Introduction
Pediatric low-grade gliomas (pLGGs) represent
one-third of all central nervous system tumors (1). Pathologically, pLGG is graded by the
World Health Organization into either grade I or II (2). The main management is via gross total
resection (GTR) whenever feasible. Deeply seated and locally
infiltrative tumors pose a challenge to effective surgical
resection, as surgery can further compromise certain functions,
such as vision in the case of hypothalamic-optic pathway gliomas
(OPGs). Diagnosis may depend only on the radiological findings
(3).
Although overall survival (OS) rates have reached as
high as 98% at 5 years, approximately one-half of the affected
patients will require adjuvant therapy, with a progression-free
survival (PFS) rate of ~50% (4).
Lower survival rates have been observed in infants <1 year of
age at diagnosis, for those with disseminated tumors or spinal cord
tumors, and for those without complete surgical excision, with a
mean event-free survival (EFS) rate of ~54% for the three groups.
Infants <1 year of age with OPG have a lower EFS rate than
infants with tumors of the cerebellum and cerebral hemispheres
(5).
Patients with neurofibromatosis type 1
(NF1)-associated pLGGs have a higher EFS rate than those with
non-NF1–associated pLGGs (6). The
majority of NF1-associated pLGGs are located in anatomical regions
where surgery is associated with complications and may lead to
severe sequelae, mainly in hypothalamic-optic pathway primary sites
(7).
First-line chemotherapy with the
vincristine/carboplatin (VC) regimen achieves a 5-year PFS rate of
53%, and this is higher for NF1-associated tumors. Progression
after first-line therapy is associated with the risk of developing
subsequent progression (4). There
is no current standard salvage regimen available for progressive
LGG (8). The present study aimed
to analyze the different salvage regimens used in progressive LGG
and to determine their efficacy in terms of the clinical and
radiological response, and the impact on survival.
Patients and methods
Patient population
The present retrospective study included 70
pediatric patients with pLGG (<18 years of age) who developed
progression (radiological and/or clinical) and received one of the
following salvage chemotherapy regimens: Weekly vinblastine,
monthly carboplatin or a re-challenge with VC. All patients had a
pathological confirmation of LGG or had undergone magnetic
resonance imaging that was suggestive of LGG without biopsy (either
in patients with NF1 or non-NF1-associated pLGG) and were not
amenable for biopsy due to a poor general condition or risky
procedure. All patients received the Children Oncology Group
protocol (COG A9952) (NCT00002944) as first-line therapy,
consisting of 10 weeks of VC induction followed by a maintenance
period of eight cycles of VC (1.5 mg/m2 intravenous
vincristine and 175 mg/m2 intravenous carboplatin)
(9). Treatment was administered in
the Children's Cancer Hospital Egypt (Cairo, Egypt) between July
2007 and December 2019, and the patients were followed-up until
June 2021.
Treatment regimens
The salvage regimen was selected according to the
time elapsed since the end of therapy and the social situation of
the patient. A VC regimen was considered if the progression
occurred >1 year from the end of the first-line therapy. Monthly
carboplatin and weekly vinblastine were considered if progression
occurred <1 year from the end of therapy. If the social issue
was difficulty regarding transportation, monthly carboplatin was
the preferred regimen to decrease the number of visits to the
hospital.
The present study aimed to compare the efficacy of
different salvage chemotherapeutic regimens in patients with
unresectable progressive LGG, as measured by the disease response
rate at 6, 12 and 24 months from the end of the salvage therapy,
and also the 2-year EFS rate. The retrieval and collection of data
were performed using the stage 6 Cerner computer system by the
health care information and management systems society
analytics.
The following data were collected: Age, sex, initial
presentation, duration of symptoms, family history, NF status,
initial Karnofsky performance status (KPS) score (10), tumor location and extension to the
surrounding structures. The visual acuity and field were assessed
using the method of following objects and visual evoked potential
testing in infants. Unique visual charts and instruments were used
in older children to calculate optical power to be translated into
seven grades as follows: Normal vision, ≥0.8; mild vision loss,
≥0.3 and <0.8; moderate visual loss, ≥0.125 and <0.3; severe
visual loss, ≥0.05 and <0.125; profound visual loss, ≥0.02 and
<0.05; near-total loss (near blindness), <0.02 till >no
light perception (NLP); and total vision loss (NLP) (11).
Initial management data, such as the extent of
surgical resection [GTR, subtotal resection (STR) and biopsy],
pathological subtypes and grading, first-line chemotherapy (number
of cycles and treatment duration) were collected. Data for the type
of response post-therapy were collected both clinically and
radiologically. The clinical response was defined as measuring
variations in the quality of life, reduction in the frequency of
seizures, reduction in steroid dosage and modification of the KPS
score. The radiological response was defined according to the
Response Assessment in Pediatric Neuro-Oncology criteria (12) as follows: i) Complete response:
Complete disappearance of the target lesion and all areas of
metastatic disease on T2-weighted and T2-weighted fluid-attenuated
inversion recovery (FLAIR) imaging; ii) major response: A ≥75%
reduction in the target lesion, but an insufficient response to
qualify as a complete response; iii) partial response: A ≥50%
reduction in the target lesion, typically on T2-weighted and
T2-weighted FLAIR imaging; iv) stable disease: An increase or a
decrease in the target lesion that is not sufficient to qualify as
progressive disease or responsive disease; and v) progressive
disease: A >25% increase in the target lesion, usually assessed
on T2-weighted and T2-weighted FLAIR imaging, or the development or
substantial growth (>25%) of new or metastatic lesions. Both an
increased and decreased enhancement (one or both) do not contribute
to the response type (12). The
date of maximum radiological response was also collected.
Recurrence and progression data (type of progression
either clinical and/or radiological, timing, tumor location and
extent, visual re-assessment upon progression in the optic pathway
and suprasellar gliomas) were collected and analyzed. The salvage
chemotherapy regimen (type, number of cycles and treatment
duration), response assessment, date of maximum response, visual
re-assessment post-salvage chemotherapy, and KPS at the time of
progressive disease and post-salvage were also collected as
essential response indicators. Complete response, major response,
partial response and stable disease were considered together to
indicate non-progressive disease. The toxicity of the different
salvage regimens was also revised using the Common Terminology
Criteria for Adverse Events (CTCAE) IV (13).
