Introduction
Malignant pleural mesothelioma (MPM) is a malignant
disease that is primarily caused by asbestos exposure and has a
poor prognosis (1). MPM is
pathologically classified into three types: epithelial, sarcomatoid
(desmoplastic as a subtype), and biphasic (2). The sarcomatoid and biphasic types
together are called the non-epithelial type and are rarer than the
epithelial type. The prognoses for non-epithelial MPM are worse
than that for the epithelial type because they respond poorly to
existing cytotoxic chemotherapies, more effective treatments have
been long awaited. Immune checkpoint therapy (ICT) with nivolumab
is reported to be effective for the treatment of epithelial MPM
[objective response rate (ORR), 29.4%; 2-year overall survival
rate, 35.3%] and is expected to be a new treatment option for MPM
(3). However, evidence for the
treatment of non-epithelial MPM is scarce, and the combined results
of the two existing clinical trials contained only 18 patients with
non-epithelial MPM who received nivolumab (3,4).
There are even fewer reports on the course of treatment in clinical
practice; to the best of our knowledge, only a few cases have been
reported (5,6). The collation of reliable evidence
regarding ICT for non-epithelial MPMs is urgent. Here, we report
three clinical cases of patients with sarcomatoid MPM (sMPM)
treated with nivolumab at Kyoto University Hospital, Japan.
Case report
Case 1
A 73-year-old man was admitted to another hospital
with left irregular pleural thickening (PT) and pleural effusion
(PE). A surgical pleural biopsy was performed in that hospital.
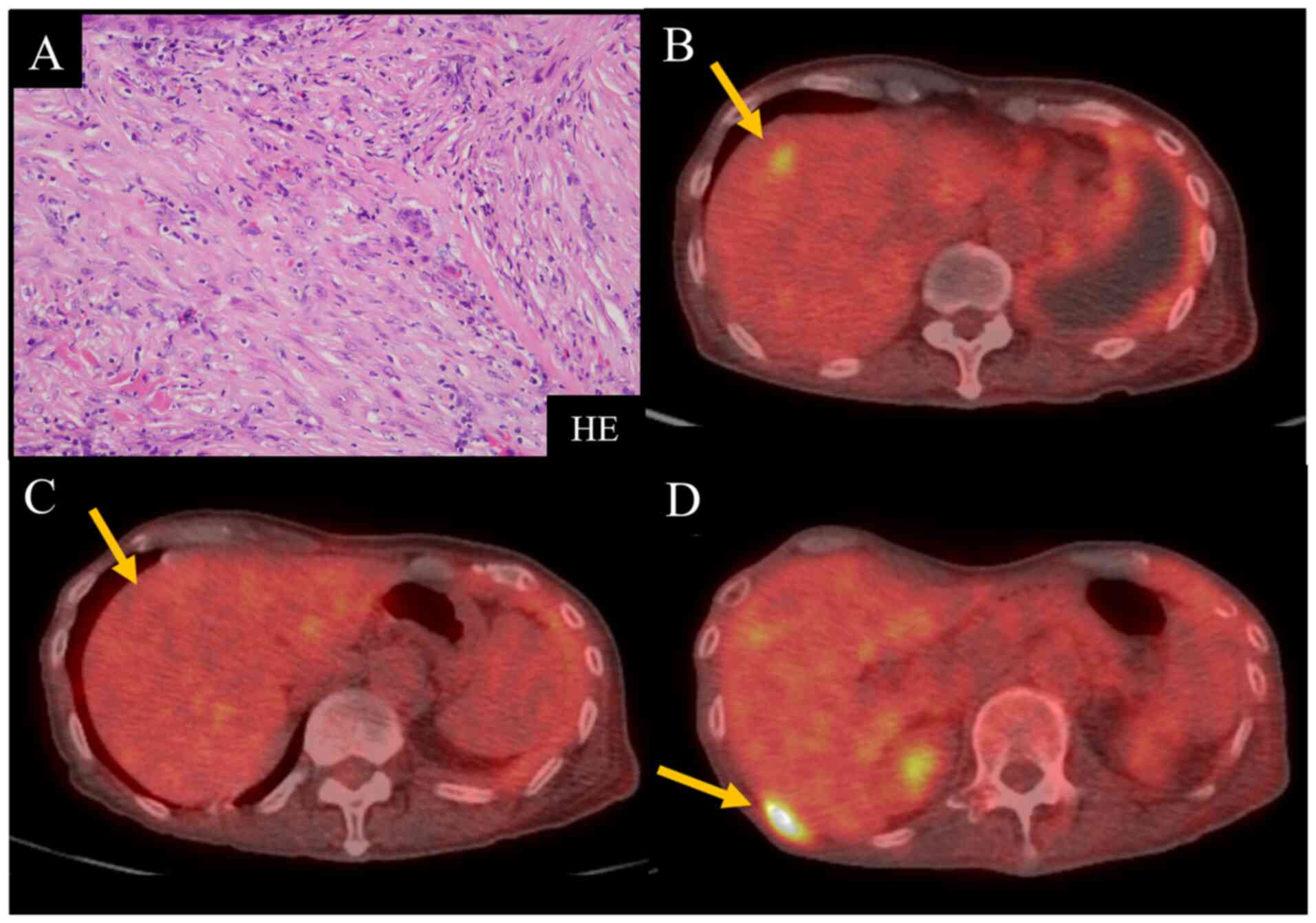
