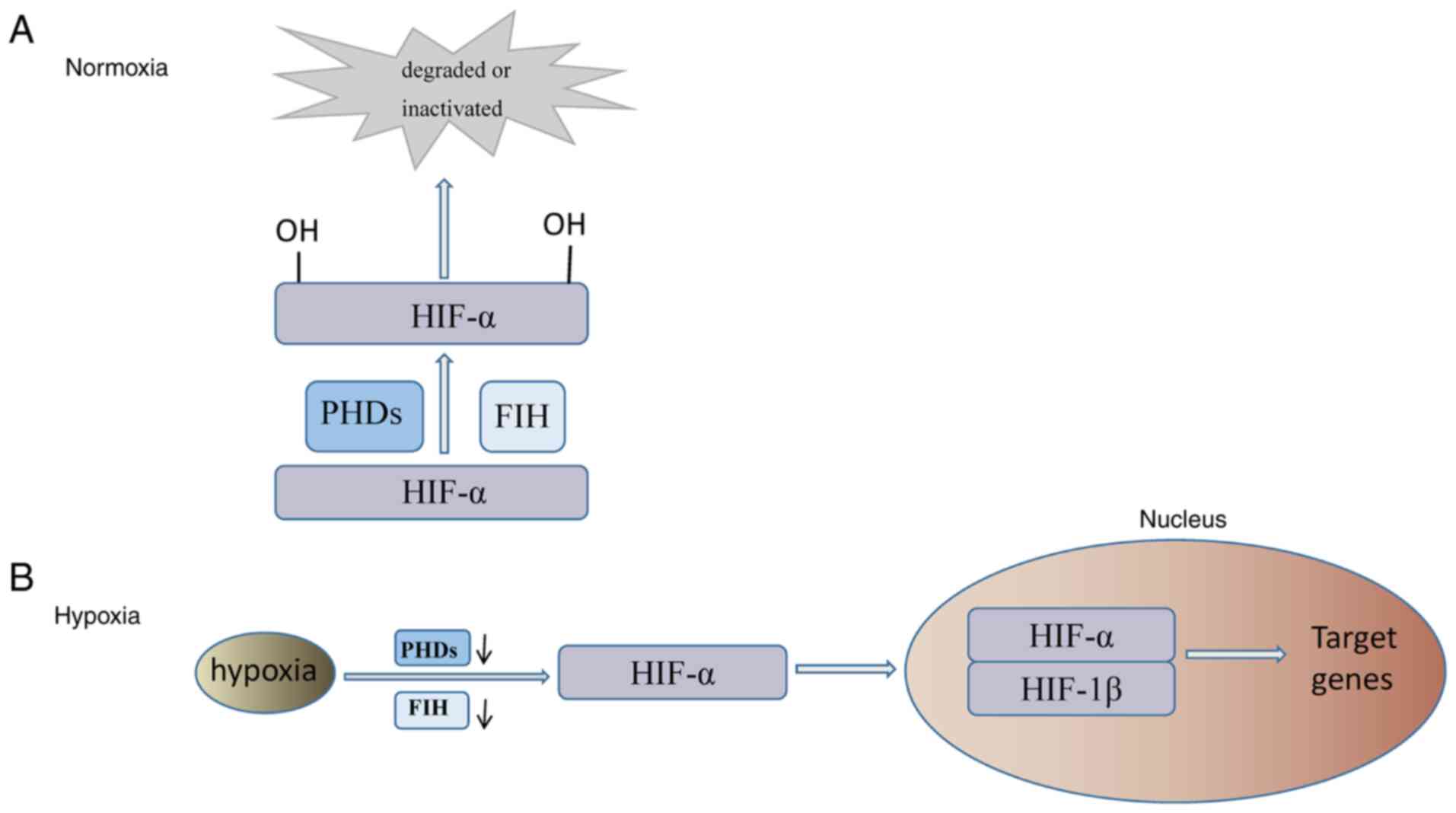
Under normal oxygen conditions, HIF-α is modified into a
hydroxylated HIF-α subunit by HIF prolyl hydroxylases (PHDs) or
factor inhibiting HIF (FIH) and then degraded or inactivated, as
shown in Fig. 2A (31,32).
Diminished PHD and FIH activity during periods of hypoxia
stabilizes HIF-α and results in its translocation to the nucleus,
where HIF-α and HIF-1β form a heterodimer, a structurally active
transcription factor, as represented in Fig. 2B (28,31,32).
Hypoxia is one of the most common conditions
encountered within the tumor microenvironment (29). Impairment in diffusion,
abnormalities in tumor microvessels and altered microcirculation
lead to deficient or even abolished oxygen supply in the tumor
microenvironment (33,34). Therefore, the HIF signaling pathway
plays an important role in tumors. First, HIF-1 is a key mediator
of tumor metabolism. Specifically, HIF-1 is an important mediator
of the tumor-associated metabolic switch, also known as the Warburg
effect, in which tumor cells generate energy mainly by
non-oxidative breakdown of glucose rather than conventional
oxidative phosphorylation (24,35,36).
Second, it regulates the tumor microenvironment and promotes tumor
progression. Hypoxia is a critical parameter that modulates stromal
and/or endothelial/tumor cell interactions, and intratumoral
hypoxia leads to the release of factors that recruit
tumor-associated macrophages, myelogenous suppressor cells and
other immune cells, which arrive at the tumor site and secrete
proinflammatory cytokines or other mediators to induce tumor
angiogenesis, promote cancer cell metastasis or produce
immunosuppression (37–40). Third, HIF-α promotes tumor
metastasis through the epithelial-to-mesenchymal transition (EMT)
and cancer stem cells (CSCs) (41–43).
HIF-α-mediated EMT results in the enrichment of a stem-like side
population of cells in thyroid cancer (42), and HIF-α promotes mammary tumor
growth and metastasis, partly through the regulation of CSCs
(43). Fourth, HIF is involved in
tumor resistance. Previous studies have shown that HIF-1 and
reactive oxygen species enhance the development of resistance to
chemotherapeutics, such as doxorubicin and etoposide, in lung,
cervical carcinoma and melanoma cell lines (44,45).
The circRNA and HIF regulatory pathways have been
extensively studied in recent years. The present review focused on
the biological functions (Table I)
and mechanisms of action (Fig. 3)
of circRNA in various tumors under hypoxia, and evaluated its
potential as a new diagnostic and prognostic biomarker.
 | Table I.Functional characteristics of
circRNAs in multiple human cancer types under hypoxia. |
Table I.
