Introduction
Osteoarthritis (OA) is a complex chronic disease
characterized by stiffness, arthralgia, and swelling, which is the
primary cause of disability amongst the elderly (1,2).
Chondrocytes are a type of cell in cartilage tissues that primarily
function to generate and maintain the extracellular matrix (ECM)
components (3). During OA,
chondrocytes undergo multiple changes, such as in their secretory
profiles and regarding their cell viability (4). Aberrant apoptosis and inflammatory
responses in chondrocytes are related to cartilage degradation in
OA (5,6). Therefore, probing the mechanism of
chondrocyte dysfunction may be useful in understanding OA
pathogenesis.
Long noncoding RNAs (lncRNAs) exert important roles
in a variety of biological processes, including metabolism,
immunity, differentiation, and apoptosis (7,8). A
growing number of studies have shown that lncRNAs play vital
effects in osteogenesis, chondrogenesis, and OA (9,10).
More recently, the dysregulation of lncRNAs has been studied in OA
(11,12). LncRNA ZNFX1 antisense RNA 1 was
shown to inhibit apoptosis and matrix synthesis, and facilitate
chondrocyte growth and migration in OA (13). In human chondrocytes, lncRNA
urothelial cancer associated 1 increased matrix metallopeptidase 13
expression by suppressing microRNA (miRNA/miR)-204-5p (14). Reports on Just proximal to
X-inactive specific transcript (JPX), a lncRNA, have primarily
focused on its regulatory effects in various cancers, including
hepatocellular carcinoma, colorectal cancer, lung cancer, oral
squamous cell carcinoma, and myeloid malignancies (15–19).
Interestingly, Gál et al (20) found that JPX expression was
upregulated in adult patients with allergic rhinitis compared with
the adult control group. Chen et al (21) showed that JPX was upregulated in
allergic rhinitis and knockdown of JPX improved the imbalance
between Treg/Th17 observed in allergic rhinitis. Nevertheless,
whether JPX participates in OA development and the underlying
molecular mechanisms involved remain to be determined.
An increasing number of studies have demonstrated
that miRNAs are involved in the regulation of cell viability,
differentiation, inflammation, lipid metabolism, apoptosis,
oncogenesis, and other core cellular activities (22). It has been shown that miRNAs may
serve as novel therapeutic targets for OA (23). LncRNAs can act as competitive
endogenous (ce)RNAs by functioning as miRNA sponges, leading to the
suppression of miRNAs (24,25).
For example, lncRNA plasmacytoma variant translocation 1 aggravated
ECM degradation by inhibiting miR-140 expression in OA (26). LINC00461 enhanced chondrocyte
proliferation and cell cycle progression by downregulating
miR-30a-5p expression in OA (27).
LncRNA HOX transcript antisense RNA (HOTAIR) knockdown promoted
proliferation and inhibited ECM degradation of OA chondrocytes by
increasing the activity of the miR-107/C-X-C motif chemokine ligand
12 axis (28). Kurowska et
al (29) found that miR-25-3p
expression was downregulated in patients with rheumatoid arthritis.
Wang et al (30) found that
miR-25-3p expression was downregulated in osteoarthritic cartilage
compared with healthy cartilage. Moreover, miR-25-3p expression was
decreased in TNF-α-induced rat chondrocytes, and miR-25-3p
negatively regulated IGFBP7 to promote chondrocyte proliferation
and reduce chondrocyte apoptosis in OA (31). However, whether lncRNA JPX
regulates miR-25-3p in OA remains to be assessed.
The first peptidyl cis-trans prolyl isomerase
(PPIase) was isolated in 1984 by Fischer et al (32). The inhibition of PPIase activity
protects cells from apoptosis (33). Lebedev et al (34) reported that PPIF was involved in
mitochondrial permeability transition. PPIF-mediated necrosis
participates in the pathological process of myocardial and/or
cerebral ischemia/reperfusion injury (35). Inhibition of PPIF increases cell
viability of TNF-α treated osteoblast-like cells (36). However, as a member of the PPIase
family, the potential regulatory role of peptidylprolyl isomerase D
(PPID) has not been elucidated.
In the present study, we investigated the potential
effects of lncRNA JPX in OA. The function of JPX in cell viability
and apoptosis of chondrocytes, and the specific mechanism of
modulating miR-25-3p activity were assessed.
Materials and methods
Specimen collection
A total of 20 OA patients and 16 non-OA patients
(patients without symptoms of OA during total hip replacement) from
Sunshine Union Hospital (Weifang, China) were collected in the
present study. Patients provided signed consent to the collection
of samples and the present study was approved by the Ethics
Committee of Sunshine Union Hospital.
Cell culture and treatment
Human C28/I2 chondrocytes were obtained from BeNa
Culture Collection. C28/I2 cells were cultured in DMEM/F12 (Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin-streptomycin solution (Thermo Fisher Scientific, Inc.)
and maintained in a humidified incubator supplied with 5%
CO2 at 37°C. To generate a model of OA, C28/I2 cells
were treated with IL-1β recombinant protein (5, 10, and 20 ng/ml)
for 24 h (37). C28/I2 cells
without IL-1β treatment were used as the control.
Cell transfection
Small interfering (si)RNAs targeting JPX (50 nM;
si-JPX#1, 5′-GTTGCAAGGCGTCCGAAGTAT-3′ and si-JPX#2,
5′-GTCCGAAGTATGAGTCCACTA-3′), and negative control (50 nM; si-NC,
5′-TTCTCCGAACGTGTCACGT-3′), miR-25-3p mimic (40 nM; sense,
5′-CAUUGCACUUGUCUCGGUCUGA-3′ and antisense,
5′-AGACCGAGACAAGUGCAAUGUU-3′) and mimic negative control (40 nM;
mimic NC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′), miR-25-3p inhibitor (40 nM;
5′-UCAGACCGAGACAAGUGCAAUG-3′) and inhibitor negative control (40
nM; inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from
Shanghai GenePharma, Co., Ltd. A PPID overexpression vector pcDNA™
3.1/V5-HisB-PPID (pc-PPID, 75 ng) and an empty pcDNA3.1 vector
(pc-NC, 75 ng) were obtained from Thermo Fisher Scientific, Inc.
