Introduction
Hepatocellular carcinoma (HCC) is a common solid
tumor, accounting for 841,000 new cases and 782,000 deaths each
year, making it the sixth most common cancer type and the fourth
leading cause of cancer death worldwide (1–3).
Despite the recent advancements in surgical techniques, HCC still
exhibits a high recurrence rate after tumor resection, which may
hinder its treatment and the long-term survival of patients
(4). Histological grade is a
well-known prognostic factor for metastasis and recurrence
following hepatic resection and transplantation. Previous studies
have shown that poorly differentiated HCC is associated with worse
overall survival and more frequent metastases and recurrence
compared with highly and moderately differentiated HCC (5,6).
Therefore, an accurate preoperative diagnosis based on tumor
pathological grade is of great importance for determining
therapeutic strategies and assessing prognosis.
Magnetic resonance imaging (MRI), a constantly
evolving technique, allows for uniform sampling of the whole tumor
in HCC pathological grading. For instance, diffusion-weighted
imaging (DWI), a non-invasive procedure with the absence of
ionizing radiation, is effective in evaluating the microscopic
mobility of water molecules within the tissue. However,
microstructural barriers (such as cell membranes, organelles and
microcirculation of blood in capillaries) restrict water diffusion,
which changes the distribution into a non-Gaussian one. The
standard DWI-derived apparent diffusion coefficient (ADC) value
cannot accurately measure the real diffusivity, as it assumes a
Gaussian distribution of displacements of diffusing spins
corresponding to water molecules freely moving in the containing
medium (7,8). Hence, MRI techniques with extended
diffusion models, such as biexponential DWI and diffusion kurtosis
imaging (DKI), may be able to offer precise information about water
diffusion (9,10). Intravoxel incoherent motion (IVIM)
with biexponential mode was first proposed by Le Bihan et al
(11) in 1986 to quantitatively
assess the microscopic translational motions that occur in each
image voxel on MRI. In addition, using a biexponential model may
distinguish the diffusion of water molecules from the
microcapillary perfusion of tissues and obtain diffusion
parameters, including the true diffusion (D), pseudo-diffusion (D*)
and perfusion fraction (f). An alternative method, DKI, initially
described by Jensen and Helpern (12) and Jensen et al (13), recommended the characterization of
the non-Gaussian nature of water diffusion. The method, derived
mathematically through the use of a polynomial model with a
dimensionless factor called K, could provide additional
microstructural information about tissue heterogeneity and
cellularity with high b-values, and was successfully applied in
subsequent diffusion studies (14,15).
While preliminary studies have assessed the correlation between DWI
and histological grade in HCC (16–19),
Nasu et al (20) report
inconclusive or conflicting results. Considering that mono and
biexponential DWI and DKI are able to illustrate different tissue
properties, this can be used to explore and compare their roles in
the differentiation of histopathological grading in HCC. However,
to the best of our knowledge, a limited number of studies have
evaluated the associations between quantitative parameters derived
from standard DWI, DKI and IVIM and histopathological grading in
HCC. Therefore, the purpose of the present prospective study was to
quantitatively correlate the ability of various diffusion
parameters derived from DWI, IVIM and DKI for detecting and grading
HCC.
Materials and methods
Patient population
The present study was approved by the Institutional
Review Board of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China; approval no. nsmc17-10). Written informed
consent was obtained from each participant before the study. All
methods were performed in accordance with the Declaration of
Helsinki. Between January 2017 and March 2020, a total of 96
patients who received routine and contrast MRI, DWI, IVIM and DKI
at the North Sichuan Medical College (Nanchong, China) for the
detection of clinically suspected HCC, which was later confirmed
and graded following histopathological examination either after
post-surgical resection or through stereotactic biopsy specimen
evaluation, were enrolled in the present study. Between
pathological examination and MRI, the mean standard deviation was 4
days (range, 1–8 days). Upon review, 18 patients were excluded from
the study for the following reasons: i) HCC tumor diameter <0.5
cm (n=3); ii) poor image quality or motion artifact (n=6); and iii)
radiotherapy, chemotherapy, ablation or trans-catheter arterial
chemo–embolization received prior to MRI examination (n=9).
Finally, 78 patients (25 females and 53 males; mean age, 56 years;
age range, 32–79 years) were enrolled for analysis, and every
patient had only one HCC lesion, including 59 cases that received
specimen resection and 19 that received stereotactic biopsy. The
tumors from these 78 subjects were classified as well
differentiated (n=22), moderately differentiated (n=41) or poorly
differentiated (n=15) HCC according to the criteria of Pathology
and Genetics Tumors of the Digestive System (21). The clinicopathological
characteristics of the patients are provided in Table I.
 | Table I.Comparison of baseline
characteristics between different pathological grades of HCC. |
Table I.
Comparison of baseline
characteristics between different pathological grades of HCC.
|
| Histological
grading |
|
|---|
|
|
|
|
|---|
| Baseline
characteristics | wHCC (n=22) | mHCC (n=41) | pHCC (n=15) | P-value |
|---|
| Age, years | 57.31±10.82 | 55.46±11.26 | 54.13±11.61 | 0.68 |
| Sex (F/M) | 8/14 | 13/28 | 4/11 | 0.82 |
| Tumor size, cm | 5.07±2.11 | 4.80±1.73 | 4.69±1.59 | 0.79 |
MRI protocol
Whole liver MRI scans were performed using a 3.0T
system (Discovery™ MR750; GE Healthcare) with a 32-channel
phased-array coil. Each patient was required to fast for 6–8 h
prior to MRI. Conventional MRI, DWI, IVIM, DKI and
contrast-enhanced MRI were performed together. The main imaging
parameters are summarized below.
Axial 3D LAVA MRI (Discovery™ MR750; GE Healthcare)
before and after contrast enhancement was performed using the
following parameters: Repetition time/echo time (TR/TE),
3.6-4.4/1.7-1.9 msec; section thickness, 4.0 mm; intersection gap,
1 mm; matrix, 192×192; field of view (FOV), 32×32 cm; flip angle,
12°; and number of excitations (NEX), 1. Axial fast-recovery fast
spin-echo T2-weighted with fat suppressed images were obtained
using the following parameters: TR/TE, 4,500-6,000/85-100 msec;
section thickness, 4.0 mm; intersection gap, 1; matrix, 512×512;
and FOV, 34×34 cm.