Histopathological findings showed atypical spindle cells
distributed with inflammatory cells in a fibrous organization that
was rich in collagen fibers, which invaded the striated muscle and
adipose tissue (Fig. 1A). The
patient was diagnosed with desmoplastic MPM. The patient was
referred to Kyoto University Hospital for consideration of the
multidisciplinary treatment since surgical treatment could not be
performed at the referring hospital. However, the referring
hospital is undisclosed in this report because this hospital is not
affiliated with any of this study authors. After reviewing the
case, we decided to treat the patient with systemic drug therapy.
One cycle of systemic chemotherapy with carboplatin [area under the
concentration-time curve (AUC)=5] and pemetrexed (400
mg/m2) (Carbo/Pem) was administered; however, the PT did
not improve, and liver metastasis was confirmed. We deemed the
Carbo/Pem treatment ineffective and initiated nivolumab as a
second-line treatment. After five cycles of nivolumab, positron
emission tomography (PET)/computed tomography (CT) using
18F-fluorodeoxyglucose (FDG) showed a reduction in liver
metastases and a decrease in FDG uptake in the same area (Fig. 1B and C). Nivolumab treatment was
judged to have achieved a partial response (PR) and was continued;
however, new bone metastases appeared, and liver metastases
reappeared after 12 cycles (Fig.
1D). Consequently, nivolumab treatment was judged to have
resulted in progressive disease (PD) and discontinued. The patient
was then treated with chemotherapy, but the disease worsened, and
he died 4 months after completing nivolumab treatment. During the
