Introduction
Currently, molecular targeted therapies have been
recommended as standard treatments for patients with non-small cell
lung cancer (NSCLC) carrying driver gene alterations, such as
epidermal growth factor receptor (EGFR) mutations. As the first
third-generation EGFR tyrosine kinase inhibitor (TKI), osimertinib
is generally preferred as the first-line standard of care
therapeutic for untreated EGFR-mutated (ex19del or L858R) advanced
NSCLC due to its superior efficacy and tolerability (1). Despite a median progression-free
survival (mPFS) time of 18.9 months, patients inevitably develop
acquired drug resistance to osimertinib after the initial clinical
benefit (2). However, only a small
number of osimertinib-resistant mechanisms have been clarified,
including MET 14 exon-skipping mutation (15%) and EGFR C797S
mutation (7%) (3). To date, most
osimertinib-resistant mechanisms have not been identified. Hence,
there is a huge unmet medical need to develop novel therapeutic
strategies to tackle the resistance to osimertinib.
Since 2019, China has continuously developed
domestic third-generation EGFR TKI agents, including aumolertinib
(formerly almonertinib; HS-10296) by Jiangsu Hansoh Pharmaceutical
Group Co., Ltd. and furmonertinib by Shanghai Allist Pharmaceutical
Technology Co., Ltd., as well as D0316 by Betta Pharma Co., Ltd.
(4–6). In 2020, the Chinese Society of
Clinical Oncology (CSCO) guidelines approved aumolertinib as a
second-line treatment for patients with advanced NSCLC and T790M
mutation. In 2021, CSCO guidelines recommended aumolertinib as a
first-line treatment for patients with advanced NSCLC and
EGFR-sensitive mutations (7).
Recently, some reports have emerged on the alternatives for
overcoming resistance to osimertinib, such as use of brigatinib
alone or in combination with cetuximab (8,9).
Also, CSCO guidelines on NSCLC recommended furmonertinib (another
third-generation EGFR-TKI in China) to treat advanced
T790M-positive NSCLC after failure of other EGFR-TKI treatment.
However, furmonertinib was not listed into the catalogue of drugs
for Basic National Medical Insurance of China in 2021. By contrast,
aumolertinib was covered in the catalogue. Thus, aumolertinib was
administered by Jiujiang University Affiliated Hospital (Jiujiang,
China) in an attempt to treat several patients with
osimertinib-resistant advanced NSCLC. The present study reports the
details of 3 patients who developed resistance to osimertinib and
favorably responded to subsequent aumolertinib treatment.
Case report
Case 1
A 70-year-old female patient was admitted to
Jiujiang University Affiliated Hospital in January 2021, with
complaints of a cough and expectoration for 2 months. The patient
had a persistent dry cough but no fever, night sweats or
hemoptysis. The medical history included type 2 diabetes and
Parkinson's disease for 11 years, with no history of smoking. No
superficial lymphadenopathy was palpable, and no abnormal breath
sounds were heard. The patient's Eastern Cooperative Oncology Group
