Introduction
Head and neck cancers (HNCs) are the sixth most
frequently occurring type of solid cancer. Most HNCs (90%)
originate from mucosal tissues and are known as head and neck
squamous cell carcinoma (HNSCC) or upper aerodigestive system
cancer (1,2). The incidence and sub-anatomical
distribution of HNSCC may vary according to the geographic location
and ethnicity of the population. Its etiology includes exposure to
various carcinogens, smoking, alcohol consumption, poor oral
hygiene and viral infections (3,4).
Several types of cancer are linked to genetic factors, and in
addition to environmental factors, genetic predisposition may also
play a role in HNSCC.
Oral cavity cancer is the most frequently diagnosed
subgroup of HNSCC worldwide. According to the 2014 World Cancer
Report, the numbers of new cases of HNSCC and associated mortality
were 700,000 and 370,000, respectively (5). Among these, the numbers of laryngeal
cancer cases were 157,000 and 83,000, respectively (5). Similarly, the mortality rate for the
60,000 patients newly diagnosed with HNSCC in the USA in 2017 was
30%, and most of these cancers developed from the oropharynx and
oral cavity (6). In Turkey, the
most frequently diagnosed HNSCC is laryngeal cancer with an
incidence of 7/100,000 between 2010–2014, and it is the eighth most
common cancer in Turkish men (7).
Host and tumor factors may serve a role in the
clinical behavior of laryngeal cancer, including the histological
grade, localization, extension, tumor size and lymph node
metastasis (8,9). Lymph node metastasis is an important
risk factor for prognosis that is mostly associated with the
localization and T stage of the tumor. Lymph node metastasis
markedly reduces the survival rate; however, the curative treatment
of laryngeal cancer with lymph node metastasis is possible
(10–12). The only parameter available to
guide the selection of treatment modality is the Tumor-Lymph
Node-Distant Metastasis (TNM) stage. Notably, no histological or
biological parameters are currently used when making treatment
decisions. Certain genetic factors may also play a role in the
pathogenesis and prognosis of LSCC (13). Despite increased genetic and
molecular knowledge, no clinical application that defines the
treatment and prognosis of laryngeal cancer has yet been
established (13).
Improved understanding of the molecular mechanisms
underlying LSCC lymph node metastasis and the identification of
potential molecular targets would be favorable. Interpreting the
associations between the differentially expressed genes in advanced
stages may facilitate the search for predictive markers that could
help in the determination of potential treatment routes. The
present study was designed to detect possible genetic alterations
in a homogeneous group of patients with locoregionally advanced
LSCC who underwent total laryngectomy and neck dissection. Patients
with and without lymph node metastasis were selected to examine the
differential gene expression in the normal mucosa (non-tumoral
mucosa), tumor and lymph node tissues. The main purpose of the
study was to identify the commonly expressed genes in this
homogenous group of Turkish patients with locoregionally advanced
laryngeal cancer. A further aim was to determine the predictive
role of these genes in lymph node metastasis and overall
prognosis.
Materials and methods
Ethics
The present study was performed after obtaining
approval from the Local Ethics Committee of the Dışkapı Yıldırım
Beyazıt Training and Research Hospital, University of Health
Sciences (18/42/14; Ankara, Turkey).
Tissue samples and patients
A total of 16 patients who had undergone total
laryngectomy and neck dissection for locoregionally advanced LSCC
at the Dışkapı Yıldırım Beyazıt Training and Research Hospital
(Ankara, Turkey) between January 2013 and January 2016 were
randomly chosen from the hospital's database. Their medical
records, follow-up data and formalin-fixed paraffin-embedded tissue
samples of the normal mucosa, tumors and lymph nodes were obtained.
Patients were excluded from the study if they had a history of
cancer, the presence of tumor-positive surgical margins or any
other connective tissue diseases. Eight patients with
histologically positive neck lymph nodes were assigned to Group 1,
and eight patients with negative lymph nodes were assigned to Group
2. All the specimens were re-examined by experienced head and neck
pathologists, and the patients were classified with stage 3 or 4
cancer according to the TNM Classification of Malignant Tumors, 7th
edition (14).
Gene array experiments were conducted on three
different tissue specimens from each patient, namely tumor tissue,
lymph nodes and normal mucosal tissue surrounding the tumor. The
mucosal tissue samples were collected ≥1 cm from the tumor margins.
This is consistent with previous studies in which mucosal specimens
morphologically free of carcinoma in situ or dysplasia were
evaluated as normal mucosal tissue (15,16).
All metastasis-negative lymph nodes were evaluated for
micrometastases using serial sections. Moreover, 2-mm tissue
specimens were collected from the centers of the tumor and lymph
nodes, with and without metastasis, from the paraffin blocks.
Nucleic acid isolation and microarray
analysis
Tissues from the paraffin blocks were treated with a
PureLink™ FFPE RNA Isolation Kit (Thermo Fisher Scientific, Inc.)
to isolate RNA. Using this kit, following deparaffinization, the
samples were treated with proteinase K and centrifuged in spin
columns to remove cell debris according to the manufacturer's
protocol. Isolated total RNA was eluted from the spin columns and
stored at 4°C.
The Transcriptor First Strand cDNA Synthesis Kit
(Roche Applied Science) was used to obtain complementary DNA (cDNA)
from total RNA for further analysis. The prepared solution
including the cDNA, Oligo(dT), hexamer, RNase inhibitor, dNTPs and
reverse transcriptase was incubated for 1 h at 16°C and 10 min at
65°C. After the incubation with double-stranded cDNA, amplified RNA
(aRNA) was synthesized by the in vitro transcription (IVT)
method using a GeneChip® 3′-IVT Express Kit (cat. no.
901229; Affymetrix; Thermo Fisher Scientific, Inc.). A solution was
prepared comprising IVT biotin label, buffer, enzyme mixture and
double-stranded cDNA, and the IVT reaction was performed at 40°C
for 16 h. The labeled aRNA was subsequently purified using
aRNA-binding magnetic microbeads in a binding buffer, and after
washing, the RNA was eluted with elution buffer. After elution, the
labeled aRNAs were incubated with Mg2+ ions for aRNA
fragmentation. The aRNA fragments were hybridized with a GeneChip
PrimeView Gene Expression Array (cat. no. 901838; Affymetrix;
Thermo Fisher Scientific, Inc.) for 16 h at 45°C, and streptavidin
phycoerythrin dye was used to stain the array. After staining, the
array was scanned with the Affymetrix GeneChip Scanner 3000 (Thermo
Fisher Scientific, Inc.) to obtain raw data. The raw data have been
deposited in the NCBI Gene Expression Omnibus and are accessible
through GEO Series accession number GSE201777.
Statistical analysis
Statistical analyses were performed using IBM SPSS
for Windows version 22.0 (IBM Corp.). Numerical variables are
expressed as the mean ± standard deviation. Categorical variables
are presented as numbers and percentages. Overall survival (OS),
disease-free survival (DFS) and disease-specific survival (DSS)
probabilities were estimated using the Kaplan-Meier product limit
estimator. The differences between independent groups, according to
the survival curves, were compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
result. After the GeneChips were scanned with the Affymetrix
scanner, the raw data were analyzed using Transcriptome Analysis
Console 4.0 software (Affymetrix; Thermo Fisher Scientific, Inc.).