Statistical analysis
The data are presented as the median and
interquartile range, counts and percentages. OS time was defined as
the time from the date of the first progression to the date of
death from any cause or the last follow-up. EFS time was defined as
the time from the date of the first progression to the date of the
second progression of the tumor, death or the last follow-up.
Kaplan-Meier curves were used for survival analysis, while the
log-rank test was used to compare the curves. The χ2 or
Fisher's exact tests were used to analyze categorical variables
(such as the radiological and clinical responses), and the
Kruskal-Wallis test measured the KPS score outcome. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using R Statistical Environment
(version 4.0.2) supported by the R Core Team and the R Foundation
for Statistical Computing.
Results
Patient characteristics
The present study included 70 patients (43 males and
27 females), with a male-to-female ratio of 1.6:1. The age of the
patients ranged from 0.3–11 years (median, 4 years). Initially, the
duration of symptoms ranged from 4–6.7 months (median, 4.5 months).
In total, 14 patients (14/70; 20.0%) had the clinical stigmata of
NF1. The median number of cycles of first-line chemotherapy was
eight. As the majority of the included patients had tumors located
on the midline, no one was subjected to GTR initially or upon
progression. Initially, 48 patients (48/70; 68.6%) underwent either
STR (two patients) or only a biopsy (46 patients), and the
remaining patients were diagnosed radiologically. The most common
primary tumor sites were the suprasellar/optic chiasm (54.3%),
thalamus (17.1%), cerebral cortex (11.4%) and isolated optic nerve
(10%), whereas other sites had lesser frequency. Following
first-line therapy, 11 patients (15.7%) exhibited a major response,
a partial response was documented in 23 patients (32.9%) and 24
patients (34.3%) had stable disease. The median time to maximum
response was 3.47 months (range, 2.8-8.5 months). A total of 12
patients (17.1%) had progressive disease post-induction. The median
KPS score of the studied patients following first line therapy was
80.
Upon progression, 27 patients (38.6%) exhibited
clinical progression, 26 patients (37.1%) exhibited radiological
progression, and 17 patients (24.3%) exhibited both clinical and
radiological progression. In total, 67 patients (95.7%) had
localized disease and three patients (4.3%) exhibited spinal cord
metastatic disease. In addition, five patients (7.1%) underwent
STR.
The VC, monthly carboplatin and weekly vinblastine
regimens were used in 17 (24.3%), 22 (31.4%) and 31 (44.3%)
patients, respectively. The median time to progression was 24.5
months (range, 11.7-42.3 months). All clinicopathological data are
summarized in Table I.
 | Table I.Clinicopathological data of the
studied patients (n=70). |
Table I.
Clinicopathological data of the
studied patients (n=70).
| Characteristics | Value |
|---|
| Median age (IQR),
years | 4 (0.3-11.0) |
| Age group, n (%) |
|
| <1
years | 12 (17.1) |
| 1–8
years | 50 (71.4) |
| >8
years | 8 (11.4) |
| NF1 status, n
(%) |
|
|
Positive | 14 (20.0) |
|
Negative | 56 (80.0) |
| Sex, n (%) |
|
|
Male | 43 (61.4) |
|
Female | 27 (38.6) |
| Family history of
cancer, n (%) |
|
|
Positive | 9 (12.9) |
|
Negative | 61 (87.1) |
| NF1 status, n
(%) |
|
|
Positive | 14 (20.0) |
|
Negative | 56 (80.0) |
| Initial surgery, n
(%) |
|
|
Yes | 48 (68.6) |
| No | 22 (31.4) |
| Extent of
resection, n (%)a |
|
|
STR | 2 (4.2) |
|
Biopsy | 46 (95.8) |
| Site, n (%) |
|
|
Suprasellar/OPG | 38 (54.3) |
|
OPG | 2 (2.9) |
|
Isolated optic nerve | 7(10.0) |
|
Cerebral cortex | 8 (11.4) |
|
Spine | 2 (2.9) |
|
Thalamus | 12 (17.1) |
| Brain
stem | 1 (1.4) |
| Tumor pathology, n
(%) |
|
|
Pilocytic | 19 (27.1) |
|
Pliomyxoid | 16 (22.9) |
|
Ganglioglioma | 5 (7.1) |
|
Others | 12 (17.1) |
| Type of initial
response, n (%) |
|
| Major
response | 11 (15.7) |
| Partial
response | 23 (32.9) |
| Stable
disease | 24 (34.3) |
|
Progressive disease | 12 (17.1) |
| Type of
progression, n (%) |
|
|
Clinical | 27 (38.6) |
|
Radiological | 26 (37.1) |
|
Both | 17 (24.3) |
| Extent of the tumor
on progression, n (%) |
|
|
Local | 67 (95.7) |
|
Metastatic | 3 (4.3) |
| Re-surgery, n
(%) |
|
| No | 65 (92.9) |
|
Yes | 5 (7.1) |
| Type of salvage, n
(%) |
|
|
Rechallenge with VC | 17 (24.3) |
| Monthly
carboplatin | 22 (31.4) |
| Weekly
vinblastine | 31 (44.3) |
Prognostic factors
Age was found to have a significant impact on the
outcome post-salvage, with a 2-year EFS rate for patients aged
<1, 1–8 and >8 years of 19.4% (95% CI, 5.7-66.4), 68.4% (95%
CI, 17.9-81.1) and 87.5% (95% CI, 56.3-83.2), respectively
(P<0.001). The OS rate in same age groups was 38.1% (95% CI,
66.0-90.0), 77.1% (95% CI, 67.3-100.0) and 87.5% (95% CI,
67.3-100.0), respectively (P<0.001). However, sex, tumor
pathology, primary tumor site and radiological response did not
affect the outcome, regardless of the salvage regimen used
(Table II). The effect of the
extent of resection upon progression could not be assessed due to
the small number of patients who underwent surgical
intervention.
 | Table II.Association between prognostic
factors of included patients and survival. |
Table II.