After addition of the transfection reagent
[Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)]
and constructs, cells were cultured for 48 h, after which the
subsequent experiments were performed.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was isolated from chondrocytes using
TRIzol® reagent (Thermo Fisher Scientific, Inc.), and
then converted to cDNA using a PrimeScript RT kit (Takara Bio,
Inc.) or TaqMan™ MicroRNA Reverse Transcription Kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, qPCR analysis was performed for miRNAs using a
MiScript SYBR-Green PCR kit (Qiagen GmbH) or SYBR Premix Ex Taq™
Kit (Takara Bio, Inc.) on an ABI 7300HT system. The qPCR
thermocycling conditions were as follows: Pre-denaturation at 95°C
for 2 min; followed by 40 cycles of denaturation at 95°C for 15
sec, and annealing and extension at 60°C for 30 sec. GAPDH was used
as the internal control for JPX and PPID, and U6 was used as the
internal control for miR-25-3p. The expression levels of miR-25-3p
and lncRNA JPX were quantified using the 2−ΔΔCq method
(38). The sequences of the
primers were: JPX forward, 5′-TTGCAAGGCGTCCGAAGTAT-3′ and reverse,
5′-AGGCGATCAGCGAGAAAGAA-3′; miR-25-3p forward,
5′-ACACTCCAGCTGGGCATTGCACTTGTCTCG-3′ and reverse,
5′-ACACTCCAGCTGGGCATTGCACTTGTCTCG-3′; PPID forward,
5′-GTGAAAAACCTGCTAAATTGTGCG-3′ and forward,
5′-ATCCGCATCCTCAGGGAAATC-3′; U6 forward,
5′-GGAACGATACAGAGAAGATTAGC-3′ and reverse,
5′-TGGAACGCTTCACGAATTTGCG-3′; GAPDH forward,
5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′.
Western blot assay
Total protein was extracted from chondrocytes using
RIPA lysis buffer containing protease inhibitors. The concentration
of proteins was measured using a BCA kit (Beyotime Institute of
Biotechnology). Then, 50 µg protein lysate was loaded per lane on a
10% SDS-gel, resolved using SDS-PAGE, and transferred onto a PVDF
membrane. After blocking with 5% fat-free milk for 1 h, the PVDF
membranes were incubated with the following primary antibodies:
Anti-PPID antibody (cat. no. ab3562; 1:1,000; Abcam), anti-Bax
antibody (cat. no. ab32503; 1:1,000; Abcam), anti-Bcl-2 (cat. no.
ab32124; 1:1,000; Abcam), anti-cleaved (c)-caspase-9 (cat. no.
20750; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH (cat.
no. ab9485; 1:1,000; Abcam) at 4°C overnight. Subsequently, the
membranes were incubated with goat anti-rabbit IgG H&L (HRP)
secondary antibody (cat. no. ab205718; 1:10,000; Abcam) at room
temperature for 1 h. The protein bands were visualized using the
BeyoECL Plus kit (Beyotime Institute of Biotechnology) and were
semi-quantified with ImageJ version 1.52v (National Institutes of
Health).
Cell viability assay
Cell viability was analyzed using a Cell Counting
Kit (CCK)-8 assay (Beyotime Institute of Biotechnology) according
to the manufacturer's protocol. After 48 h of transfection, cells
were plated to a 96-well plate and cultured at 37°C, and incubated
until they had adhered. Then, the CCK-8 reagent was added, and
cells were further incubated for 4 h. Finally, the absorbance was
measured using a microplate reader at 450 nm.
Apoptosis analysis
Apoptosis was measured using an Annexin V-FITC
Apoptosis Detection Kit (Beyotime Institute of Biotechnology).
After 48 h of transfection, cells were stained with Annexin V-FITC
and PI for 15 min at 25°C in the dark. Cell apoptosis was scanned
using a BD FACSCalibur™ flow cytometer (BD Biosciences) and
analyzed using FlowJo 10.0.6 software (FlowJo, LLC).
Dual-luciferase reporter assay
The binding sites between miR-25-3p and JPX or the
3′UTR PPID were predicted using starBase (https://starbase.sysu.edu.cn/). Cells were plated in a
24-well plate. When cell confluence reached 80%, the WT (or Mut)
pmirGLO luciferase reporter gene vector of JPX (or PPID 3′UTR) and
miR-25-3p mimic (or mimic NC) were co-transfected into cells with
Lipofectamine 2000. After 48 h of transfection, the luciferase
activity was measured using a dual luciferase assay kit according
to the manufacturer's protocol (Promega Corporation) and normalized
to Renilla luciferase activity.
RNA immunoprecipitation (RIP)
assay
RIP assays were performed to verify the targeted
relationship between miR-25-3p, JPX, and PPID using the RIP Kit
(Millipore Sigma) according to the manufacturer's protocol. Cells
were lysed using the lysis buffer and incubated with magnetic beads
pre-coated with Ago2 antibody (cat. no. ab186733; 1:1,000; Abcam)
for 6 h at 4°C. IgG (cat. no. SAB5600195; 1:1,000; MilliporeSigma)
was used as the control. Subsequently, beads were washed with RNA
binding buffer, and the levels of JPX, miR-25-3p, and PPID were
detected by RT-qPCR.
starBase database analysis
The starBase database is a widely-used open-source
platform for studying ncRNA interactions from CLIP-seq,
degradome-seq, and RNA-RNA interactome data (39,40).
Here, starBase was used to predict the binding sites between lncRNA
JPX, miR-25-3p, and PPID.
Statistical analysis
All data are presented as the mean ± SD. All
experiments were performed in triplicate. A one-way ANOVA followed
by a Tukey's post hoc test was used to analyze the differences
between multiple groups. A Student's t-test was used to analyze the
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of lncRNA JPX on cell viability
and apoptosis of IL-1β-treated chondrocytes
The RT-qPCR results showed that JPX was notably
upregulated in OA cartilage tissues compared with the NC cartilage
tissues (Fig. 1A). JPX expression
was significantly increased in chondrocytes after treatment with 10
and 20 ng/ml IL-1β (Fig. 1B).
Thus, 10 ng/ml IL-1β was used for the follow-up experiments. To
probe the effect of JPX on chondrocytes, JPX siRNAs were
transfected into chondrocytes. Transfection of JPX siRNAs markedly
decreased JPX expression levels, especially si-JPX#1 (Fig. 1C). JPX knockdown significantly
decreased JPX expression in IL-1β-treated chondrocytes (Fig. 1D). Thus, the effect of JPX
knockdown on IL-1β-treated chondrocytes was assessed. IL-1β
treatment reduced cell viability and increased cell apoptosis of
chondrocytes (Fig. 1E-G); JPX
knockdown significantly enhanced cell viability and inhibited cell
apoptosis of IL-1β-stimulated chondrocytes (Fig. 1E-G). Additionally, the protein
expression levels of Bcl-2, Bax, and c-caspase-9 were determined
using western blotting. Bcl-2 expression levels in the IL-1β group
were significantly decreased whereas the expression levels of Bax
and c-caspase-9 were significantly increased (Fig. 1H and I). JPX knockdown increased
Bcl-2 expression levels whilst decreasing the expression levels of
Bax and c-caspase-9 in the IL-1β-stimulated chondrocytes (Fig. 1H and I).