During these analyses, the Robust Multi-chip Analysis algorithm was
used to make background adjustments and perform quantile
normalization. Summarization indicated that all samples passed
quality control checks. No additional filtering was applied to the
data. After analysis, the differentially expressed genes between
groups that had a fold change of >2 and P<0.05 were
considered statistically significant.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) validation
Gene functions were investigated and gene expression
levels were compared between metastasis-negative and -positive
samples. Two genes were found to be significantly associated with
metastasis. For RT-qPCR, RNAs were isolated with RiboEx™ (cat. no.
301-001; GeneAll®) and Hybrid-R (catalog no: 305-101;
GeneAll) kits. Later, cDNA was synthesized from the RNA using a
WizScript™ cDNA Synthesis Kit (cat. no. W2211; Wizbio). The reverse
transciption reaction was performed in 3 steps. In the first step,
samples were incubated for 10 min at 25°C, followed by incubation
for 120 min at 37°C in the second step. In the third step, samples
were incubated at 85°C for 5 min. The following primers were used:
Transgelin forward, 5′-GGGGTTAGAGAATAGTGAAGTAGGAGTA-3′ and reverse,
5′-ACACTCACAAAACTTCCTCAAAACT-3′ (17); cofilin1 forward,
5′-GGTGCTCTTCTGCCTGAGTG-3′ and reverse, 5′-TCTTGACAAAGGTGGCGTAG-3′;
and β-actin forward, 5′-CATCCTCACCCTGAAGTACC-3′ and reverse,
5′-TGAAGGTCTCAAACATGATCTG-3′. These primers were purchased from
Oligomer Biotechnology and used with a WizPure™ qPCR Master (SYBR)
kit (cat. no: W1711; Wizbio). The qPCR analysis was performed with
initial denaturation at 95°C for 300 sec, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
60 sec. By subtracting the housekeeping gene (actin) quantification
cycle (Cq) values from the Cq values obtained for each sample, the
relative expression was calculated as ΔCq. For the survival
analysis, ΔCq values >-2 were accepted as low levels of
expression and any ΔCq values <-2 were accepted as high
expression values. The gene expression levels were identified by
comparing the ΔCq of metastasis-negative and -positive samples, and
calculated as 2−ΔΔCq or fold-change values (18).
Results
Patient characteristics and survival
analysis
The mean age of the 16 patients was 56.2±5.9 years
(range, 44–72 years). The patients were all men and were followed
up for a mean period of 47.8±25.2 months. In Group 1, one patient
had a supraglottic tumor, seven patients had transglottic tumors,
and all patients had stage 4 tumors. All patients in Group 1 died;
six patients died due to locoregional recurrence (LRR) and/or
distant metastasis and two patients died due to other reasons. In
Group 2, one patient had supraglottic tumor and seven had
transglottic tumors; two patients had stage 3 tumors, and the
others had stage 4 tumors. Five patients in Group 2 were alive, one
died due to LRR and two died due to other reasons. The patient
characteristics summarized in Table
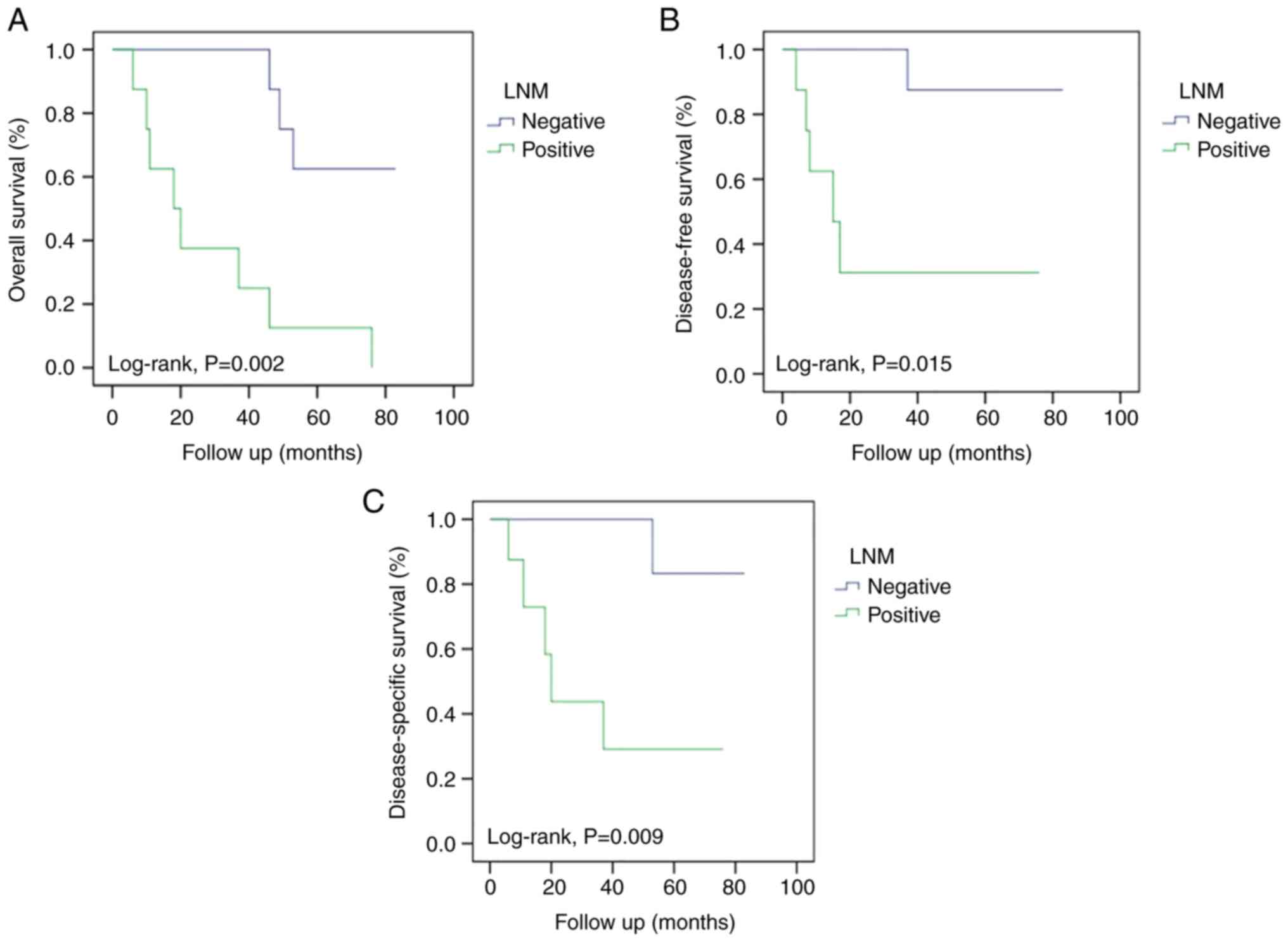
I. The 5-year OS rates were 12.5 and 62.5% in Groups 1 and 2,
respectively (P=0.002). The DFS rates were 31.3% in Group 1 and
87.5% in Group 2 (P=0.015). The DSS rates were 29.2% in Group 1 and
83.3% in Group 2 (P=0.009). The survival rates were higher in Group
2 than in Group 1, as shown by the survival curves in Fig. 1.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Sex | Age, years |
Alcohol/smoking | Site | pT | pN | Pathologic
stage | NED, LRR, DM | Mortality | Cause of death |
|---|
| Group 1 |
|
|
|
|
|
|
|
|
|
|
|
1-1 | M | 55 | N/P | Transglottic | 4 | 2c | 4 | LRR | Dead | LRR |
|
1-2 | M | 58 | N/P | Supraglottic | 4 | 1 | 4 | DM | Dead | DM |
|
1-3 | M | 49 | N/P | Transglottic | 4 | 3 | 4 | NED | Dead | OR |
|
1-4 | M | 49 | N/P | Transglottic | 4 | 2c | 4 | LRR+DM | Dead | LRR+DM |
|
1-5 | M | 51 | N/P | Transglottic | 4 | 2c | 4 | LRR+DM | Dead | LRR+DM |
|
1-6 | M | 54 | N/P | Transglottic | 4 | 1 | 4 | LRR+DM | Dead | LRR+DM |
|
1-7 | M | 57 | N/P | Transglottic | 3 | 1 | 4 | NED | Dead | OR |
|
1-8 | M | 44 | N/P | Transglottic | 4 | 2c | 4 | LRR | Dead | LRR |
| Group 2 |
|
|
|
|
|
|
|
|
|
|
|
2-1 | M | 72 | N/P | Transglottic | 4 | 0 | 4 | NED | Alive | - |
|
2-2 | M | 52 | P/P | Supraglottic | 4 | 0 | 4 | LRR | Dead | LRR |
|
2-3 | M | 56 | N/P | Transglottic | 4 | 0 | 4 | NED | Alive | - |
|
2-4 | M | 57 | N/P | Transglottic | 3 | 0 | 3 | NED | Dead | OR |
|
2-5 | M | 63 | N/P | Transglottic | 4 | 0 | 4 | NED | Alive | - |
|
2-6 | M | 55 | P/P | Transglottic | 4 | 0 | 4 | NED | Alive | - |
|
2-7 | M | 51 | N/P | Transglottic | 4 | 0 | 4 | NED | Alive | - |
|
2-8 | M | 62 | P/P | Transglottic | 3 | 0 | 3 | NED | Dead | OR |
Microarray and qPCR analyses
The average expression values of various RNAs in the
three types of tissue were compared between metastasis-positive and
-negative patients. The comparisons indicated that 68 genes were
differentially expressed in these tissues. Among these, 18 were
ribosomal proteins and 15 were associated with mitochondrial
pathways. A third group of genes were classified based on protein
synthesis and proliferation. Other genes that were identified but
not included in these groups included transgelin (TAGLN) and
cofilin 1 (CFL1) (Table SI;
Fig. 2).