Functional characteristics of
circRNAs in multiple human cancer types under hypoxia.
| First author,
year | Cancer type | circRNA name | Expression | Host gene name | Functional
roles | Target miRNA | HIFs | Study model | (Refs.) |
|---|
| Ma et al,
2021 | Lung cancer | circAGFG1 | Upregulated | AGFG1 | Viability (+) | miR-28-5p | HIF-1α | In vivo/in
vitro | (23) |
|
|
|
|
|
| Proliferation
(+) |
|
|
|
|
|
|
|
|
|
| Migration (+) |
|
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
|
|
|
|
|
| Apoptosis (−) |
|
|
|
|
| Feng et al,
2020 | Lung cancer | hsa-circ- | Upregulated | SFMBT2 | Migration (+) | miR-622 | HIF-1α | In
vitro | (47) |
|
|
| 0000211 |
|
| Invasion (+) |
|
|
|
|
| Chi et al,
2019 | Lung cancer | circPIP5K1A | Upregulated | PIP5K1A | Proliferation
(+) | miR-600 | HIF-1α | In vivo/in
vitro | (51) |
|
|
|
|
|
| Migration (+) |
|
|
|
|
| Cao et al,
2020 | Breast cancer | circRNF20 | Upregulated | RNF20 | Proliferation
(+) | miR-487a | HIF-1α | In vivo/in
vitro | (24) |
|
|
|
|
|
| Apoptosis (−) |
|
|
|
|
| Liang et al,
2017 | Breast cancer | circDENND4C | Upregulated | - | Proliferation
(+) | - | HIF-1α | In
vitro | (53) |
| Wang et al,
2020 | Breast cancer | circPVT1 | Upregulated | - | Proliferation
(+) | miR-29a-3p | HIF-1α | In vivo/in
vitro | (54) |
|
|
|
|
|
| Migration (+) |
|
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Darbeheshti et
al, 2021 | Breast cancer | circ_0047303 | Upregulated | ZNF521 | Proliferation
(+) | - | HIF-1 | In
vitro | (55) |
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Liu et al,
2020 | Gastric cancer | circ-MAT2B | Upregulated | - | Proliferation
(+) | miR-515-5p | HIF-1α | In vivo/in
vitro | (25) |
| Darbeheshti et
al, 2021 | Gastric cancer | circHIPK3 | Upregulated | HIPK3 | Proliferation
(+) | miR-653-5p/ | HIF-2α | In vivo/in
vitro | (55) |
|
|
|
|
|
| Migration (+) | miR-338-3p- |
|
|
|
|
|
|
|
|
| Invasion (+) | NRP1 |
|
|
|
| Zhai et al,
2019 | Hepatoce- | hsa-circ- | Upregulated | B3GNTL1 | Proliferation
(+) | miR-640 | HIF-1α | In vivo/in
vitro | (59) |
|
| llular
carcinoma | 0046600 |
|
| Migration (+) |
|
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Tu et al,
2021 | Hepatoce | circ-0003006 | Upregulated | FLNB | Proliferation
(+) | miR-542-3p | HIF-1α | In vivo/in
vitro | (60) |
|
| llular
carcinoma |
|
|
| Migration (+) |
|
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Tan et al,
2019 | Hepatoce- | circ-EPHB4 | Downregulated | EPHB4 | Apoptosis (+) | - | HIF-1α | In vivo/in
vitro | (61) |
|
| llular
carcinoma |
|
|
| Migration (−) |
|
|
|
|
|
|
|
|
|
| Invasion (−) |
|
|
|
|
| Liu and Xu,
2021 | Pancreatic
cancer | circ_03955 | Upregulated | - | Proliferation
(+) | miR-3662 | HIF-1α | In vivo/in
vitro | (64) |
|
|
|
|
|
| Apoptosis (−) |
|
|
|
|
| Ou et al,
2019 | Pancreatic
cancer | circ_0000977 | Upregulated | NOL10 | Immune escape
(+) | miR-153 | HIF-1α | In vivo/in
vitro | (67) |
| Chen et al,
2020 | Colorectal
cancer | circ-ERBIN | Upregulated | - | Proliferation
(+) | miR-138-5p/ | HIF-1α | In vivo/in
vitro | (70) |
|
|
|
|
|
| Migration (+) | 4EBP-1 |
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Zhou et al,
2020 | Colorectal
cancer | circRNA_ | Upregulated | - | Proliferation
(+) | miR-217 | HIF-1α | In vivo/in
vitro | (71) |
|
|
| 100859 |
|
| Apoptosis (−) |
|
|
|
|
|
|
|
|
|
| Migration (+) |
|
|
|
|
|
|
|
|
|
| Invasion (+) |
|
|
|
|
| Qian et al,
2020 | Cervical
cancer | circ-HIPK3 | Upregulated | HIPK3 | Proliferation
(+) | miR-338-3p | HIF-1α | In vivo/in
vitro | (72) |
|
|
|
|
|
| Migration (+) |
|
|
|
|
| Chen et al,
2019 | Cervical
cancer | circRNA | Downregulated | - | Proliferation
(−) | miR-135b-5p | HIF1AN | In vivo/in
vitro | (73) |
|
|
| CDR1as |
|
| Migration (−) |
|
|
|
|
|
|
|
|
|
| Invasion (−) |
|
|
|
|
| Su et al,
2019 | Glioma | hsa_circ_ | Upregulated | DENND2A | Migration (+) | miR-625-5p | HIF-1α | In
vitro | (74) |
|
|
| 0002142 |
|
| Invasion (+) |
|
|
|
|
Role of circRNA in lung cancer under
hypoxia
Lung cancer is the most commonly diagnosed cancer
and the primary cause of cancer-associated mortality worldwide
(1). Given the high mortality and
markedly low survival rate of lung cancer, the determination of
specific biomarkers for the diagnosis and treatment of lung cancer
remains critical. Previous studies found that, under hypoxia,
circRNAs may become important biomarkers of lung cancer, since they
participate in the malignant progression of lung cancer, including
metastasis, cell proliferation, tumor growth and drug resistance)
(23,46–48).
In lung cancer tissues, HIF-α expression was
significantly elevated compared with that in adjacent normal lung
control tissues, suggesting the existence of tumor hypoxia during
the development of lung cancer (23,46,47).
Li et al (49) determined
the protein levels of HIF-1α and downstream target genes, such as
pyruvate dehydrogenase kinase, glucose transporter 1,
adrenomedullin vascular endothelial growth factor A and ribosomal
protein L28, in hypoxic A549 cells, and they were significantly
higher than those in normoxic A549 cells. Notably, there was no
change in HIF-1α expression level, suggesting that hypoxia
regulates HIF-1α at the translational level (46). Hypoxia is common in lung cancer
with lymph node metastasis (50).
Cheng et al (46) examined
the circFAM120A levels in 11 paired tumor and adjacent non-cancer
tissues from patients with lymph node-positive lung adenocarcinoma
(LAC) using reverse transcription-quantitative PCR (RT-qPCR). The
expression of circFAM120A was significantly reduced in all tumor
specimens, suggesting that circFAM120A may be a potential marker of
LAC hypoxia, and may be able to predict the risk of lung cancer
metastasis.