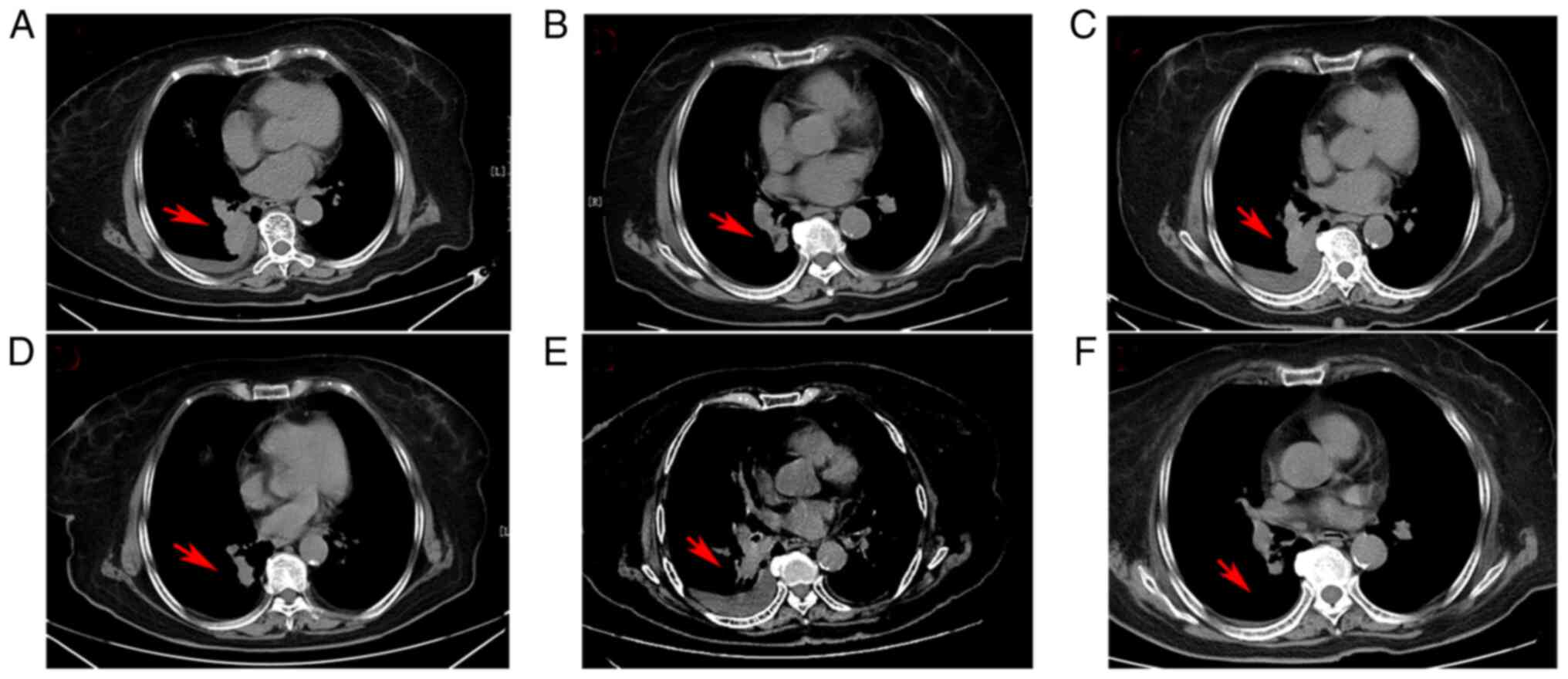
(ECOG) performance score was 0 points (10). Chest computed tomography (CT)
imaging upon admission reported a large mass (3.6×3.1 cm) occupying
the inferior lobe of the right lung and an accompanying small
pleural effusion (Fig. 1A).
Positron emission tomography/CT imaging showed no bone metastases
at the time of diagnosis, and no metastases were noted during a
brain magnetic resonance imaging (MRI) examination. The definite
pathological diagnosis was confirmed as invasive lung
adenocarcinoma via percutaneous lung puncture. High-throughput
sequencing revealed an EGFR exon 21 mutation (L858R). The
Tumor-Node-Metastasis (TNM) stage was IVB (cT4N0M1c) (American
Joint Committee on Cancer TNM eighth version) (11).
The CSCO NSCLC guidelines (2021 version) have
recommended both osimertinib and aumolertinib as first-line
treatments for patients with stage IV NSCLC harboring
EGFR–sensitive mutations (12).
However, aumolertinib is not included under Chinese basic medical
insurance, whereas osimertinib is. At 2 weeks post-admission, the
patient was started on osimertinib at the standard dose of 80 mg
once daily. After 2 months, the first efficacy evaluation revealed
that the lung lesion had decreased in size markedly, and the
pleural effusion had disappeared, indicating that partial remission
(PR) had been achieved (Fig. 1B).
Thereafter, efficacy was regularly assessed every 1–2 months. After
5 months of osimertinib treatment, however, a chest CT scan
revealed that the primary lung foci appeared significantly larger,
and the pleural effusion had increased in size again (Fig. 1C). The clinical efficacy result was
assessed as progressive disease (PD), suggesting the existence of
osimertinib resistance. Furthermore, EGFR gene mutant status was
examined in a peripheral blood sample, revealing an EGFR exon
20-T790M mutation. Due to the frailty and advanced age of the
patient, chemotherapy plus antiangiogenic therapy was refused.
After sufficient communication, the patient was
treated with oral aumolertinib (110 mg per day) beginning in July
2021. Unexpectedly, a substantial PR response was achieved again
after 2 months (Fig. 1D). However,
at this time, the patient experienced mild acute pancreatitis, and
aumolertinib therapy was paused. The patient recovered completely
from the acute pancreatitis within 2 weeks. In September 2021, the
patient complained of mild chest tightness and chest discomfort.
Chest CT imaging revealed that the primary lung foci and pleural
effusion had progressed again (Fig.
1E), and aumolertinib therapy was re-instated. The patient
achieved PR once again (Fig. 1F).
To date, the patient is continuing on aumolertinib treatment and
stable disease has been recorded. The first and second PR periods
for aumolertinib were 2.0 and 8 months, respectively. The total PFS
time (defined as the time from the initial treatment of
aumolertinib to disease progression) was 10 months (at the time of
writing this study).