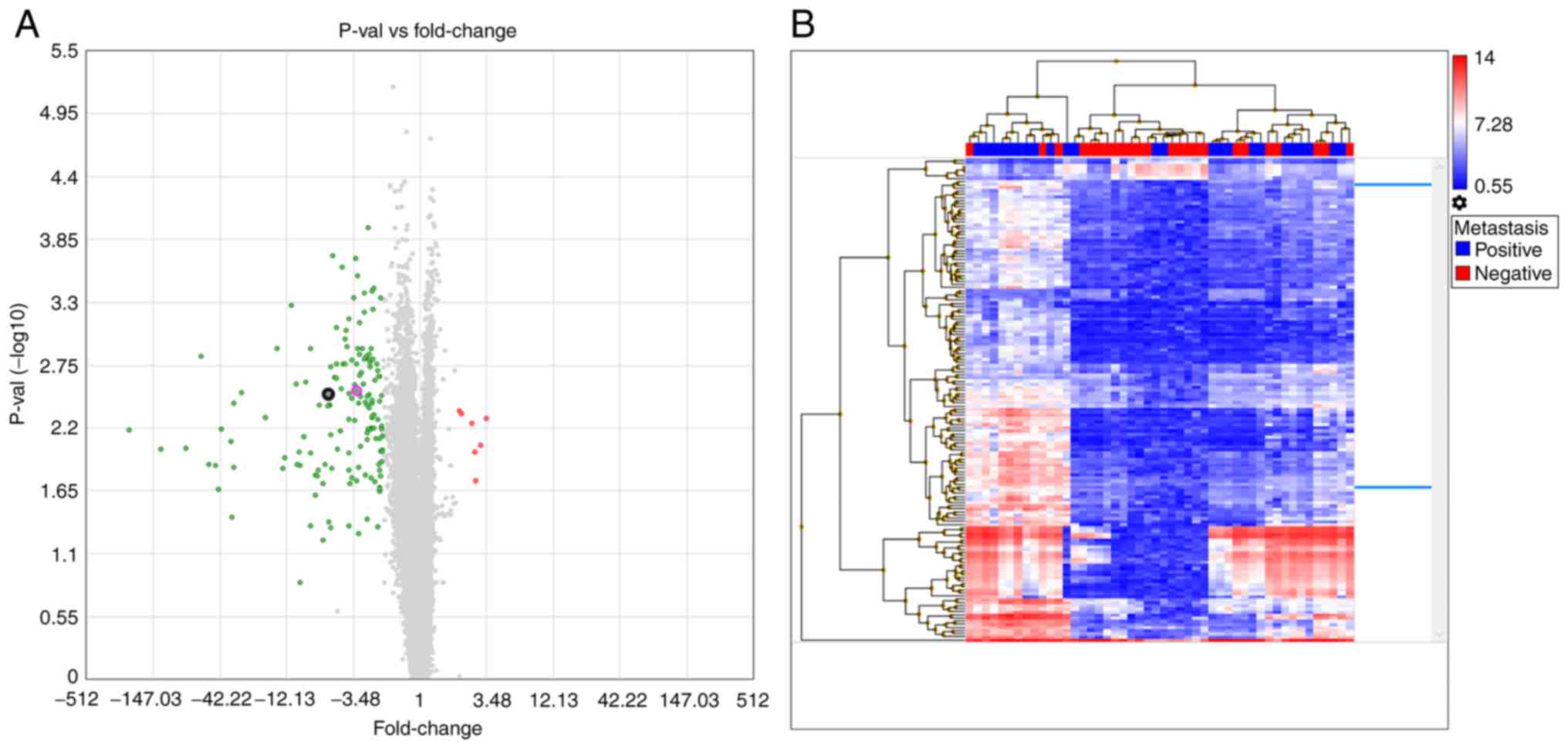
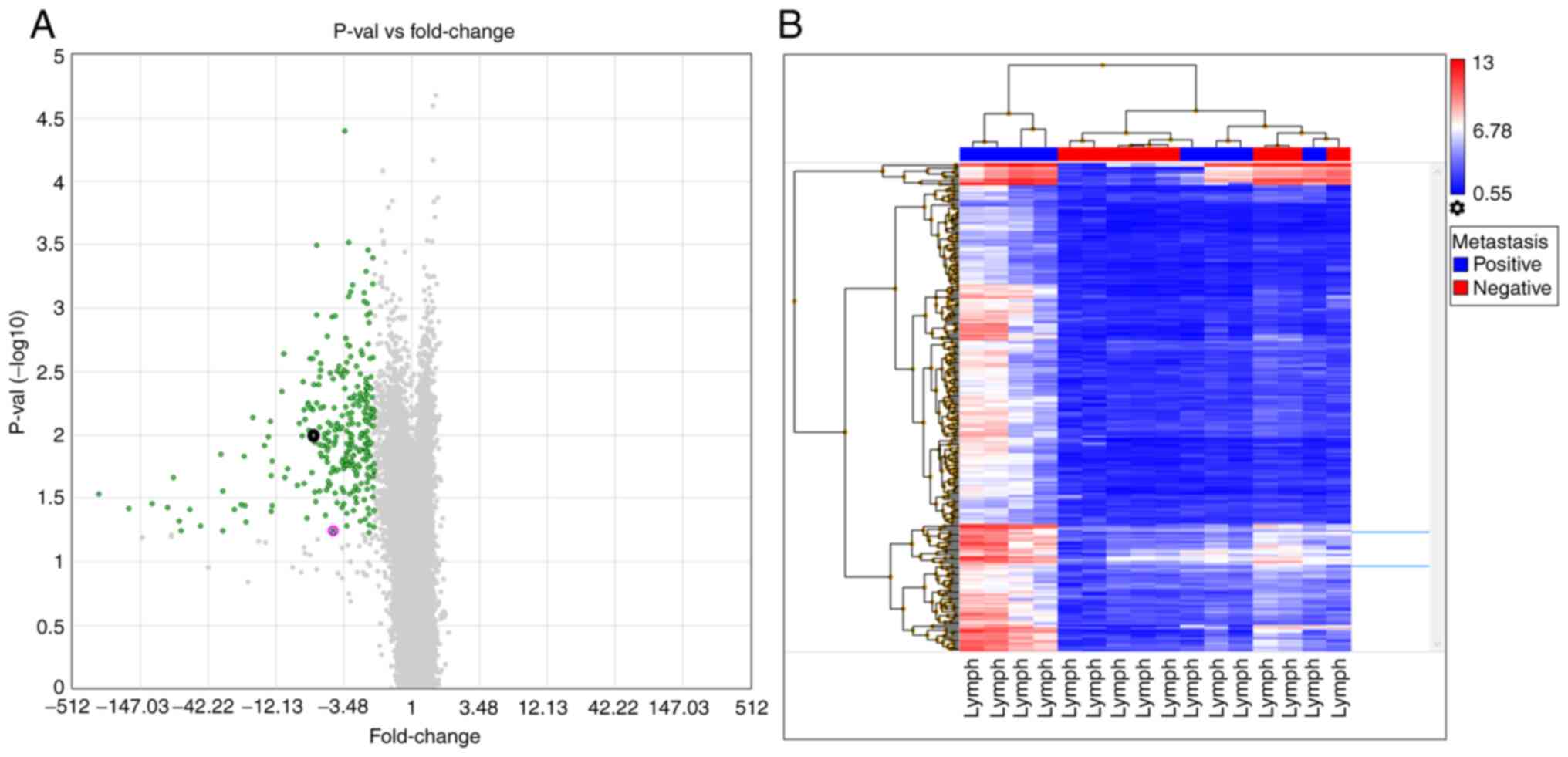
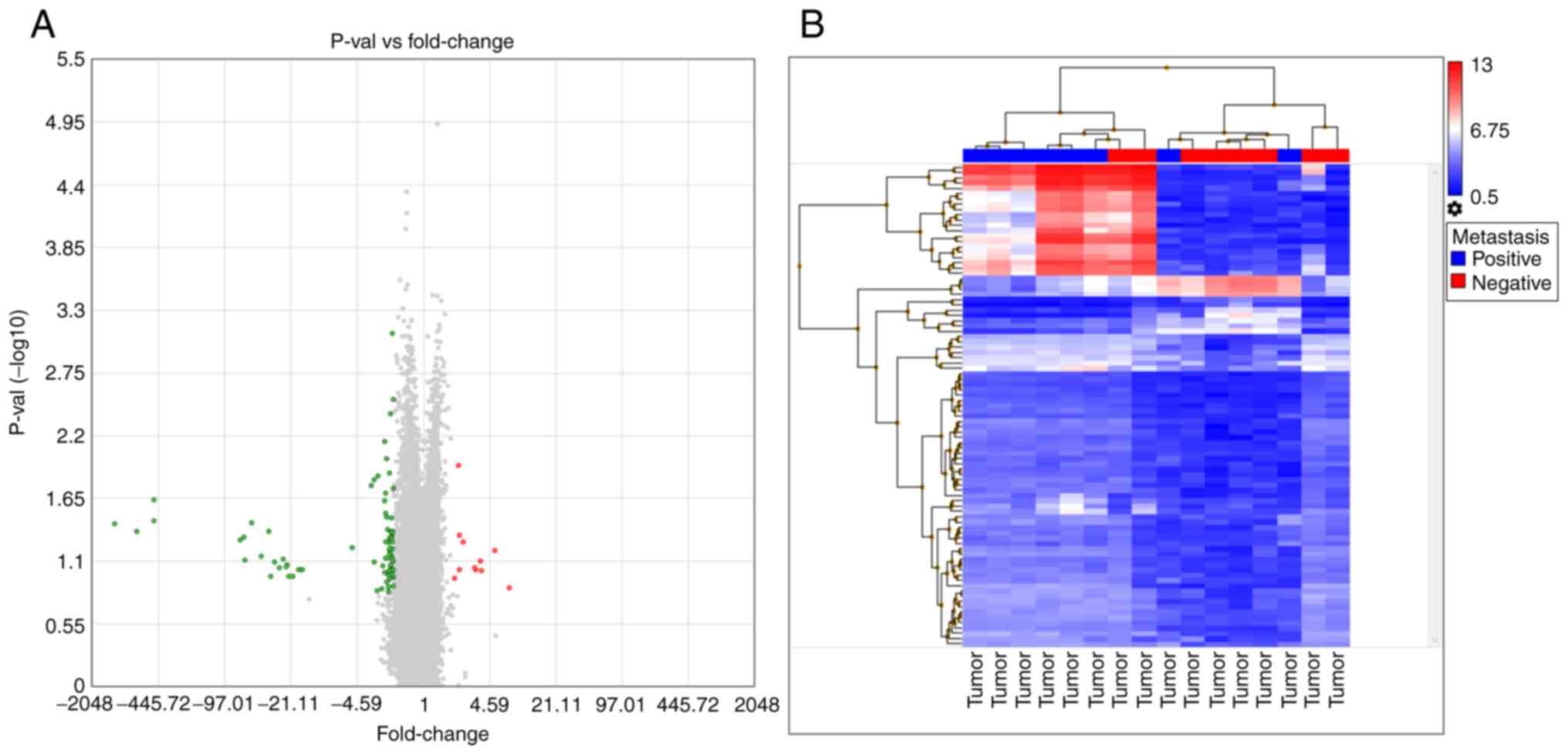
The lymph node, mucosal and tumor tissues yielded
similar results when compared between metastasis-negative and
-positive patients. When lymph node tissues were investigated in
the lymph node metastasis-positive and -negative groups, 312 genes
showed a ≥2-fold expression difference (Table SII). A volcano plot of this
analysis indicated that all the differentially expressed genes were
downregulated in metastasis-negative tissues. In addition, a
hierarchical analysis indicated similar signal distributions
between the groups (Fig. 3). When