Ma et al (23) found that miR-28-5p suppressed the
proliferation, migration and invasion, and accelerated the
apoptosis of NSCLC cells by targeting HIF-1α. In H1299 cells
transfected with anti-miR-28-5p alone or in combination with
si-circAGFG1, miR-28-5p interference elevated the expression of
HIF-1α, and the introduction of small interfering RNA (siRNA)
circAGFG1 reduced the expression of HIF-α, which confirmed that
circAGFG1 enhanced the enrichment of HIF-1α via sponging miR-28-5p
in NSCLC cells. circAGFG1 notably upregulated in NSCLC tumor
tissues compared with the findings in adjacent normal tissues.
circAGFG1 was significantly upregulated in NSCLC tumor tissues
compared with the levels found in normal adjacent tissues. In vivo
and in vitro experiments confirmed that circAGFG1 promoted the
proliferation, migration and invasion of NSCLC cells, and
accelerated cell apoptosis under hypoxia (23). Similarly, Feng et al
(47) reported higher expression
of hsa-circ-0000211 in LAC tissues than in normal tissues.
hsa-circ-0000211 upregulated HIF-1α expression by targeting
miR-622, and inhibition of hsa-circ-0000211 inhibited LAC migration
and invasion. The mechanism of action is that hsa-circ-0000211
promotes LAC cell migration and invasion by regulating the
miR-622/HIF-1α network. In addition, circPIP5K1A (hsa_circ_0014130)
expression was increased in NSCLC cells (51). circPIP5K1A knockdown suppressed
NSCLC cell metastasis and proliferation by promoting the expression
of miR-600 (51). Overexpression
of miR-600 inhibited the HIF-1α-mediated metastasis and
proliferation of NSCLC cells by downregulating the EMT-related
proteins, Snail and vimentin, and by upregulating E-cadherin
(51). Regarding the mechanism of
action, miR-600 was the circPIP5K1A target, and miR-600 interacted
with the 3′ untranslated region (UTR) of HIF-1α circPIP5K1A, which
functioned as a miR-600 sponge to facilitate NSCLC proliferation
and metastasis by promoting HIF-1α (51).
The above findings suggest that, in a hypoxic
environment, circRNAs can promote lung cancer proliferation and
metastasis through different pathways; thus, these circRNAs may
become candidate markers and therapeutic targets for lung
cancer.
Role of circRNA in breast cancer (BC) under
hypoxia
BC is emerging as the leading cause of
cancer-associated mortality, and the second highest cause of
mortality in women worldwide (1,52).
Compelling evidence has demonstrated the potential functions of
circRNAs in BC tumorigenesis (24,53–55).
circDENND4C is a HIF1α-associated circRNA induced
under hypoxia in BC cells (53).
Liang et al (53) found
that the expression of circDENND4C was increased after hypoxia
induction or decreased after knocking down HIF-1α. Compared with
that in the negative control group, silencing of circDENND4C or
HIF-1α resulted in decreased proliferation of cancer cells under
hypoxia (53). In addition,
RT-qPCR showed that circDENND4C expression was higher in tumor
tissues than in normal adjacent tissues, and the expression level
of circDENND4C was positively correlated with the HIF-1α mRNA
level. In addition, the circDENND4C level was correlated with tumor
size, which suggested that the larger the tumor became, the more
likely it was to suffer hypoxia-mediated injury (53). Using RT-qPCR, Cao et al
(24) demonstrated that circRNF20
was overexpressed in BC tissues compared with its expression levels
in normal tissue. Survival analysis conducted by Kaplan-Meier and
log-rank test revealed that high circRNF20 expression was
associated with a low survival rate in patients with BC. The
RT-qPCR results showed that circRNF20 gene knockout increased the
expression of miR-487a and downregulated the expression level of
HIF-1α, while circRNF20 overexpression decreased the expression of
miR-487a and enhanced the expression of HIF-1α. miR-487a
overexpression decreased the HIF-1α level, while miR-487a silencing
upregulated the HIF-1α level (24). Cell proliferation and flow
cytometry assays showed that circRNF20 gene knockout inhibited cell
proliferation and increased cell apoptosis, while circRNF20
overexpression had the opposite effects. Therefore, these findings
indicate that circRNF20 promotes BC tumor growth and proliferation,
and inhibits tumor cell apoptosis. Mechanistically, circRNF20
harbors miR-487a, acting as a miRNA sponge, and then miR-487a
targets the 3′ UTR of HIF-1α (24).
Wang et al (54) reported that circPVT1 was remarkably
increased in BC tissues compared with its level in adjacent normal
tissues, while the miR-29a-3p levels were considerably decreased.
Kaplan-Meier analysis revealed that patients with BC with low
miR-29A levels had a shorter median survival, and overexpressed
circPVT1 was correlated with positive lymph node examination and
tumor size. The authors also found that overexpression of circPVT1
or downregulation of miR-29a-3p significantly increased the
anterior gradient 2 (AGR2) and HIF-1α levels, and promoted the
proliferation, migration and invasion of BC cells. The mechanism of
action is that circPVT1 acts as a BC oncogene, binds to miR-29a-3p,
and promotes cell proliferation, invasion, migration, and
inhibition of apoptosis through a miR-29a-3p-mediated AGR2-HIF-1α
axis (54).
The aggressive and highly metastatic nature of
triple-negative BC (TNBC) causes poor outcomes in patients with
TNBC. The HIF-1 signaling pathway is a prominent pathway that
contributes to angiogenesis and metastasis progression in tumors
(37). Darbeheshti et al
(55) found that circ_0047303 was
significantly upregulated in TNBC compared with its expression in
normal adjacent tissues by fluorescence RT-qPCR. The results
indicated that circ_0047303, the highest upregulated circRNA, could
potentially sponge/inhibit multiple downstream miRNAs that function
in the HIF-1 signaling pathway. Kaplan-Meier analysis suggested
that patients with high circ_0047303 expression had shorter
disease-free survival. By regulating the function of HIF-1,
circ_0047303 expression was associated with the clinicopathological
characteristics of the patients, and was positively correlated with
tumor size, tumor stage and lymph node metastasis. Therefore, this
circRNA was considered to have a prognostic value (55).
These findings suggest that circRNAs may be
promising biomarkers for BC diagnosis, and even potential
therapeutic targets for BC treatment through the HIF signaling
pathway under hypoxic conditions.
Role of circRNA in GC under hypoxia
GC is a major public health problem worldwide, and
is responsible for >1 million new cases and estimated 783,000
mortalities in 2018. GC is the fifth most commonly diagnosed type
of cancer, and the third leading cause of cancer-associated
mortality (1,56). Thus, it is crucial to study
biomarkers and therapeutic targets for GC.
Liu et al (25) found that circ-MAT2B and HIF-1α
circ-MAT2B gene knockout could significantly inhibit cell viability
and DNA synthesis in GC cells. In addition, it could inhibit
glucose uptake, lactic acid synthesis and tumor growth in vivo.
Moreover, high expression of circ-MAT2B was positively correlated
with tumor size, lymph node metastasis and TNM stage, and the
overall survival (OS) and DFS of patients with high circ-MAT2B
expression were shorter than those of patients with low circ-MAT2B
expression (26). HIF-1α is a
driver of glycolysis and uncontrolled proliferation of tumor cells,
activating various oncogenic pathways (57). HIF-1α was upregulated in GC, which
was due to the overexpression of circMAT2B. Exogenous expression of
HIF-1α could effectively rescue the glycolysis reduction caused by
circ-mat2b gene knockout, suggesting that HIF-1α is necessary for
the function of circ-mat2b in GC (25). The mechanism of action is that
circ-mat2b was predominantly located in the cytoplasm, and acted as
a ceRNA to sponge miR-515-5p and increase HIF-1α-mediated silencing
of miR-515-5p. In addition, overexpression of HIF-1α could rescue
the attenuated aggressive phenotype of GC cells caused by
circ-mat2b knockdown. Importantly, HIF-1α was able to directly bind
to the circ-mat2b promoter and transcriptionally activate
circ-mat2b, thus forming a positive feedback loop (25).
