Introduction
Lung cancer is one of the primary causes of
cancer-related deaths worldwide (1). Lung cancer can be divided into two
major sub-types: Non-small cell lung cancer (NSCLC) and small cell
lung cancer (SCLC). Among all lung cancer patients, NSCLC accounts
for ~85% of cases (2), far higher
than the prevalence of SCLC. Despite the abundance of research on
NSCLC, its pathogenesis has remained insufficiently understood and
the five-year survival rate remains <20% (3,4).
Therefore, further research is required to explore the pathogenesis
of NSCLC, to identify early diagnostic biomarkers, and for
development of novel treatment strategies.
The possibility of gene-based therapies for disease
treatment has sparked widespread interest, ~97% of the human genome
encodes ‘noncoding RNAs’ that regulate DNA-RNA-protein interactions
(5). MicroRNAs (miRNAs) are a
class of small non-coding single-stranded RNA molecules that
consist of 19–25 nucleotides; they inhibit target gene expression
by directly binding to the 3′-UTR (6,7).
Increasing evidence indicates that miRNAs participate in multiple
biological processes, such as cell cycle regulation,
differentiation, proliferation, and migration (8), thereby implicating them in the
initiation and development of various diseases, particularly
tumorigenesis (9). Various miRNAs
have been considered potential NSCLC biomarker candidates, such as
miR-19b-3a, miR-133a, and miR-148a (10–12).
Previous research has revealed that miR-29a-3p suppresses tumor
growth in a variety of malignancies. In endometrial cancer,
miR-29a-3p inhibits cells' malignant properties by regulating the
VEGFA/CDC42/PAK1 signaling pathway (13). miR-29a-3p expression is lower in
colorectal carcinoma cells compared with normal colon epithelial
cells and inhibits cell function by targeting RPS15A (14). Moreover, miR-29a-3p inhibited the
proliferation, migration, and invasion of liver cancer cells by
specifically targeting COL4A1 (15). However, the role of miR-29a-3p in
NSCLC has yet to be determined.
The Wnt/β-catenin signaling pathway is an
evolutionary cell-to-cell coordination mechanism that is essential
for a wide range of physiological processes, such as stem cell
regeneration, proliferation, and migration. Hypo or
hyper-activation of various signaling pathways is linked to the
progression of various human disorders, most notably cancers
(16). Alterations in the
Wnt/β-catenin pathway contribute to the development/progression of
several types of cancer, including NSCLC (17). Numerous studies have demonstrated
the dysregulation of miRNAs in several types of cancer (18–20),
and that aberrantly expressed miRNAs are related to dysregulation
of the Wnt/β-catenin signaling (6). Numerous miRNAs inactivate the
Wnt/β-catenin signaling pathway to affect the progression of NSCLC.
miR-590 has been found to suppress the progression of NSCLC via
inhibition of the Wnt/β-catenin signaling pathway (21). miR-433 inhibits tumorigenesis
through inactivation of the Wnt/β-catenin signaling pathway
(22). miR-19b-3p inhibits the
progression of NSCLC by inhibiting the activation of Wnt/β-catenin
signaling (23). A previous study
revealed that miR-29a-3p inactivated the Wnt/β-catenin pathway to
suppress gastric cancer (GC) progression (8). However, it remains unknown whether
miR-29a-3p affects NSCLC progression by regulating the
Wnt/β-catenin signaling pathway.
The present study aimed to investigate the role of
miR-29a-3p in NSCLC cells by assessing proliferation, migration,
and invasion. Furthermore, whether miR-29a-3p affected NSCLC
progression via regulation of the Wnt/β-catenin signaling pathway
was identified. The data provided a theoretical basis for
investigating the protective effects of miR-29a-3p on NSCLC and its
mechanism.
Materials and methods
Cell culture and transfection
The human NSCLC cell lines (A549, H1299, and H460
cells) and human normal lung epithelial cells (BEAS-2B) were
purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd.
All cells were cultured in RPMI1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). Media was supplemented with 10% FBS (HyClone,
Cytiva), 100 U/ml penicillin, and 100 µg/ml streptomycin. All cells
were cultured at 37°C in a humidified incubator with 5%
CO2.
For cell transfection, 5×104 A549 cells
were seeded into 24-well plates and grown to 30–50% confluence.
miR-29a-3p mimic, miR-29a-3p inhibitor and corresponding negative
controls (NCs; 5 µl 20 µM; Guangzhou RiboBio Co., Ltd.) were
diluted with 30 µl riboFECT™ CP buffer (Guangzhou RiboBio Co.,
Ltd.) before incubating with 3 µl riboFECT™ CP reagent (Guangzhou
RiboBio Co., Ltd.) for 15 min at room temperature. Then, the
mixture was added to the complete medium to a final volume of 500
µl/well. Cells were cultured for 24 h at 37°C for subsequent
experiments. The transfected miRNA sequences were: miR-29a-3p mimic
forward, 5′-UAGCAGGAUCUGAAAUCGGUUA-3′ and reverse,
5′-ACCGAUUUCAUAUCCUGCUAUU-3′; miR-29a-3p inhibitor,
5′-UAACCGAUUUCAUAUCCUGCUA-3′; and miR NC,
5′-CAGUACUUUUGUGUAGUACAA-3′. All transfections were performed
strictly in line with the instructions included with the riboFECT™
CP Reagent (Guangzhou RiboBio Co., Ltd.). In addition, a subset of
A549 cells transfected with miR-29a-3p mimic were treated with 20
mM LiCl, an activator of the Wnt signaling pathway (24), for 24 h.
Animal model
A total of 15 female BALB/C nude mice (4–6 weeks
old, weighing 16–20 g) were purchased from Shanghai Laboratory
Animal Company and stored in a specific pathogen-free environment
(21°C, 60% humidity), with a 12 h light/dark cycle with free access
to water and food. All mice were randomly divided into 3 groups
(n=5 per group): control, NC, and miR-29a-3p groups. A549 cells
(1×107 cells/mouse) transfected with miR-29a-3p mimic or
NC-mimic were subcutaneously injected into the right flank of mice
to establish murine xenograft models. Tumor length and width were
measured using a caliper every week, and tumor size was determined
using the following formula: π/6 × length × width2 at
the end of the experiments. After 4 weeks, all mice were deeply
anesthetized with 2% isoflurane and sacrificed by cervical
dislocation. After confirming that the mice had stopped breathing,
tumors were removed, weighed and collected for subsequent analyses.