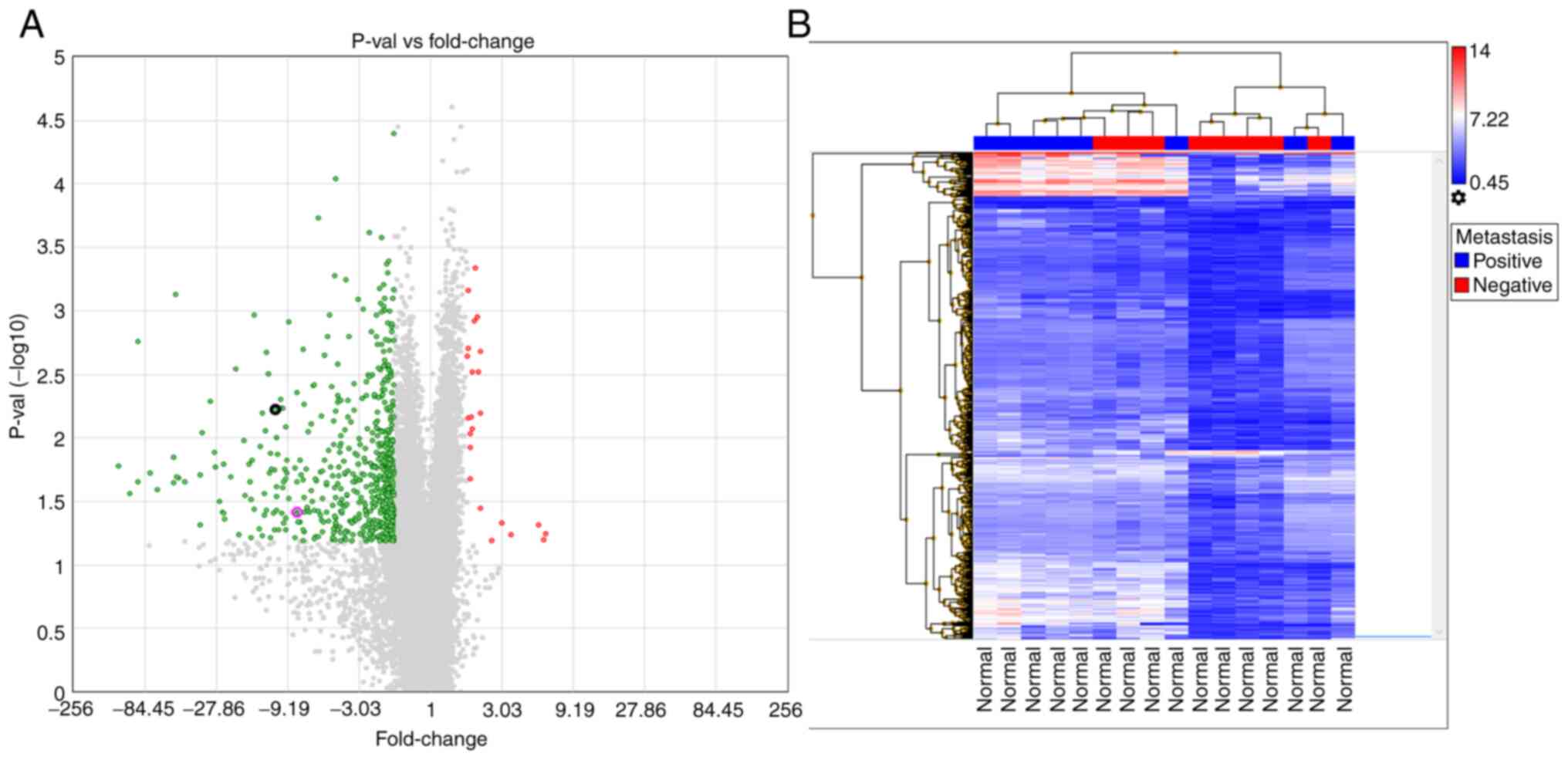
the normal mucosal tissues were investigated, 691 genes showed a
≥2-fold difference in expression (Table SIII). The volcano plot of this
analysis indicated that 24 of the genes were upregulated in the
metastasis-negative tissues. Moreover, the hierarchical analysis
indicated similar signal distributions between the groups (Fig. 4). When the tumor tissues were
investigated, 93 genes showed a ≥2-fold difference in expression
(Table SIV). The volcano plot of
this analysis indicated that 11 of the genes were upregulated in
the metastasis-negative group. Hierarchical analysis indicated
similar signal distributions between the groups (Fig. 5).