Association between prognostic
factors of included patients and survival.
| Variable | 2-year EFS rate
(%) | P-value | 2-year OS rate
(%) | P-value |
|---|
| Age, years |
| <0.001 |
| <0.001 |
|
<1 | 19.4 |
| 38.1 |
|
|
1-8 | 68.4 |
| 77.1 |
|
|
>8 | 87.5 |
| 87.5 |
|
| Sex |
| >0.99 |
| 0.3 |
|
Male | 60.8 |
| 75.6 |
|
|
Female | 64.6 |
| 70.2 |
|
| Sitea |
| 0.7 |
| 0.7 |
|
Cerebral cortex | 60 |
| 60 |
|
| Optic
pathway glioma | 60.8 |
| 71.4 |
|
|
Others | 66.5 |
| 80 |
|
| Pathology |
| 0.5 |
| 0.3 |
|
Pilocytic | 70.7 |
| 82 |
|
|
Pilomyxoid | 60.6 |
| 73.7 |
|
|
Ganglionglioma | 60 |
| 60 |
|
|
Others | 41.7 |
| 50 |
|
| Radiological
response |
| 0.6 |
| 0.8 |
|
Responders (major response and
partial response) | 75 |
| 75.5 |
|
| Stable
disease | 66.4 |
| 75 |
|
|
Progressive disease | 52.3 |
| 65.5 |
|
The median number of cycles for the VC and monthly
carboplatin regimens was four and eight cycles, respectively,
whereas the median number of weeks on the vinblastine arm was 48
weeks. The VC, monthly carboplatin and weekly vinblastine protocols
exhibited a median time to maximum response of 3.3, 4.5 and 3.5
months, respectively. A total of three patients (4.3%) were not
evaluated, as they succumbed to the disease before the end of
therapy.
VC, weekly vinblastine and monthly carboplatin
exhibited a radiological response (major and partial response) of
58.8, 46.5 and 27.3%, respectively (P=0.25; data not shown).
Non-progressive disease was reported in 89.3% of patients treated
with weekly vinblastine, in 88.2% of patients treated with VC and
in 77.3% of patients treated with the monthly carboplatin regimen
(Table III). No single patient
achieved a complete radiological response.
 | Table III.Different types of response with
varying salvage regimen. |
Table III.
Different types of response with
varying salvage regimen.
| Salvage
regimen | Partial response, n
(%) | Major response, n
(%) | Stable disease, n
(%) | Progressive
disease, n (%) | Total, n |
|---|
| Rechallenge with
VC | 4 (23.5) | 6 (35.3) | 5 (29.4) | 2 (11.8) | 17 |
| Monthly
carboplatin | 2 (9.1) | 4 (18.2) | 11 (50.0) | 5 (22.7) | 22 |
| Weekly
vinblastine | 5 (17.9) | 8 (28.6) | 12 (42.8) | 3 (10.7) | 28 |
The KPS score did not differ significantly following
the salvage regimens, with a median score of 80, 70 and 80 for the
VC, monthly carboplatin and weekly vinblastine arms, respectively
(P=0.99; data not shown).
In total, 47 patients had suprasellar and OPGs. In
addition, 13 patients were re-initiated on the VC protocol (four of
them had radiological progression), five of the 13 patients (38.5%)
exhibited visual improvement, six (46.2%) exhibited stable vision
and two (15.4%) had deteriorated vision. A total of 13 patients
received monthly carboplatin (four of them had radiological
progression). Visual improvement, stability and deterioration were
reported in two, four and seven patients, respectively. A total of
18 patients (18/44; 40.9%) received weekly vinblastine (two of them
had documented radiological progression). In addition, five of
these 18 patients (27.8%) exhibited visual improvement, eight
(44.4%) exhibited stable vision and five (27.8%) experienced
deteriorated vision. There was no significant difference in the
visual outcome between the three regimens (P=0.16; data not
shown).
A total of four patients (4/9; 44.4%) with
NF1-associated OPG and 10 patients (10/35; 28.6%) with
non-NF1-associated OPG had documented visual deterioration
following salvage therapy (P=0.43; data not shown).
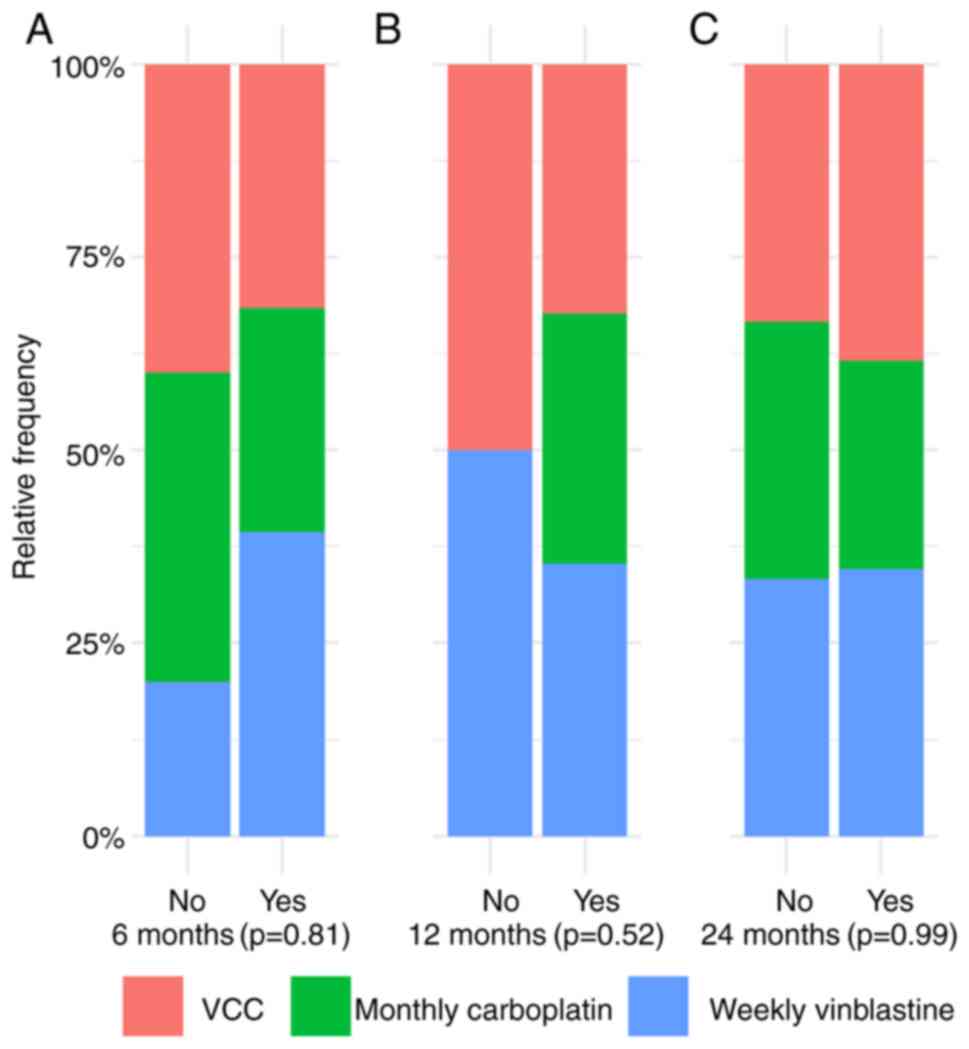
The disease status was assessed in patients with OPG
at 6, 12 and 24 months following the end of salvage treatment. At 6
months, 12 (12/13; 92.3%), 11 (11/13; 84.6%) and 15 patients
(15/18; 83.3%) exhibited non-progressive disease on the VC, monthly
carboplatin and weekly vinblastine arms, respectively. A total of
two patients on the weekly vinblastine arm were not assessed.