Conventional DWI was expressed by the following
equation (7):
Sb/S0=exp (−b × ADC), where Sb and
S0 are the signal intensities in the diffusion gradient
factors of b and 0, respectively. ADC could be calculated by
fitting the signal with b-values of 0 and 800 sec/mm2 to
this model.
The IVIM model was expressed as follows (10): Sb/S0=(1-f)
exp (−b × D) + f exp [-b × (D + D*)], where Sb and
S0 are the signal intensities in the diffusion gradient
factors of b and 0, respectively. Three parameters, namely D, D*
and f, could be derived from IVIM by fitting the MRI signal
acquired at 14 b-values (b=0, 5, 10, 15, 20, 25, 50, 80, 150, 300,
500, 600, 800 and 1,000 sec/mm2), a section thickness of
3 mm, an intersection gap of 1 mm, a FOV of 320×320 mm and a NEX of
3 to a biexponential model. F is the perfusion fraction, while D is
the diffusion coefficient representing pure molecular diffusion,
and D* is the pseudo-diffusion coefficient representing incoherent
microcirculation within the voxel. For the IVIM model, a two-step
fitting method was used to calculate the increase in robustness of
the fitting with a lower calculation error, as follows: b >400
sec/mm2 was fitted for the single parameter D, since D*
is significantly larger than D; thus, the influence of
pseudo-diffusion on signal decay could be neglected when the
b-value was >400 sec/mm2 (22).
The signal intensities of three b-values (b=0, 1,000
and 2,000 sec/mm2) with 15 diffusion directions for
every b-value, a section thickness of 4 mm, an intersection gap of
1 mm, a FOV of 320×320 mm, a NEX of 6 and an acquisition time of 8
min were used. DKI parameters, including MD and mean diffusional
kurtosis (MK), were obtained with the following equation (11,12):
Sb/S0=exp [(−b × D) + (b2 ×
D2 × K/6)], where Sb and S0 are
the signal intensities acquired with diffusion gradient factors of
b and 0, respectively. D represents corrected ADC and K represents
diffusion kurtosis. For the DKI model, empirical evidence indicates
that maximum b-values of 2,000-3,000 sec/mm2 are
appropriate, and it is more efficient and convenient to simply use
the b-values of 0, 1,000 and 2,000 sec/mm2 (23,24).
For the axial 3D LAVA-Flex dynamic contrast-enhanced
scan sequence (Discovery™ MR750; GE Healthcare), 20 ml
gadolinium-diethylenetriaminepentaacetic acid (Magnevist; Schering
AG) was rapidly administered intravenously at a speed of 2–3 ml/sec
for a total dose of 0.2 mmol/kg of body weight using a double-tube
high-pressure injector (MEDRAD® Spectris Solaris EP MR
injection system; Bayer AG), alongside 20-ml sterile saline flush.
For early, hepatic arterial, venous and delayed phases, the scans
were set at 16, 30, 60 and 120 sec post-contrast injection,
respectively.
Image analysis
The compiled data were computed using DKI, as well
as mono and bi-exponential models. All original MR images were
transferred to the workstation (Advantage Workstation v.4.4; GE
Healthcare) for post-processing. To avoid selection bias to a
greater extent, two radiologists with experience in hepatobiliary
and gastrointestinal MRI for 12 and 8 years, respectively,
performed the DKI, IVIM and DWI. Both were blinded to the results
of the histopathological analysis. Regions of interest (ROIs) were
manually traced on each slice of the DKI, IVIM and DWI (MK, MD, D,
D*, f and ADC) to include the majority of the solid part of the
tumor. Conventional pre- and post-contrast T2-weighted,
contrast-enhanced and T1-weighted MR images were used for reference
and to avoid regions of hemorrhage, cystic degeneration or necrotic
areas. The mean ROI area was 67 mm2 (range, 55–80
mm2). The ROI was drawn multiple times (range, 2–4) on
each lesion, depending on tumor size, and the mean value was
calculated for analysis. The technique used is represented in
Fig. 1.
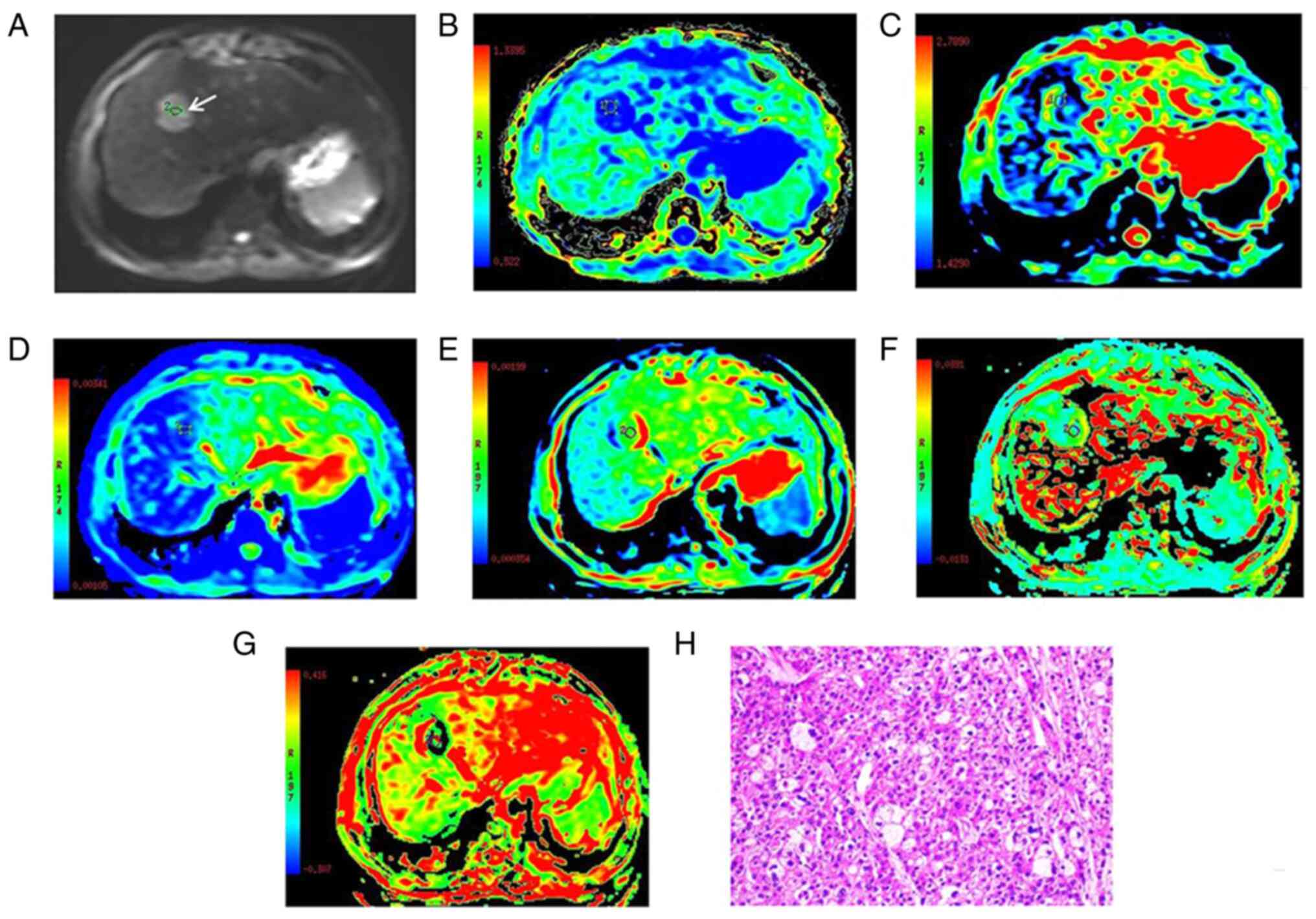 | Figure 1.Images of a 52-year-old male with
well-differentiated HCC at the anterior superior segment of the
right lobe of liver, which are representative of all the patients
with well-differentiated HCC enrolled in the present study. (A) DWI
with a b-value of 800 sec/mm2. (B-G) Parametric maps of
(B) MK, (C) MD, (D) ADC, (E) D, (F) D* and (G) f calculated from