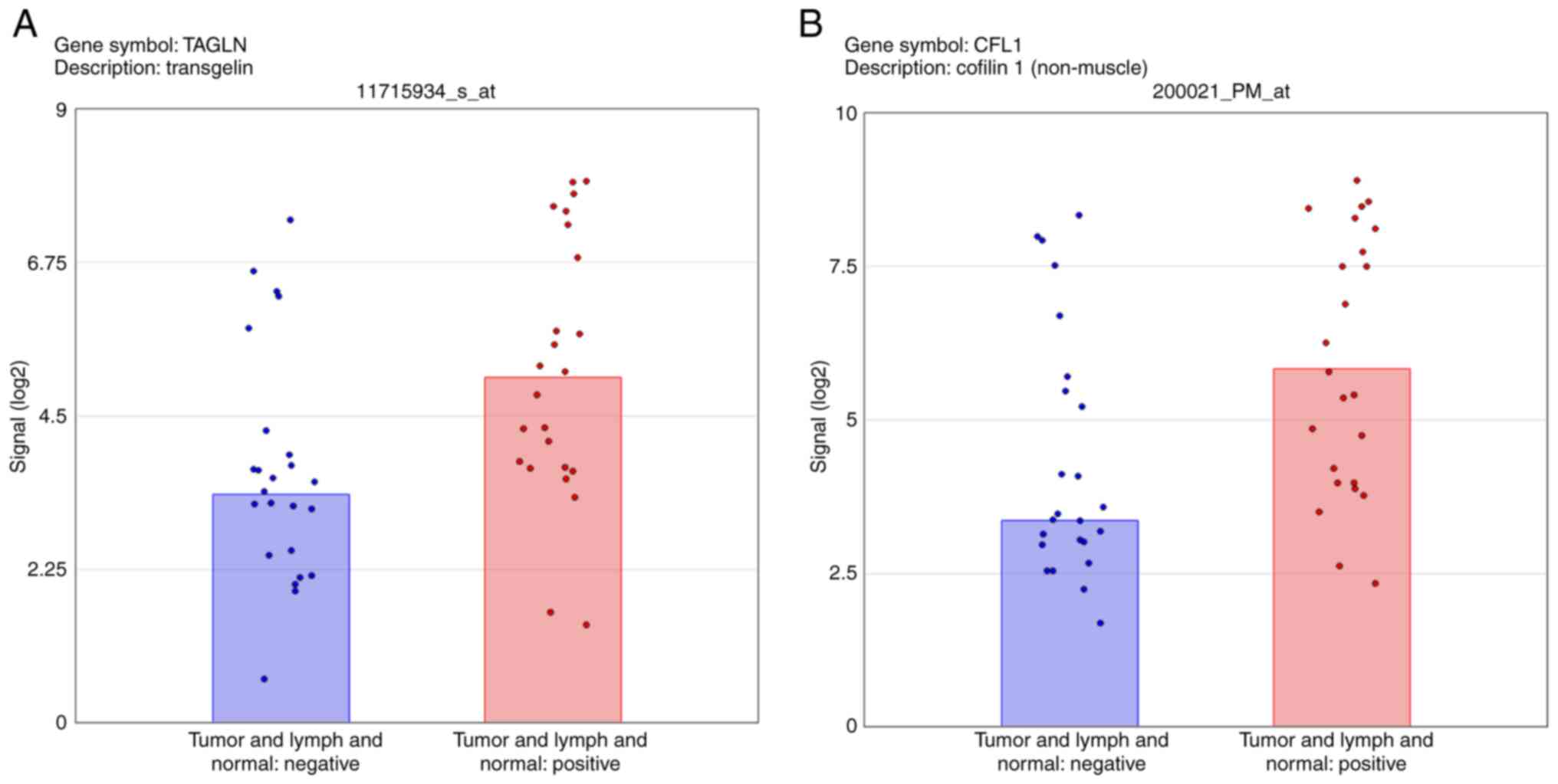
The analysis of average expression in the three
tissues revealed the upregulation of TAGLN and CFL1 in Group 1, and
these genes were then selected for RT-qPCR validation (Fig. 6). The microarray data for the
mucosa and lymph node tissues revealed the differential expression
of CFL1 and TAGLN according to lymph node metastasis status. By
contrast, the microarray data for the tumor tissue did not show a
significant difference in the expression of these genes. The PCR
ΔΔCq values of TAGLN also indicated significant differences between
the mucosa and lymph nodes, and the ΔΔCq values of CFL1 in the
mucosa and tumor tissue validated the microarray data.