course of treatment, there were no side effects that could be
attributed to nivolumab.
Case 2
A 66-year-old man was referred to our hospital with
a large left-sided PE. PET/CT showed an irregular mass extending to
the left pleura with increased FDG uptake. The patient was
diagnosed with sarcomatoid or desmoplastic MPM by ultrasound-guided
pleural biopsy. As the first-line treatment, Carbo (AUC=5)/Pem (400
mg/m2) was administered; however, after one cycle, the
left PT worsened. Carbo/Pem was determined to be ineffective, and
nivolumab was administered as the second-line treatment. After
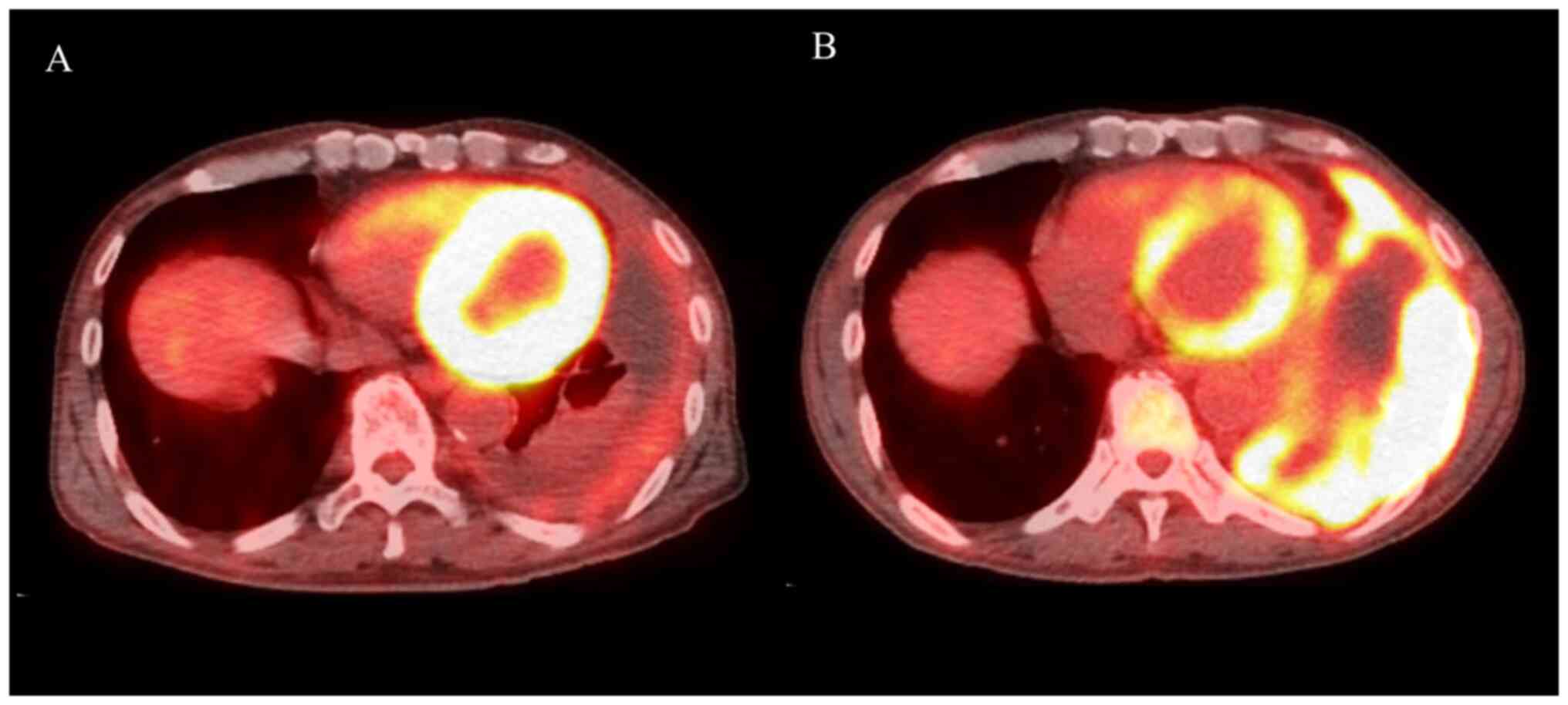
three cycles of nivolumab, PET-CT revealed a decrease in the left
PT and PE, with a decrease in FDG uptake (Fig. 2A). In this case, the patient
developed complicated eosinophilia and eosinophilic PE, which
improved over time after nivolumab treatment. Further details on
the progress of this case were previously reported (5). Then, we determined that nivolumab
produced a good PR and continued the treatment; however, after nine
cycles, the left pleural lesion thickening re-occurred with
increased FDG uptake (Fig. 2B). We
considered that the nivolumab treatment had resulted in PD and
therefore discontinued it. Two months after treatment completion,
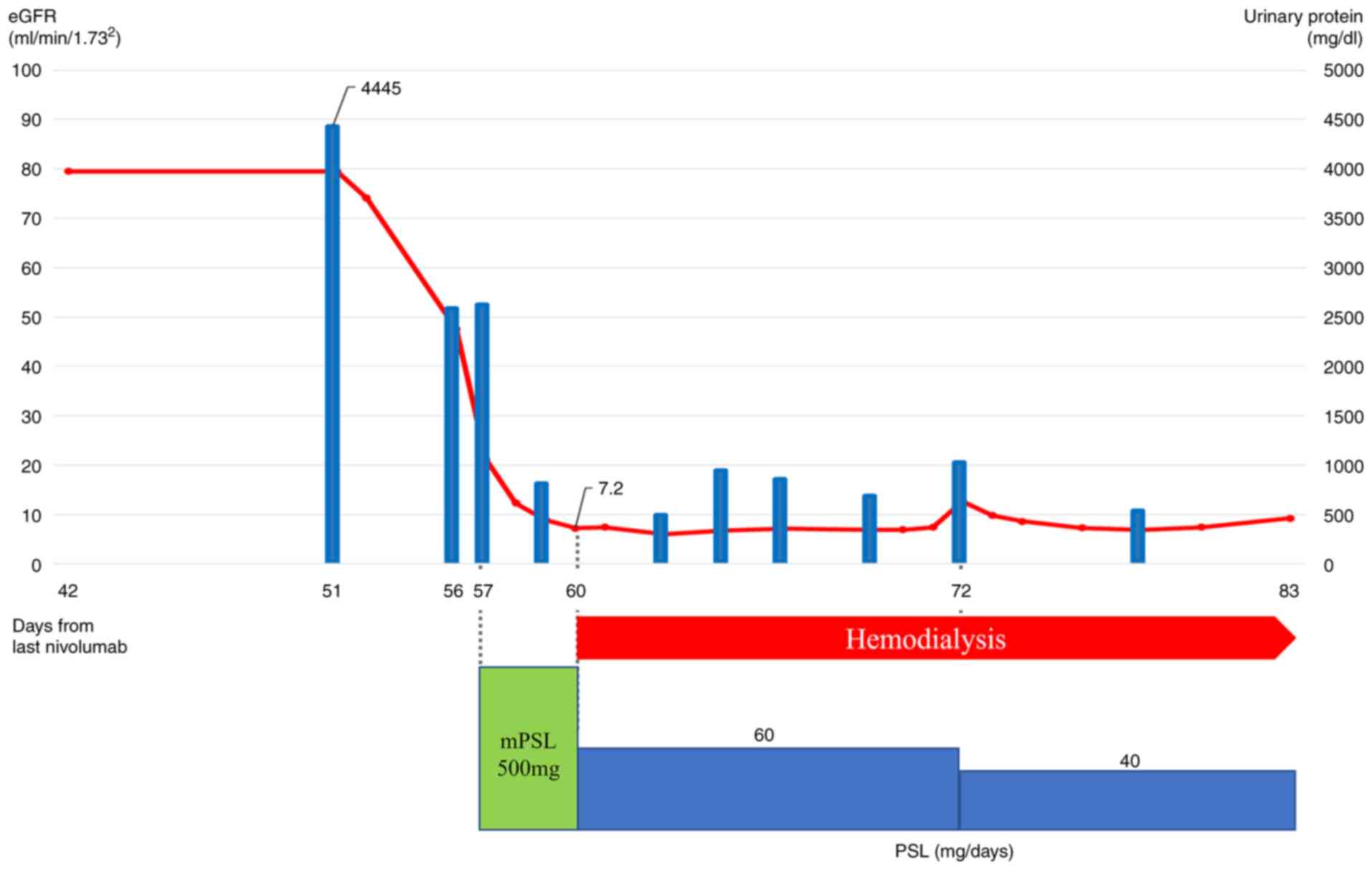
the patient developed acute kidney injury and nephrotic syndrome
due to immune-related adverse events (Fig. 3, baseline data as Table SI). Steroid therapy was started
with dialysis, and although the patient showed signs of recovery,
he later developed gastrointestinal bleeding and died. The autopsy
revealed that the bleeding was due to metastasis to the small