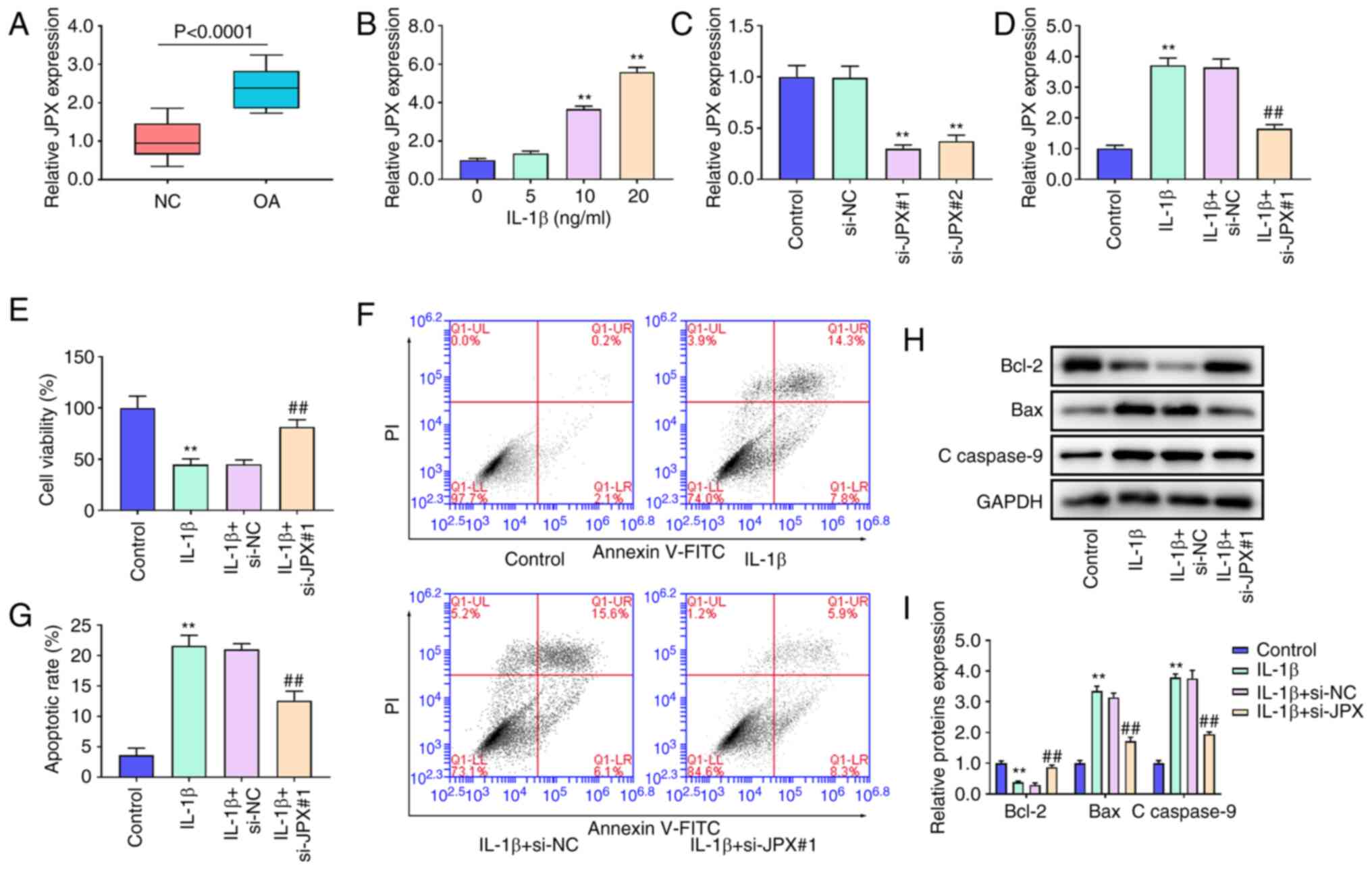 | Figure 1.Effect of lncRNA JPX on cell
viability and apoptosis in IL-1β-treated chondrocytes. (A) lncRNA
JPX expression in OA and NC cartilage tissues were measured using
RT-qPCR. (B) JPX expression in chondrocytes stimulated with 0, 5,
10, or 20 ng/ml IL-1β was detected using RT-qPCR. (C) JPX
expression in chondrocytes transfected with the JPX siRNAs was
detected using RT-qPCR. (D) JPX expression in IL-1β-stimulated
chondrocytes after transfection with JPX siRNA was detected using
RT-qPCR. (E) The cell viability of chondrocytes was assessed using
a Cell Counting Kit-8 assay. (F and G) Cell apoptosis of
chondrocytes was measured using flow cytometry. (H and I) The
protein expression levels of apoptosis-related proteins Bcl-2, Bax
and c-caspase-9 were detected using western blotting. **P<0.01
vs. control group; ##P<0.01 vs. IL-1β+si-NC group..
lncRNA, long non-coding RNA; JPX, Just proximal to X-inactive
specific transcript; RT-qPCR, reverse transcription-quantitative
PCR; OA, osteoarthritic; NC, negative control; siRNA, small
interfering RNA; c-caspase, cleaved-caspase. |
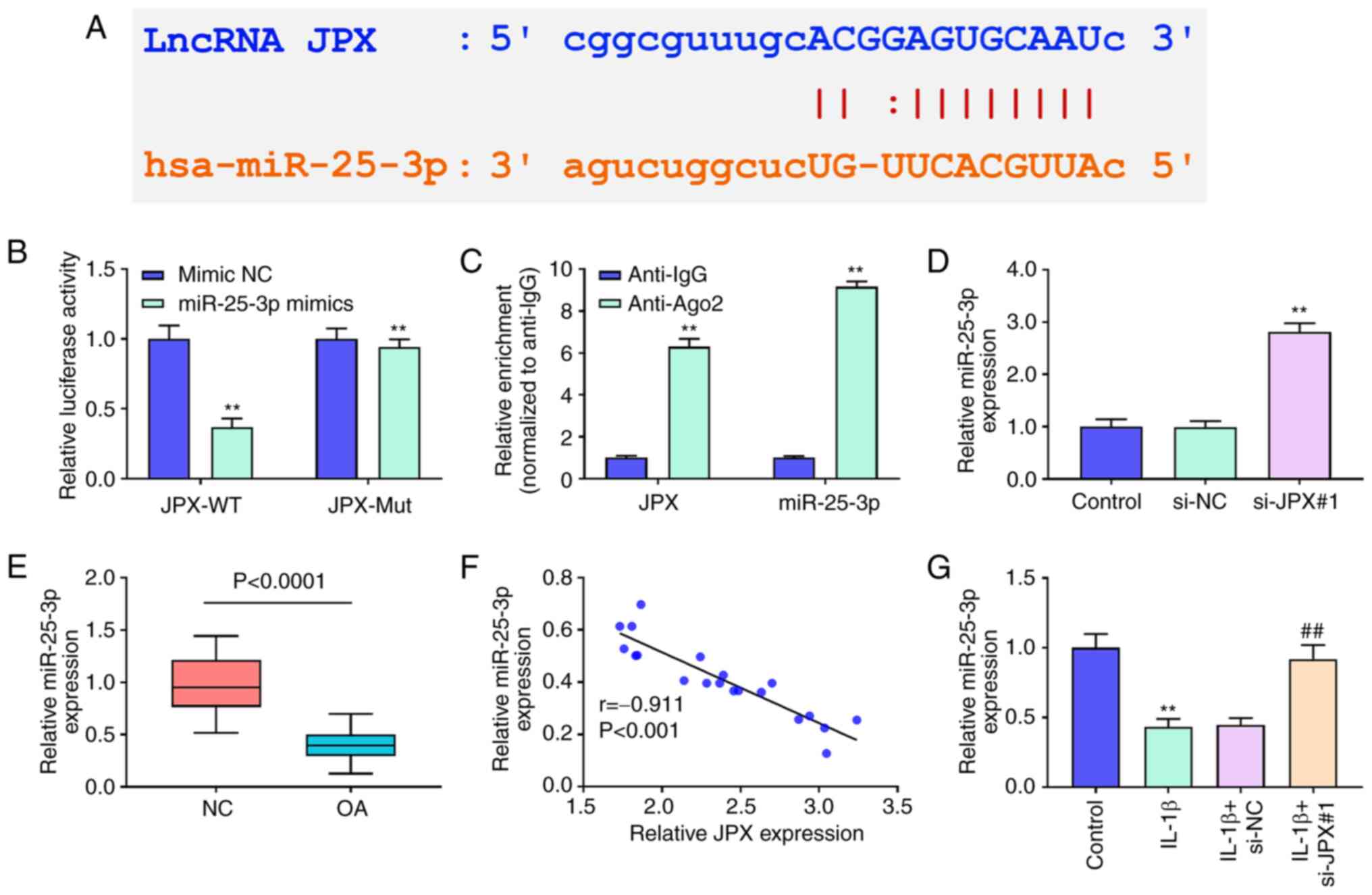
miR-25-3p is a target of JPX in
chondrocytes
starBase analysis predicted that JPX could target
miR-25-3p (Fig. 2A). A
dual-luciferase reporter assay and RIP assay were used to confirm
the targeting relationship between JPX and miR-25-3p. miR-25-3p
mimics decreased luciferase activity in the JPX-WT group (Fig. 2B). The results of RIP revealed that
JPX and miR-25-3p were significantly enriched using anti-Ago2
(Fig. 2C). JPX knockdown