This animal experiments were performed in accordance with the
protocols approved by the Ethics Committee of the Experimental
Animal Center of The Affiliated Hospital of Shaoxing
University.
Immunohistochemical staining
NSCLC tumor tissue samples taken from the model mice
were fixed in 4% neutral formalin, embedded in paraffin, and cut
into 4-µm thick sections for immunohistochemical staining. Tissue
sections were incubated with an anti-Ki-67 primary antibody (1:200;
cat. no. ab16667, Abcam) overnight at 4°C. Then, secondary antibody
goat anti-rabbit IgG H&L (1:1,000; cat. no. ab6721, Abcam) was
added for 20 min 3′-Diaminobenzidine was used as a chromogen
substrate.
Cell Counting Kit-8 (CCK-8) assay
Stably transfected A549 cells were plated in a
96-well plate (1×104 cells/well) for 24 h. Subsequently,
10 µl CCK-8 (Beyotime Institute of Biotechnology) solution was
added to each well, and cells were further incubated for 2 h. Then,
the absorbance was measured on a microplate reader at 450 nm
(Bio-Rad Laboratories, Inc.).
EdU assay
A BeyoClick™ EdU kit (Beyotime Institute of
Biotechnology) was used to detect cell proliferation. Cells were
incubated with 50 µM EdU solution for 2 h at 37°C. After fixing
with 4% paraformaldehyde for 15 min at room temperature and
permeabilizing with 0.2% Triton X-100 for 10 min, cells were
stained with click reaction solution in the dark for 30 min and
counterstained with Hoechst 33342 for 30 min at room temperature.
EdU positive cells were counted using a fluorescence microscope
(×20 magnification; Olympus Corporation).
Wound healing assays
A549 cells were digested with 0.25% trypsin (Beijing
Solarbio Science & Technology Co., Ltd.) and seeded in six-well
plates (2×105 cells/well). When cells had proliferated
to 100% confluence, cell monolayers were scratched using a sterile
pipette tip. Cells were then washed with serum-free medium three
times. The healing of the wound was imaged using a light microscope
(Olympus Corporation) after 0 and 24 h, respectively. The wound
healing rate was quantified using Image-Pro Plus version 6.0 (Media
Cybernetics, Inc.).
Transwell invasion assay
Transwell invasion assays were performed to assess
the invasive ability of A549 cells. The upper chamber of the
Transwell insert (8 µM pore; Corning, Inc.) was coated with
Matrigel (Corning, Inc.) in advance of cell seeding. A549 cells
were suspended in serum-free PRMI-1640 media to a density of
1×105 cells/ml. Then, 600 µl medium containing 20% FBS
was added to the lower chamber in a 24-well plate, 200 µl of the
cell suspension was placed in the upper chamber of the insert.
After incubating for 24 h at 37°C, cells in the upper chamber were
removed, and those in the lower chamber were fixed with 4%
anhydrous methanol for 30 min at room temperature, and stained with
0.1% crystal violet for 20 min at room temperature (22±2°C). Cells
that had invaded were counted under a light microscope (×100
magnification; Olympus Corporation) in 6 randomly selected
non-overlapping fields.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The cDNA template was obtained using a TIANGEN Kit (cat. no.
KR118; Tiangen Biotech Co., Ltd.) using 10 µl of the total RNA
extract according to the manufacturer's protocol. qPCR was
performed using SYBR-Green reagent (Lifeint) on an Mx3000P fast
real-time PCR System (Agilent Technologies, Inc.). The
thermocycling conditions used were: 95°C for 3 min; followed by 40
cycles of 95°C for 12 sec, and 62°C for 40 sec. The sequences of
the primers used were: miR-29a-3p forward,
5′-UAACCGAUUUCAAAUGGUGCUA-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′;
and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′, and reverse
5′-AACGCTTCACGAATTTGCGT-3′. The relative expression was determined
using the 2−ΔΔCq method (25). U6 was used as the internal control.
The raw data is available from the corresponding author.
Western blotting
A549 cells were lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology) and collected by
centrifugation (14,500 × g for 10 min at 4°C) to extract the total
protein. A BCA protein concentration kit (Beyotime Institute of
Biotechnology) was used to quantify protein concentration. A total
of 10 µg total protein sample was loaded per a lane on 10%
SDS-gels, resolved using SDS-PAGE, and transferred to PVDF
membranes (Beyotime Institute of Biotechnology) for 2 h at 65 V.