intestine. Here, we have reported Case 2 again to additionally
describe the occurrence of side effects after the previous
publication (5) and to make
conclusions on the treatment choice as part of a case series that
included the other two cases.
Case 3
An 82-year-old man was admitted to our hospital with
right PT and PE. Surgical pleural biopsy was performed.
Histopathological findings showed atypical cells with large oval or
spindle nuclei multiplying into a seat form with necrosis. These
tumor cells were immunoreactive to calretinin but not to TTF-1 or
p40 (Fig. 4). Based on these
findings, the patient was diagnosed with sMPM. He was also
diagnosed with neoplastic fever before biopsy. The patient was
started on Carbo (AUC=4.5)/Pem (375 mg/m2), but the
fever persisted along with high levels of C-reactive protein, and
his performance status declined. After one cycle, no improvement
was observed on the chest radiograph. Carbo/Pem was determined to
be ineffective, and nivolumab was administered as the second-line
treatment. After two cycles of nivolumab, the fever resolved and
PET/CT showed a decrease in the right PT and PE, as well as a
decrease in FDG uptake (Fig. 5A and
B). However, before the start of the third cycle, the patient
developed liver dysfunction due to immune-related adverse events
(Fig. 6, baseline data as Table SII). Although the liver damage
improved with steroid treatment, nivolumab treatment was
discontinued because of the adverse event. Thereafter, the patient
was placed on a treatment-free follow-up, and the tumor remained
stable for 6 months. The patient died of aspiration pneumonia 1
month after the tumor began to re-grow (Fig. 5C).