the diffusion kurtosis imaging, intravoxel incoherent motion and
DWI data. (H) Histologically, the HCC was confirmed as well
differentiated by hematoxylin and eosin staining (magnification,
×200). The tumor (white arrow in A) exhibited a high signal
intensity on DWI. The MK, MD, ADC, D, D* and f values for the
regions of interest of the HCC were 0.54, 1.71×10−3
mm2/sec, 1.17×10−3 mm2/sec,
1.09×10−3 mm2/sec, 35.12×10−3
mm2/sec and 0.19, respectively, which were indicative of
well-differentiated HCC. HCC, hepatocellular carcinoma; DWI,
diffusion-weighted imaging; MK, mean diffusional kurtosis; MD, mean
diffusivity; ADC, apparent diffusion coefficient; D, true diffusion
coefficient; D*, pseudo-diffusion coefficient; f, perfusion
fraction. |
Biopsy and histopathological
examination
The tissue cylinders from the core biopsy were
obtained by using an 18G cutting needle and biopsy gun (Magnum
Bard). The penetration length ranged from 1.0 to 2.2 cm, which was
selected according to the size and anatomical position of the
lesion. The standard practice is to obtain 2–3 tissue cylinders.
Subsequent specimens were obtained from various areas within the
lesion by manually moving the outer needle to sample at random.
Tumor tissue specimens, which were obtained by
post-surgical resection or stereotactic biopsy, were formalin–fixed
(concentration, 10%; room temperature; duration, 6–48 h) and
paraffin-embedded. The sections (thickness, 3 µm) were then stained
with hematoxylin (room temperature; duration, 20 min) and eosin
(room temperature; duration, 1 min) for pathological evaluation
(Optical microscope, Leica DM2000 LED; Leica Microsystems;
magnification, ×200). A single pathologist, who had 15 years of
experience in evaluating histopathological slices and was blinded
to the findings of MRI, interpreted all the lesions. Each carcinoma
was categorized cytologically as well-, moderately or poorly
differentiated, according to the criteria of Pathology and Genetics
Tumors of the Digestive System (20).
Statistical analysis
Statistical analysis was performed using SPSS v.
23.0 (IBM Corp.) and MedCalc v. 16.2.1 (MedCalc Software bvba)
software. Data are expressed as the mean ± standard deviation (SD)
of the three replicates. The data consistency of DKI-, IVIM- and
DWI-derived parameters between both radiologists were evaluated by
intraclass correlation coefficient (ICC) and Bland-Altman plot. ICC
was indicative of good reliability when the measurements were
>0.75, moderate reliability when the measurements were
≥0.4-<0.75, and poor reliability when they were <0.4. When
the ICC was <0.75, repeat measurements of the corresponding
DKI-, IVIM- and DWI-derived parameters were performed by the same
reviewers (radiologists). Thereafter, the mean of 4 measurements
was used as the final result for further analysis. The Mann-Whitney
U-test and one-way ANOVA were used to compare differences in the
DWI, IVIM and DKI parameters among different tumor grades.
Spearman's correlation coefficient was performed to analyze the
correlation between each parameter and pathological grade. In
addition, Z test analysis was used to compare the receiver
operating characteristic (ROC) curves of MK, MD, D, ADC, D* and f
values to determine their performance in predicting highly
differentiated HCC. The diagnostic accuracy, sensitivity and
specificity were calculated with an optimal cutoff point determined
by the point of the largest Youden index for each parameter. The
tests were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics and MRI
appearance
The clinical and demographic characteristics of the
78 HCC patients (25 females and 53 males; mean age, 55.73±11.12
years; range, 32–79 years) are summarized in Table I. Out of these 78 subjects, 22
patients were pathologically diagnosed with well-differentiated
HCC, 41 with moderately differentiated HCC and 15 with poorly
differentiated HCC. The largest diameters of lesions ranged from
1.9 to 10.4 cm (mean, 4.9 cm). Fig.
1 shows MR diffusion images of a histopathologically confirmed
case of well-differentiated HCC, which are representative of all
patients with well-differentiated HCC enrolled in the present
study.