The microarray analysis of the average of the three
tissue types indicates a 3.29-fold difference between the lymph
node metastasis-negative and -positive groups for TAGLN (Fig. 2A). When examined individually (the
most abundant probe was chosen to demonstrate differences if there
was more than one probe targeted for a gene), the mucosal tissue
showed an 11.06-fold increase in TAGLN expression in Group 1
compared with Group 2 (Fig. 4A)
and the lymph tissue showed a 4.23-fold increase (Fig. 3A); however, no significant
difference in TAGLN was observed in the tumor tissues (Fig. 5A). The PCR data validated these
microarray results, with fold differences in the mucosa, lymph node
and tumor tissues of 22.87-, 154.64- and 2.17-fold, respectively.
By contrast, the CFL1 microarray analysis revealed a 5.53-fold
increase in Group 1 compared with Group 2 for the average in the
three tissues (Fig. 2A), a
7.91-fold change in the mucosa (Fig.
4A) and a 6.06-fold change in lymph node (Fig. 3A) tissues. Additionally, no
significant difference in CFL1 between the groups was observed in
the tumor tissues (Fig. 5A). The
PCR data for CFL1 validated the results for mucosal tissues with a
2.03-fold change.
OS, DFS and DSS analyses indicated that survival
between the lymph node metastasis-positive and -negative groups was
differed significantly. In addition, survival comparison between
patients with high- and low-level TAGLN gene expression indicated
an association between high TAGLN expression and decreased OS, DFS
and DSS in all tissues. However, a significant association was only
observed with high TAGLN expression in the lymph nodes. By
contrast, higher CFL1 expression levels in tumor and mucosal
tissues were associated with increased survival. However,
statistically significant differences based on the expression of
CFL1 were only observed for DFS and DSS in the tumor tissues
(Table II).
 | Table II.Survival analysis according to lymph
node status and gene expression. |
Table II.
Survival analysis according to lymph
node status and gene expression.
|
| OS | DFS | DSS |
|---|
|
|
|
|
|
|---|
| Variable | Mean survival time
(95% CI) | P-value | Mean survival time
(95% CI) | P-value | Mean survival time
(95% CI) | P-value |
|---|
| Lymph node
status |
|
|
|
|
|
|
|
Negative | 71.1
(60.4–81.8) | 0.002 | 77.3
(66.7–87.8) | 0.015 | 78.3
(70.0–86.7) | 0.009 |
|
Positive | 30.8
(14.7–46.8) |
| 31.1
(8.4–53.8) |
| 38.7
(19.0–58.4) |
|
| TAGLN tumor
tissue |
|
|
|
|
|
|
|
High | 48.5
(32.6–64.4) | 0.879 | 53.5
(33.7–73.3) | 0.605 | 58.0
(40.8–75.0) | 0.561 |
|
Low | 56.8
(29.1–84.4) |
| 58.8
(29.5–88.0) |
| 63.0
(39.2–86.8) |
|
| TAGLN mucosal
tissue |
|
|
|
|
|
|
|
High | 43.8
(28.4–59.1) | 0.178 | 47.0
(26.6–67.3) | 0.093 | 52.0
(34.4–69.5) | 0.087 |
|
Low | 70.3
(57.1–83.4) |
| 79.0
(79.0–79.0) |
| 79.0
(79.0–79.0) |
|
| TAGLN lymph
node |
|
|
|
|
|
|
|
High | 33.7
(19.1–48.2) | 0.018 | 32.4
(11.8–52.9) | 0.005 | 39.6
(22.3–56.9) | 0.005 |
|
Low | 71.3
(60.6–82.0) |
| 83.0
(83.0–83.0) |
| 83.0
(83.0–83.0) |
|
| CFL1 tumor
tissue |
|
|
|
|
|
|
|
High | 56.0
(38.0–74.0) | 0.270 | 74.7
(59.3–90.1) | 0.021 | 75.0
(60.2–89.8) | 0.039 |
|
Low | 43.6
(23.2–64.0) |
| 33.1
(11.8–54.5) |
| 43.6
(24.9–62.2) |
|
| CFL1 mucosal
tissue |
|
|
|
|
|
|
|
High | 59.4
(43.6–75.3) | 0.412 | 63.9
(41.4–86.3) | 0.397 | 69.0
(52.5–85.5) | 0.411 |
|
Low | 43.3
(24.6–62.1) |
| 49.2
(27.1–71.3) |
| 52.0
(31.7–72.4) |
|
| CFL1 lymph
node |
|
|
|
|
|
|
|
High | 49.3
(24.4–74.1) | 0.622 | 52.0
(24.8–79.2) | 0.644 | 53.5
(27.2–79.8) | 0.643 |
|
Low | 49.0
(34.3–63.7) |
| 53.8
(34.2–73.3) |
| 58.7
(42.5–75.0) |
|
Discussion
In several types of cancer, it has been shown that
there are various genetic markers that may predict regional
metastasis. However, only a few studies have reported these
differences in LSCC. In a case-control study, it was suggested that
the presence of ‘risk alleles’ of the nucleotide excision repair
genes ERCC excision repair 1 (ERCC1), ERCC5, ERCC6 and RAD23
homolog B could significantly increase the risk of laryngeal cancer
in patients with a history of smoking and alcohol intake (19). The expression of Notch pathway
proteins has been shown to play a similar role in laryngeal cancer
prognosis (20). The
overexpression of EGFR, which is well known to occur in HNSCC, may
play a role in poor prognosis and resistance to treatment in LSCC
(21,22). It has also been reported that lower
expression levels of membrane-associated protein 17 (MAP17) in LSCC
are associated with poorer OS and laryngoesophageal
dysfunction-free survival rates (23), while conversely, the upregulation
of MAP17 and H2AX phosphorylation are associated with improved
survival rates (24). Another
study suggested that the increased interaction between endoplasmic
reticulum protein 57 and STAT3 may contribute to radioresistance in
LSCC (25). Yang et al
(26) concluded that the
overexpression of diaphanous related formin 1 (DIAPH1) regulates
apoptosis via the ataxia telangiectasia and
Rad3-related/p53/caspase-3 signaling pathway in LSCC. The authors
suggested that DIAPH1 acts as an oncogene and is a potential
therapeutic target. Li et al (27) suggested that the low expression of
cell adhesion molecule 1 and contactin associated protein 2 genes
and high expression of folate receptor γ and kynureninase genes may
be indicators of chemosensitivity in LSCC. Another study indicated
that the downregulation of zinc finger E-box-binding homeobox 2
protein, which is associated with proliferation, migration,
invasion, cell cycle progression, apoptosis and
epithelial-mesenchymal transition (EMT), could play a promising
role in LSCC treatment (28). In
addition, the DNA methylation of CpG islands, HOX transcript
antisense RNA, CLKF-like MARVEL transmembrane domain containing 3,
MYC target 1, zinc finger protein 667 (ZNF667)-antisense RNA 1 and
ZNF667, has been revealed to cause epigenetic alterations in LSCC
(29–32). Zhang et al observed that the
downregulation of dachshund family transcription factor was
associated with advanced clinical stage in patients with LSCC
(33). In another study, the
upregulation of CDR1 antisense RNA was shown to be related to tumor
progression (34). Although
several studies have focused on the genomics and proteomics of
LSCC, there are no effective predictive molecular targets or
markers for LSCC (13,35–39).