Case 2
In May 2018, a 64-year-old female patient presented
to Jiujiang University Affiliated Hospital due to dizziness for 2
weeks. The patient complained of mild nausea and vomiting,
tiredness and headaches. No pulmonary symptoms were documented,
such as a cough, expectoration, dyspnea or chest pain. The patient
had a history of hypertension for >10 years and no history of
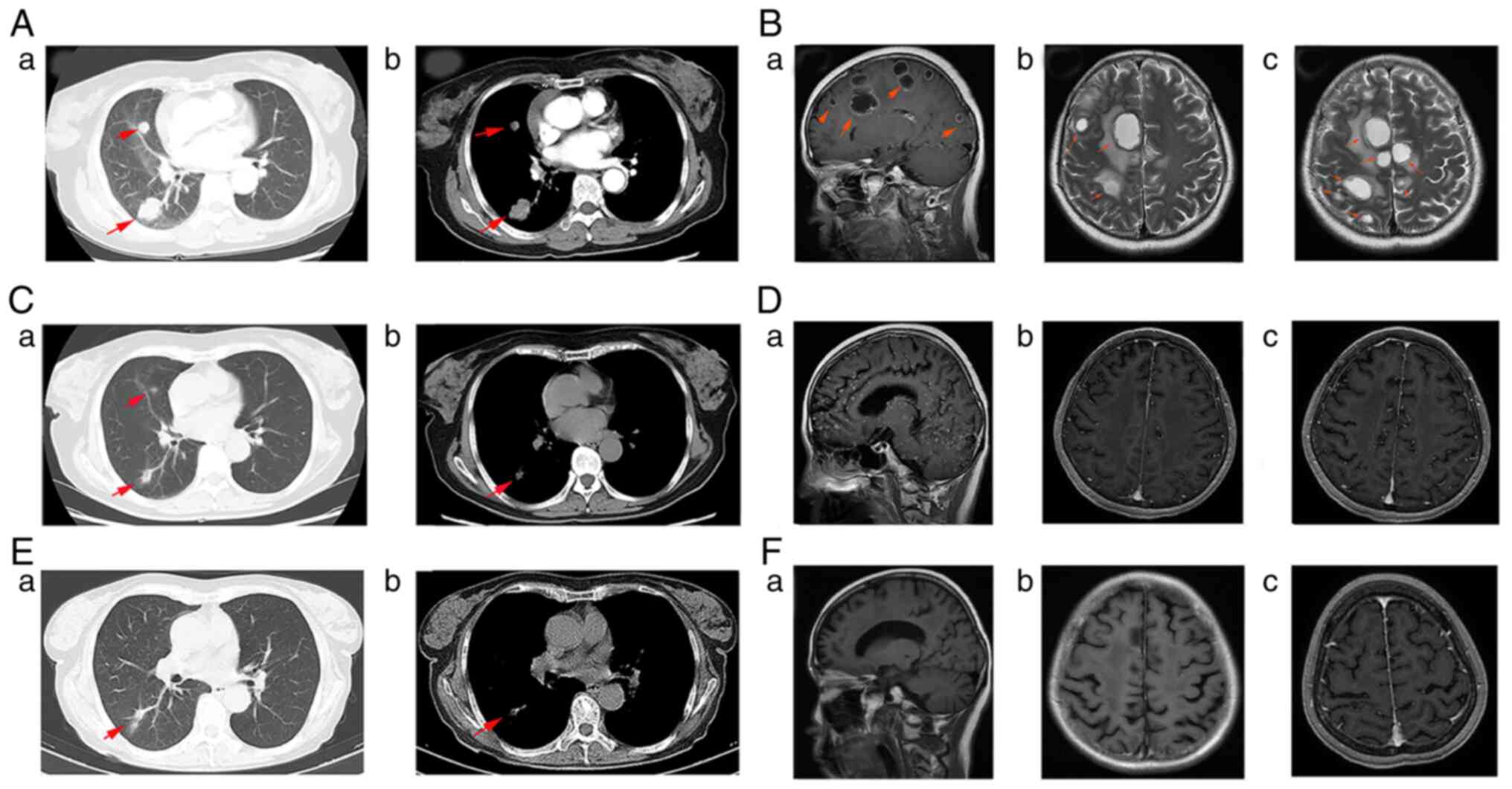
smoking. Chest CT showed two solid masses in the upper and lower
lobes of the right lung, with a maximum diameter of 3.5×2.2 cm
(Fig. 2Aa and b). Next, a plain
and enhanced MRI examination was performed, which revealed multiple
brain metastases in the bilateral cerebral hemisphere, right
cerebellar hemisphere, basal ganglia and thalamus (Fig. 2Ba-c). Color-ultrasound revealed
that the left supraclavicular lymph nodes were enlarged, with a
diameter of 7–9 mm. Spine and pelvic MRI found multiple osteolytic
bone lesions in the sternum, thoracic vertebrae, lumbar vertebrae
and ilium (data not shown). Bronchoscopy was performed, revealing a
small mass in the opening of the right intermediate bronchus. The
biopsy revealed poorly differentiated adenocarcinoma, and genetic
testing showed an EGFR 19 delete mutation. According to the TNM
staging system, the patient had stage IVB disease.