Inter-observer reproducibility
The ICCs between the two radiologists for MK, MD,
ADC, D, D*, f and ADC are shown in Table II. The results indicated that
there was good reliability between the two observers for all
parameters. Therefore, the mean measurement from the two observers
was used as the final result in the current study.
 | Table II.Analysis of reliability between the
two radiologists. |
Table II.
Analysis of reliability between the
two radiologists.
| Diffusion
parameter | ICC | 95% CI | P-value |
|---|
| MK | 0.927 | 0.889-0.953 | <0.001 |
| MD,
×10−3 mm2/sec | 0.910 | 0.863-0.942 | <0.001 |
| ADC,
×10−3 mm2/sec | 0.913 | 0.866-0.943 | <0.001 |
| D, ×10−3
mm2/sec | 0.953 | 0.928-0.970 | <0.001 |
| D*,
×10−3 mm2/sec | 0.911 | 0.864-0.943 | <0.001 |
| f, % | 0.851 | 0.776-0.903 | <0.001 |
Comparison between DKI-, IVIM- and
DWI-derived parameters, and correlation analysis
The MK value of the well-differentiated HCC group
was significantly lower than that of the moderately and poorly
differentiated HCC groups (all P<0.01). The MD, D and ADC values
of the well-differentiated HCC group were significantly higher than
those of the moderately and poorly differentiated HCC groups (all
P<0.05), whereas no significant difference was observed in D* or
f (P=0.502 and 0.853, respectively). The quantitative comparison of
the differences in the DKI-, IVIM- and DWI-derived parameters among
the 3 groups are displayed in Fig.
2 and Table III.
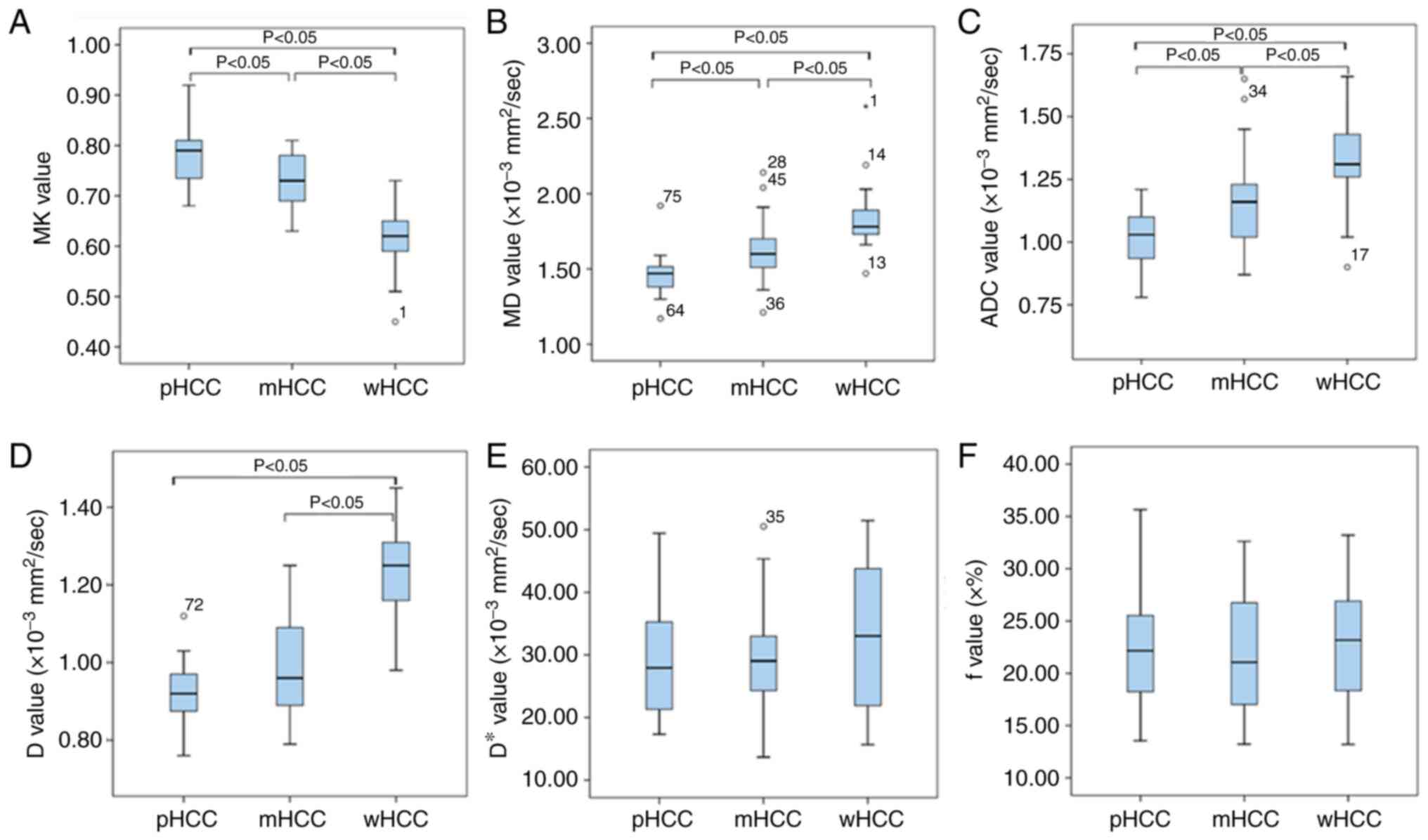 | Figure 2.(A-F) Association between
quantitative parameters and the histological grade of HCC. (A) MK,
(B) MD, (C) ADC, (D) D, (E) D* and (F) f values. HCC,
hepatocellular carcinoma; w, well-differentiated; m, moderately
differentiated; p, poorly differentiated; MK, mean diffusional
kurtosis; MD, mean diffusivity; ADC, apparent diffusion
coefficient; D, true diffusion coefficient; D*, pseudo-diffusion
coefficient; f, perfusion fraction. |
 | Table III.Parameters derived from diffusion
kurtosis imaging, intravoxel incoherent motion and
diffusion-weighted imaging of different pathological grades of
HCC. |
Table III.