Then, 5% skimmed milk was added to block membranes for 1 h at room
temperature. Subsequently, the membranes were treated with primary
antibodies overnight at 4°C. The primary antibodies used in this
study were as follows: Wnt3a (1:1,000; cat. no. ab219412; Abcam),
β-catenin (1:5,000; cat. no. ab32572; Abcam), and GAPDH (1:2,500;
cat. no. ab9485; Abcam). The PVDF membranes were treated with a
horseradish peroxidase-conjugated goat anti-rabbit Immunoglobulin G
H&L antibody (1:2,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. The signals were visualized using an enhanced
chemiluminescence system (Nano-Drop 8000, Thermo Fisher Scientific,
Inc.). Images of the signals were obtained using a ChemiDoc™
imaging system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were independently performed at
least 3 times. Data are presented as the mean ± standard deviation
and compared using GraphPad Prism version 7.0 (GraphPad Software,
Inc.). Comparisons between multiple groups of parametric data were
performed using a one-way ANOVA followed by a post hoc Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
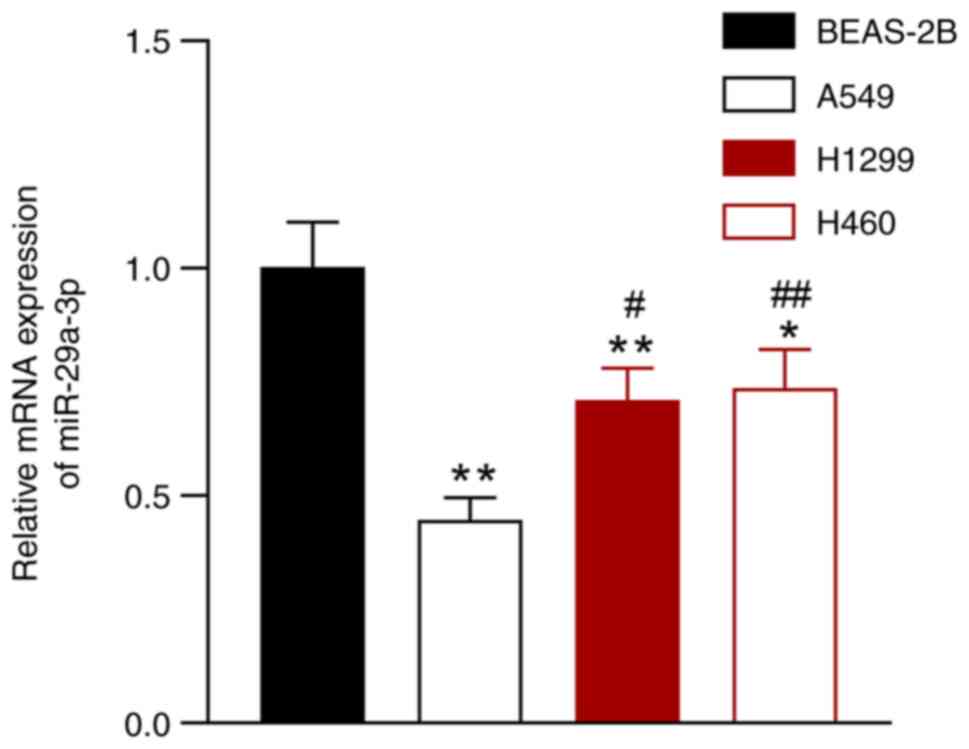
miR-29a-3p expression is reduced in
NSCLC cells
The expression of miR-29a-3p was evaluated in NSCLC
cell lines (A549, H1299, and H460) and in a normal lung epithelial
cell line (BEAS-2B). Compared with the BEAS-2B cells, the
expression of miR-29a-3p was downregulated in A549, H1299, and H460
cells, and the most significant difference was observed between
A549 cells and BEAS-2B cells (P<0.05; Fig. 1). Thus, A549 cells were selected
for all subsequent experiments.
miR-29a-3p inhibits the proliferation,
migration, and invasion of NSCLC cells
To further explore the effects of miR-29a-3p on
NSCLC cells, miR-29a-3p mimics or inhibitor were transfected into
A549 cells. Compared with the control cells, miR-29a-3p mimic
successfully promoted miR-29a-3p expression in A549 cells, and the
miR-29a-3p inhibitor exerted the opposite effect (P<0.05;
Fig. 2A). Compared with the
control cells, A549 cell proliferation, migration, and invasion was
significantly increased after transfection with miR-29a-3p
inhibitor, but markedly decreased in cells transfected with
miR-29a-3p mimic (P<0.05; Fig.
2B-E).
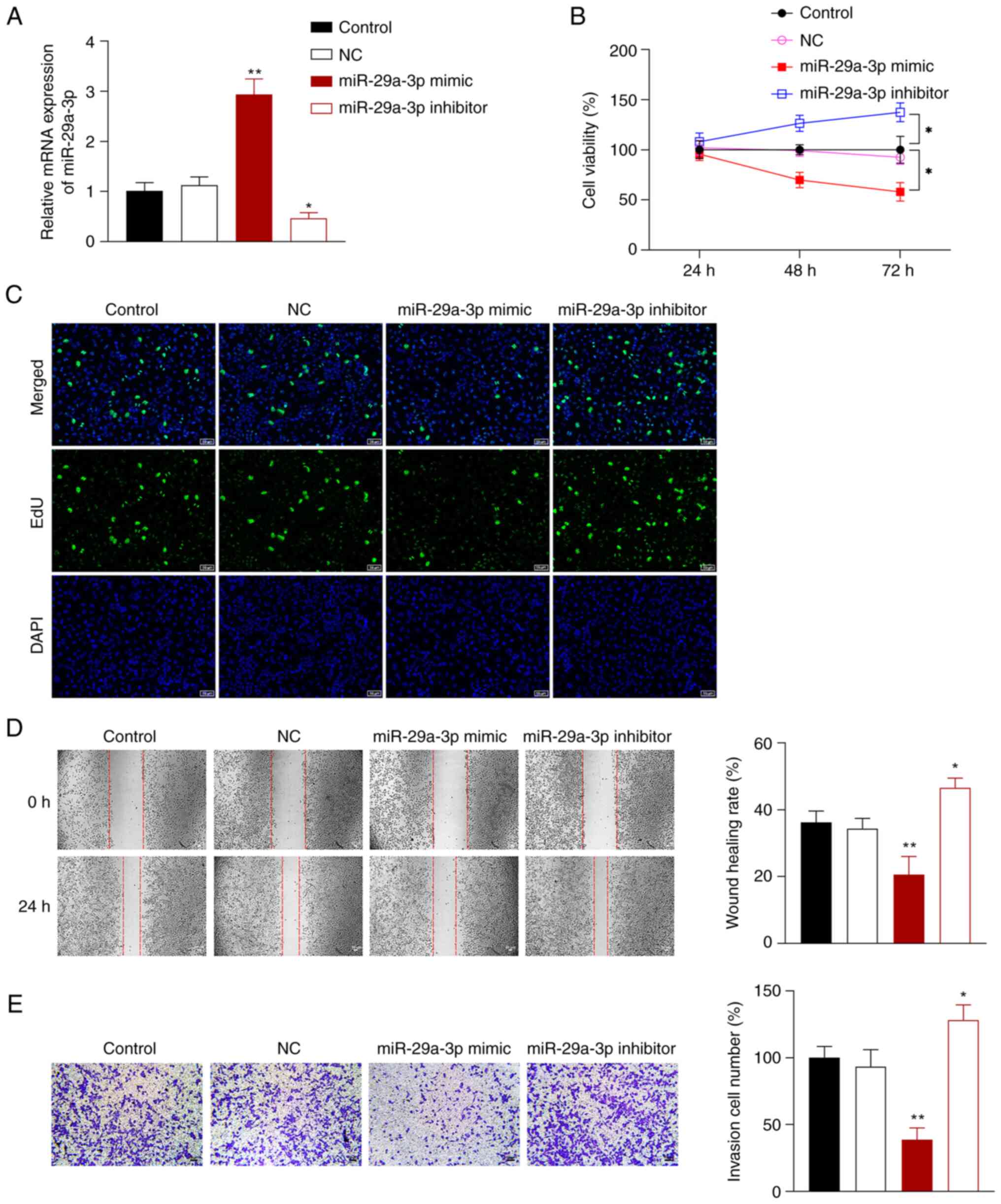 | Figure 2.miR-29a-3p inhibits NSCLC cell
proliferation, migration, and invasion. (A) Reverse
transcription-quantitative PCR was used to measure the expression
of miR-29a-3p in A549 cells. (B) A Cell-Counting Kit-8 assay was
used to measure cell proliferation after 24, 48, and 72 h. (C) An
EdU assay was used to assess proliferation in the transfected cells
(scale bar, 50 µm). (D) A wound healing assay was used to assess
cell migration (scale bar, 50 µm). (E) Transwell assays were used
to assess cell invasion (scale bar, 50 µm). A549 cells were
transfected with NC, miR-29a-3p mimic or inhibitor.