Moreover, it is not possible to predict lymph node metastasis using
the currently available genetic information in LSCC (40).
The literature suggests several potential biomarkers
(19–34). CFL1 is an important protein that
contributes to cell migration processes and serves a crucial role
in actin filament dynamics as well as in oxidant-induced apoptosis
(41). The results of the present
study suggest that CFL1 may serve as a biomarker for LSCC. Lu et
al (42) reported that CFL1
plays an important role in the development of prostate cancer and
lymph node metastasis, while Polachini et al (43) affirmed that CFL1 modulates cell
invasion in oral cavity squamous cell cancers. In addition,
Madak-Erdogan et al (44)
found that upregulated CFL1 expression was associated with tumor
aggressiveness and poor prognosis in patients with estrogen
receptor α-negative breast cancer. Furthermore, Zhang et al
presented results indicating that TAGLN and CFL1 genes are involved
in the development of esophageal squamous cell cancer, leading to
the suggestion that CFL1 can be used as a biomarker in the early
diagnosis of this type of cancer (45). Although the microarray differential
expression analyses and gene expression-dependent DFS and DSS
results for CFL1 in the present study showed similar significance
to those in previous studies, the PCR analysis did not indicate a
strong association. These results suggest that it is necessary to
analyze a larger group of patients to understand whether CFL1 is
suitable for use as a lymph node metastasis biomarker.
According to the results of the present study, TAGLN
is another gene that may contribute to the clinical behavior of
LSCC. No study has established a relationship between TAGLN and
LSCC. However, TAGLN has been shown to have effects on numerous
different types of cancer. TAGLN, also known as smooth muscle 22α,
is an actin-binding protein abundantly expressed on smooth muscle
cells (46). It has been reported
that the increased expression of TAGLN may trigger the development
of metastasis and affect the prognosis of colorectal cancer
(47,48). Xu et al (49) suggested that TAGLN can be used in
the postoperative follow-up period to screen for recurrence in
colorectal cancer, and as a prognostic marker. Furthermore,
Dvorakova et al (50) demonstrated
that TAGLN expression was higher in breast cancer patients with
lymph node metastasis compared with those without metastasis. In
addition, Wu et al (51)
suggested that TAGLN may be a potential biomarker and therapeutic
target gene in lung adenocarcinoma, while Bu et al (52) argued that TAGLN overexpression in
tissues and salivary secretion is an independent prognostic factor
in oral cavity cancer and has the potential to be used as a
reliable biomarker. A clear relationship between TAGLN and LSCC was
revealed by the results of the present study, which showed that
higher TAGLN expression was associated with poorer prognosis. These
results were confirmed by microarray, PCR and survival analyses. In
order to invade and metastasize, it is necessary for cancerous
cells to exhibit certain properties, including reorganization of
the actin cytoskeleton, an increase or change of metalloproteinases
in the extracellular fluid, and focal adhesion signaling (53,54).
Additionally, EMT is an important process occurring in cancer cells
with metastatic properties, which plays a major role in resistance
to cancer treatment (55). Lin
et al (56) suggested that
TAGLN expression in different tissues and tumors is consistent with
its involvement in EMT, by which tumor cells exhibit a more
aggressive phenotype. In accordance with these data and the results
of the present study, the overexpression of TAGLN has the potential
to be an important poor prognostic factor for cancers of epithelial
origin, including LSCC.
In the present study, the DFS and DSS analyses
suggested that changes in CFL1 and TAGLN expression may have
important effects on survival. Increased TAGLN expression in lymph
node tissues was indicative of poor survival. The increase in TAGLN
expression in the mucosal tissue of patients in the
metastasis-positive group, 11.06-fold in the microarray and
22.87-fold in the RT-qPCR results, appears to be a strong predictor
of regional metastasis. The survival analysis supported the
microarray and RT-qPCR results and strengthened the potential of
TAGLN as a novel biomarker for poor prognosis and metastasis.
Similarly, the mean survival time of patients with high CFL1
expression in the tumor tissue was longer than that of patients
with low tumor CFL1 expression, which indicates that CFL1 may be a
useful biomarker for good prognosis. However, these results require
confirmation in a different cohort and a larger sample group.
Moreover, protein-level analyses should be included in further
studies to reveal the significance of these genes at the protein
level. Another limitation of the present study is the inclusion of
the samples from paraffin-embedded tissues; it is speculated that
fresh tissues may give a higher resolution for expression level
analysis.
In conclusion, TAGLN and CFL1 may serve important
roles in the mechanism of regional lymph node metastasis in
advanced LSCC. The downregulation of CFL1 and upregulation of TAGLN
are associated with regional metastasis. However, their combined
effect and their relationship with metastasis merit further
discussion and investigation in further studies. Although this
relationship and its effects are not yet fully understood, the
findings of the present study may increase the possibility of
obtaining an early diagnosis for metastatic advanced LSCC and
predicting a poor prognosis for the disease. If the present results
are confirmed by the analysis of large populations, these genes may
be used as biomarkers for the prediction of future regional
metastasis. Further studies with a larger group of patients should
be conducted to understand the mechanisms and clinical value of
these two genes in LSCC. Moreover, these data could be collected at
different stages of lymph node metastasis to elucidate the combined
effects of TAGLN and CFL1.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Sevilay Karahan
(Department of Biostatistics, Hacettepe University School of
Medicine, Ankara, Turkey) for helping with the statistical
analysis.
Funding
The present study was supported by the Ankara Yıldırım Beyazıt
University Scientific Research Unit fund (project no. 1867).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available in the Gene Expression Omnibus repository
(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE201777).
Authors' contributions
ÖB, MDA, GS and FAP were responsible for the study
concept and ÖB, MDA, GS, FAP and UH were responsible for study
design. EÇT, UH, EŞ and MHK supervised the study. ÖB, MDA and GS
provided resources and ÖB, MDA, UH and FPA provided materials. ÖB,
MDA, GS, UH, FPA, EÇT, EŞ and MHK performed data collection and/or
processing. ÖB, MDA, GS, UH, FPA and MHK contributed to the
analysis and/or interpretation of the data. ÖB, MDA, UH and FPA
searched the literature. ÖB and MDA wrote the manuscript and EÇT,
FPA and MHK critically reviewed it. All authors read and approved
the final version of the manuscript. ÖB and MDA confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was performed at Dışkapı Yıldırım Beyazıt
Training and Research Hospital after approval by the local Ethics
Committee (18/42/14) and was conducted in compliance with the
principle of the Declaration of Helsinki. Written informed consent
was obtained from all patients or their family members.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CFL1
|
cofilin 1
|
|
cDNA
|
complementary DNA
|
|
Cq
|
quantification cycle
|
|
DFS
|
disease-free survival
|
|
DSS
|
disease specific survival
|
|
HNC
|
head and neck cancer
|
|
IVT
|
in vitro transcription
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
OS
|
overall survival
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TAGLN
|
transgelin
|
References
|
1
|
Vigneswaran N and Williams MD:
Epidemiologic trends in head and neck cancer and aids in diagnosis.