However, one patient on the VC and weekly vinblastine arms, and two
patients on the monthly carboplatin arm exhibited progressive
disease (P=0.81; data not shown).
At 12 months, non-progressive disease was documented
in 11 patients in both the VC and monthly carboplatin regimens. In
addition, 12 patients on the weekly vinblastine arm did not exhibit
progressive disease (P=0.52). Furthermore, two patients on the
monthly carboplatin and weekly vinblastine arm were not evaluated
due to clinical deterioration, which did not permit the
radiological assessment.
At 24 months, 10 (83.3%), 7 (77.7%) and 9 (81.8%)
patients in the VC, monthly carboplatin and weekly vinblastine
arms, respectively, did not exhibit progressive disease (P=0.99;
data not shown).
In addition, one patient on the VC arm, four
patients on the monthly carboplatin and seven patients on the
weekly vinblastine arms were not assessed due to disease-related
severe morbidity with marked clinical deterioration.
There was no significant difference in the rate of
non-progressive OPG between the three arms at the 6-, 12- and
24-month evaluations (P=0.81 at 6 months, P=0.52 at 12 months and
P=0.99 at 24 months) (Fig. 1).
All patients with NF1-associated OPG did not exhibit
any progression until 24 months after the salvage regimens. There
was no significant difference observed in the progression rate
following salvage therapy at the time of data collection beyond 24
months of follow-up between the patients with NF1-associated and
non-NF1-associated OPG; one patient (1/9) and seven patients (7/28)
developed progressive disease in the NF1-associated and
non-NF1-associated groups, respectively (P=0.99; data not
shown).
When taking into consideration all patients with or
without NF1-associated OPG, irrespective of the site, no
significant difference was observed in the rate of disease
progression between the NF1-associated and non-NF1-associated
groups at 6, 12 and 24 months from the end of treatment (P=0.60,
P=0.32 and P=0.29, respectively; data not shown). In addition, one
patient with NF1-associated disease (1/14; 7.1%) developed disease
progression versus 21 patients (21/53; 39.6%) in the non-NF1
group.
Toxicity of therapy
The primary toxic effects observed with weekly
vinblastine were hematological, mainly neutropenia. Grade 3 and 4
fever and neutropenia were documented in 5% of patients, with
confirmed infection in 2% of cases. Grade 3–4 peripheral neuropathy
was noted in three patients. Monthly carboplatin treatment was
associated with grade 3–4 hematological toxicity, mainly
neutropenia, in 20% of patients. The VC regimen was associated with
allergies in 15% of cases, with grade 4 hypersensitivity reported