*P<0.05, **P<0.01 vs. control group.
NC, negative control; NSCLC, non-small cell lung cancer; miR,
microRNA. |
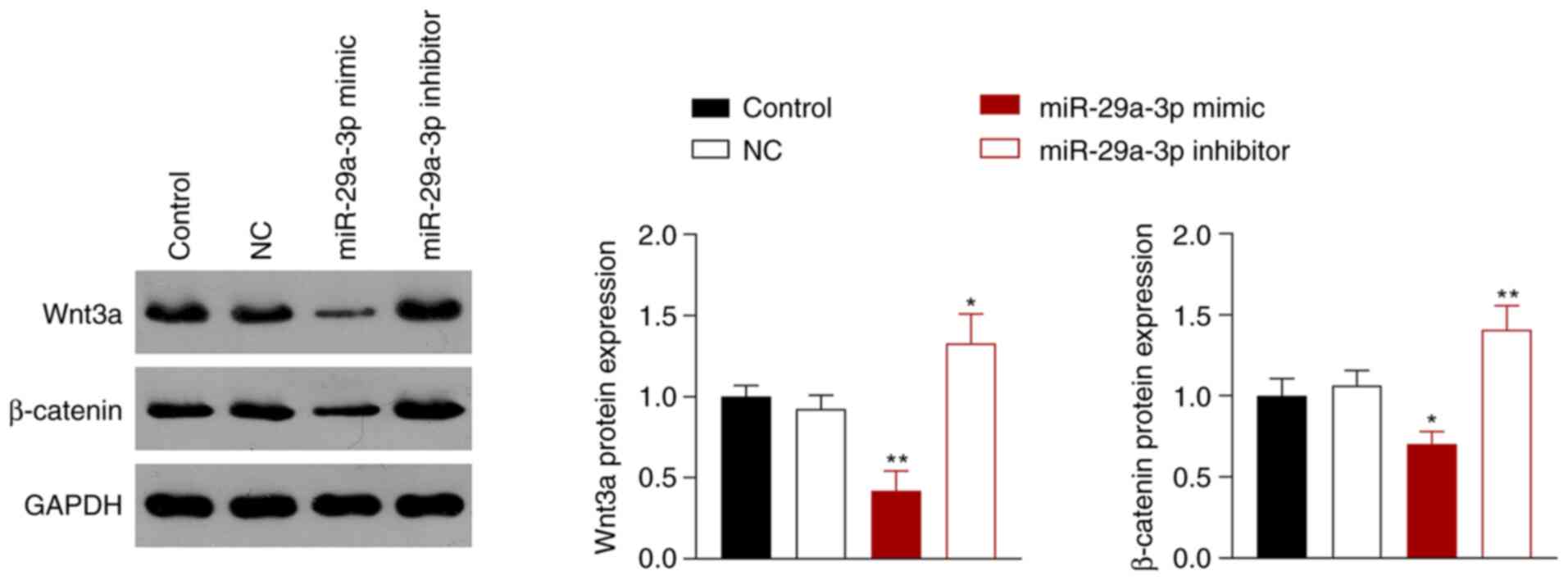
miR-29a-3p inhibits the activity of
the Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is involved in
the occurrence and development of NSCLC (26), which is associated with aberrantly
expressed miRNAs (27). Thus,
whether miR-29a-3p inhibited NSCLC via regulation of the
Wnt/β-catenin signaling pathway was next assessed. miR-29a-3p
overexpression inhibited the Wnt/β-catenin pathway in A549 cells by
reducing the protein expression levels of Wnt3a and β-catenin
compared with the control cells, whereas miR-29a-3p inhibition had
the opposite effect (P<0.05; Fig.
3).
miR-29a-3p suppresses NSCLC cell
proliferation, migration, and invasion by inhibiting the activity
of the Wnt/β-catenin signaling pathway
To investigate whether miR-29a-3p suppressed NSCLC
cell progression via inhibiting the activity of the Wnt/β-catenin
pathway, A549 cells transfected with miR-29a-3p mimic were treated
with LiCl (an activator of the Wnt signaling pathway). miR-29a-3p
mimic upregulated the expression of miR-29a-3p in A549 cells
compared with the control cells; however, the addition of LiCl did
not change this effect (P<0.01; Fig. 4A). Compared with the control group,
miR-29a-3p overexpression reduced the proliferation, migration, and
invasion of A549 cells, and this reduction was reversed by addition
of LiCl (P<0.05; Fig. 4B-E).
Based on the western blotting data, the ability of miR-29a-3p to
decrease the expression of Wnt3a and β-catenin protein was
significantly reversed by LiCl (P<0.01; Fig. 4F).