The role of circRNAs in GC metastasis under hypoxia
is unclear. Previous results showed that the expression of HIF-2α
was significantly upregulated in well-differentiated GC cells.
HIF-2α gene knockdown reduced the expression of circHIPK3 in GC
cells, and further overexpression of circHIPK3 enhanced the
migration and invasion abilities of GC cells (55). These results suggest that HIF-2α
mainly promotes the upregulation of circHIPK3 expression in GC
cells under a long-term hypoxic microenvironment, thus promoting GC
metastasis (55). The mechanism of
action is that circHIPK3 induces the upregulation of neuropilin 1
(NRP1) expression in GC cells by using miR-653-5p and miR-338-3p
sponges in a long-term hypoxic microenvironment. NRP1 may promote
the migration and invasion of GC cells through the ERK/AKT
signaling pathway. A previous study found that the OS of patients
with GC with high expression of NPR1 was shorter than that of
patients with GC with low expression of NPR1, which also indicated
that NPR1 is upregulated by circHIPK3 and may lead to poor
prognosis of patients with GC (55).
In conclusion, circRNA may be used as a potential
biomarker of long-term hypoxia in GC, as well as a sensitive and
specific indicator of diagnosis and prognosis.
Role of circRNA in hepatocellular carcinoma
(HCC) under hypoxia
HCC is a malignant tumor with a high incidence.
Despite the development of advanced diagnostic and treatment
techniques for HCC, the outcomes remain unsatisfactory due to
disease relapse, late diagnosis and drug resistance (1,58).
Therefore, it is of great importance for patients with HCC to
identify biomarkers associated with early diagnosis, therapeutic
intervention and prognosis, and to study the molecular mechanism of
HCC occurrence and metastasis.
Zhai et al (59) and Tu et al (60) found that the expression level of
hsa-circ-0046600 and circ-0003006 in HCC tissues was significantly
higher than that in adjacent normal tissues, and was positively
correlated with tumor size, TNM stage, pathological vascular
invasion and lymph node metastasis. Further experiments showed that
hsa-circ-0046600 knockdown inhibited SK-HEP-1 cell migration,
indicating that hsa-circ-0046600 was overexpressed in HCC cells and
promoted the migration of these cells. The expression of miR-640
was significantly downregulated in HCC tissues, and the expression
of hsa-circ-0046600 was negatively correlated with that of miR-640.
HIF-1α was the downstream protein of hsa-circ-0046600. Therefore,
the-circ-0046600 mainly affected the malignant biological behavior
of HCC cells by competitively binding to miR-640 HCC by promoting
hypoxia-induced expression of HIF-1α and other proteins (59). Similarly, circ-0003006 was
demonstrated to promote HCC progression in vitro and in vivo by
sponging miR-542-3p to release the inhibition of HIF-1α (60).
In HCC, the expression of circEPHB4 was
downregulated, while the overexpression of circEPHB4 inhibited the
survival of HCC cells, induced apoptosis, and inhibited cell
migration and invasion (61). Tan
et al (61) found that
induced overexpression of circEPHB4 increased the apoptosis rate of
HCC cells, inhibited cell proliferation and prevented HCC cell
invasion. In addition, circ-EPHB4 levels were negatively correlated
with tumor weight, size and metastasis in vitro, suggesting that
circ-EPHB4 inhibits tumor genesis, development and metastasis. The
results showed that circ-EPHB4 inhibited tumor genesis and
metastasis by inhibiting HIF-1α expression, which in turn inhibited
the HIF-1α PI3K-AKT signaling pathway and the HIF-1α/ZEB1 axis
(61). These findings provide a
novel perspective for the study of HCC, and suggest that circRNA
has antitumor effects on HCC.
Role of circRNA in pancreatic cancer under
hypoxia
Pancreatic cancer (PC) is a common malignant tumor
worldwide that presents a serious threat to human health. PC's
survival is the poorest amongst all solid cancer types, with a
median survival duration of <6 months (62,63).
Thereby, the identification of novel and effective biomarkers for
PC diagnosis and therapeutic targets is urgently required.
Liu and Xu (64)
showed that the upregulation of circ_03955 in PC was positively
correlated with the adverse clinical outcome of patients with PC.
In vitro and in vivo experiments showed that circ_03955 could
promote the proliferation and inhibit the apoptosis of PC cells, as
well as promote the Warburg effect. Its mechanism is as follows:
circ_03955 acts as a sponge for miR-3662 to promote cancer through
the miR3662/HIF-1α axis (64).
Disruption of the circ_03955/miR-3662/HIF-1α loop may prevent the
development and progression of PC.
Previous studies have shown that hypoxia can induce
the upregulation of HIF1a, metalloproteinase ADAM10 and the
downregulation of MHC class I chain-related molecule A, leading to
the reduction of natural killer group 2D in natural killer (NK)
cells, and tumor cell escaping immune surveillance and NK
cell-mediated lysis (65,66). One of the reasons for the malignant
progression of PC is the ability of tumor cells to evade
immune-mediated lysis (67). Ou
et al (67) found that
HIF-1α mRNA expression was significantly upregulated in PC tissues
compared with that in non-cancer tissues. Hypoxia has been
considered an essential inducer of tumor cell resistance to immune
effectors-mediated lysis (65).
Hypoxia was reduced, whereas HIF-1α knockdown partially restored
the killing effect of NK cells, suggesting that HIF-1α could
modulate the immune resistance and immune escape of Panic-1 cells
to NK cell-mediated lysis upon hypoxic conditions (67). The expression of circ_0000977 could
be induced by hypoxia, and circ_0000977 knockdown enhanced the
killing effect of NK cells on PC cells under hypoxia through HIF-1α
and ADAM10. Therefore, circ_0000977 could regulate the
HIF-1α-mediated immune escape of PC cells (67). The mechanism of action is that the
circ_0000977/miR-153 axis modulates the HIF-1α-mediated immune
escape of PC cells through the miR-153 downstream targets HIF-1α
and ADAM10 (67). These findings
indicate that the circ_0000977/miR-153/HIF1A/ADAM10 axis could
potentially be used as an immune-sensitizer in the treatment and/or
prevention of cancer.
Role of circRNA in colorectal cancer (CRC)
under hypoxia
CRC is one of the most common gastrointestinal
malignancies, and the incidence of the disease has rapidly
increased in recent years (68).
Although comprehensive therapies have been used, the prognosis of
colon cancer remains poor, which may be due to the lack of early
diagnosis and effective targeted therapy agents (69).