in 5% of patients. Grade 3 or 4 peripheral neuropathy was reported
in 15% of cases, leading to temporary discontinuation of therapy,
and grade 3–4 neutropenia in 10% of patients (data not shown).
Survival outcomes
A total of 16 patients (16/70) were deceased at the
time of the analysis. Of note, 15 patients succumbed due to
progressive disease and one patient succumbed due to septic shock
following salvage therapy with monthly carboplatin. All patients
with metastatic disease are still alive.
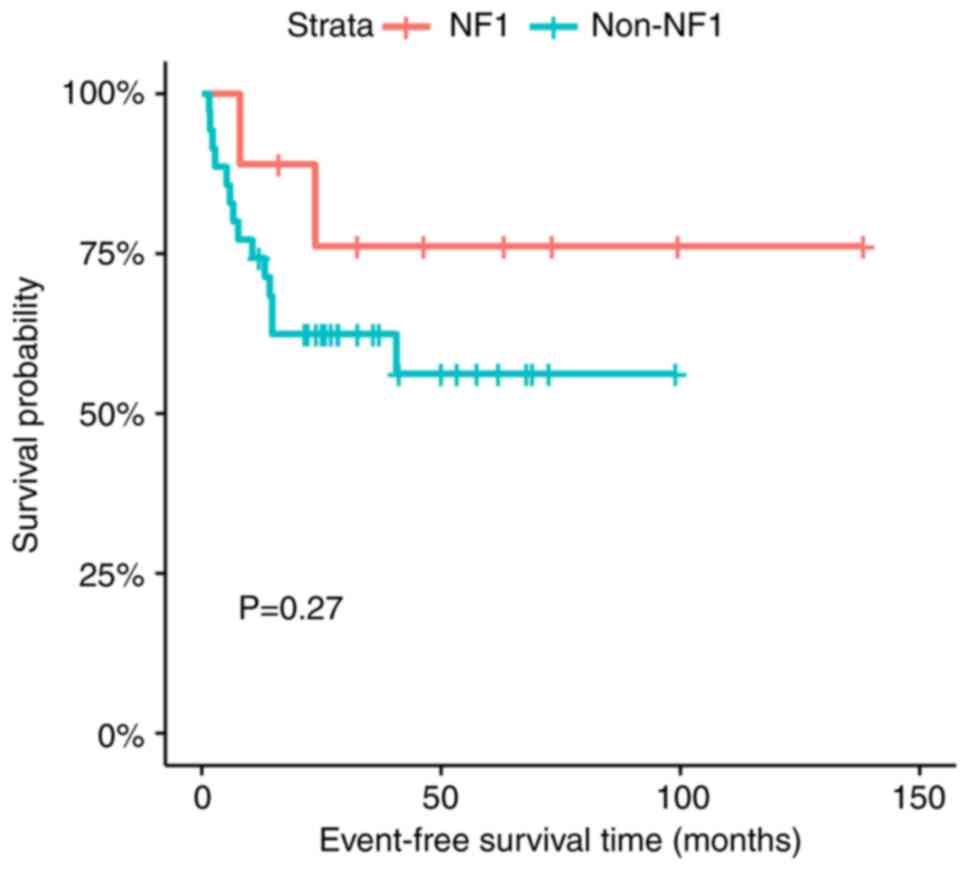
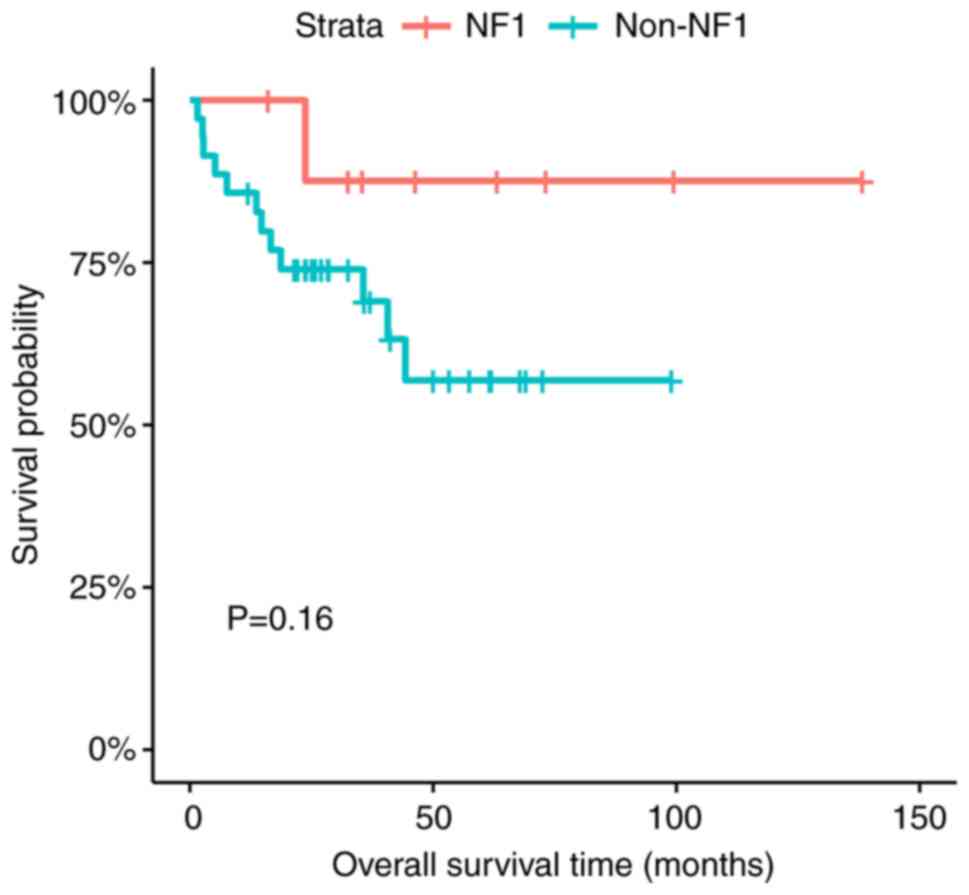
There was no statistically significant difference in
the EFS and OS between the patients with NF1-associated and
non-NF1-associated OPG (P=0.27 for EFS and P=0.16 for OS) (Figs. 2 and 3).
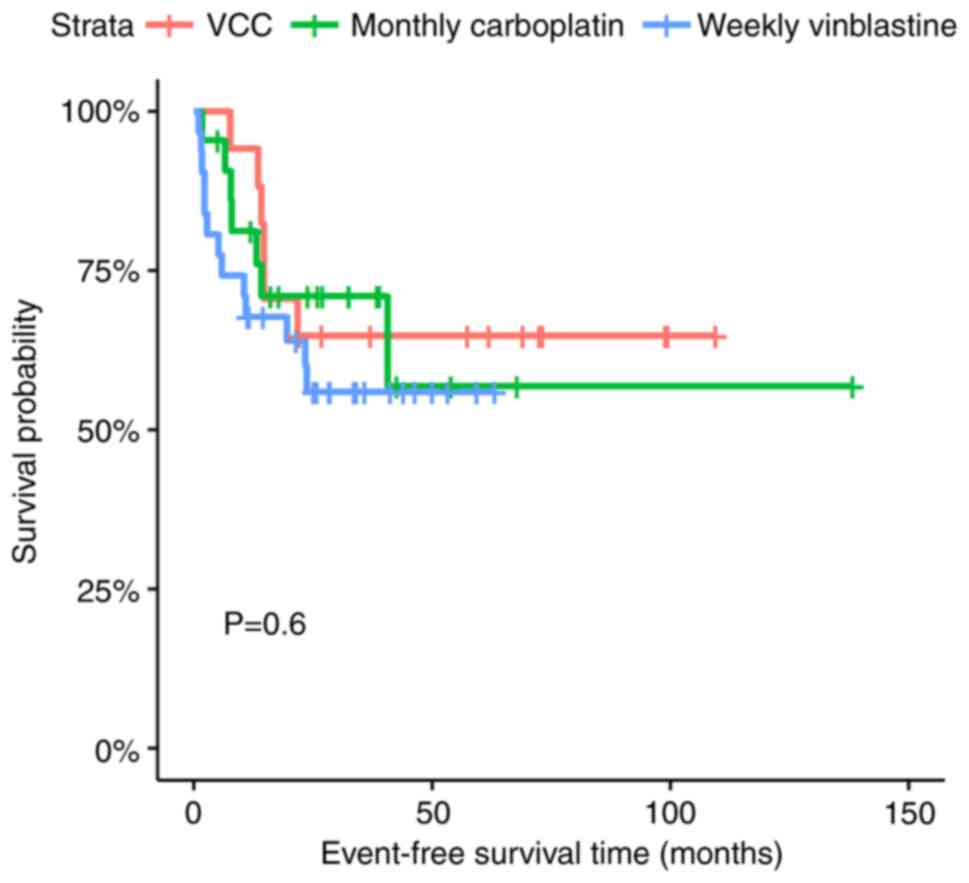
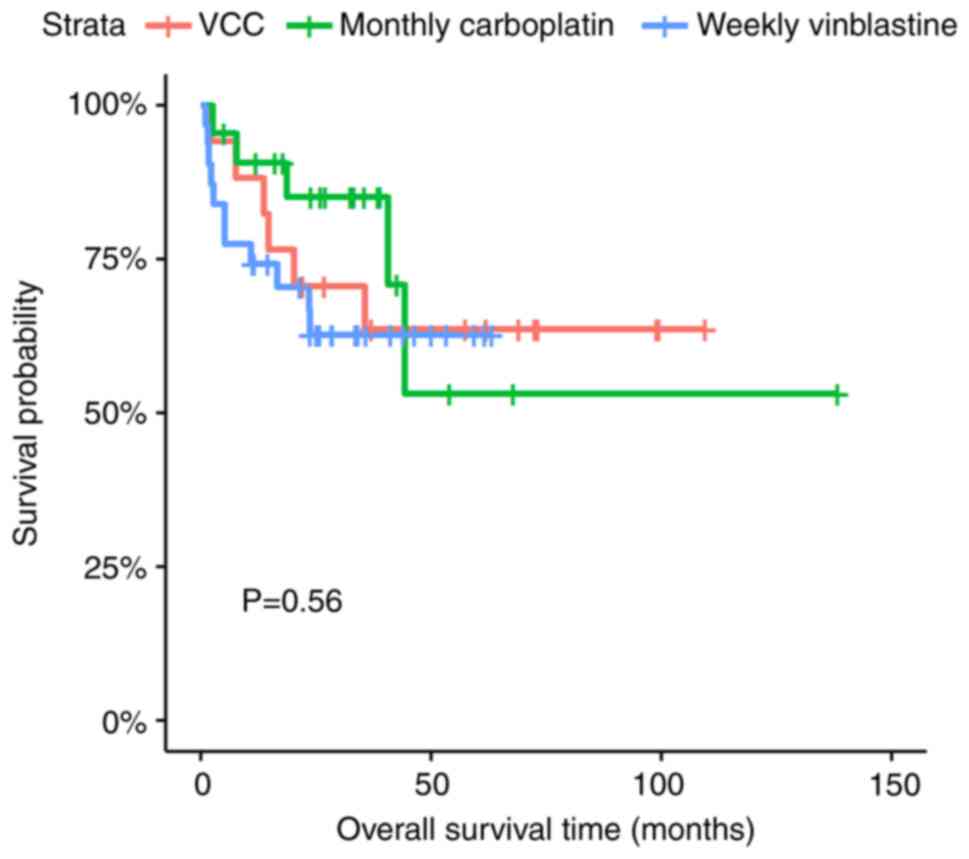
The 2-year EFS rates of the VC, monthly carboplatin
and weekly vinblastine regimens were 64.7% (95% CI, 45.5-91.9),
71.0% (95% CI, 53.8-93.6) and 56.0% (95% CI, 40.4-77.5), while the
2-year OS rates for the three regimens were 70.6% (95% CI,
51.9-95.9), 85.0% (95% CI, 70.6-100) and 62.7% (95% CI, 47.2-83.1),
respectively (P=0.60 for EFS and P=0.56 for OS) (Figs. 4 and 5).
In the present study, 22 patients (22/70; 31.4%)
developed disease progression following salvage therapy, 16 of
which did not survive (data not shown).
Discussion
Progressive LGG is considered a challenging disease
to treat. To the best of our knowledge, there are no data available
in the literature comparing the efficacy of different salvage
strategies. Scheinemann et al (14) reported irrelevant differences in
the PFS after the second, third and fourth lines of chemotherapy. A
pilot study by Bouffet et al (15) reported the efficacy of weekly
vinblastine in patients with progressive LGG. Another phase II
study confirmed the efficacy and safety of monthly carboplatin in
progressive LGG (16). The present
study provided data on the efficacy of different salvage regimens
in order to reach a consensus for the standard salvage regimens in
progressive LGG. The median age at diagnosis was close to that
reported in the study by Gururangan et al (16) (4 years), but lower than the median
age reported in the study by Bouffet et al (15) (7 years).
Age was a significant prognostic factor for survival
in the present study, with patients <1 year of age exhibiting
the worst outcome, which coincided with the data in the study by
Kandels et al (5), where an
age <1 year was a risk factor for progression and a lower PFS
time. Ater et al (17)
reported that an age <3 years and a residual tumor >3
cm2 in volume were independent poor prognostic factors.
The poor outcome of the younger age group may be associated with