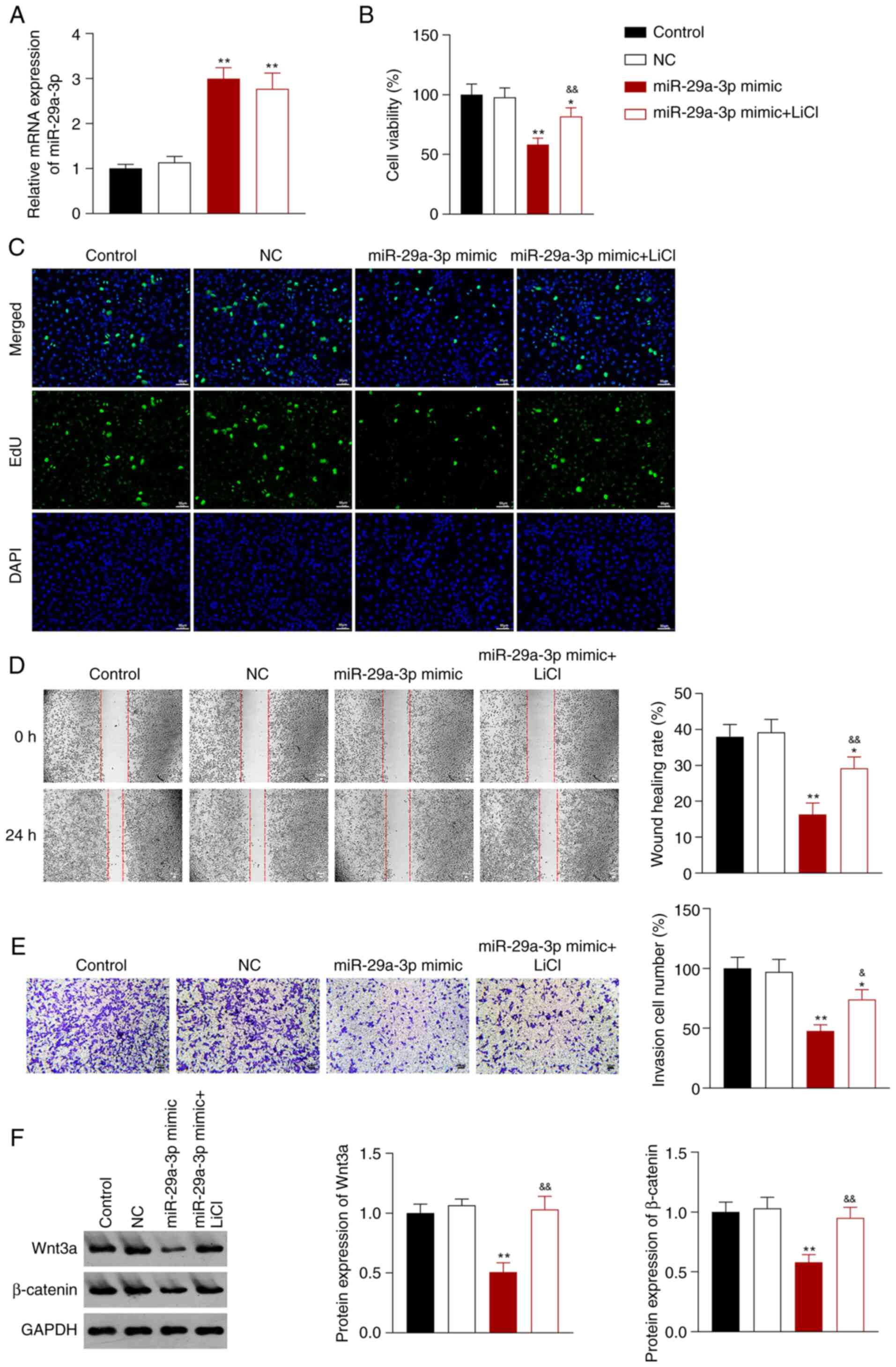 | Figure 4.miR-29a-3p inhibits the malignant
characteristics of A549 NSCLC cells by decreasing the activity of
the Wnt/β-catenin signaling pathway. (A) Reverse transcription
quantitative-PCR was used to measure the expression of miR-29a-3p
in A549 cells. (B) A Cell Counting Kit-8 assay was used to assess
A549 cell proliferation 48 h after transfection. (C) Fluorescence
staining using an EdU assay on A549 cells (scale bar, 50 µm). (D) A
wound healing assay was used to assess the migratory ability of the
transfected A549 cells (scale bar, 50 µm). (E) A Transwell invasion
assay was to assess the invasive ability of A549 cells (scale bar,
50 µm). (F) Western blotting was used to measure the expression of
Wnt3a and β-catenin in A549 cells. A549 cells were transfected with
NC, or miR-29a-3p mimic, and subsequently treated with LiCl.
*P<0.05, **P<0.01 vs. control group;
&P<0.05, &&P<0.01 vs.