According to the NSCLC guideline of CSCO, the
patient received osimertinib monotherapy (80 mg) once daily from 2
weeks post-admission. Concurrently, anti-resorptive therapy using 4
mg zoledronic acid was administered every 4 weeks. After 2 weeks,
the patient reported that the symptoms of dizziness and
light-headedness were resolved. Subsequent multiple reviews showed
a great reduction in tumor volume of the primary pulmonary and
brain lesions (data not shown), indicating a PR response. However,
19 months later, the patient was admitted to the emergency room
with a numb face and aphasia. Reflexes were decreased in the lower
extremities. Chest CT did not confirm pulmonary lesion progression
(Fig. 2Ca and b), but brain MRI
depicted extensive small tumor infiltrations (Fig. 2Da-c). The EGFR gene testing of a
peripheral blood sample showed a T790M mutation. PD was indicated
and the patient refused the recommended chemotherapy.
After communicating fully with family members, the
patient received aumolertinib treatment (110 mg daily) from January
2020. After 1 month, language functions were considerably
recovered. A regular evaluation of efficacy was planned every 1–2
months. As the patient's compliance was not very good, the first
evaluation of efficacy was performed 5 months after beginning
aumolertinib therapy. Chest CT imaging revealed that the pulmonary
cancer lesion was stable (Fig. 2Ea and
b), while brain MRI showed that the extensive small
infiltrations of the brain had greatly diminished (Fig. 2Fa-c). From this point, efficacy was
assessed every 1–2 months; however, the patient succumbed to an
intracerebral hemorrhage in December 2020. At that time, brain CT
and MRI revealed that the intracerebral hemorrhage was in the basal
ganglia in the left hemisphere, but no intracranial space-occupying
lesions were clearly detected, with the exception of a few obscure
small infiltrations of metastases. The metastases remained in PR
status. Notably, the blood pressure before admission to the
hospital was 180/110 mmHg and the patient experienced agitation for
2 min before lapsing into a coma. These symptoms strongly indicated
that the intracerebral hemorrhage was not from metastasis but from
the poor control of hypertension. The PFS time following
aumolertinib was 11 months.
Case 3
A 75-year-old female patient was referred to
Jiujiang University Affiliated Hospital in January 2020. The
patient complained of a cough and expectoration with right-sided