The clinical characteristics of the three patients
are summarized in Table I.
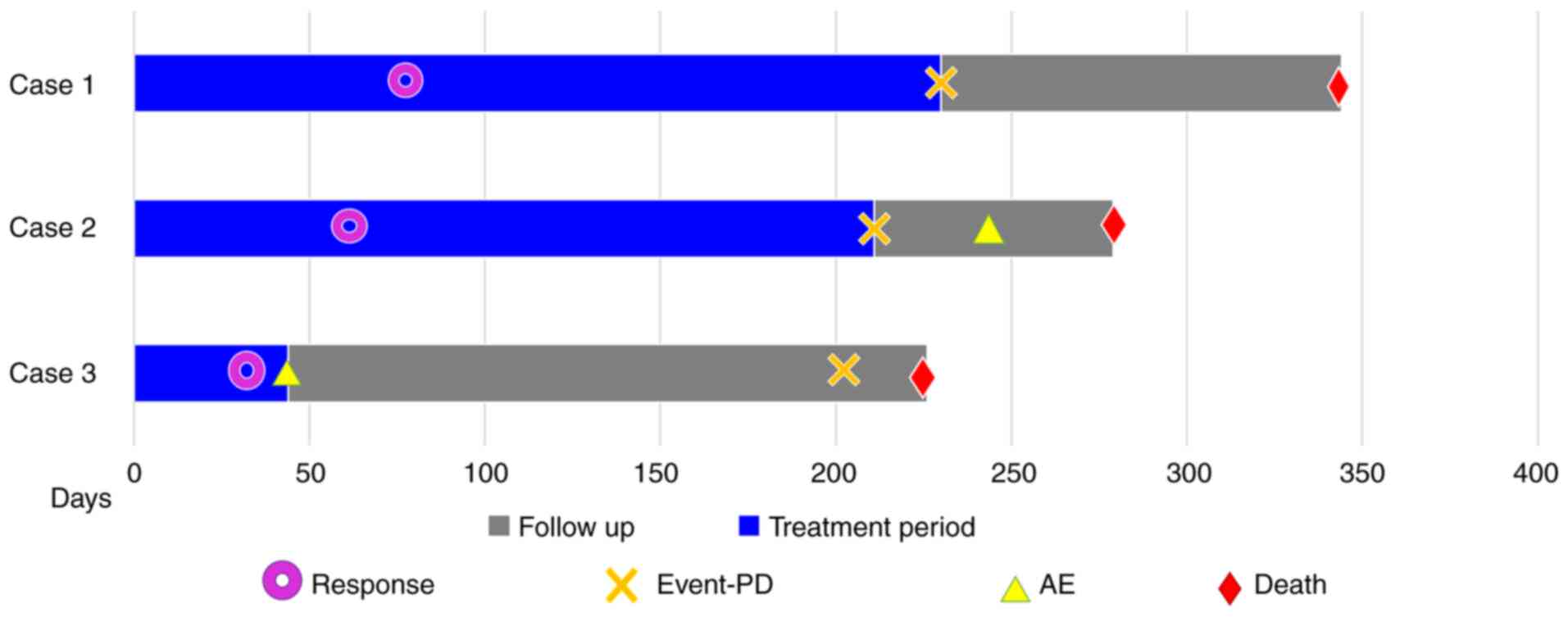
Fig. 7 shows the progress of the
patients after starting nivolumab therapy. The three patients had
varied histories of smoking, and two of them had a history of known
asbestos exposure. Notably, the time to disease progression for the
three cases following nivolumab treatment was 223, 211, and 202
days, respectively, which is similar to the median progression-free
survival reported in the two previous trials (3,4),
despite the difference in the number of cycles of nivolumab
administration among the three patients, with 12, 9, and 2 cycles,
respectively. We tested the expression of programmed death ligand 1
(PD-L1) in tumor cells, using DAKO 22C3 tumor proportion scoring
method (Fig. 8). The results were
positive in all the three cases, with Case 3 having more than 50%
PD-L1 positivity (Cases 1 and 2 were 1–24% positive).
 | Table I.Summary of the clinical
characteristics of the 3 cases. |
Table I.
Summary of the clinical
characteristics of the 3 cases.
| Variable | Case 1 | Case 2 | Case 3 |
|---|
| Age,
yearsa | 73 | 66 | 82 |
| Sex | Male | Male | Male |
| Smoking, p-y | 4 | 58 | None |
| Asbestos | Yes | Uncertain | Yes |
| Histopathological
diagnosis | Desmoplastic |
Sarcomatoid/desmoplastic | Sarcomatoid |
| Stage (UICC ver.