miR-29a-3p mimic group. NC, negative control; NSCLC, non-small cell
lung cancer; miR, microRNA. |
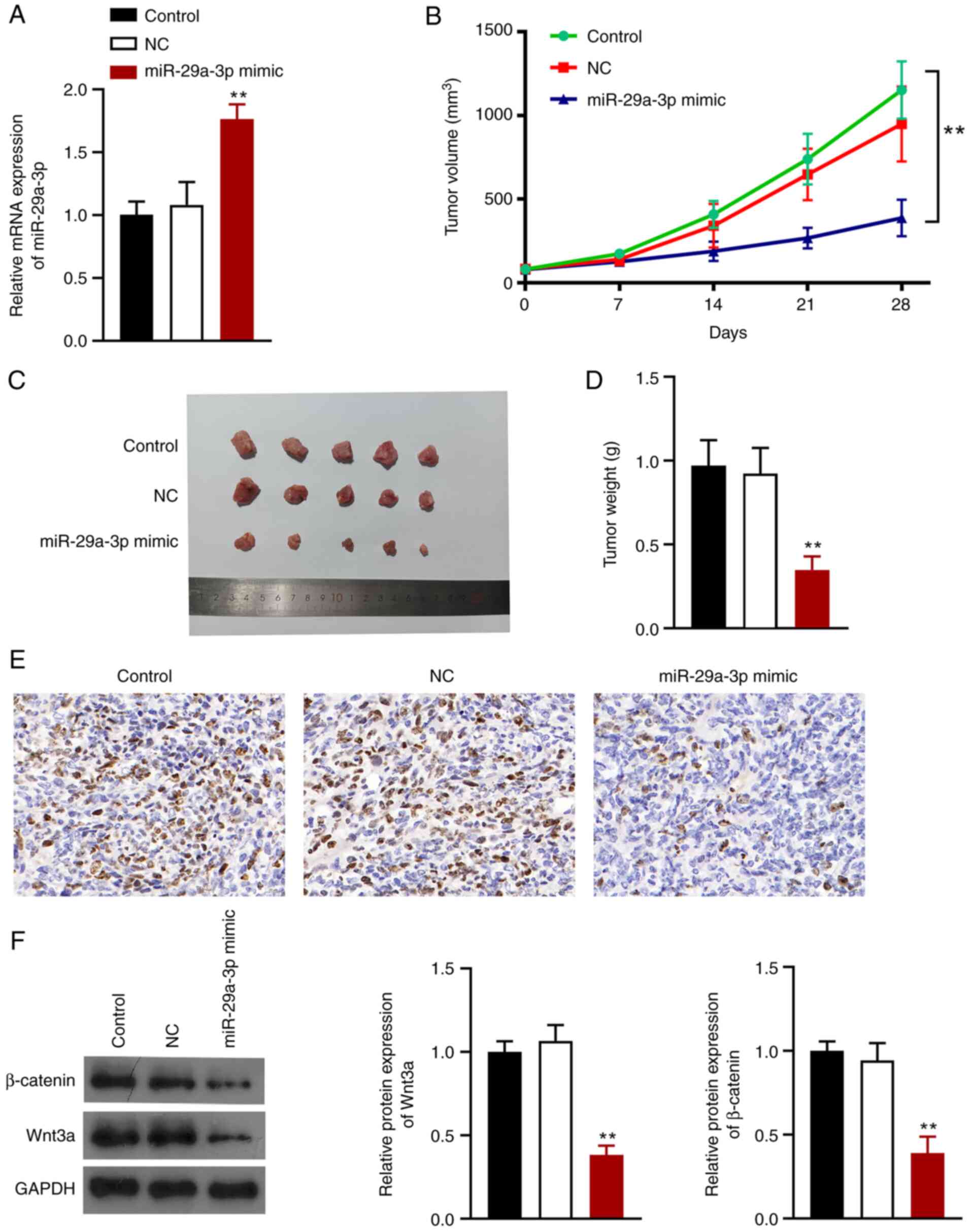
miR-29a-3p suppresses tumor growth in
vivo by inhibiting the Wnt/β-catenin signaling pathway
A mouse lung tumor xenograft model was established
to explore the effects of miR-29a-3p on NSCLC in vivo.
BALB/c nude mice were injected with cells transfected with
miR-29a-3p mimic or miRNA-NC. RT-qPCR analysis showed that
miR-29a-3p expression was successfully upregulated in mice
(P<0.01; Fig. 5A). Tumor volume
and weight were smaller after miR-29a-3p overexpression (Fig. 5B-D). Ki67 (a marker of cell
proliferation) expression was significantly reduced when miR-29a-3p
was overexpressed (Fig. 5E)
Additionally, miR-29a-3p overexpression reduced the expression of
Wnt3a and β-catenin in the tumors (P<0.01; Fig. 5F).
Discussion
NSCLC is a subtype of lung cancer associated with a
poor prognosis and high morbidity and mortality rates (4). The potential value of miR-29a-3p as a
diagnostic and/or prognostic marker in several types of cancer has
been shown, including NSCLC (28–30).
The present study revealed that miR-29a-3p expression was
significantly reduced in the NSCLC cells and mouse model miR-29a-3p
overexpression inhibited lung tumor growth and NSCLC cell
proliferation, migration, and invasion. Further experiments
demonstrated that miR-29a-3p inhibited the malignant
characteristics of NSCLC by targeting the activity of the
Wnt/β-catenin signaling pathway.
miRNAs play important roles in tumor progression
through tumor suppressor inactivation or regulation of pertinent
pathways (31). miR-29a-3p has
been recognized as a critical miRNA in various types of cancer, and
typically functions as a tumor suppressor by directly targeting
oncogenic genes (29,32–34).
miR-29a-3p expression was shown to be downregulated in some cancer
types, such as cervical (35),
endometrial (13), and gastric
(36) cancer, and this
downregulation was associated with worse outcomes. Studies have
reported that miR-29a-3p suppresses cell proliferation and
migration in various types of cancer. In hepatocellular carcinoma,
miR-29a-3p suppresses cell proliferation and migration by
downregulating IGF1R expression (37). Overexpression of miR-29a-3p reduced
HeLa cell migration and proliferation in cervical cancer (38). In this study, the expression of
miR-29a-3p in NSCLC cells and normal lung epithelial cells was
first examined. The results showed that miR-29a-3p expression was
markedly reduced in NSCLC cells compared with normal lung
epithelial cells. This result indicated that miR-29a-3p may also
serve as a tumor suppressor in NSCLC. To further analyze the
effects of miR-29a-3p on NSCLC, miR-29a-3p was overexpressed in
NSCLC cells, and the acquisition of malignant properties was
determined. The results showed that miR-29a-3p overexpression
suppressed proliferation, migration, and invasion of NSCLC cells
and inhibited tumor growth in NSCLC mice, whereas miR-29a-3p
inhibition reversed this effect. These results further imply that
miR-29a-3p functions as a tumor suppressor in NSCLC.
miR-29a-3p directly targets the Wnt/β-catenin
signaling pathway in several types of cancer including
ameloblastoma (39) and gastric
cancer (8). To further investigate
the underlying mechanisms involved, the association between
miR-29a-3p and Wnt/β-catenin signaling pathway in NSCLC was further
examined next. The Wnt/β-catenin signaling pathway is required for
numerous core biological processes, whilst also driving tumor
initiation and progression (40).