significantly increased the expression levels of miR-25-3p
(Fig. 2D). The results of RT-qPCR
showed that miR-25-3p expression in OA patients was notably
decreased compared with that in healthy individuals (Fig. 2E). The correlation analysis
revealed that miR-25-3p levels in OA patients were negatively
related with JPX levels (Fig. 2F).
Additionally, miR-25-3p expression levels were significantly
decreased by IL-1β (Fig. 2G).
Together, these results suggested that lncRNA JPX could target
miR-25-3p in chondrocytes.
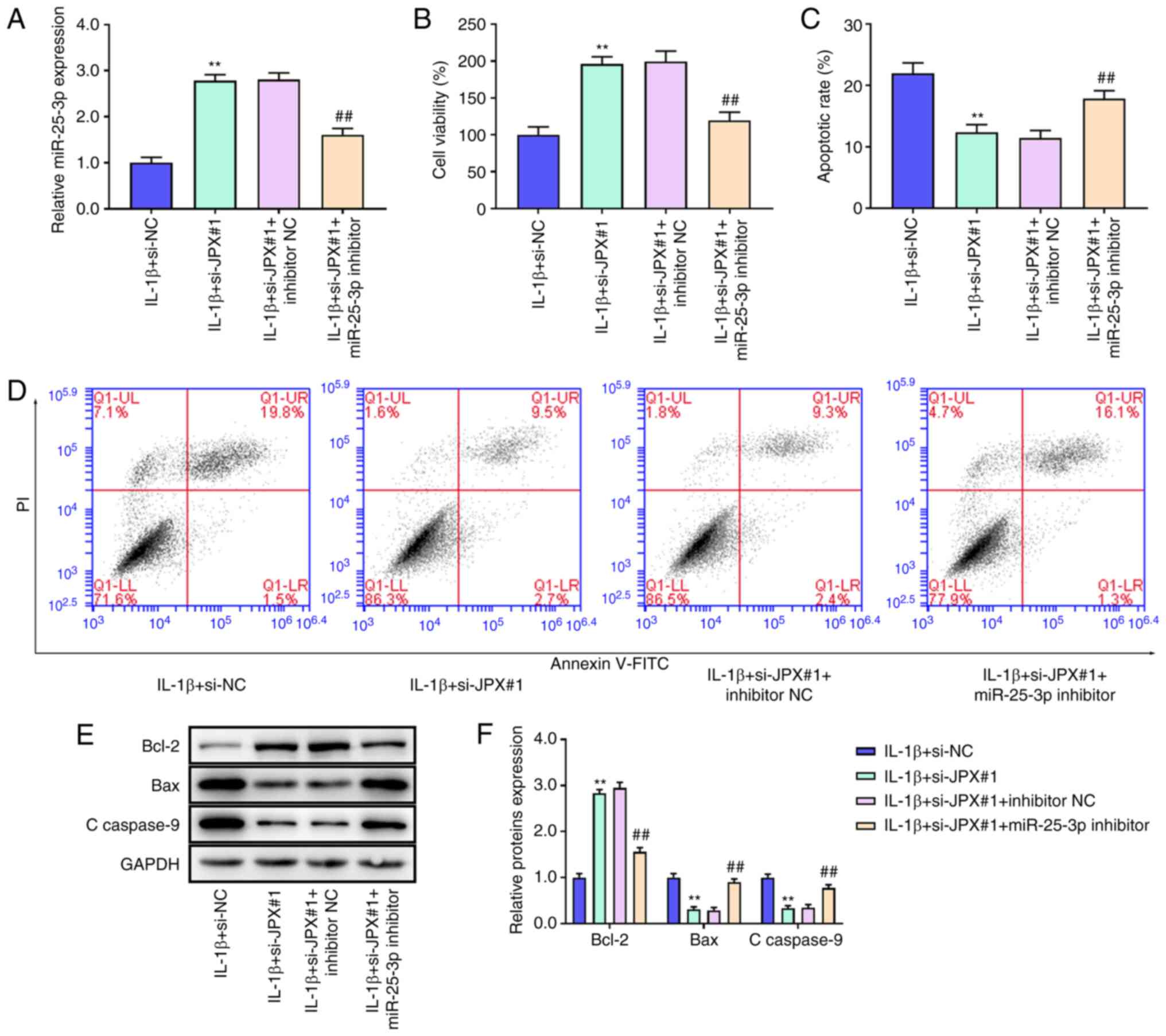
Inhibition of miR-25-3p abrogates the
effects of JPX knockdown on IL-1β-stimulated chondrocytes
Next, whether JPX regulated miR-25-3p to affect the
activity and apoptosis of IL-1β-stimulated chondrocytes was
assessed. miR-25-3p inhibitor notably reversed the JPX
knockdown-induced increase in miR-25-3p expression (Fig. 3A). CCK-8 results showed that JPX
knockdown increased cell viability of IL-1β-stimulated chondrocytes
(Fig. 3B). JPX knockdown notably
reduced cell apoptosis in the IL-1β-stimulated chondrocytes
(Fig. 3C and D). However, cell
viability was decreased and apoptosis was increased in the
IL-1β+si-JPX#1+miR-25-3p inhibitor group compared with the
IL-1β+si-JPX#1+inhibitor NC group (Fig. 3B-D). Western blotting results
showed that JPX knockdown significantly increased Bcl-2 levels and
decreased the levels of Bax and c-caspase-9 (Fig. 3E and F); the effect of JPX
knockdown was abrogated by the miR-25-3p inhibitor (Fig. 3E and F). Together, these findings
demonstrated that inhibition of miR-25-3p abrogated the effects of
JPX knockdown on IL-1β-stimulated injury in chondrocytes.