A previous study has shown that circ-Erbin was
highly expressed in CRC cells, and circ-Erbin overexpression
facilitated the proliferation, migration and metastasis of CRC in
vitro and in vivo (70). Chen
et al (70) found that
circ-Erbin overexpression was promoted through angiogenesis by
increasing the expression of HIF-1α in CRC (37,70).
The main mechanism is that, as the sponge of miR-125A-5p and
miR-138-5p, circ-Erbin mediates HIF-1α activation through the
miR-125a-5p-5p/miR-138-5p/eukaryotic translation promoter
4E-binding protein 1 (4EBP-1) axis, and promotes the
cap-independent protein translation of HIF-1α in CRC cells
alonsgise targeted 4EBP-1. Similarly, another study identified that
circRNA_100859 was overexpressed in colon cancer tissues and
inhibited cell apoptosis (71).
circRNA_100859 is a miR-217 sponge. miR-217 directly targets
HIF-1α, and the circRNA_100859-miR-217-HIF-1α axis is associated
with stage, histological grade and HIF-1α mutations in CRC
(71). Thus, circRNA_100859 may be
used as a new biomarker for the diagnosis and prognosis of CRC.
Role of circRNA in other cancer types under
hypoxia
A number of previous studies have shown that
circRNAs play an important role in gynecological tumors (72,73)
and gliomas (74) under conditions
of hypoxia.
Cervical cancer (CC) is the fourth most common
malignancy and the second most frequent cause of cancer-associated
death in women (1,75). The high mortality of CC is related
to the molecular mechanism responsible for its occurrence and
development. Qian et al (72) found that circ-HIPK3 expression was
significantly elevated in CC cells and tissues. circ-HIPK3
silencing repressed tumor growth and metastasis, and induced
apoptosis in CC cells. circ-HIPK3 sponged miR-338-3p and miR-338-3p
to upregulate HIF-1α and CC progression. miR-338-3p silencing or
HIF-1α overexpression rescued the circ-HIPK3 knockdown-mediated
inhibition of CC malignant characteristics. Functionally,
circ-HIPK3 promoted CC cell proliferation, clone formation,
migration and invasion, while inhibited cell apoptosis by sponging
miR-338-3p to upregulate HIF-1α expression, and contributed to EMT
in CC cells. Mechanismly, circ-HIPK3 acts as a ceRNA of miR-338-3p
to promote cell proliferation and metastasis in CC via regulating
HIF-1α-mediated EMT (72).
circRNAs and HIF1-α inhibitors (HIF1AN) are closely correlated with
cancer. Chen et al (73)
showed that CDR1as expression was significantly lower in ovarian
cancer tissues than in normal ovarian tissues by using RT-qPCR
(73). In vitro and in vivo,
CDR1as overexpression inhibited the proliferation, invasion and
migration of ovarian cancer cells. Silencing CDR1as increased the
expression of miR-135b-5p, and decreased the expression of HIF1AN,
thus increasing the proliferation capacity of ovarian cancer cells
(73). Mechanistically, CDR1as,
acting as a sponge of miR-135b-5p, promotes the expression of
HIF1AN and therefore plays a role in tumor inhibition (73). These results provide new
information for the diagnosis and treatment of ovarian cancer.
Glioma, which originates from glial cells, is the
most prevalent and malignant tumor of the central nervous system
(76). Despite the existence of
potential treatments for glioma, glioma tumors are highly invasive
and led to a high mortality rate (76,77).
Therefore, it is necessary to conduct in-depth research on the
pathological molecular mechanism of this disease to identify new
treatment methods. circDENND2A (hsa_circ_0002142) has been
suggested to be a hypoxia-responsive circRNA in glioma via
bioinformatic analysis (74).
Hypoxia induced the expression of circDENND2A, and promoted the
migration and invasion of glioma cells, which was achieved through
the sponge action of miR-625-5p (74). Notably, glioma tissues
overexpressing HIF-1α showed high expression of circDENND2A and low
expression of miR-625-5p under hypoxia. These results suggest that
there is a DENND2A/miR-625-5p axis in glioma tissues, which is
associated with HIF-1α (74).
Understanding the mechanism of this interaction may provide a
promising target for the treatment of glioma metastasis in a
hypoxic microenvironment.
Association of circRNA with tumor drug
resistance under hypoxia
HIF, a marker of hypoxia, activates downstream
oncogene transcription containing hypoxia response elements to
regulate tumor metabolism and metastasis (78). HIF may also lead to chemotherapy
and radiotherapy resistance through a variety of mechanisms
(48,79). Therefore, in the clinic, HIF
expression is associated with poor prognosis and treatment
recurrence. In recent years, it has been found that therapeutic
resistance induced by hypoxia can be reversed by circRNA
intervention (Table II).
 | Table II.Mechanisms of drug resistance of
circRNAs under hypoxia in various human cancer types. |
Table II.
Mechanisms of drug resistance of
circRNAs under hypoxia in various human cancer types.
| First author,
year | Cancer type | circRNA name | Expression | Host gene name | Resistance
type | Target miRNA | HIF | Study model | (Refs.) |
|---|
| Yu et al,
2021 | Lung cancer | circASXL1 | Upregulated | ASXL1 | Platinum
resistance | miR-206 | HIF-1α | In vivo | (48) |
| Xu et al,
2020 | Lung cancer | circAKT3 | Upregulated | AKT3 | Platinum
resistance | miR-516B-5p | HIF-1α | In vivo | (82) |
| Xu et al,
2020 | Breast cancer | circELP3 | Upregulated | ELP3 | Platinum
resistance | - | - | In vivo | (81) |
| Zeng et al,
2021 | Gastric cancer | circNRIP1 | Upregulated | NRIP1 | Fluorouracil
resistance | mir138-5P | HIF-1α | In vivo | (83) |
| Yang et al,
2017 | Pancreatic
cancer | circZNF91 | Upregulated | - | Gemcitabine
resistance | mir-23b-3p | HIF-1α | In vivo | (84) |
| Li et al,
2018 | Hepatocellular
carcinoma | circRNA ZNF292
(CZNF292) | Upregulated | - |
Radioinsensitivity | - | HIF-1α | In vivo | (80) |
Platinum drugs are platinum compounds that are
first-line drugs for the treatment of cancer, including lung cancer
(26,80). However, platinum drug sensitivity
is repressed with chemoresistance progression. Compared with that
in normoxic cells, the expression of circASXL1 and crcAKT3 was
upregulated, while the expression of miR-206 and miR-516B-5p was
downregulated, in hypoxic lung cancer tissues and cells (48,81).
Functionally, knocking out the circASXL1 and circAKT3 genes
increased the sensitivity of cells to cisplatin (DDP), inhibited
HIF-1α-dependent glycolysis, and weakened the increase in
chemotherapy resistance in ASXL1 and AKT3-induced lung cancer
cells. In terms of the mechanism, circASXL1 has binding sites for
miR-206, while AKT3 has binding sites for miR-516b-5p. Notably, the
knockdown-mediated inhibition of cisplatin resistance and
glycolysis were reversed in lung cancer cells with inhibitors of
miR-206 or miR-516b-5p. In addition, the circASXL1/miR-206 or
miR-516B-5p/STAT3 axis could inhibit lung cancer growth in vivo
(48,81). These results indicated that there
may be multiple pathways leading to lung cancer drug resistance,
and further research is necessary to completely reverse
chemotherapy drug resistance under hypoxia. Similarly, circELP3 has
been associated with disease progression and hypoxia-induced DDP
resistance in BC (27). Decreasing
the level of circELP3 via siRNA reduced the in vitro proliferation
and DDP resistance of BC cells, and promoted cell apoptosis.