the high prevalence of canonical BRAF alterations (97% of cases),
particularly BRAF V600E in midline infantile LGG, as reported by
Stucklin et al (18).
In the present study, sex, tumor site, pathological
subtypes and radiological response did not affect the outcome. In
total, 20% of the studied patients had clinical stigmata of NF1,
similar to the study by Gururangan et al (16), although with a lower frequency than
the studies by Ater et al (17) and Manoharan et al (19), which had 30 and 33% of patients
with progressive LGG associated with NF1, respectively.
The three salvage regimens used in the present study
according to frequency were weekly vinblastine, followed by monthly
carboplatin and then VC which differs from the findings of the
study by Moorman et al (8),
which collected data from multiple North American centers, where
the VC regimen was the most common, followed by weekly vinblastine
then monthly carboplatin across different tumor sites.
Packer et al (20) reported that non-progressive disease
occurred in 74% of patients on the VC regimen, which was lower than
the rate observed in the present study. Ater et al (17) found that 76% of patients (NF1 and
non-NF1) had non-progressive disease following the VC regimen.
Non-progressive disease was reported in 86% of patients in the
studies by Bouffet et al (15) and Dodgshun et al (21), which used weekly vinblastine and
monthly carboplatin, respectively. These results were similar to
those of the weekly vinblastine arm outcome (89.3%), but higher
than those of the monthly carboplatin arm (77.3%), observed in the
present study.
Patients with NF1-associated disease did not exhibit
any sign of radiological progression until 1 year after salvage
therapy in the three regimens. Stokland et al (6) concluded that NF1 was not a risk
factor for progression, which is in accordance with the findings of
the present study. There was no difference in the response rate
between patients with NF1-associated and non-NF1-associated disease
in the three regimens, coinciding with the findings of Bouffet
et al (22). Scheinemann
et al (14) reported
radiological progression in 50% of patients with NF1-associated
disease following treatment with second-line salvage regimens
(vinblastine, vincristine/etoposide, or thioguanine, procarbazine,
lomustine and vincristine).
Gururangan et al (16) and Packer et al (20) did not find a significant difference
in the EFS rate of patients with OPG associated with or without
NF1, as in the present study. By contrast, Stokland et al
(6) reported a significantly
improved PFS rate for patients with OPG associated with NF1
compared with that in patients with non-NF1-associated disease (70
vs. 46.7%; P<0.001).