chest pain for 3 weeks, with no history of smoking. A chest CT
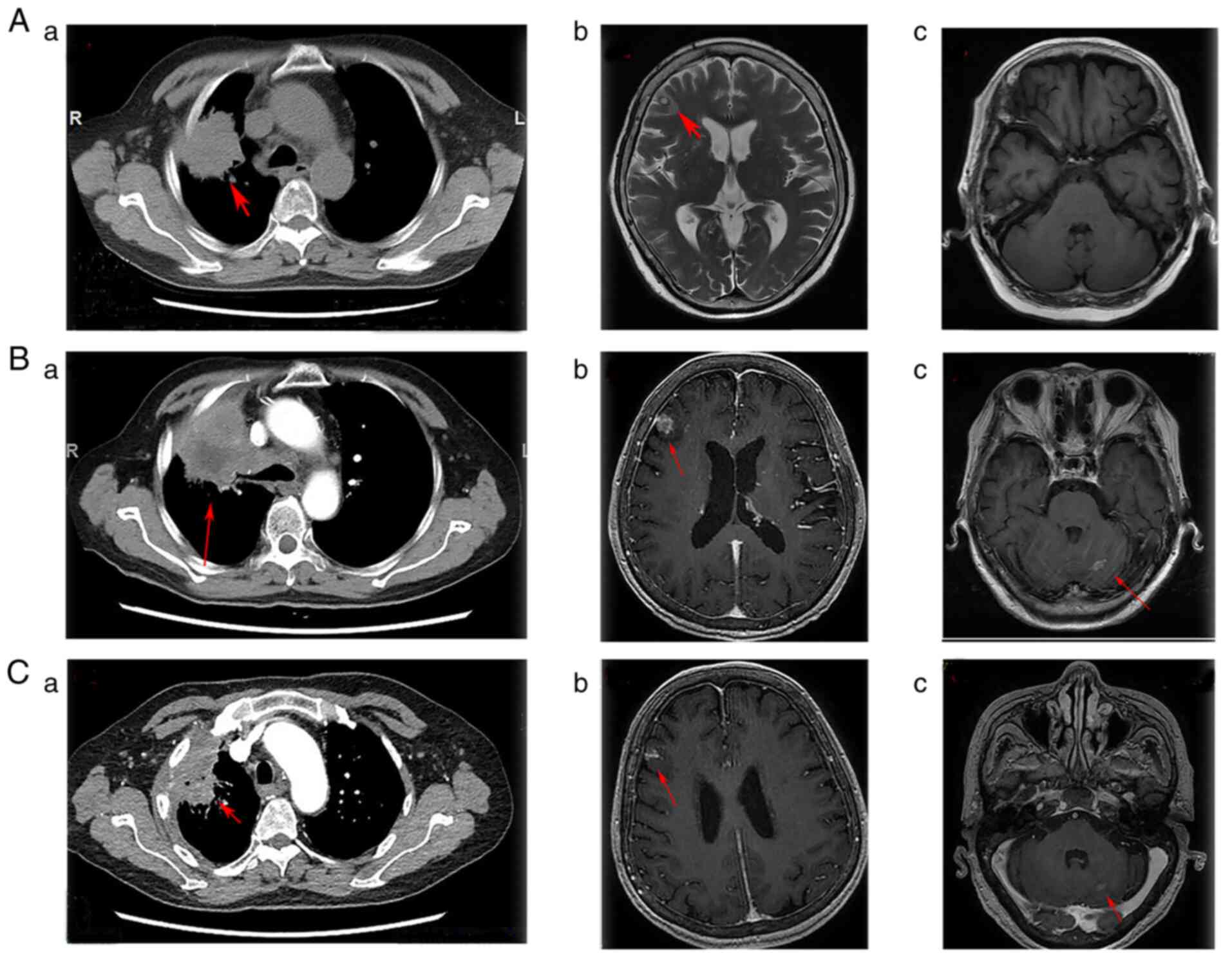
examination showed a large lesion in the right upper lung with a
diameter of 4.8×4.1 cm and lymphadenopathy in the mediastinum
(Fig. 3Aa). The brain MRI findings
demonstrated a solid lesion in the right frontal lobe (0.8×1.0 cm)
(Fig. 3Ab and c). A CT-guided
percutaneous fine-needle aspiration biopsy was performed, which
confirmed invasive adenocarcinoma with an EGFR 21 exon L858R
mutation based on next-generation sequencing (NGS). The clinical
TNM stage was IVB (cT4N3M1c), and the ECOG score was 1 point. The
patient began osimertinib treatment (80 mg daily) in January 2020
and, 1 month later, the clinical pulmonary symptoms were greatly
relieved. A regular review was performed every 3 months thereafter,
and the results of cranial MRI and thoracic/abdominal CT
examinations showed a PR response (data not shown).
However, in August 2021, the patient experienced
increased chest pain and a cough, with mild dizziness. Chest CT
imaging demonstrated that the right upper lung lesion had increased
to 6.7×5.3 cm (Fig. 3Ba). Brain
MRI confirmed that the right frontal lobe lesion had grown to
1.4×1.1 cm, and a novel lesion measuring 0.8×0.6 cm had appeared in
the left cerebellum (Fig. 3Bb and
c). The imaging examination indicated a response result of PD.
The patient underwent a second percutaneous fine-needle aspiration
biopsy, which again revealed invasive adenocarcinoma and an EGFR
exon 20 T790M mutation based on NGS. After communicating with their
family, the patient declined to accept chemotherapy. Subsequently,
the patient started to receive aumolertinib treatment (110 mg
daily) in August 2021. The scheduled evaluation of efficacy was
every 1–2 months, but the compliance of the patient was poor and
therefore the first efficacy assessment was only accepted 4 months
after starting aumolertinib treatment. A chest CT examination
showed that the pulmonary lesion had decreased to 4.6×2.5 cm
(Fig. 3Ca). Similarly,
cranio-cerebral MRI imaging revealed that the right frontal lobe
lesion had reduced significantly in size to 0.8×0.5 cm, and the
left cerebellum lesion to 0.4×0.3 cm (Fig. 3Cb). The clinical efficacy was
assessed as PR. Following this, the patient accepted the efficacy
evaluation regularly every 1–2 months. The patient has continued to
receive aumolertinib treatment to date. The PFS time following
aumolertinib was 9 months (at the time of writing this study).