Furthermore, interfering with circELP3 suppressed tumor xenograft
growth in nude mice in vivo (27).
In a previous study, hypoxia induced the
upregulation of circNRIP1 and reduced the sensitivity of GC cells
to 5-fluorouracil (5-FU), as evidenced by the increase in multidrug
resistance 1 gene, P-glycoprotein, HIF-1α and glucose-6-phosphate
levels, glucose consumption, lactate production and cell survival
(82). Silencing of circNRIP1
enhanced the sensitivity of GC cells to 5-FU under hypoxic
conditions. The mechanism of action is that circNRIP1, as a
miR138-5P sponge, regulates HIF-1α-dependent glycolysis through the
circNRIP1/mir138-5P/HIF-1α axis to enhance hypoxia-induced 5-FU
resistance (82). In PC, circZNF91
overexpression could significantly promote chemotherapy resistance
in PC cells, while knockdown of circZNF91 retarded the hypoxic
exosome-transmitted chemoresistance (83). Mechanistically, the hypoxia-induced
extracellular domain ZNF91 can competitively bind to miR-23b-3p,
which suppresses the inhibitory effect of miR-23b-3p on the
expression of the deacetylase Sirtuin 1 (SIRT1). Therefore,
upregulation of SIRT1 enhances the deacetylation-dependent
stability of the HIF-1α protein, resulting in glycolysis and
gemcitabine resistance in receptor PC cells. Therefore, the
aforementioned signaling axis may be used in the future to treat
tumor chemoresistance induced by hypoxia.
In HCC cells, cZNF292 was hypoxia-induced in a
time-dependent manner, independently of HIF-1α (79). CZNF292 knockdown could enhance the
radiosensitivity of hepatoma cells under hypoxic or normoxic
conditions. CZNF292 gene knockout may lead to ataxia telangiectasia
mutated phosphorylation and decreased DNA-PKCs kinase activity,
which may reduce the DNA repair ability of HCC cells, which may be
the main reason for their radiosensitization (79). These findings provide a theoretical
and experimental foundation for the application of circRNAs as
radiosensitizers in tumor radiotherapy.
Discussion
circRNAs mainly regulate tumor proliferation,
metastasis, invasion and chemical resistance through the
Wnt/β-catenin, MAPK/ERK, PI3K/AKT pathways (84). Hypoxia is one of the most common
characteristics of the tumor microenvironment. Whereas transient or
acute hypoxia occurs in tumors with inadequate blood perfusion,
chronic hypoxia, which limits oxygen diffusion, occurs in enlarged
tumors (85). Under hypoxia, HIFs
regulate tumor metabolism through the mTOR-HIF-1α signaling
pathway, which is a key mediator of Warburg metabolism (86,87).
HIF-1 induces mesenchymal transformation from epithelial tumor
cells via the Wnt/β-catenin and Wnt/HIF-catenin signaling pathways
(88,89). Tumor inflammation is regulated by
NF-κB, signal transduction and STAT3 (87,90).
Therefore, circRNAs and HIFs have multiple overlapping pathways
that jointly promote tumor proliferation, metastasis, angiogenesis
and drug resistance under hypoxia. Further investigation of such
common pathways should aim to explore tumor prevention and targeted
therapy.
The impact of hypoxia on chemoresistance can be
attributed to several factors. First, due to the low drug
concentration in hypoxic cells, as it accumulates in areas away
from functioning blood vessels (4). Second, the majority of anticancer
drugs target proliferating cells; however, hypoxic cells experience
nutrient starvation and impaired cell proliferation compared with
aerobic cells, and thus they have less effect on hypoxic cells
(91,92). Besides knocking down relevant
circRNA target genes or adding downstream miRNA inhibitors to
reverse tumor resistance, HIFs-targeted tumor therapy has the
potential to improve therapeutic efficacy. Different strategies
targeting hypoxic cancer cells and/or HIF include hypoxic-activated
precursor drugs and inhibition of HIF dimerization, mRNA or protein
expression, DNA binding ability and transcriptional activity
(93). Recent advances in clinical
immunotherapy and studies on the regulation of tumor immune
response by HIF suggest that combined immunotherapy and inhibition
of HIF may be an effective treatment (28,93).
In conclusion, circRNAs and HIFs act together to
form signaling pathway axes, and promote tumor growth,
proliferation, metastasis and chemical resistance under hypoxic
conditions. Further studies should be conducted to identify key
targets in their interactions that can inhibit tumor growth and,
most importantly, reverse tumor resistance. The interactions
between circRNAs and HIFs are complex, and there are numerous
cross-pathway interferences, which increases the difficulty of
future studies. Further in-depth investigation is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Science and
Technology Project of Zigong City, Sichuan Province (grant no.
2021ZCYKY11).
Availability of data and materials
Not applicable.
Authors' contributions
QL performed the literature search and selection. ZD
was responsible for the conception, analysis and design of the
study. QL and WL were major contributors to the writing of the
manuscript. HP and SX participated in the coordination of the study
and reviewed the manuscript. QL and WL were responsible for the
revision of the manuscript. HW and SX were responsible for the
literature search and assisted in the design of the study. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Research on
Cancer, . Latest global cancer data, 2020. GLOBOCAN database.
Available from:. https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/July
1–2021
|
|
3
|
Marzhoseyni Z, Shojaie L, Tabatabaei SA,
Movahedpour A, Safari M, Esmaeili D, Mahjoubin-Tehran M, Jalili A,
Morshedi K, Khan H, et al: Streptococcal bacterial components in
cancer therapy. Cancer Gene Ther. 29:141–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuzillet C, Tijeras-Raballand A, de
Mestier L, Cros J, Faivre S and Raymond E: MEK in cancer and cancer
therapy. Pharmacol Ther. 141:160–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajappa A, Banerjee S, Sharma V and
Khandelia P: Circular RNAs: Emerging role in cancer diagnostics and
therapeutics. Front Mol Biosci. 7:5779382020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie R, Zhang Y, Zhang J, Li J and Zhou X:
The role of circular RNAs in immune-related diseases. Front
Immunol. 11:5452020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Shen Y, Li Z, Ruan Y, Li T, Xiao
B and Sun W: The biogenesis and biological functions of circular
RNAs and their molecular diagnostic values in cancers. J Clin Lab
Anal. 34:e230492020.PubMed/NCBI
|
|
13
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hentze MW and Preiss T: Circular RNAs:
Splicing's enigma variations. EMBO J. 32:923–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bramham CR and Wells DG: Dendritic mRNA:
Transport, translation and function. Nat Rev Neurosci. 8:776–789.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z
and Sharpless NE: Expression of linear and novel circular forms of
an INK4/ARF-associated non-coding RNA correlates with
atherosclerosis risk. PLoS Genet. 6:e10012332010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Guo S, Li W and Yu P: The circular
RNA Cdr1as, via miR-7 and its targets, regulates insulin
transcription and secretion in islet cells. Sci Rep. 5:124532015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Wang C, Chen J, Wei D, Yu F and Sun
J: circAGFG1 sponges miR-28-5p to promote non-small-cell lung
cancer progression through modulating HIF-1α level. Open Med
(Wars). 16:703–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao L, Wang M, Dong Y, Xu B, Chen J, Ding
Y, Qiu S, Li L, Karamfilova Zaharieva E, Zhou X and Xu Y: Circular
RNA circRNF20 promotes breast cancer tumorigenesis and Warburg
effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 11:1452020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Liu H, Zeng Q, Xu P, Liu M and Yang
N: Circular RNA circ-MAT2B facilitates glycolysis and growth of
gastric cancer through regulating the miR-515-5p/HIF-1α axis.