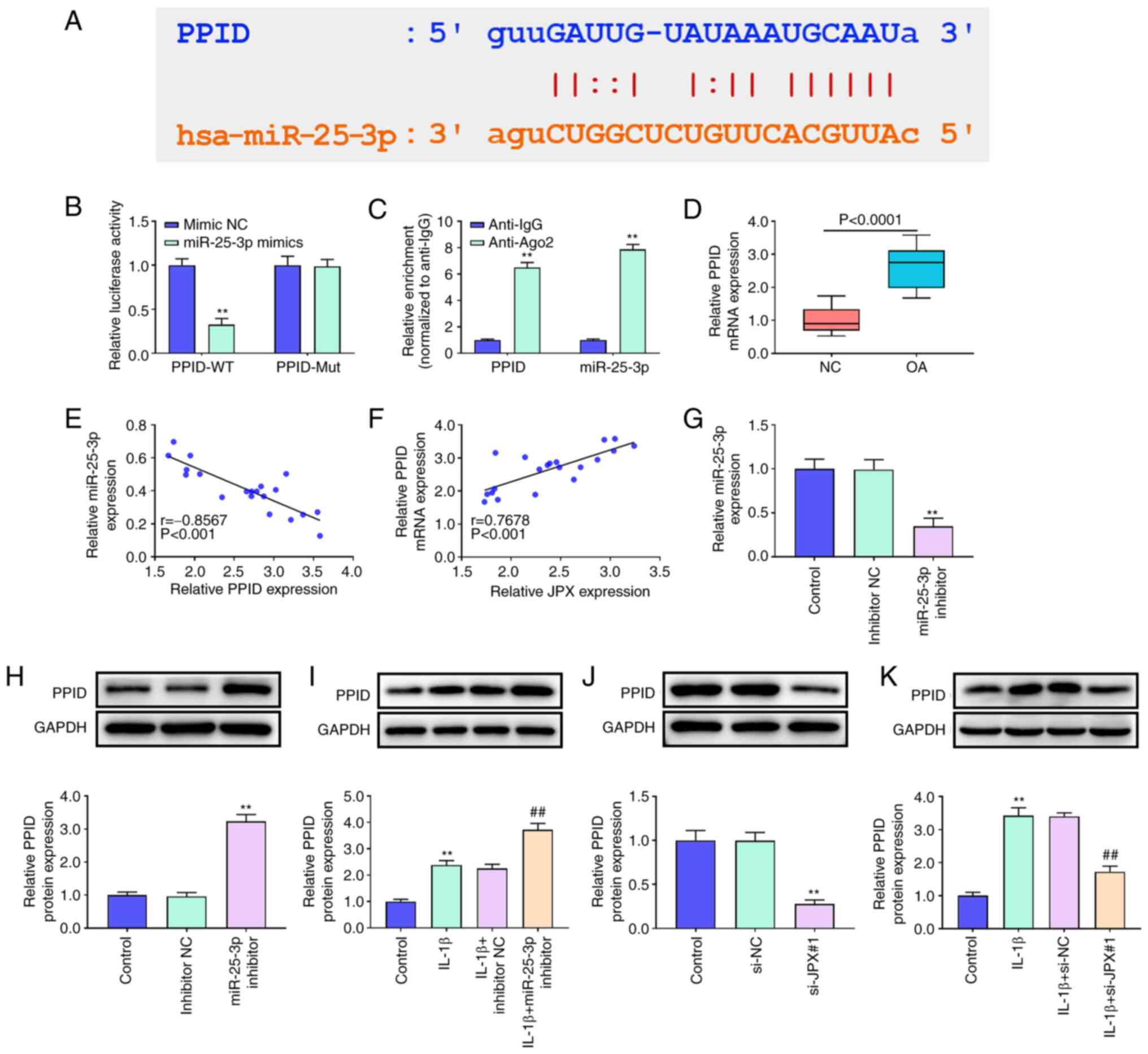
PPID is a target of miR-25-3p in
chondrocytes
starBase analysis predicted that PPID was a target
of miR-25-3p (Fig. 4A). The
results of the dual-luciferase reporter assay and RIP analysis
confirmed the targeted relationship between miR-25-3p and PPID
(Fig. 4B and C). The results of
RT-qPCR showed that PPID expression in OA patients was
significantly higher than that in the healthy individuals (Fig. 4D). The correlation analysis showed
that the PPID levels in OA patients were negatively associated with
miR-25-3p and positively related to JPX levels (Fig. 4E and F). Next, the effect of
miR-25-3p and JPX on PPID expression was assessed. miR-25-3p
inhibitor significantly decreased miR-25-3p expression levels
(Fig. 4G). Additionally, IL-1β
treatment significantly increased PPID expression in chondrocytes
(Fig. 4I and K). The protein
expression levels of PPID were significantly increased by
transfection of the miR-25-3p inhibitor whereas JPX knockdown
significantly decreased PPID expression irrespective of IL-1β
treatment (Fig. 4H and J). These
results suggested that PPID is a target of miR-25-3p and it can be
regulated by lncRNA JPX in chondrocytes.