Wnt3a and β-catenin are Wnt signaling pathway-related proteins,
that can enter the nucleus to regulate gene transcription. The
invasive and metastatic process of several types of cancer, such as
colorectal cancer, can be promoted via activation of the
Wnt/β-catenin signaling pathway (41). A previous study reported that the
Wnt/β-catenin pathway was associated with tumor progression in
NSCLC (17). miRNAs could
stimulate early-stage NSCLC progression by simultaneously
stimulating Wnt/β-catenin signaling (6). Moreover, it has been reported that
miR-29a-3p regulates the migration and invasion in ameloblastoma
via the Wnt/β-catenin pathway (39). The Wnt/β-catenin pathway was
inactivated in a miR-29a-3p-dependent manner in GC cells (8). Therefore, it was speculated that
miR-29a-3p may play a profound role in NSCLC cells by regulating
the Wnt/β-catenin signaling pathway. The results of the present
study demonstrated that overexpressing miR-29a-3p reduced the
expression of Wnt3a and β-catenin, meanwhile, and the malignant
characteristics were inhibited. Subsequently, rescue assays were
performed using LiCl, which is an activator of the Wnt signaling
pathway. LiCl partially reversed the decrease in cell
proliferation, invasion, and migration induced by miR-29a-3p
overexpression. This results is in agreement with previous studies
which showed that activation of Wnt/β-catenin signaling facilitates
tumor growth in lung cancer (40).
The results showed that miR-29a-3p had a tumor suppressive role in
NSCLC by restraining Wnt/β-catenin signaling.
In conclusion, miR-29a-3p inhibits NSCLC tumor
growth and cell proliferation, migration, and invasion by retarding
the Wnt/β-catenin signaling pathway. The results of the present
study provide a potential avenue for developing targeted
therapeutics in the management of NSCLC. However, the present study
has some limitations. First, the effects of miR-29a-3p on NSCLC
were explored in A549 cells, future studies should use a wider
array of NSCLC cell lines to confirm or disprove the
generalizability of the results. Second, clinical information is
still required to verify whether miR-29a-3p affects the prognosis
of NSCLC patients. Finally, additional experiments are required to
explore how miR-29a-3p targets the Wnt/β-catenin signaling
pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KZ and BH conceived and designed the study. KZ, XH,
WH, and CS acquired the data. KZ, XH and WH analyzed and
interpreted the data. XH, WH, and CS performed the statistical
analysis. KZ wrote the manuscript. KZ and BH revised the
manuscript. KZ, XH, WH, CS and BH confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments were performed in accordance
with the protocols approved by the Ethics Committee of the
Experimental Animal Center of The Affiliated Hospital of Shaoxing
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-SCLC
|
|
miR/miRNA
|
microRNA
|
|
GC
|
gastric cancer
|
References
|
1
|
Han H, Pan B, Liang F, Wu L, Liu X, Yang Y
and Chen J: MiR-224 promotes lymphatic metastasis by targeting
ANGPTL1 in non-small-cell lung carcinoma. Cancer Biomark.
34:431–441. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu W, Zhang X, Zhang W, Xiong M, Lin Y,
Chang M, Xu L, Lu Y, Liu Y and Zhang J: 19-Hydroxybufalin inhibits
non-small cell lung cancer cell proliferation and promotes cell
apoptosis via the Wnt/β-catenin pathway. Exp Hematol Oncol.
10:482021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang R, Liu J, Li K, Yang G, Chen S, Wu J,
Xia X, Ren H and Pang Y: An SETD1A/Wnt/β-catenin feedback loop
promotes NSCLC development. J Exp Clin Cancer Res. 40:3182021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YG, Li J, Nie F and Jin G: LINC00961
functions as an anti-oncogene in non-small cell lung carcinoma by
regulation of miR-3127. Am J Transl Res. 14:888–898.
2022.PubMed/NCBI
|
|
5
|
Xie Y, Xue C, Guo S and Yang L:
MicroRNA-520a suppresses pathogenesis and progression of
non-small-cell lung cancer through targeting the RRM2/Wnt axis.
Anal Cell Pathol (Amst). 2021:96524202021.PubMed/NCBI
|
|
6
|
Fan X, Tao S, Li Q, Deng B, Tan QY and Jin
H: The miR-23a/27a/24-2 cluster promotes postoperative progression
of early-stage non-small cell lung cancer. Mol Ther Oncolytics.
24:205–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye J, Feng H and Peng Z: miR-23a-3p
inhibits sepsis-induced kidney epithelial cell injury by
suppressing Wnt/β-catenin signaling by targeting wnt5a. Braz J Med
Biol Res. 55:e115712022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han S, Wang Z, Liu J, Wang HD and Yuan Q:
miR-29a-3p-dependent COL3A1 and COL5A1 expression reduction assists
sulforaphane to inhibit gastric cancer progression. Biochem
Pharmacol. 188:1145392021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei Z, Wang W, Li Q, Du L and He X: The
microRNA miR-19a-3p suppresses cell growth, migration, and invasion
in multiple myeloma via the Wnt/β-catenin pathway. Transl Cancer
Res. 10:1053–1064. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Li D and Yin GQ: MiR-19b-3p promotes
tumor progression of non-small cell lung cancer via downregulating
HOXA9 and predicts poor prognosis in patients. Histol Histopathol.
Mar 11–2022.(Epub ahead of print).
|
|
11
|
Yu B, Pang J and You J: Effects and
mechanism of miR-133a on invasion and migration of lung cancer
cells. Am J Transl Res. 14:728–739. 2022.PubMed/NCBI
|
|
12
|
Zhang Y and Hu X: miR-148a promotes cell
sensitivity through downregulating SOS2 in radiation-resistant
non-small cell lung cancer cells. Oncol Lett. 23:1352022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng A, Luo L, Ren F, Zhang L, Zhou H and
Gao X: miR-29a-3p inhibits endometrial cancer cell proliferation,
migration and invasion by targeting VEGFA/CD C42/PAK1. BMC Cancer.
21:8432021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Z, Cui H, Wang Y and Yao W:
Downregulation of RPS15A by miR-29a-3p attenuates cell
proliferation in colorectal carcinoma. Biosci Biotechnol Biochem.
83:2057–2064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Wang Y and Ding H: COL4A1,
negatively regulated by XPD and miR-29a-3p, promotes cell
proliferation, migration, invasion and epithelial-mesenchymal
transition in liver cancer cells. Clin Transl Oncol. 23:2078–2089.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayat R, Manzoor M and Hussain A: Wnt
signaling pathway: A comprehensive review. Cell Biol Int.
46:863–877. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Z, Zhong M, Zhou L, Le Y, Wang H and
Fang Z: Low-density lipoprotein receptor-related protein 8
facilitates the proliferation and invasion of non-small cell lung
cancer cells by regulating the Wnt/β-catenin signaling pathway.