Parameters derived from diffusion
kurtosis imaging, intravoxel incoherent motion and
diffusion-weighted imaging of different pathological grades of
HCC.
|
| Histological
grading |
|
|
|---|
|
|
|
|
|
|---|
| Diffusion
parameter | wHCC (n=22) | mHCC (n=41) | pHCC (n=15) | F-value | P-value |
|---|
| MK | 0.62±0.06 | 0.73±0.05 | 0.78±0.06 | 43.10 | <0.001 |
| MD,
×10−3 mm2/sec | 1.84±0.22 | 1.62±0.18 | 1.47±0.17 | 18.45 | <0.001 |
| ADC,
×10−3 mm2/sec | 1.31±0.18 | 1.16±0.27 | 1.02±0.13 | 12.20 | 0.001 |
| D, ×10−3
mm2/sec | 1.24±0.11 | 0.99±0.12 | 0.92±0.10 | 43.64 | <0.001 |
| D*,
×10−3 mm2/sec | 32.20±11.11 | 29.56±8.39 | 29.05±9.76 | 0.70 | 0.502 |
| f, % | 22.89±6.21 | 21.99±6.09 | 22.25±5.69 | 0.16 | 0.853 |
The correlation coefficients among all the
parameters and histopathological grades are shown in Table IV. Correlation analysis showed
that the MK (r=0.705; P<0.001) values decreased from poor to
moderately to well differentiated HCC, while the MD (r=0.570;
P<0.001), ADC (r=0.423; P<0.001) and D (r=0.687; P<0.001)
values were increased. MK was negatively correlated with the degree
of differentiation, while MD, D and ADC were positively correlated
with it. However, the values of D* and f were not significantly
correlated with the degree of differentiation.
 | Table IV.Spearman's correlation coefficients
of the parameters derived from diffusion kurtosis imaging,
intravoxel incoherent motion and diffusion-weighted imaging with
histopathological grades. |
Table IV.
Spearman's correlation coefficients
of the parameters derived from diffusion kurtosis imaging,
intravoxel incoherent motion and diffusion-weighted imaging with
histopathological grades.
|
| Histological
grading |
|
|
|---|
|
|
|
|
|
|---|
| Diffusion
parameter | wHCC (n=22) | mHCC (n=41) | pHCC (n=15) | Correlation
coefficient | P-value |
|---|
| MK | 0.62±0.06 | 0.73±0.05 | 0.78±0.06 | −0.705 | <0.001 |
| MD,
×10−3 mm2/sec | 1.84±0.22 | 1.62±0.18 | 1.47±0.17 | 0.570 | <0.001 |
| ADC,
×10−3 mm2/sec | 1.31±0.18 | 1.16±0.27 | 1.02±0.13 | 0.423 | <0.001 |
| D, ×10−3
mm2/sec | 1.24±0.11 | 0.99±0.12 | 0.92±0.10 | 0.687 | <0.001 |
| D*,
×10−3 mm2/sec | 32.20±11.11 | 29.56±8.39 | 29.05±9.76 | 0.120 | 0.284 |
| f, % | 22.89±6.21 | 21.99±6.09 | 22.25±5.69 | 0.042 | 0.705 |
Diagnostic performance of multiple
parameters
The ROC curves of multiple parameters for evaluating
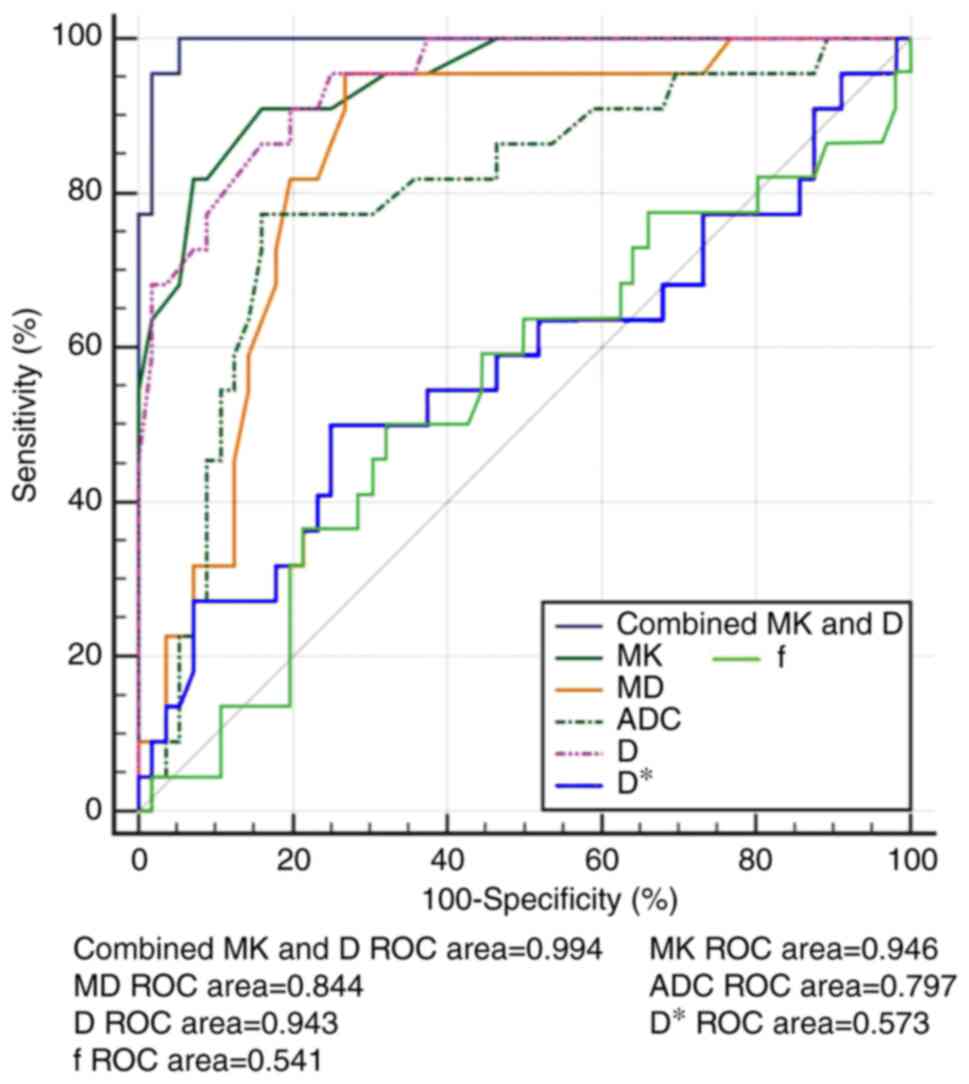
highly differentiated HCC are shown in Fig. 3. The diagnostic accuracy,
sensitivity and specificity of DKI-, IVIM- and DWI-derived
parameters with the optimal cutoff point being determined by the
point of the largest Youden index are shown in Table V. The comparison among the ROC
curves of the MK, MD, D, ADC, D* and f values for predicting highly
differentiated HCC indicated that MK and D may be the best
indicators for predicting highly differentiated HCC, as the AUC of
the MK and D values were significantly higher than those of the ADC
value (Z=2.247 and 2.428, P=0.025 and 0.016, respectively; Fig. 3), whereas no statistically
significant differences in the AUC values of MK or D were observed
(Z=0.072; P=0.942; Fig. 3).