Methods
H&E and immunohistochemistry staining
The needle lung biopsy was fixed for 6 h with 10%
neutral formalin at 35–37°C. Routine sampling, dehydration and
embedding were performed, followed by sectioning into 4-µm thick
samples, which were stained using hematoxylin and eosin at room
temperature for 1 h, and assessed under a light microscope.
Immunohistochemical staining was also performed.
Sequencing
The Tiangen paraffin-embedded tissue DNA extraction
kit (cat. #DP304-02; Tiangen Biotech Co., Ltd.) was used to extract
DNA from the sample to be sequenced. A Qseq1 Bioanalyzer was used
to verify the quality/integrity of the processed samples. The type
of sequencing was 150 bp for length and paired-end for direction.
The NextSeq 500/550 Mid Output v2 kit (300 cycles; cat.
#FC-404-2003; Berry Genomics, Co., Ltd.) was used in conjunction
with a sequencer to complete the high-throughput sequencing process
and obtain sample sequence information. The loading concentration
of the final library was 1.7 pM measured by Micro quantitative
detector. The sequencing data analysis process is as follows: i)
Data quality control: fastp, version 0.23.0 (https://github.com/OpenGene/fastp); ii) data
comparison: bwa, version 0.7.17 (http://bio-bwa.sourceforge.net); iii) variation
detection: GATK, version 3.8 (https://software.broadinstitute.org/gatk); iv)
variation annotation: SnpEff, version 5.0 (https://pcingola.github.io/SnpEff/); v) Report
generation: self-built software (http://www.yunkanghealth.com/technology).
Discussion
As the first third-line EGFR TKI, osimertinib has
been universally approved as the first-line treatment for patients
with advanced NSCLC carrying EGFR-sensitive mutations. The FLAURA
study showed that the PFS time following use of osimertinib as a
front-line therapy was 18.9 months (2). However, acquired drug resistance
occurs eventually and inevitably. Currently, only a minority of
patients with osimertinib-resistant NSCLC have the opportunity to
receive savolitinib treatment against MET 14 exon-skipping
mutations (13).
Platinum-containing chemotherapy remains the most common treatment
for most osimertinib-resistant patients (14). Recently, a retrospective study
compared osimertinib plus bevacizumab vs. chemotherapy plus
bevacizumab in patients with EGFR-mutant NSCLC after the failure of
osimertinib, and concluded the superiority of osimertinib plus
bevacizumab over chemotherapy plus bevacizumab (7.0 vs. 4.9 months
mPFS time) (15). In addition, in
certain case reports, erlotinib together with bevacizumab has been
reported to overcome osimertinib resistance (16). The present study reported the
switch to aumolertinib treatment in patients with advanced NSCLC
harboring EGFR-sensitive mutations who were resistant to
osimertinib.
Aumolertinib is the second third-generation EGFR TKI
and breaks the monopoly situation on the use of osimertinib
worldwide. The APOLLO study enrolled 244 patients with EGFR
T790M-positive NSCLC who received aumolertinib treatment. The
overall response rate (ORR) and disease control rate (DCR) were
68.9 and 93.4%, respectively, and the mPFS time was 12.4 months.
For the 23 patients with assessable central nervous system (CNS)
metastases, the CNS-ORR and CNS–DCR were 60.9 and 91.3%,
respectively. These results indicate that aumolertinib is an
effective third-generation EGFR TKI for patients with EGFR
T790M-positive advanced NSCLC after disease progression following
first- and second-generation EGFR TKI therapy (17). In the mouse model of NSCLC brain
and spinal cord metastases, aumolertinib easily penetrates the
blood-brain barrier and inhibits brain and spinal cord metastases
(18). In the AENEAS study,
aumolertinib, as a first-line treatment for locally advanced or
metastatic EGFR-mutated NSCLC, achieved a PFS time of 19.3 months,
which is marginally higher than the time of 18.9 months achieved
with osimertinib in the FLAURA study (4). A recent network meta-analysis showed
that in terms of brain metastases, third-generation EGFR-TKIs
showed obvious superiority, with aumolertinib and osimertinib both
optimally prolonging PFS time in patients with brain metastases
(19). These studies offer strong
support for the potent anticancer activity of aumolertinib against
EGFR-mutant NSCLC cells, in particular for brain metastases.
In a case report by Shen et al (20), almonertinib overcame osimertinib
resistance associated with the L718Q mutation in a patient with
metastatic NSCLC. Wu et al (21) and Zhang et al (22) reported successful treatment with
aumolertinib after osimertinib-induced interstitial lung disease
and cardiotoxicity. These results suggested a potential agent for
reversing drug osimertinib-resistance and indicated a better safety
profile for aumolertinib compared with that for osimertinib. In the
present case reports, the three patients developed osimertinib
resistance with T790M mutation and without other additional genetic
mutations, which indicated no available targeted therapy.