8) | cT3N0M0 stage1B | cT3N0M0 stage1B | cT4N0M0 stage3B |
| Pre-treatment | Carbo/Pem | Carbo/Pem | Carbo/Pem |
| PD-L1 TPS (22C3),
% | 1-24 | 1-24 | >50 |
| Effect | PR | PR | PR |
| Time to response,
days | 83 | 57 | 34 |
| Nivolumab cycles,
n | 12 | 9 | 2 |
| Time to progression,
days | 223 | 211 | 202 |
| Overall survival,
days | 344 | 279 | 226 |
Discussion
Nivolumab showed some efficacy against sMPM in the
three cases treated at our hospital. In a previous study on the use
of nivolumab to treat MPM (the MERIT study), three cases of sMPM
were included. Nivolumab was reported to be effective in two of
these cases, suggesting that the treatment may be more effective in
sarcomatoid than in epithelial MPM (3). Historically, sMPM has been less
likely than epithelial MPM to respond to cytotoxic chemotherapy
(7). Thus, immunotherapy is
expected to become an increasingly important treatment,
particularly for sMPM.
In this study, we report three cases of sMPM in
which the tumor cells tested positive for PD-L1 expression. In
non-small cell lung cancer (NSCLC) tumors, PD-L1 expression is
known to be a biomarker of the therapeutic efficacy of
anti-programmed cell death protein 1 (PD-1) inhibitors (8). In MPM, high expression levels of
PD-L1 have been associated with non-epithelial histology and poor
prognosis (9–11). However, these results were reported
before the introduction of ICT. Long-term follow-up data from the
MERIT study showed that PD-L1-positive tumors tended to have a
higher ORR to nivolumab in patients with MPM (12). In the same report, progression-free
survival and overall survival tended to be better for patients with
non-epithelial tumors compared to those with epithelial tumors.
Currently, PD-L1 expression is not recognized as a biomarker for
predicting the efficacy of PD-1 inhibitors in MPM. However, based
on this evidence, sMPMs may be more likely to have higher PD-L1
expression levels than epithelial MPMs and may benefit from
ICT.
Recently, the combination of nivolumab and
ipilimumab (Nivo/Ipi), an anti-cytotoxic T-lymphocyte-associated
protein 4 inhibitor, became available for the treatment of various
carcinomas. In NSCLC, the effectiveness of Nivo/Ipi has been shown
to be almost equal, regardless of PD-L1 expression (13). However, there are concerns
regarding the toxicity of Nivo/Ipi. Although manageable, the
toxicity profile was less favorable than that of nivolumab
monotherapy. For NSCLC with high PD-L1 expression, there is no
consensus on the benefit of Nivo/Ipi over anti-PD-1 monotherapy.
Therefore, anti-PD-1 monotherapy will continue to be a viable, less
toxic, and generally effective option for NSCLC with high
expression levels of PD-L1. Nivo/Ipi has also been reported to be
effective against MPM, for which it has shown greater efficacy than
systemic chemotherapy, including platinum-based agents, as a
first-line treatment (14). This
evidence indicates that the use of ICT in the treatment of MPM is
expected to become increasingly important, and Nivo/Ipi will play a
leading role. However, the side effect concerns are similar to
those for NSCLC. There is a lack of evidence on which ICT treatment
strategy should be used in sMPM, as is expected to respond to PD-1
inhibitors alone. We believe that this report is significant
because it contributes to the body of knowledge on nivolumab
treatment for sMPM, on which few reports exist. Yet, this study
included only three cases from a single institution, which
potentially limits the validity of our findings. For a more
reliable report, it is necessary to gather similar cases from
multiple centers and study them in more detail.
In conclusion, we described three cases of nivolumab
treatment for sMPM, which has rarely been reported before. PD-1
monotherapy may be more effective in treating sMPM than it is in
treating epithelial MPM, and nivolumab treatment is a promising
treatment option.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KHa and HO conceived and designed this case report.
KHa collected the clinical data. AY performed the histological
examination and PD-L1 test. KHa and HO drafted the initial
manuscript of the report. KHa, HO, AY, HirosY, TO, KHo, MY, HA, TF,
HironY, YS and TH performed analysis and interpretation of data.