Bioengineered. 13:6807–6818. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi Q, Miao Y, Kong Y, Xu Y, Zhou J, Dong Q
and Liu H: MiR-579 inhibits lung adenocarcinoma cell proliferation
and metastasis via binding to CRABP2. Comput Math Methods Med.
2022:91116812022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng L, Wan TM, Iyer DN, Huang Z, Sin RW,
Man AT, Li X, Foo DC, Lo OS and Law WL: High levels of tumor
miR-187-3p-a potential tumor-suppressor microRNA-are correlated
with poor prognosis in colorectal cancer. Cells. 11:24212022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai F, Xiu Z, Yang Q, Zhong Z, Zhong C and
Qiu Y: MicroRNA-375 inhibits laryngeal squamous cell carcinoma
progression via targeting CST1. Braz J Otorhinolaryngol. Jul
6–2022.(Epub ahead of print). View Article : Google Scholar
|
|
21
|
Hao X and Su A: MiR-590 suppresses the
progression of non-small cell lung cancer by regulating YAP1 and
Wnt/β-catenin signaling. Clin Transl Oncol. 24:546–555. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Zhang R, Zhu Y and Hao R:
Exosome-derived microRNA-433 inhibits tumorigenesis through
incremental infiltration of CD4 and CD8 cells in non-small cell
lung cancer. Oncol Lett. 22:6072021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Wang L, Guo J, Zuo S, Wang Z and
Hua S: MYPT1, regulated by miR-19b-3p inhibits the progression of
non-small cell lung cancer via inhibiting the activation of
wnt/β-catenin signaling. Life Sci. 278:1195732021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Meng Z, Zou T, Wang G, Su Y, Yao S
and Sun X: MiR-374a activates Wnt/β-catenin signaling to promote
osteosarcoma cell migration by targeting WIF-1. Pathol Oncol Res.
26:533–539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Zhang J, Yun C, Dong C and Tian Y:
Effects of afatinib on development of non-small-cell lung cancer by
regulating activity of Wnt/β-catenin signaling pathway. Comput Math
Methods Med. 2022:52130162022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang P, Li L, Wang B, Ran X, Yang Z, Liu
Y and Zhu B: miR-489-3p promotes malignant progression of non-small
cell lung cancer through the inactivation of Wnt/β-catenin
signaling pathway via regulating USP48. Respir Res. 23:932022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin G, Lin L, Lin H and Xu Y, Chen W, Liu
Y, Wu J, Chen S, Lin Q, Zeng Y and Xu Y: C1QTNF6 regulated by
miR-29a-3p promotes proliferation and migration in stage I lung
adenocarcinoma. BMC Pulm Med. 22:2852022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Li X, Wei C, Zhao C, Wang S and
Gao J: High expression of SETDB1 mediated by miR-29a-3p associates
with poor prognosis and immune invasion in breast invasive
carcinoma. Transl Cancer Res. 10:5065–5075. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Lin LZ, Guan JS, Chen CM, Zuo Q and
Lin BQ: TCM combined western medicine treatment of advanced NSCLC:
A preliminary study of mIRNA expression profiles. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 36:1076–1081. 2016.(In Chinese). PubMed/NCBI
|
|
31
|
Yan Y, Du C, Duan X, Yao X, Wan X, Jiang
Z, Qin Z, Li W, Pan L, Gu Z, et al: Inhibiting collagen I
production and tumor cell colonization in the lung via miR-29a-3p
loading of exosome-/liposome-based nanovesicles. Acta Pharm Sin B.
12:939–951. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Xu M, Wang Z, Huang P, Huang C, Chen
Z, Tang G, Zhu X, Cai M and Qin S: The EMT-induced lncRNA NR2F1-AS1
positively modulates NR2F1 expression and drives gastric cancer via
miR-29a-3p/VAMP7 axis. Cell Death Dis. 13:842022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Xiang X, Liu L, Yang H, Cen D and
Tang G: Bioinformatics analysis of hub genes and potential
therapeutic agents associated with gastric cancer. Cancer Manag
Res. 13:8929–8951. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muluhngwi P and Klinge CM: Identification
and roles of miR-29b-1-3p and miR29a-3p-regulated and non-regulated
lncRNAs in endocrine-sensitive and resistant breast cancer cells.
Cancers (Basel). 13:35302021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu H, Tang Y, He C, Tian Y and Ni R:
Prognostic value of lncRNA HOXA-AS3 in cervical cancer by targeting
miR-29a-3p and its regulatory effect on tumor progression. J Obstet
Gynaecol Res. Jul 11–2022.(Epub ahead of print). View Article : Google Scholar
|
|
36
|
Pan H, Ding Y, Jiang Y, Wang X, Rao J,
Zhang X, Yu H, Hou Q and Li T: LncRNA LIFR-AS1 promotes
proliferation and invasion of gastric cancer cell via
miR-29a-3p/COL1A2 axis. Cancer Cell Int. 21:72021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Liu S, Cao L, Zhang T, Yue D, Wang
L, Ping Y, He Q, Zhang C, Wang M, et al: miR-29a-3p suppresses cell
proliferation and migration by downregulating IGF1R in
hepatocellular carcinoma. Oncotarget. 8:86592–86603. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Zhang W, Yan L, Zheng P and Li J:
miR-29a-3p directly targets Smad nuclear interacting protein 1 and
inhibits the migration and proliferation of cervical cancer HeLa
cells. PeerJ. 8:e101482020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu S, Liu D, Liu J, Liu J and Zhong M:
miR-29a-3p promotes migration and invasion in ameloblastoma via
Wnt/β-catenin signaling by targeting catenin beta interacting
protein 1. Head Neck. 43:3911–3921. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han SH, Han JH, Chun WJ, Lee SS, Kim HS
and Lee JW: Nobiletin inhibits non-small-cell lung cancer by
inactivating WNT/β-catenin signaling through downregulating
miR-15-5p. Evid Based Complement Alternat Med. 2021:77829632021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi Y, Ge C, Fang D, Wei W, Li L, Wei Q
and Yu H: NCAPG facilitates colorectal cancer cell proliferation,
migration, invasion and epithelial-mesenchymal transition by
activating the Wnt/β-catenin signaling pathway. Cancer Cell Int.
22:1192022. View Article : Google Scholar : PubMed/NCBI
|