Therefore, aumolertinib was employed to treat the patients with
osimertinib-resistant advanced NSCLC. Encouragingly, the 3 patients
achieved PFS times of 10+, 11 and 9+ months.
Although the patient in case 2 succumbed to hypertension-induced
intracerebral hemorrhage, aumolertinib remained effective in the
three cases, with a mPFS time of >9 months. Notably, the patient
in case 1 had an episode of acute pancreatitis, from which they
quickly recovered and which did not occur again during the period
following aumolertinib treatment, indicating the lack of connection
of aumolertinib with the onset of acute pancreatitis.
The possible mechanisms of a successful challenge
with aumolertinib in osimertinib-resistant NSCLC may, in part, be
ascribed to the following factors. One is that aumolertinib carries
lower half maximal inhibitory values for T790M and L858R mutations,
and T790M and Del 19 mutations, respectively, than osimertinib
(i.e., 0.29 vs. 0.46 nmol/l, and 0.21 vs. 0.29 nmol/l) (23). The other is that aumolertinib may
partly overcome the drug resistance of osimertinib in advanced
NSCLC (21). Finally, the mean
plasma concentration of aumolertinib (110 mg/day) is slightly
higher than that of osimertinib (80 mg/day) (155.5 vs. 138.98
ng/ml) (24). Additionally, for
patients with advanced NSCLC and EGFR exon 20 insertion mutations,
aumolertinib has been reported to achieve the PFS time of 10.0
months (25), while osimertinib
leads to a mere mPFS time of 2.3 months (26). Therefore, to some extent,
aumolertinib and osimertinib belong to completely different EGFR
TKI categories despite being parts of the same third-generation
EGFR TKI group.
In conclusion, in the present report, aumolertinib
challenge is described as an optional treatment after osimertinib
failure for patients with EGFR-sensitive mutations. The limitation
of the present study is its small sample size, and the conclusions
of this retrospective study need to be further explored and
validated.
Acknowledgements
The authors would like to thank Professor Wang
Liangliang (Jiujiang University Affiliated Hospital, Jiujiang,
China) for performing the pathological detection and molecular
examination.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD designed and revised the manuscript. XD drafted
the manuscript and analyzed the patient data. ZL and YS acquired
the raw data, obtained the medical images, advised on patient
treatment and analyzed patient data as the patients' medical intern
and primary medical oncologist, respectively. XD, JD, ZL and YS
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was granted by the Medical Ethics
Committee of Jiujiang University Affiliated Hospital (Jiujiang,
China; approval no. jjuhmer-a-2020-01).
Patient consent for publication
Patients from cases 1 and 3 gave written consent for
the publication of the current study. The son of the patient from
case 2 gave written consent for publication of the current
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in Untreated EGFR-Mutated
Advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmid S, Li JJN and Leighl NB: Mechanisms
of osimertinib resistance and emerging treatment options. Lung
Cancer. 147:123–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastaticnon-small-cell lung cancer with EGFR
Exon 19 Deletion or L858R Mutations. J Clin Oncol. Sep
20–2022.(Epub ahead of print).
|
|
5
|
Deeks ED: Furmonertinib: First Approval.
Drugs. 81:1775–1780. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jian H, Wang K, Cheng Y, Ding L, Wang Y,
Shi Z, Zhang L, Wang Y and Lu S: Phase I trial of a third
generation EGFR mutant-selective inhibitor (D-0316) in patients
with advanced non-small cell lung cancer. Oncologist. 27:163–e213.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv W, Cheng H, Shao D, Wei Y, Zhu W, Wu K,
Jiang W, Hu L, Sha Z, Zhong B and Pei X: Treatment patterns and
survival of patients with advanced non-small cell lung cancer
guided by comprehensive genomic profiling: Real-World
single-institute study in China. Front Oncol. 11:6307172021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang Y, Liu S, Jiang Y, Hua L and Wen L:
Effective treatment of pulmonary adenocarcinoma harboring triple
EGFR mutations of L858R, T790M, cis-G796s/cis-C797s by osimertinib,
brigatinib, and bevacizumab combination therapy: A case report.