KHa and HO confirmed the authenticity of all the raw data. All
authors contributed to manuscript revision and have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent for the participation in
the study was obtained from the patients.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the concentration-time
curve
|
|
Carbo/Pem
|
carboplatin and pemetrexed
|
|
FDG
|
18F-fluorodeoxyglucose
|
|
ICT
|
immune checkpoint therapy
|
|
MPM
|
malignant pleural mesothelioma
|
|
Nivo/Ipi
|
nivolumab and ipilimumab
|
|
NSCLC
|
non-small cell lung cancer
|
|
ORR
|
objective response rate
|
|
PD
|
progressive disease
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
programmed death ligand 1
|
|
PE
|
pleural effusion
|
|
PET
|
positron emission tomography
|
|
PR
|
partial response
|
|
PT
|
pleural thickening
|
|
sMPM
|
sarcomatoid MPM
|
|
TTF-1
|
thyroid transcription factor-1
|
References
|
1
|
Schumann SO, Kocher G and Minervini F:
Epidemiology, diagnosis and treatment of the malignant pleural
mesothelioma, a narrative review of literature. J Thorac Dis.
13:2510–2523. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galateau-Salle F, Churg A, Roggli V and
Travis WD; world health organization committee for tumors of the
Pleura, : The 2015 world health Organization classification of
tumors of the pleura: Advances since the 2004 classification. J
Thorac Oncol. 11:142–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okada M, Kijima T, Aoe K, Kato T, Fujimoto
N, Nakagawa K, Takeda Y, Hida T, Kanai K, Imamura F, et al:
Clinical efficacy and safety of nivolumab: Results of a
multicenter, open-label, single-arm, Japanese phase II study in
Malignant Pleural mesothelioma (MERIT). Clin Cancer Res.
25:5485–5492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scherpereel A, Mazieres J, Greillier L,
Lantuejoul S, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette
C, Locatelli-Sanchez M, et al: Nivolumab or nivolumab plus
ipilimumab in patients with relapsed malignant pleural mesothelioma
(IFCT-1501 MAPS2): A multicentre, open-label, randomised,
non-comparative, phase 2 trial. Lancet Oncol. 20:239–253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamazoe M, Ozasa H and Kim YH:
Effectiveness of nivolumab on sarcomatoid malignant pleural
mesothelioma with eosinophilia and eosinophilic pleural effusion. J
Thorac Oncol. 14:e251–e253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsubouchi K, Inoue S, Ibusuki R, Iwasaki T
and Harada T: Remarkable response to nivolumab in sarcomatoid
malignant pleural mesothelioma with high PD-L1. Respirol Case Rep.
8:e005362020. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mansfield AS, Symanowski JT and Peikert T:
Systematic review of response rates of sarcomatoid malignant
pleural mesotheliomas in clinical trials. Lung Cancer. 86:133–136.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen BH, Montgomery R, Fadia M, Wang J
and Ali S: PD-L1 expression associated with worse survival outcome
in malignant pleural mesothelioma. Asia Pac J Clin Oncol. 14:69–73.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brosseau S, Danel C, Scherpereel A,
Mazières J, Lantuejoul S, Margery J, Greillier L, Audigier-Valette
C, Gounant V, Antoine M, et al: Shorter survival in malignant
pleural mesothelioma patients with high PD-L1 expression associated
with sarcomatoid or biphasic histology subtype: A series of 214
cases from the bio-MAPS cohort. Clin Lung Cancer. 20:e564–e575.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang F and Gong W: Prognostic and
clinicopathological utility of programmed death-ligand 1 in
malignant pleural mesothelioma: A meta-analysis. Int
Immunopharmacol. 83:1064812020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujimoto N, Okada M, Kijima T, Aoe K, Kato
T, Nakagawa K, Takeda Y, Hida T, Kanai K, Hirano J and Ohe Y:
Clinical efficacy and safety of nivolumab in Japanese patients with
malignant pleural mesothelioma: 3-year results of the MERIT study.
JTO Clin Res Rep. 2:1001352021.PubMed/NCBI
|
|
13
|
Hellmann MD, Paz-Ares L, Caro RB, Zurawski
B, Kim SW, Costa EC, Park K, Alexandru A, Lupinacci L, de la Mora
Jimenez E, et al: Nivolumab plus ipilimumab in advanced
non-small-cell lung cancer. N Engl J Med. 381:2020–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|