There are limited data available regarding the
visual outcome post-OPG/suprasellar primary tumor progression. In
the present study, visual deterioration was reported in 32% of
progressive OPG tumors in all salvage regimens, which was lower
than the rate reported in the study by Shofty et al
(23), where 47.2% of these
patients had deteriorated vision and 13.8% of the studied patients
had improved vision with the VC regimen.
In the population in the present study, visual
improvement was highest with the VC regimen (38.5%), followed by
the weekly vinblastine (27.8%) and monthly carboplatin (15.4%)
regimens, although without statistical significance. Bouffet et
al (22) reported visual
deterioration in one patient. The study by de Haas et al
(24), which used VC, vinblastine
and radiotherapy for progressive OPG, reported that six patients
(6/33) had become blind, nine children had deteriorated vision and
18 children had stable vision. The patients with preserved vision
were equally distributed between the chemotherapy and radiotherapy
groups (24). Visual improvement
was observed in 20% of patients with OPG, as documented by
Lassaletta et al (25),
when using single-agent vinblastine therapy.
In the present study, visual deterioration in
patients with NF1-associated OPG occurred in 44.4% of patients,
which was a higher rate than that in the non-NF1 counterparts
(28.6%), and was also in accordance with the study by Scheinemann
et al (14). This previous
study reported severe visual impairment in 35% of patients, while
in the NF1 group, 75% of the patients had severely impaired vision.
The discrepancy between the absence of radiological progression in
patients with NF1-associated OPG, regardless of the site, until 1
year post-therapy, and the visual deterioration in almost half of
patients with NF1-associated disease, emphasizes the hypothesis
that the radiological response does not usually match the visual
outcome, as reported by Ullrich et al (26).
In the present study, there was no significant
difference in the progression rate between patients with
NF1-associated and non-NF1-associated disease at 6, 12 and 24
months of salvage therapy. Gururangan et al (16) reported a 3-year PFS rate of 72 and
62% for patients with non-NF1-associated and NF1-associated glioma,
respectively (P=0.39). By contrast, Bouffet et al (22) found a significant difference in PFS
rates between patients with NF1 (75%) and non-NF1 (37%) disease
(P=0.04). Ater et al (17)
reported the COG 9952 data, with a superior EFS rate in patients
with NF1-asociated in comparison to those with non-NF1-associated
disease (P<0.001).
In the present study, the 2-year EFS rate of
patients on the weekly vinblastine arm (56%) was lower than that of
the patients on the other two regimens, although with no
significant difference. Kandels et al (4) reported 3-year PFS rates of 48 and 8%
following salvage vinblastine monotherapy in patients with
NF1-associated and non-NF1-associated disease, respectively.
Lassaletta et al (25)
reported a 5-year OS and PFS rate of 94.4 and 53.2%, respectively,
using vinblastine single-agent therapy.
In the present study, the 2-year EFS and OS rates of
the monthly carboplatin group were 71 and 85%, respectively, which
is comparable to the results in the study by Dodgshun et al
(21), which reported a 3-year PFS
of 65% with monthly carboplatin treatment.
In the present study, 22 patients (22/70; 31.4%)
developed disease progression following salvage therapy, of which
16 did not survive; this rate of progression was lower than that
observed in the study by Kandels et al (4), which reported 58% progression
following salvage therapy, and only 8/86 of the patients succumbed
to the disease.
There are some limitations to the present study due
to its retrospective design, such as the relatively small number of
patients in each therapy group, the absence of a precise method for
visual assessment in young patients and the lack of fixed clinical
indication for recruitment in each therapy group. Although there
was some selection bias as the salvage regimen was selected
according to the time elapsed from the end of therapy and the
social situation of the patient, the homogeneity of the study
population in each group regarding age, midline location and
inaccessibility for surgery decreased this bias.
In conclusion, according to the present results,
there is no standard regimen for pLGG. Due to its benign course,
the outcome of progressive disease is good. The present study
showed that an age <1 year was associated with a poor survival
rate. Other prognostic factors, such as sex, tumor site, pathology
and radiological response did not affect the outcome. There was no
significant difference in efficacy between the three regimens. The
NF1 status did not affect the rate of progression following salvage
therapy, although it was associated with poor visual outcomes,
regardless of the salvage regimen. One of the important causes of
tumor progression is underlying molecular abnormality, so molecular
testing is mandatory to detect chemo-resistant tumors that may
benefit from targeted therapy. Randomized prospective clinical
trials are required to detect the most effective salvage
chemotherapy regimen for progressive LGG.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AEH was involved in the conception of the study. OA
and FA were involved in data curation. AEH and FA confirmed data
accuracy. AEH was involved in the data analysis. OA was involved in
the study methodology. FA provided the software and was involved in
the data validation. OA and AEH were involved in the writing of the
original draft. OA, AEH, HT, AR and ME were involved in designing
the study, and writing, reviewing and editing the manuscript. AEH
and OA confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Institutional
Review Board of Children's Cancer Hospital Egypt 57357 (Cairo,
Egypt; approval no. CCHE IRB 12–2020). Informed verbal consent was
obtained by phone from the guardians of all patients involved in
the study, as most of the patients were from remote areas with
difficulties in transportation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors. Diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (Suppl_4):iv1–iv86.
2018. View Article : Google Scholar
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 13:803–820. 2016. View Article : Google Scholar
|
|
3
|
Wisoff JH, Sanford RA, Heier LA, Sposto R,
Burger PC, Yates AJ, Holmes EJ and Kun LE: Primary neurosurgery for
pediatric low-grade gliomas: A prospective multi-institutional
study from the children's oncology group. Neurosurgery.
68:1548–1554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryall S, Tabori U and Hawkins C: Pediatric
low-grade glioma in the era of molecular diagnostics. Acta
Neuropathol Commun. 8:302020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kandels D, Pietsch T, Bison B,
Warmuth-Metz M, Thomale UW, Kortmann RD, Timmermann B, Driever PH,
Witt O, Schmidt R and Gnekow AK: Loss of efficacy of subsequent
nonsurgical therapy after primary treatment failure in pediatric
low-grade glioma patients-report from the german siop-lgg 2004
cohort. Int J Cancer. 147:3471–3489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stokland T, Liu JF, Ironside JW, Ellison
DW, Taylor R, Robinson KJ, Picton SV and Walker DA: A multivariate
analysis of factors determining tumor progression in. Childhood
low-grade glioma: A population-based cohort study (CCLG CNS9702).