Furthermore, the combined diagnostic performance of the MK and D
values exhibited higher accuracy, sensitivity and specificity, as
the AUC of combined MK and D was significantly higher than that of
each of these parameters alone (Z=2.044 and 2.106, P=0.041 and
0.035, respectively; Fig. 3).
 | Table V.Measurements of the threshold value,
Youden index, sensitivity, specificity, accuracy and AUC of the MK,
MD, ADC, D, D* and f values for differentiating highly
differentiated HCC from non-highly differentiated HCC. |
Table V.
Measurements of the threshold value,
Youden index, sensitivity, specificity, accuracy and AUC of the MK,
MD, ADC, D, D* and f values for differentiating highly
differentiated HCC from non-highly differentiated HCC.
| Diffusion
parameter | AUC (95% CI) | Optimal cutoff
value | Youden index | Sensitivity
(%) | Specificity
(%) | Accuracy (%) |
|---|
| MK | 0.946
(0.870-0.984) | 0.69 | 0.75 | 90.9 | 83.9 | 85.90 |
| MD,
×10−3 mm2/sec | 0.844
(0.744-0.916) | 1.65 | 0.69 | 95.5 | 73.2 | 79.49 |
| ADC,
×10−3 mm2/sec | 0.797
(0.691-0.880) | 1.25 | 0.61 | 77.3 | 83.9 | 82.05 |
| D, ×10−3
mm2/sec | 0.943
(0.866-0.983) | 1.10 | 0.71 | 90.9 | 80.4 | 83.33 |
| D*,
×10−3 mm2/sec | 0.573
(0.456-0.685) | 34.11 | 0.25 | 50.0 | 75.0 | 67.95 |
| f, % | 0.541
(0.424-0.654) | 23.89 | 0.18 | 50.0 | 67.9 | 62.82 |
| Combined MK and
D | 0.994
(0.943-1.000) | 0.13 | 0.95 | 100.0 | 94.6 | 96.15 |
Discussion
Despite being an invasive procedure, computed
tomography/ultrasound-guided biopsy is also the main method of
assessing the pathological grade of HCC. However, it has several
limitations, such as requiring an appropriate site, the presence of
complications and the risk of heterogenous tumors, which renders it
challenging in routine clinical practice. It is therefore vital to
establish preoperative tumor grading based on unbiased available
data for the purpose of diagnosis (25). The ADC value derived from standard
DWI has been found to be a valuable biomarker for predicting HCC
grading, as it was reported to be inversely correlated with the
pathological grade of HCC in previous studies (16–19).
However, such ADC values were derived using a monoexponential
Gaussian model, which is unsuitable for interpreting water
diffusion (26). To the best of
our knowledge, few studies in the literature have focused on
comparing quantitative parameters derived from standard DWI, DKI
and IVIM for detecting and grading HCC. The aim of the present
study was to determine the diagnostic performance of parameters
derived from DWI, IVIM and DKI for the assessment of the
pathological grading of HCC.
The present study demonstrated that the application
of ADC, D, MK and MD has both clinical practicability and value in
differentiating HCC grades. The MD, D and ADC values were
significantly higher in the well-differentiated HCC group than in
the moderately and poorly differentiated HCC groups. As the tissues
in these groups have a high cellular density and
nuclear-to-cytoplasmic ratio, in addition to restricted
extracellular space, the diffusion of water molecules is limited
(27). Nishie et al
(28) demonstrated that the ADC
value was lower in poorly differentiated HCC than in well- and
moderately differentiated HCC, which could contribute to the
radiological diagnosis of poorly differentiated components in HCC.
However, Nasu et al (20)
found that the histopathological grade of 125 resected HCCs was not
correlated with ADC, although higher-grade HCC displayed higher DWI
and signal intensity. It was hypothesized that the pathological
grading of tumors was mainly determined by the tumor's structural
and cellular atypia, while the ADC mainly reflected structural
atypia. Cellular atypia, expressed as the nucleus-to-cytoplasm
ratio, is not fully represented by the current DWI model, as it is
concerned with the extracellular Brownian motion rather than the
intracellular water molecules. Therefore, evaluating pathological
grade by ADC value alone could lead to incomplete results.
The results of the present study showed that the MK
values in the well-differentiated HCC group were significantly
lower than those in the moderately and poorly differentiated HCC
groups, suggesting that MK values probably reflect the degree of
tissue complexity. Well-differentiated HCC tends to have a lower
cell density and abundant homogeneous nest of well-differentiated
cells, while moderately and poorly differentiated HCCs have a
higher degree of tissue complexity, microvascular invasion, and
increased cellular density and nuclear-to-cytoplasmic ratio,
including heterogeneity with necrosis and hemorrhage (21).
Furthermore, the present results demonstrated that
the D* and f values were not statistically significant in
differentiating between well-, moderately and poorly differentiated
HCC, which was in line with the findings of Zhu et al
(29). This was mainly as HCC may
have an abnormal perfusion area due to aberrant blood supply, and
this feature may overlap in its histopathological grades. This may
ultimately influence the D* and f values, which are the perfusion
parameters that reflect the vascularity of the tissue. Furthermore,
previous studies have demonstrated that the D* and f values have a
large SD and poor reproducibility, which makes them prone to
instability, thus decreasing their diagnostic potential (30,31).