Respir Med Case Rep. 36:1015822022.PubMed/NCBI
|
|
9
|
Wang Y, Han R, Zhu M, He T and He Y: Case
report: Durable response to the combination of brigatinib and
cetuximab plus icotinib in a NSCLC patient harboring EGFR
L858R-T790M-cis-G796S and L718Q resistance mutations following
progression with osimertinib. Front Oncol. 12:8753132022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Markovic SN, Molina JR,
Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, Li R, Wei J, Wang
L, Liu B, et al: Association of sex, age, and eastern cooperative
oncology group performance status with survival benefit of cancer
immunotherapy in randomized clinical trials: A systematic review
and meta-analysis. JAMA Netw Open. 3:e20125342020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallieres E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang C, Liu T, Liang W, Feng S, Su Z, Tang
H, Huang H and Chen Z: Clinical pharmacist participation in
selecting and dosing targeted drugs for a patient with ALK-positive
non-small cell lung cancer: A case report. Ann Transl Med.
9:14882021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma P, Huang R, Gu Y, Fang Y, Wu X, Chen
DS, Zhang HW, Gao W and Shu Y: Savolitinib monotherapy exerted
significant benefit in a non-small cell lung cancer patient with
osimertinib resistance harboring primary EGFR L858R mutation and
MET amplification: A case report. Anticancer Drugs. Aug
10–2022.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Lim JU: Overcoming osimertinib resistance
in advanced non-small cell lung cancer. Clin Oncol (R Coll Radiol).
33:619–626. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui Q, Hu Y, Cui Q, Wu D, Mao Y, Ma D and
Liu H: Osimertinib rechallenge with bevacizumab vs. chemotherapy
plus bevacizumab in EGFR-Mutant NSCLC patients with osimertinib
resistance. Front Pharmacol. 12:7467072021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satoh H and Kagohashi K: Response to
erlotinib and bevacizumab combination therapy after acquired
resistance to osimertinib in patients with non-small cell lung
cancer. Anticancer Drugs. 33:320–322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu S, Wang Q, Zhang G, Dong X, Yang CT,
Song Y, Chang GC, Lu Y, Pan H, Chiu CH, et al: Efficacy of
Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+
NSCLC: Updated post-national medical products administration
approval results from the APOLLO registrational trial. J Thorac
Oncol. 17:411–422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhang Y, Niu W, Ge X, Huang F,
Pang J, Li X, Wang Y, Gao W, Fan F, et al: Experimental study of
almonertinib crossing the blood-brain barrier in EGFR-Mutant NSCLC
brain metastasis and spinal cord metastasis models. Front
Pharmacol. 12:7500312021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang F, Zhang W, Shang X, Liu N, Ma X, Qin
J, Zhang Y, Liu Y and Wang X: Comparison of the efficacy and safety
of first-line treatments based on clinicopathological
characteristics for patients with advanced epidermal growth factor
receptor mutated non-small-cell lung cancer: A systematic review
and network meta-analysis. Crit Rev Oncol Hematol. 177:1037602022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen G, Shi L, Tian X, Huang D, Chen H,
Gao C, Shen X and Zhang H: Case report: Response to almonertinib in
a patient with metastatic NSCLC resistant to osimertinib due to
acquired EGFR L718Q Mutation. Front Pharmacol. 12:7318952021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Zhong W, Li A, Qiu Z, Xie R, Shi H
and Lu S: Successful treatment of EGFR T790M-mutant non-small cell
lung cancer with almonertinib after osimertinib-induced
interstitial lung disease: A case report and literature review. Ann
Transl Med. 9:9502021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Liu H and Yang J: Aumolertinib
effectively reduces clinical symptoms of an EGFR L858R-Mutant
non-small cell lung cancer case coupled with osimertinib-induced
carditoxicity: Case report and review. Front Endocrinol.
13:8339292022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JC, Camidge DR, Yang CT, Zhou J, Guo
R, Chiu CH, Chang GC, Shiah HS, Chen Y, Wang CC, et al: Safety,
efficacy, and pharmacokinetics of almonertinib (HS-10296) in
pretreated patients With EGFR-Mutated advanced NSCLC: A
multicenter, open-label, phase 1 trial. J Thorac Oncol.
15:1907–1918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Meng L, Ma Y, Li Y, Xing X, Guo C
and Dong Z: Determination of osimertinib, aumolertinib, and
furmonertinib in human plasma for therapeutic drug monitoring by
UPLC-MS/MS. Molecules. 27:44742022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng Y, Yang C, Liu W, Cai S and Guo X:
Aumolertinib-based comprehensive treatment for an uncommon site of
EGFR exon 20 insertion mutations with multiple metastases non-small
cell lung cancer: A case report. Anticancer Drugs. 33:406–412.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang GJ, Li J, Xu HY, Sun Y, Liu L, Li HS,
Yang L, Zhang Y, Li GH and Wang Y: Osimertinib for Chinese advanced
non-small cell lung cancer patients harboring diverse EGFR exon 20
insertion mutations. Lung Cancer. 152:39–48. 2021. View Article : Google Scholar : PubMed/NCBI
|