Cancer Cell Int. 20:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su Y, Yang W, Jiang N, Shi J, Chen L,
Zhong G, Bi J, Dong W, Wang Q, Wang C and Lin T: Hypoxia-elevated
circELP3 contributes to bladder cancer progression and cisplatin
resistance. Int J Biol Sci. 15:441–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prabhakar NR and Semenza GL: Adaptive and
maladaptive cardiorespiratory responses to continuous and
intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Physiol Rev. 92:967–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albadari N, Deng S and Li W: The
transcriptional factors HIF-1 and HIF-2 and their novel inhibitors
in cancer therapy. Expert Opin Drug Discov. 14:667–682. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murugesan T, Rajajeyabalachandran G, Kumar
S, Nagaraju S and Jegatheesan SK: Targeting HIF-2α as therapy for
advanced cancers. Drug Discov Today. 23:1444–1451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heikkilä M, Pasanen A, Kivirikko KI and
Myllyharju J: Roles of the human hypoxia-inducible factor (HIF)-3α
variants in the hypoxia response. Cell Mol Life Sci. 68:3885–3901.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semenza GL: Oxygen sensing,
hypoxia-inducible factors, and disease pathophysiology. Annu Rev
Pathol. 9:47–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wenger RH, Stiehl DP and Camenisch G:
Integration of oxygen signaling at the consensus HRE. Sci STKE.
2005:re122005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown JM: Evidence for acutely hypoxic
cells in mouse tumours, and a possible mechanism of reoxygenation.
Br J Radiol. 52:650–656. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marín-Hernández A, Gallardo-Pérez JC,
Ralph SJ, Rodríguez-Enríquez S and Moreno-Sánchez R: HIF-1alpha
modulates energy metabolism in cancer cells by inducing
over-expression of specific glycolytic isoforms. Mini Rev Med Chem.
9:1084–1101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Semenza GL: Cancer-stromal cell
interactions mediated by hypoxia-inducible factors promote
angiogenesis, lymphangiogenesis, and metastasis. Oncogene.
32:4057–4063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chouaib S, Kieda C, Benlalam H, Noman MZ,
Mami-Chouaib F and Rüegg C: Endothelial cells as key determinants
of the tumor microenvironment: Interaction with tumor cells,
extracellular matrix and immune killer cells. Crit Rev Immunol.
30:529–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnson BF, Clay TM, Hobeika AC, Lyerly HK
and Morse MA: Vascular endothelial growth factor and
immunosuppression in cancer: Current knowledge and potential for
new therapy. Expert Opin Biol Ther. 7:449–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Woo SR, Corrales L and Gajewski TF: Innate
immune recognition of cancer. Annu Rev Immunol. 33:445–474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishi H, Nakada T, Hokamura M, Osakabe Y,
Itokazu O, Huang LE and Isaka K: Hypoxia-inducible factor-1
transactivates transforming growth factor-beta3 in trophoblast.
Endocrinology. 145:4113–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calvani M, Comito G, Giannoni E and
Chiarugi P: Time-dependent stabilization of hypoxia inducible
factor-1α by different intracellular sources of reactive oxygen
species. PLoS One. 7:e383882012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seebacher NA, Richardson DR and Jansson
PJ: Glucose modulation induces reactive oxygen species and
increases P-glycoprotein-mediated multidrug resistance to
chemotherapeutics. Br J Pharmacol. 172:2557–2572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng X, Qiu J, Wang S, Yang Y, Guo M,
Wang D, Luo Q and Xu L: Comprehensive circular RNA profiling
identifies CircFAM120A as a new biomarker of hypoxic lung
adenocarcinoma. Ann Transl Med. 7:4422019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng D, Xu Y, Hu J, Zhang S, Li M and Xu
L: A novel circular RNA, hsa-circ-0000211, promotes lung
adenocarcinoma migration and invasion through sponging of
hsa-miR-622 and modulating HIF1-α expression. Biochem Biophys Res
Commun. 521:395–401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu L, Li J, Peng B, Cai P, Zhao B, Chen Y
and Zhu H: CircASXL1 knockdown restrains hypoxia-induced DDP
resistance and NSCLC progression by sponging miR-206. Cancer Manag
Res. 13:5077–5089. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu KH, Tsai YT, Chin SY, Lee WR, Chen YC
and Shen SC: Hypoxia stimulates the epithelial-to-mesenchymal
transition in lung cancer cells through accumulation of nuclear
β-catenin. Anticancer Res. 38:6299–6308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chi Y, Luo Q, Song Y, Yang F, Wang Y, Jin
M and Zhang D: Circular RNA circPIP5K1A promotes non-small cell
lung cancer proliferation and metastasis through miR-600/HIF-1α
regulation. J Cell Biochem. 120:19019–19030. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thompson B, Hohl SD, Molina Y, Paskett ED,
Fisher JL, Baltic RD and Washington CM: Breast cancer disparities
among women in underserved communities in the USA. Curr Breast
Cancer Rep. 10:131–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liang G, Liu Z, Tan L, Su AN, Jiang WG and
Gong C: HIF1α-associated circDENND4C promotes proliferation of
breast cancer cells in hypoxic environment. Anticancer Res.
37:4337–4343. 2017.PubMed/NCBI
|
|
54
|
Wang J, Huang K, Shi L, Zhang Q and Zhang
S: CircPVT1 Promoted the progression of breast cancer by regulating
MiR-29a-3p-mediated AGR2-HIF-1α pathway. Cancer Manag Res.
12:11477–11490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Darbeheshti F, Mahdiannasser M, Noroozi Z,
Firoozi Z, Mansoori B, Daraei A, Bastami M, Nariman-Saleh-Fam Z,
Valipour E and Mansoori Y: Circular RNA-associated ceRNA network
involved in HIF-1 signalling in triple-negative breast cancer:
circ_0047303 as a potential key regulator. J Cell Mol Med.
25:11322–11332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singh D, Arora R, Kaur P, Singh B, Mannan
R and Arora S: Overexpression of hypoxia-inducible factor and
metabolic pathways: Possible targets of cancer. Cell Biosci.