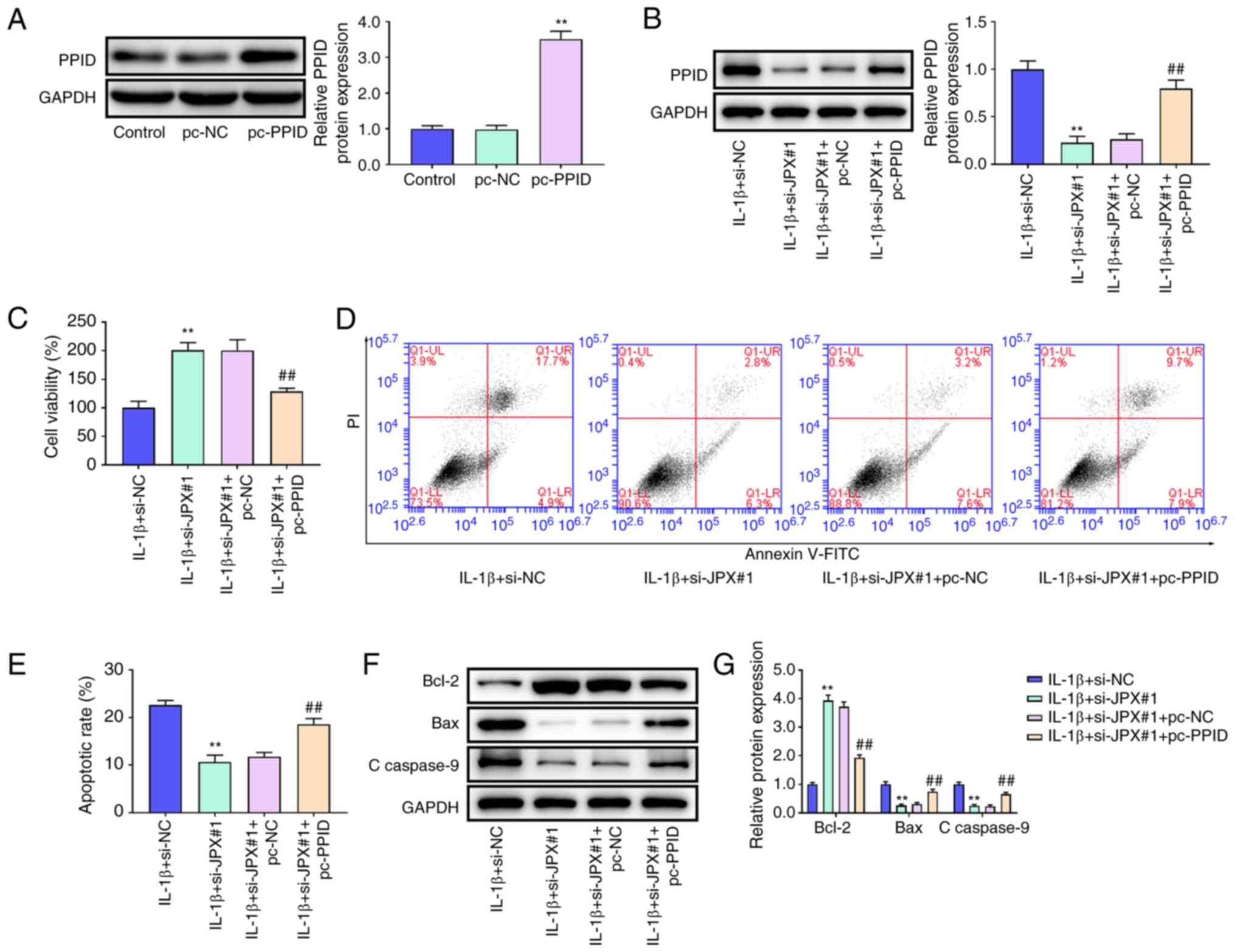
PPID overexpression abrogates the
effect of JPX knockdown in IL-1β-stimulated chondrocytes
To confirm whether JPX affected cartilage damage via
regulation of PPID, JPX siRNA and PPID overexpression plasmid were
transfected into chondrocytes. PPID was notably increased in the
pc-PPID group compared with the pc-NC group (Fig. 5A). In the IL-1β-stimulated
chondrocytes, JPX knockdown markedly decreased PPID expression
compared with the IL-1β+si-NC group. Compared with the
IL-1β+si-JPX#1+pc-NC group, the expression levels of PPID were
significantly enhanced in the IL-1β+si-JPX#1+pc-PPID group
(Fig. 5B). CCK-8 analysis showed
that JPX knockdown increased cell viability in IL1β-stimulated
chondrocytes, and the increase in cell viability following JPX
knockdown was eliminated by PPID overexpression (Fig. 5C). As shown in Fig. 5D and E, JPX knockdown significantly
decreased apoptosis of IL-1β-stimulated chondrocytes. Additionally,
the inhibitory effect of JPX knockdown on apoptosis was eliminated
by overexpression of PPID. JPX knockdown markedly increased Bcl-2
expression and reduced expression of Bax and c-caspase-9 in
IL-1β-stimulated chondrocytes, and the effects of JPX knockdown on
Bcl-2, Bax, and c-caspase-9 were eliminated by PPID overexpression
(Fig. 5F and G). These results
indicated that JPX knockdown may reduce cell injury in
IL-1β-stimulated chondrocytes by regulating PPID expression.
Discussion
lncRNAs are associated with the function and
inflammatory response of chondrocytes in OA (41,42).
JPX has been reported to play an important role in myeloid
malignancies (19). However, the
underlying mechanism of JPX in OA remains unclear. In this study,
C28/I2 cells were used to explore the effect of JPX on
IL-1β-stimulated injury. The results showed that JPX knockdown
enhanced cell viability and reduced apoptosis of IL-1β-stimulated
chondrocytes. Moreover, the results showed that JPX was associated
with the miR-25-3p/PPID axis in OA.
The cartilage damage is a characteristic and
defining feature of OA (5,43). IL-1β has been reported to
participate in OA progression (44). Therefore, chondrocyte C28/I2 cells
were treated with IL-1β to establish an in vivo OA model. The
results showed that IL-1β treatment reduced the viability of C28/I2
cells. Apoptosis is an important process related to cell viability
(45). Activation of
apoptosis-related proteins, such as Bcl-2, Bax, and caspase-3, are
reliable markers of cell apoptosis (46,47).
In this study, it was shown that IL-1β treatment facilitated
apoptosis, which was observed as an increase in Bcl-2 levels, and a
decrease in the levels of Bax and cleaved-caspase-9 in C28/I2
cells. Additionally, it was found that JPX expression was
upregulated in OA patients and IL-1β-stimulated chondrocytes, which
indicated that JPX may be associated with OA pathogenesis. The
experiments confirmed that JPX knockdown suppressed the cell injury
stimulated by IL-1β in chondrocytes, highlighting the therapeutic
potential of JPX suppression on the progression of OA.
It has been found that JPX can competitively bind to
various miRNAs as a ceRNA, such as miR-33a-5p (17), miR-155-5p (48), and miR-944 (18). In intervertebral disc degeneration,
JPX upregulates HIF-1α expression by inhibiting miR-18a-5p in
nucleus pulposus cells (49). In
the present study, it was confirmed that JPX could bind to
miR-25-3p as a ceRNA in C28/I2 cells. Additionally, miR-25-3p
expression was downregulated in OA tissues and IL-1β-stimulated
chondrocytes. miR-25-3p inhibitor reduced cell viability, promoted
apoptosis in chondrocytes, and reversed the effects of JPX
knockdown in chondrocytes. Li et al (50) found that miR-25-3p has
anti-apoptotic effects on cultured primary neurons. Suppression of
miR-25-3p reduced cell proliferation in a mouse model of polycystic
kidney disease (51). The results
of the present study are in agreement with the previous studies;
the protective effects of JPX knockdown on chondrocytes were
achieved by abrogating the effects of miR-25-3p.