Neuro Oncol. 12:1257–1268. 2010.
|
|
7
|
Campen CJ and Gutmann DH: Optic pathway
gliomas in neurofibromatosis type 1. J Child Neurol. 33:73–81.
2018. View Article : Google Scholar
|
|
8
|
Moorman B, Barbour M and Huang MA: Current
salvage treatment strategies for younger children (<10 y of Age)
with progressive low-grade glioma after initial chemotherapy in
north america: A web-based survey. J Pediatr Hematol Oncol.
43:e141–e145. 2021. View Article : Google Scholar
|
|
9
|
Ater JL, Zhou T, Holmes E, Mazewski CM,
Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, et
al: Randomized study of two chemotherapy regimens for treatment of
low-grade glioma in young children: A report from the children's
oncology group. J Clin Oncol. 30:2641–2647. 2012. View Article : Google Scholar
|
|
10
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancer.
Evaluation of Chemotherapeutic Agents. MacLeod CM: Columbia
University Press; New York, NY: pp. 191–205. 1949
|
|
11
|
Colenbrander A: Visual Standards aspects
and ranges of vision loss with emphasis on population surveys.
Proceedings of the 29th International Congress of Ophthalmology;
Sydney: 2002
|
|
12
|
Fangusaro J, Witt O, Driever PH, Bag AK,
de Blank P, Kadom N, Kilburn L, Lober RM, Robison NJ, Fisher MJ, et
al: Response assessment in pediatric low-grade glioma:
Recommendations from the response assessment in pediatric
neuro-oncology (RAPNO) working group. Lancet Oncol. 21:e305–e316.
2020. View Article : Google Scholar
|
|
13
|
National Cancer Institute (NIH), . Common
Terminology Criteria for Adverse Events (CTCAE). Version 4. NIH;
Bethesda, MD: 2010, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v4June
14–2010
|
|
14
|
Scheinemann K, Bartels U, Tsangaris E,
Hawkins C, Huang A, Dirks P, Fried I, Bouffet E and Tabori U:
Feasibility and efficacy of repeated chemotherapy for progressive
pediatric low-grade gliomas. Pediatr Blood Cancer. 57:84–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouffet E, Hargrave D, Cairney E, Garre M,
Slavc I and Baruchel S: Weekly vinblastine for
recurrent/progressive low-grade gliomas. Proceedings of
International Society of Paediatric Oncology SIOP XXXIV Meeting.
Porto; pp. p2152002
|
|
16
|
Gururangan S, Cavazos CM, Ashley D,
Herndon JE II, Bruggers CS, Moghrabi A, Scarcella DL, Watral M,
Tourt-Uhlig S, Reardon D and Friedman HS: Phase II study of
carboplatin in children with progressive low-grade gliomas. J Clin
Oncol. 20:2951–2958. 2002. View Article : Google Scholar
|
|
17
|
Ater JL, Xia C, Mazewski CM, Booth TN,
Freyer DR, Packer RJ, Sposto R, Vezina G and Pollack IF:
Nonrandomized comparison of neurofibromatosis type 1 and
non-neurofibromatosis type 1 children who received carboplatin and
vincristine for progressive low-grade glioma: A report from the
children's oncology group. Cancer. 122:1928–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stucklin ASG, Ryall S, Fukuoka K,
Zapotocky M, Lassaletta A, Li C, Bridge T, Kim B, Arnoldo A,
Kowalski PE, et al: Alterations in ALK/ROS1/NTRK/MET drive a group
of infantile hemispheric gliomas. Nat Commun. 10:43432019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manoharan N, Choi J, Chordas C, Zimmerman
MA, Scully J, Clymer J, Filbin M, Ullrich NJ, Bandopadhayay P, Chi
SN and Yeo KK: Trametinib for the treatment of
recurrent/progressive pediatric low-grade glioma. J Neurooncol.
149:253–262. 2020. View Article : Google Scholar
|
|
20
|
Packer RJ, Lange B, Ater J, Nicholson HS,
Allen J, Walker R, Prados M, Jakacki R, Reaman G, Needles MN, et
al: Carboplatin and vincristine for recurrent and newly diagnosed
low-grade gliomas of childhood. J Clin Oncol. 11:850–856. 1993.
View Article : Google Scholar
|
|
21
|
Dodgshun AJ, Maixner WJ, Heath JA,
Sullivan MJ and Hansford JR: Single-agent carboplatin for pediatric
low-grade glioma: A retrospective analysis shows equivalent
efficacy to multi-agent chemotherapy. Int J Cancer. 138:481–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bouffet E, Jakacki R, Goldman S, Hargrave
D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, et
al: Phase II study of weekly vinblastine in recurrent, refractory
pediatric low-grade glioma. J Clin Oncol. 30:1358–1363. 2012.
View Article : Google Scholar
|
|
23
|
Shofty B, Ben-Sira L, Freedman S, Yalon M,
Dvir R, Weintraub M, Toledano H, Constantini S and Kesler A: Visual
outcome following chemotherapy for progressive optic pathway
gliomas. Pediatr Blood Cancer. 57:481–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Haas V, Grill J, Raquin MA, Couanet D,
Habrand JL, Sainte-Rose C, Laithier V, Kieffer V and Kalifa C:
Relapses of optic pathway tumors after first-line chemotherapy.
Pediatr Blood Cancer. 52:575–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lassaletta A, Scheinemann K, Zelcer SM,
Hukin J, Wilson BA, Jabado N, Carret AS, Lafay-Cousin L, Larouche
V, Hawkins CE, et al: Phase II weekly vinblastine for
chemotherapy-na¨ive children with progressive low-grade glioma: A
Canadian pediatric brain tumor consortium study. J Clin Oncol.
34:3537–3543. 2016. View Article : Google Scholar
|
|
26
|
Ullrich NJ, Prabhu SP, Packer RJ, Goldman
S, Robison NJ, Allen JC, Viskochil DH, Gutmann DH, Perentesis JP,
Korf BR, et al: Visual outcomes following everolimus targeted
therapy for neurofibromatosis type 1-associated optic pathway
gliomas in children. Pediatric Blood Cancer. 68:e288332021.
View Article : Google Scholar : PubMed/NCBI
|