7:622017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xing X, Liang D, Huang Y, Zeng Y, Han X,
Liu X and Liu J: The application of proteomics in different aspects
of hepatocellular carcinoma research. J Proteomics. 145:70–80.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhai Z, Fu Q, Liu C, Zhang X, Jia P, Xia
P, Liu P, Liao S, Qin T and Zhang H: Emerging roles of
hsa-circ-0046600 targeting the miR-640/HIF-1α signalling pathway in
the progression of HCC. Onco Targets Ther. 12:9291–9302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tu Q, You X, He J, Hu X, Xie C and Xu G:
Circular RNA Circ-0003006 promotes hepatocellular carcinoma
proliferation and metastasis through sponging miR-542-3p and
regulating HIF-1A. Cancer Manag Res. 13:7859–7870. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tan Y, Du B, Zhan Y, Wang K, Wang X, Chen
B, Wei X and Xiao J: Antitumor effects of circ-EPHB4 in
hepatocellular carcinoma via inhibition of HIF-1α. Mol Carcinog.
58:875–886. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang J, Ren B, Yang G, Wang H, Chen G, You
L, Zhang T and Zhao Y: The enhancement of glycolysis regulates
pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu A and Xu J: Circ_03955 promotes
pancreatic cancer tumorigenesis and Warburg effect by targeting the
miR-3662/HIF-1α axis. Clin Transl Oncol. 23:1905–1914. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barsoum IB, Hamilton TK, Li X, Cotechini
T, Miles EA, Siemens DR and Graham CH: Hypoxia induces escape from
innate immunity in cancer cells via increased expression of ADAM10:
Role of nitric oxide. Cancer Res. 71:7433–7441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lu Y, Hu J, Sun W, Duan X and Chen X:
Hypoxia-mediated immune evasion of pancreatic carcinoma cells. Mol
Med Rep. 11:3666–3672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ou ZL, Luo Z, Wei W, Liang S, Gao TL and
Lu YB: Hypoxia-induced shedding of MICA and HIF1A-mediated immune
escape of pancreatic cancer cells from NK cells: Role of
circ_0000977/miR-153 axis. RNA Biol. 16:1592–1603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Harada S and Morlote D: Molecular
pathology of colorectal cancer. Adv Anat Pathol. 27:20–26. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vasaikar S, Huang C, Wang X, Petyuk VA,
Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al:
Proteogenomic analysis of human colon cancer reveals new
therapeutic opportunities. Cell. 177:1035–1049.e19. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen LY, Wang L, Ren YX, Pang Z, Liu Y,
Sun XD, Tu J, Zhi Z, Qin Y, Sun LN and Li JM: The circular RNA
circ-ERBIN promotes growth and metastasis of colorectal cancer by
miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1α
translation. Mol Cancer. 19:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhou P, Xie W, Huang HL, Huang RQ, Tian C,
Zhu HB, Dai YH and Li ZY: circRNA_100859 functions as an oncogene
in colon cancer by sponging the miR-217-HIF-1α pathway. Aging
(Albany NY). 12:13338–13353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qian W, Huang T and Feng W: Circular RNA
HIPK3 promotes EMT of cervical cancer through sponging miR-338-3p
to Up-regulate HIF-1α. Cancer Manag Res. 12:177–187. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen H, Mao M, Jiang J, Zhu D and Li P:
Circular RNA CDR1as acts as a sponge of miR-135b-5p to suppress
ovarian cancer progression. Onco Targets Ther. 12:3869–3879. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Su H, Zou D, Sun Y and Dai Y:
Hypoxia-associated circDENND2A promotes glioma aggressiveness by
sponging miR-625-5p. Cell Mol Biol Lett. 24:242019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Matulonis UA: Ovarian cancer. Hematol
Oncol Clin North Am. 32:13–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Y and Ye D: Cancer therapy by targeting
hypoxia-inducible factor-1. Curr Cancer Drug Targets. 10:782–796.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang W, Liu Y, Gao R, Xiu Z and Sun T:
Knockdown of cZNF292 suppressed hypoxic human hepatoma SMMC7721
cell proliferation, vasculogenic mimicry, and radioresistance. Cell
Signal. 60:122–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li K, Guo J, Wu Y, Jin D, Jiang H, Liu C
and Qin C: Suppression of YAP by DDP disrupts colontumor
progression. Oncol Rep. 39:2114–2126. 2018.PubMed/NCBI
|
|
81
|
Xu Y, Jiang T, Wu C and Zhang Y: CircAKT3
inhibits glycolysis balance in lung cancer cells by regulating
miR-516b-5p/STAT3 to inhibit cisplatin sensitivity. Biotechnol
Lett. 42:1123–1135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xu G, Li M, Wu J, Qin C, Tao Y and He H:
Circular RNA circNRIP1 sponges microRNA-138-5p to maintain
hypoxia-induced resistance to 5-fluorouracil through
HIF-1α-dependent glucose metabolism in gastric carcinoma. Cancer
Manag Res. 12:2789–2802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye
Z, Hu P, Chen D, Xu P, Chen J, et al: Hypoxic exosomal
HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic
pancreatic cancer cells via enhancing glycolysis. Oncogene.
40:5505–5517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang Z, Xie L, Han L, Qu X, Yang Y, Zhang
Y, He Z, Wang Y and Li J: Circular RNAs: Regulators of
cancer-related signaling pathways and potential diagnostic
biomarkers for human cancers. Theranostics. 7:3106–3117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vaupel P and Mayer A: Hypoxia in tumors:
Pathogenesis-related classification, characterization of hypoxia
subtypes, and associated biological and clinical implications. Adv
Exp Med Biol. 812:19–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cheng SC, Quintin J, Cramer RA, Shepardson
KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao
NA, Aghajanirefah A, et al: mTOR- and HIF-1α-mediated aerobic
glycolysis as metabolic basis for trained immunity. Science.
345:12506842014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jiang YG, Luo Y, He DL, Li X, Zhang LL,
Peng T, Li MC and Lin YH: Role of Wnt/beta-catenin signaling
pathway in epithelial-mesenchymal transition of human prostate
cancer induced by hypoxia-inducible factor-1alpha. Int J Urol.
14:1034–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lavecchia A, Di Giovanni C and Cerchia C:
Novel inhibitors of signal transducer and activator of
transcription 3 signaling pathway: An update on the recent patent
literature. Expert Opin Ther Pat. 24:383–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Brown JM: Tumor microenvironment and the
response to anticancer therapy. Cancer Biol Ther. 1:453–458. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Doktorova H, Hrabeta J, Khalil MA and
Eckschlager T: Hypoxia-induced chemoresistance in cancer cells: The
role of not only HIF-1. Biomed Pap Med Fac Univ Palacky Olomouc
Czech Repub. 159:166–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wigerup C, Påhlman S and Bexell D:
Therapeutic targeting of hypoxia and hypoxia-inducible factors in
cancer. Pharmacol Ther. 164:152–169. 2016. View Article : Google Scholar : PubMed/NCBI
|