As a member of the PPIase family, PPID knockdown
protected HaCaT keratinocytes from death following UVA irradiation
(52). In the present study, PPID
was a target gene of miR-25-3p. PPID expression was upregulated in
OA tissues and IL-1β-stimulated chondrocytes. Additionally, PPID
expression was regulated by JPX, and JPX levels were positively
correlated with PPID. Next, whether JPX could regulate PPID to
affect IL-1β-stimulated chondrocytes was assessed. The data showed
that PPID overexpression promoted apoptosis in chondrocytes and
increased the expression of Bax and c-caspase-9. Moreover, PPID
overexpression partly eliminated the influence of JPX knockdown on
chondrocytes. These findings also resulted in accelerating the
effect of PPID on OA progression, which was observed as the
reversal of the protective effect of JPX knockdown in
IL-1β-stimulated injury. Together, JPX can affect OA progression
via actively modulating PPID through competitively sponging
miR-25-3p.
The present study has some limitations. The role of
JPX in OA was only explored in vitro, thus in vivo
experiments should be performed to confirm the results in future
studies. The effect of JPX on inflammatory response, oxidative
stress, and other aspects associated with OA
development/progression need further study. Finally, the number of
patients included in the present study was low, thus the results
should be confirmed in a larger cohort.
In conclusion, the results of the present study
showed that lncRNA JPX increased the cell viability of chondrocytes
and suppressed apoptosis in OA by modulating a miR-25-3p/PPID axis,
thereby reducing the cell damage in OA. These findings highlight
the JPX/miR-25-3p/PPID axis as a potentially novel therapeutic
target in OA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZR designed the study. LT and ZD performed the
experiments. JS, HZ and DL analyzed the data. ZR wrote the
manuscript. ZR, LT and ZD confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Sunshine Union Hospital. All patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNAs
|
long noncoding RNAs
|
|
OA
|
osteoarthritis
|
|
PPID
|
peptidylprolyl isomerase D
|
|
mir/miRNA
|
microRNA
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RIP
|
RNA immunoprecipitation
|
|
ECM
|
extracellular matrix
|
|
JPX
|
Just proximal to X-inactive specific
transcript
|
|
ceRNA
|
competitive endogenous RNA
|
|
PPIase
|
peptidyl cis-trans prolyl
isomerase
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Sacitharan PK: Ageing and osteoarthritis.
Subcell Biochem. 91:123–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhi L, Zhao J, Zhao H, Qing Z, Liu H and
Ma J: Downregulation of LncRNA OIP5-AS1 induced by IL-1β aggravates
osteoarthritis via regulating miR-29b-3p/PGRN. Cartilage. 3
(2_suppl):1345S–1355S. 2020.PubMed/NCBI
|
|
3
|
Bolduc JA, Collins JA and Loeser RF:
Reactive oxygen species, aging and articular cartilage homeostasis.
Free Radic Biol Med. 132:73–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charlier E, Deroyer C, Ciregia F, Malaise
O, Neuville S, Plener Z, Malaise M and de Seny D: Chondrocyte
dedifferentiation and osteoarthritis (OA). Biochem Pharmacol.
165:49–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pap T and Korb-Pap A: Cartilage damage in
osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat
Rev Rheumatol. 11:606–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Theocharis AD, Manou D and Karamanos NK:
The extracellular matrix as a multitasking player in disease. FEBS
J. 286:2830–2869. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao L, Wang Y, Wang Q and Huang J: LncRNA
FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by
acting as a sponge of miR-206 to modulate CCND1 expression. Biomed
Pharmacother. 106:1220–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wawrzyniak O, Zarębska Ż, Rolle K and
Gotz-Więckowska A: Circular and long non-coding RNAs and their role
in ophthalmologic diseases. Acta Biochim Pol. 65:497–508.
2018.PubMed/NCBI
|
|
10
|
Wei JW, Huang K, Yang C and Kang CS:
Non-coding RNAs as regulators in epigenetics (Review). Oncol Rep.
37:3–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Zhang P, Sun X, Zhou L and Zhao
J: Long non-coding RNA DANCR regulates proliferation and apoptosis
of chondrocytes in osteoarthritis via miR-216a-5p-JAK2-STAT3 axis.
Biosci Rep. 38:BSR201812282018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao G, Kang Y, Lin R, Hu S and Zhang Z, Li
H, Liao W and Zhang Z: Long Non-coding RNA HOTTIP promotes CCL3
expression and induces cartilage degradation by sponging
miR-455-3p. Front Cell Dev Biol. 7:1612019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye D, Jian W, Feng J and Liao X: Role of
long noncoding RNA ZFAS1 in proliferation, apoptosis and migration
of chondrocytes in osteoarthritis. Biomed Pharmacother.
104:825–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Bu X, Zhang Y, Zhao X, Kong Y, Ma
L, Niu S, Wu B and Meng C: LncRNA-UCA1 enhances MMP-13 expression
by inhibiting miR-204-5p in human chondrocytes. Oncotarget.
8:91281–91290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma X, Yuan T, Yang C, Wang Z, Zang Y, Wu L
and Zhuang L: X-inactive-specific transcript of peripheral blood
cells is regulated by exosomal Jpx and acts as a biomarker for
female patients with hepatocellular carcinoma. Ther Adv Med Oncol.
9:665–677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khajehdehi M, Khalaj-Kondori M and
Hosseinpour Feizi MA: Expression profiling of cancer-related long
non-coding RNAs revealed upregulation and biomarker potential of
HAR1B and JPX in colorectal cancer. Mol Biol Rep. 49:6075–6084.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan J, Fang S, Tian H, Zhou C, Zhao X,
Tian H, He J, Shen W, Meng X, Jin X and Gong Z: lncRNA
JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis
of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer.
19:92020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao Y, Chen S, Lu N, Yin Y and Liu Z:
LncRNA JPX overexpressed in oral squamous cell carcinoma drives
malignancy via miR-944/CDH2 axis. Oral Dis. 27:924–933. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimta AA, Tomuleasa C, Sahnoune I, Calin
GA and Berindan-Neagoe I: Long Non-coding RNAs in myeloid
malignancies. Front Oncol. 9:10482019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gál Z, Gézsi A, Semsei ÁF, Nagy A, Sultész
M, Csoma Z, Tamási L, Gálffy G and Szalai C: Investigation of
circulating lncRNAs as potential biomarkers in chronic respiratory
diseases. J Transl Med. 18:4222020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Ke X, Wang X, Kang H and Hong S:
LncRNA JPX contributes to Treg/Th17 imbalance in allergic rhinitis
via targeting the miR-378g/CCL5 axis. Immunopharmacol
Immunotoxicol. 44:519–524. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mellis D and Caporali A: MicroRNA-based
therapeutics in cardiovascular disease: Screening and delivery to
the target. Biochem Soc Trans. 46:11–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swingler TE, Niu L, Smith P, Paddy P, Le
L, Barter MJ, Young DA and Clark IM: The function of microRNAs in
cartilage and osteoarthritis. Clin Exp Rheumatol. 37
(Suppl):S40–S47. 2019.PubMed/NCBI
|
|
24
|
Chen K, Zhu H, Zheng MQ and Dong QR:
LncRNA MEG3 inhibits the degradation of the extracellular matrix of
chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 axis.