Nonetheless, Granata et al (32) demonstrated that the IVIM-derived f
value could significantly differentiate high-grade HCC from
low-grade HCC, and was positively correlated with the pathological
grade of HCC, which was inconsistent with the present results; this
reason may be related to differences in study subjects, MRI model
and the selection of b values. Therefore, the association between
the IVIM-derived f value and histological grade remains
unclear.
Furthermore, these results demonstrated that the
diagnostic performance of the MK and D values derived from DKI and
IVIM, respectively, in differentiating well differentiated HCC from
other types of HCC was higher than that of the DWI-derived ADC
value. In addition, the combined diagnostic performance of MK and D
values exhibited higher accuracy, sensitivity and specificity. Even
though DWI reflected the characteristics of biological tissue
through the movement of water molecules, the complex
microstructures in biological tissues, including membranes,
organelles and micro-capillary perfusion, can markedly influence
water diffusion, for which the ADC value cannot accurately display
the real diffusivity. Moreover, due to its intrinsic properties,
the ADC value contains the combined information on both tissue
cellularity (D) and perfusion (f) (33). Different grades of HCC, with their
complex microstructure and presence of perfusion gradient, can
affect the ADC values, resulting in signal loss, and thus decreased
diagnostic performance during HCC evaluation. Therefore, the
diagnostic value of ADC is controversial for detecting and grading
HCC. The IVIM-derived D value is solely dependent on a molecular
diffusion coefficient without any contributions from the
microcirculation. Therefore, the diagnostic performance of D in
differentiating well-differentiated HCC from other HCC types was
higher than that of the ADC value. Furthermore, DKI was reported to
quantify the non-Gaussian nature of water diffusion in biological
tissues (11), which suggests that
it can more accurately reflect the diffusivity of water molecules
in the tissue, while providing additional microstructural
information about tissue heterogeneity and cellularity using high
b-values (30).
The present study had several limitations. First,
the sample size was relatively small and did not include cases of
undifferentiated carcinoma. Further studies with a larger patient
population are recommended. Secondly, 24.36% (19/78) of HCC cases
were confirmed by histopathological analysis. However, the biopsy
tissue may not be representative of the whole tumor, due to the
cytopathological heterogeneity of tumors. Therefore, this sampling
bias could have contributed to the disparity between each parameter
and the cytopathological analysis. Furthermore, certain tumors were
also present in the left lobe of the liver, and the ROIs in those
tumors were prone to adjacent organ functions, such as
gastrointestinal peristalsis, and heart and diaphragm motion.
Finally, the present study did not evaluate the difference in
prognosis between the histopathological grades of HCC based on the
DKI, IVIM and ADC parameters, which should be a topic for further
investigation.
In conclusion, the present study indicated that the
MK and D values, derived from DKI and IVIM, respectively, are
feasible and helpful in distinguishing the histological grade of
HCC and were superior to ADC. However, the conventional DWI using
two b values with acceptable diagnostic efficiency and short
scanning time remains a consideration for routine clinical
application.
Acknowledgements
The authors would like to thank the English native
speaker Professor Morgan A. McClure (Department of Radiology,
Affiliated Hospital of North Sichuan Medical College, Nanchong,
China) for helping to correct the grammar and structure of the
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
HWL, YD, GWY and YJL contributed to the concept and
design of the study; LHZ, HCY, XF, XXZ, RHF, JY, AB and HFY
contributed to the literature search, data collection, statistical
analysis and data interpretation; YD, JY and AB revised the
manuscript. HWL, YD, YJL and GWY confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of the Affiliated Hospital of North Sichuan
Medical College (Nanchong, China; approval no. nsmc17-10). Written
informed consent was obtained from all the patients enrolled in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izzo F, Palaia R, Albino V, Amore A, di
Giacomo R, Piccirillo M, Leongito M, Nasto A, Granata V, Petrillo A
and Lastoria S: Hepatocellular carcinoma and liver metastases:
Clinical data on a new dual-lumen catheter kit for surgical sealant
infusion to prevent perihepatic bleeding and dissemination of
cancer cells following biopsy and loco-regional treatments. Infect
Agent Cancer. 10:112015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piccirillo M, Granata V, Albino V, Palaia
R, Setola SV, Petrillo A, Tatangelo F, Botti G, Foggia M and Izzo
F: Can hepatocellular carcinoma (HCC) produce unconventional
metastases? Four cases of extrahepatic HCC. Tumori. 99:e19–e23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poon RT, Fan ST, Lo CM, Liu CL and Wong J:
Long-term survival and pattern of recurrence after resection of
small hepatocellular carcinoma in patients with preserved liver
function: Implications for a strategy of salvage transplantation.
Ann Surg. 235:373–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Decaens T, Roudot-Thoraval F, Badran H,
Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J,
Dharancy S, et al: Impact of tumor differentiation to select
patients before liver transplantation for hepatocellular carcinoma.
Liver Int. 31:792–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L, Rui JA, Wang SB, Chen SG, Qu Q,
Chi TY, Wei X, Han K, Zhang N and Zhao HT: Factors predictive for
long-term survival of male patients with hepatocellular carcinoma
after curative resection. J Surg Oncol. 95:298–303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heijmen L, Ter Voert EE, Nagtegaal ID,
Span P, Bussink J, Punt CJ, de Wilt JH, Sweep FC, Heerschap A and
van Laarhoven HW: Diffusion-weighted MR imaging in liver metastases
of colorectal cancer: Reproducibility and biological validation.