Cartilage. 13 (2_Suppl):1274S–1284S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang P, Sun J, Liang C, Gu B, Xu Y, Lu H,
Cao B and Xu H: lncRNA IGHCγ1 Acts as a ceRNA to regulate
macrophage inflammation via the miR-6891-3p/TLR4 axis in
osteoarthritis. Mediators Inflamm. 2020:97430372020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao N, Peng S, Wu H, Liu W, Cai D and
Huang D: Long noncoding RNA PVT1 promotes chondrocyte extracellular
matrix degradation by acting as a sponge for miR-140 in
IL-1β-stimulated chondrocytes. J Orthop Surg Res. 17:2182022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Ma L, Wang C, Wang L, Guo Y and
Wang G: Long noncoding RNA LINC00461 induced osteoarthritis
progression by inhibiting miR-30a-5p. Aging. 12:4111–4123. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu J, Wu Z and Xiong Y: Knockdown of long
noncoding RNA HOTAIR inhibits osteoarthritis chondrocyte injury by
miR-107/CXCL12 axis. J Orthop Surg Res. 16:4102021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurowska W, Kuca-Warnawin E, Radzikowska
A, Jakubaszek M, Maślińska M, Kwiatkowska B and Maśliński W:
Monocyte-related biomarkers of rheumatoid arthritis development in
undifferentiated arthritis patients-a pilot study. Reumatologia.
56:10–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Ning Y, Zhou B, Yang L, Wang Y and
Guo X: Integrated bioinformatics analysis of the
osteoarthritis-associated microRNA expression signature. Mol Med
Rep. 17:1833–1838. 2018.PubMed/NCBI
|
|
31
|
He X and Deng L: Potential of miR-25-3p in
protection of chondrocytes: Emphasis on osteoarthritis. Folia
Histochem Cytobiol. 59:30–39. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fischer G, Berger E and Bang H: Kinetic
beta-deuterium isotope effects suggest a covalent mechanism for the
protein folding enzyme peptidylprolyl cis/trans-isomerase. FEBS
Lett. 250:267–270. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galat A: Peptidylprolyl cis/trans
isomerases (immunophilins): Biological diversity-targets-functions.
Curr Top Med Chem. 3:1315–1347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lebedev I, Nemajerova A, Foda ZH, Kornaj
M, Tong M, Moll UM and Seeliger MA: A novel in vitro CypD-Mediated
p53 aggregation assay suggests a model for mitochondrial
permeability transition by chaperone systems. J Mol Biol.
428:4154–4167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu LQ, Tian J, Luo XJ and Peng J:
Targeting the pathways of regulated necrosis: A potential strategy
for alleviation of cardio-cerebrovascular injury. Cell Mol Life
Sci. 78:63–78. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gan X, Zhang L, Liu B, Zhu Z, He Y, Chen
J, Zhu J and Yu H: CypD-mPTP axis regulates mitochondrial functions
contributing to osteogenic dysfunction of MC3T3-E1 cells in
inflammation. J Physiol Biochem. 74:395–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Z, Lan J and Gao X: Feprazone
Mitigates IL-1β-Induced cellular senescence in chondrocytes. ACS
Omega. 6:9442–9448. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:(Database Issue). D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang JH, Li JH, Shao P, Zhou H, Chen YQ
and Qu LH: starBase: A database for exploring microRNA-mRNA
interaction maps from Argonaute CLIP-Seq and Degradome-Seq data.
Nucleic Acids Res. 39:(Database Issue). D202–D209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pearson MJ and Jones SW: Review: Long
Noncoding RNAs in the regulation of inflammatory pathways in
rheumatoid arthritis and osteoarthritis. Arthritis Rheumatol.
68:2575–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cen X, Huang XQ, Sun WT, Liu Q and Liu J:
Long noncoding RNAs: A new regulatory code in osteoarthritis. Am J
Transl Res. 9:4747–4755. 2017.PubMed/NCBI
|
|
43
|
Brooks P: Inflammation as an important
feature of osteoarthritis. Bull World Health Organ. 81:689–690.
2003.PubMed/NCBI
|
|
44
|
Yu T, Qu J, Wang Y and Jin H: Ligustrazine
protects chondrocyte against IL-1β induced injury by regulation of
SOX9/NF-κB signaling pathway. J Cell Biochem. 119:7419–7430. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zuniga MC, Raghuraman G and Zhou W:
Physiologic levels of resistin induce a shift from proliferation to
apoptosis in macrophage and VSMC co-culture. Surgery. 163:906–911.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao C, Cao X, Fu Z, Tian J, Dong W, Xu J,
An K, Zhai L and Yu J: Boschniakia rossica polysaccharide triggers
laryngeal carcinoma cell apoptosis by regulating expression of
Bcl-2, Caspase-3, and P53. Med Sci Monit. 23:2059–2064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim JA, Kim JC, Min JS, Kang I, Oh J and
Ahn JK: HSV-1 ICP27 induces apoptosis by promoting Bax
translocation to mitochondria through interacting with 14-3-3θ. BMB
Rep. 50:257–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin XQ, Huang ZM, Chen X, Wu F and Wu W:
XIST Induced by JPX suppresses hepatocellular carcinoma by sponging
miR-155-5p. Yonsei Med J. 59:816–826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang H, Wang G, Liu J, Lin M, Chen J, Fang
Y, Li Y, Cai W and Zhan D: LncRNA JPX regulates proliferation and
apoptosis of nucleus pulposus cells by targeting the
miR-18a-5p/HIF-1α/Hippo-YAP pathway. Biochem Biophys Res Commun.
566:16–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li R, Wen Y, Wu B, He M, Zhang P, Zhang Q
and Chen Y: MicroRNA-25-3p suppresses epileptiform discharges
through inhibiting oxidative stress and apoptosis via targeting
OXSR1 in neurons. Biochem Biophys Res Commun. 523:859–866. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu G, Kang X, Guo P, Shang Y, Du R, Wang
X, Chen L, Yue R and Kong F: miR-25-3p promotes proliferation and
inhibits autophagy of renal cells in polycystic kidney mice by
regulating ATG14-Beclin 1. Ren Fail. 42:333–342. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jandova J, Janda J and Sligh JE:
Cyclophilin 40 alters UVA-induced apoptosis and mitochondrial ROS
generation in keratinocytes. Exp Cell Res. 319:750–760. 2013.
View Article : Google Scholar : PubMed/NCBI
|