Eur Radiol. 23:748–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kele PG and van der Jagt EJ: Diffusion
weighted imaging in the liver. World J Gastroenterol. 16:1567–1576.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan Q, Deng YS, Zhou JX, Yu YD, Bao YY,
Lei Q, Chen HJ, Peng YH, Mei YJ, Zeng QS and Li XC: Intravoxel
incoherent motion diffusion-weighted MR imaging in assessing and
characterizing solitary pulmonary lesions. Sci Rep. 7:432572017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan J, Yeung DK, Mok GS, Bhatia KS, Wang
YX, Ahuja AT and King AD: Non-Gaussian analysis of diffusion
weighted imaging in head and neck at 3T: A pilot study in patients
with nasopharyngeal carcinoma. PLoS One. 9:e870242014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Bihan D, Breton E, Lallemand D, Grenier
P, Cabanis E and Laval-Jeantet M: MR imaging of intravoxel
incoherent motions: Application to diffusion and perfusion in
neurologic disorders. Radiology. 161:401–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jensen JH and Helpern JA: MRI
quantification of non-Gaussian water diffusion by kurtosis
analysis. NMR Biomed. 23:698–710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jensen JH, Helpern JA, Ramani A, Lu H and
Kaczynski K: Diffusional kurtosis imaging: the quantification of
non-gaussian water diffusion by means of magnetic resonance
imaging. Magn Reson Med. 53:1432–1440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weber RA, Hui ES, Jensen JH, Nie X,
Falangola MF, Helpern JA and Adkins DL: Diffusional kurtosis and
diffusion tensor imaging reveal different time-sensitive
stroke-induced microstructural changes. Stroke. 46:545–550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J, Zhuo C, Qin W, Wang D, Ma X, Zhou Y
and Yu C: Performances of diffusion kurtosis imaging and diffusion
tensor imaging in detecting white matter abnormality in
schizophrenia. Neuroimage Clin. 7:170–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo W, Zhao S, Yang Y and Shao G:
Histological grade of hepatocellular carcinoma predicted by
quantitative diffusion-weighted imaging. Int J Clin Exp Med.
8:4164–4169. 2015.PubMed/NCBI
|
|
17
|
Chen J, Wu M, Liu R, Li S, Gao R and Song
B: Preoperative evaluation of the histological grade of
hepatocellular carcinoma with diffusion-weighted imaging: A
meta-analysis. PLoS One. 10:e01176612015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Li C, Wang R, Ren J, Yang J and
Zhang Y: Combined application of gadoxetic acid disodium-enhanced
magnetic resonance imaging (MRI) and diffusion-weighted imaging
(DWI) in the diagnosis of chronic liver disease-induced
hepatocellular carcinoma: A meta-analysis. PLoS One.
10:e01442472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woo S, Lee JM, Yoon JH, Joo I, Han JK and
Choi BI: Intravoxel incoherent motion diffusion-weighted MR imaging
of hepatocellular carcinoma: Correlation with enhancement degree
and histologic grade. Radiology. 270:758–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nasu K, Kuroki Y, Tsukamoto T, Nakajima H,
Mori K and Minami M: Diffusion-weighted imaging of surgically
resected hepatocellular carcinoma: Imaging characteristics and
relationship among signal intensity, apparent diffusion
coefficient, and histopathologic grade. AJR Am J Roentgenol.
193:438–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamiton Stanley R..Lauri A: altonen.
Pathology & genetics tumors of the digestive system (the Master
Translator: Yu JY, Cu QC)[M]. BeiJing. (People's Medical Publishing
House). 2022006.
|
|
22
|
Le Bihan D, Turner R and MacFall JR:
Effects of intravoxel incoherent motions (IVIM) in steady-state
free precession (SSFP) imaging: Application to molecular diffusion
imaging. J Magn Reson Med. 10:324–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glenn GR, Helpern JA, Tabesh A and Jensen
JH: Quantitative assessment of diffusional kurtosis anisotropy. NMR
Biomed. 28:448–459. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lätt J, Nilsson M, Malmborg C, Rosquist H,
Wirestam R, Ståhlberg F, Topgaard D and Brockstedt S: Accuracy of
q-space related parameters in MRI: Simulations and phantom
measurements. IEEE Trans Med Imaging. 26:1437–1447. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stigliano R, Marelli L, Yu D, Davies N,
Patch D and Burroughs AK: Seeding following percutaneous diagnostic
and therapeutic approaches for hepatocellular carcinoma. What is
the risk and the outcome? Seeding risk for percutaneous approach of
HCC. Cancer Treat Rev. 33:437–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iima M and Le Bihan D: Clinical intravoxel
incoherent motion and diffusion MR imaging: Past, present, and
future. Radiology. 278:13–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Moigne F, Boussel L, Haquin A, Bancel
B, Ducerf C, Berthezène Y and Rode A: Grading of small
hepatocellular carcinomas (≤2 cm): Correlation between histology,
T2 and diffusion-weighted imaging. Br J Radiol. 87:201307632014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishie A, Tajima T, Asayama Y, Ishigami K,
Kakihara D, Nakayama T, Takayama Y, Okamoto D, Fujita N, Taketomi
A, et al: Diagnostic performance of apparent diffusion coefficient
for predicting histological grade of hepatocellular carcinoma. Eur
J Radiol. 80:e29–e33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu SC, Liu YH, Wei Y, Li LL, Dou SW, Sun
TY and Shi DP: Intravoxel incoherent motion diffusion-weighted
magnetic resonance imaging for predicting histological grade of
hepatocellular carcinoma: Comparison with conventional
diffusion-weighted imaging. World J Gastroenterol. 24:929–940.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo M, Zhang L, Jiang XH and Zhang WD:
Intravoxel Incoherent Motion Diffusion-weighted Imaging: Evaluation
of the differentiation of solid hepatic lesions. Transl Oncol.
10:831–838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun H, Xu Y, Xu Q, Shi K and Wang W:
Rectal cancer: Short-term reproducibility of intravoxel incoherent
motion parameters in 3.0T magnetic resonance imaging. Medicine
(Baltimore). 96:e68662017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Granata V, Fusco R, Catalano O, Guarino B,
Granata F, Tatangelo F, Avallone A, Piccirillo M, Palaia R, Izzo F
and Petrillo A: Intravoxel incoherent motion (IVIM) in
diffusion–weighted imaging (DWI) for Hepatocellular carcinoma:
Correlation with histologic grade. Oncotarget. 7:79357–79364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chandarana H, Lee VS, Hecht E, Taouli B
and Sigmund EE: Comparison of biexponential and monoexponential
model of diffusion weighted imaging in evaluation of renal lesions:
Preliminary experience. Invest Radiol. 46:285–291. 2011. View Article : Google Scholar : PubMed/NCBI
|