Oral Maxillofac Surg Clin North Am. 26:123–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mifsud M, Eskander A, Irish J, Gullane P,
Gilbert R, Brown D, de Almeida JR, Urbach DR and Goldstein DP:
Evolving trends in head and neck cancer epidemiology: Ontario,
Canada 1993–2010. Head Neck. 39:1770–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dok R and Nuyts S: HPV positive head and
neck cancers: Molecular pathogenesis and evolving treatment
strategies. Cancers (Basel). 8:412016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
World Health Organization (WHO), . World
Cancer Report 2014. WHO; Geneva: pp. 422–435. 2014
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Republic of Turkey Ministry of Health,
General Directorate of Public Health, . Cancer Statistics.
https://hsgm.saglik.gov.tr/depo/birimler/kanser-db/istatistik/2014-RAPOR._uzuuun.pdfSeptember
3–2019
|
|
8
|
Leoncini E, Vukovic V, Cadoni G, Pastorino
R, Arzani D, Bosetti C, Canova C, Garavello W, La Vecchia C, Maule
M, et al: Clinical features and prognostic factors in patients with
head and neck cancer: Results from a multicentric study. Cancer
Epidemiol. 39:367–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen EE, LaMonte SJ, Erb NL, Beckman KL,
Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS,
Stein KD, et al: American cancer society head and neck cancer
survivorship care guideline. CA Cancer J Clin. 66:203–239. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Timmermans AJ, de Gooijer CJ,
Hamming-Vrieze O, Hilgers FJ and van den Brekel MW: T3-T4 laryngeal
cancer in The Netherlands cancer institute; 10-year results of the
consistent application of an organ-preserving/-sacrificing
protocol. Head Neck. 37:1495–1503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Layland MK, Sessions DG and Lenox J: The
influence of lymph node metastasis in the treatment of squamous
cell carcinoma of the oral cavity, oropharynx, larynx, and
hypopharynx: N0 versus N+. Laryngoscope. 115:629–639. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Miguel-Luken MJ, Chaves-Conde M and
Carnero A: A genetic view of laryngeal cancer heterogeneity. Cell
Cycle. 15:1202–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Compton CC, Byrd DR, Garcia-Aguilar J,
Kurtzman SH, Olawaiye A and Washington MK: Larynx. AJCC Cancer
Staging Atlas: A companion to the seventh editions of the AJCC
cancer staging manual and handbook TNM classification of malignant
tumours. Springer; New York, NY: pp. 79–90. 2012, View Article : Google Scholar
|
|
15
|
Ciolofan MS, Vlăescu AN, Mogoantă CA,
Ioniță E, Ioniță I, Căpitănescu AN, Mitroi MR and Anghelina F:
Clinical, histological and immunohistochemical evaluation of larynx
cancer. Curr Health Sci J. 43:367–375. 2017.PubMed/NCBI
|
|
16
|
Sanguansin S, Kosanwat T, Juengsomjit R
and Poomsawat S: Diagnostic value of cytokeratin 17 during oral
carcinogenesis: An immunohistochemical study. Int J Dent.
2021:40895492021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sayar N, Karahan G, Konu O, Bozkurt B,
Bozdogan O and Yulug IG: Transgelin gene is frequently
downregulated by promoter DNA hypermethylation in breast cancer.
Clin Epigenetics. 7:1042015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbasi R, Ramroth H, Becher H, Dietz A,
Schmezer P and Popanda O: Laryngeal cancer risk associated with
smoking and alcohol consumption is modified by genetic
polymorphisms in ERCC5, ERCC6 and RAD23B but not by polymorphisms
in five other nucleotide excision repair genes. Int J Cancer.
125:1431–1439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krikelis D, Kotoula V, Bobos M, Fountzilas
E, Markou K, Karasmanis I, Angouridakis N, Vlachtsis K, Kalogeras
KT, Nikolaou A and Fountzilas G: Protein and mRNA expression of
notch pathway components in operable tumors of patients with
laryngeal cancer. Anticancer Res. 34:6495–6503. 2014.PubMed/NCBI
|
|
21
|
Demiral AN, Sarioglu S, Birlik B, Sen M
and Kinay M: Prognostic significance of EGF receptor expression in
early glottic cancer. Auris Nasus Larynx. 31:417–424. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nijkamp MM, Span PN, Terhaard CHJ,
DoornaertPA H, Langendijk JA, van den EndePLA, de Jong M, van der
Kogel AJ, Bussink J and Kaanders JHAM: Epidermal growth factor
receptor expression in laryngeal cancer predicts the effect of
hypoxia modification as an additive to accelerated radiotherapy in
a randomised controlled trial. Eur J Cancer. 49:3202–3209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Miguel-Luken MJ, Chaves-Conde M, de
Miguel-Luken V, Muñoz-Galván S, López-Guerra JL, Mateos JC, Pachón
J, Chinchón D, Suarez V and Carnero A: MAP17 (PDZKIP1) as a novel
prognostic biomarker for laryngeal cancer. Oncotarget.
6:12625–12636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Miguel-Luken MJ, Chaves-Conde M,
Quintana B, Menoyo A, Tirado I, de Miguel-Luken V, Pachón J,
Chinchón D, Suarez V and Carnero A: Phosphorylation of gH2AX as a
novel prognostic biomarker for laryngoesophageal dysfunction-free
survival. Oncotarget. 7:31723–31737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choe MH, Min JW, Jeon HB, Cho DH, Oh JS,
Lee HG, Hwang SG, An S, Han YH and Kim JS: ERp57 modulates STAT3
activity in radioresistant laryngeal cancer cells and serves as a
prognostic marker for laryngeal cancer. Oncotarget. 6:2654–2666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Zhou L, Zhang Y, Zheng J, Zhou J,
Wei Z and Zou J: DIAPH1 Is upregulated and inhibits cell apoptosis
through ATR/p53/caspase-3 signaling pathway in laryngeal squamous
cell carcinoma. Dis Markers. 2019:67164722019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Wang R, He S, Shen X, Kong F, Li S,
Zhao H, Lian M and Fang J: The identification of induction
chemo-sensitivity genes of laryngeal squamous cell carcinoma and
their clinical utilization. Eur Arch Otorhinolaryngol.
275:2773–2781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Ma L, Wu Z, Wang G, Huang Q, Shen Z
and Yu R: Zinc finger Ebox binding homeobox 2 functions as an
oncogene in human laryngeal squamous cell carcinoma. Mol Med Rep.
19:4545–4552. 2019.PubMed/NCBI
|
|
29
|
Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J
and Liu M: Long intergenic noncoding RNA HOTAIR is overexpressed
and regulates PTEN methylation in laryngeal squamous cell
carcinoma. Am J Pathol. 182:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng W, Cui W, Zhao L, Chi W, Cao H and
Wang B: Aberrant methylation and downregulation of ZNF667-AS1 and
ZNF667 promote the malignant progression of laryngeal squamous cell
carcinoma. J Biomed Sci. 26:132019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen Z, Chen X, Li Q, Zhou C, Xu Y, Yu R,
Ye H, Li J and Duan S: Elevated methylation of CMTM3 promoter in
the male laryngeal squamous cell carcinoma patients. Clin Biochem.
49:1278–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Li W, Liu YY, Fu S, Qiu GB, Sun KL
and Fu WN: Promoter hypermethylation-induced transcriptional
down-regulation of the gene MYCT1 in laryngeal squamous cell
carcinoma. BMC Cancer. 12:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Ren X, Wang B, Cao J, Tian L and
Liu M: Effect of DACH1 on proliferation and invasion of laryngeal
squamous cell carcinoma. Head Face Med. 14:202018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Hu H and Zhao Y and Zhao Y:
CDR1as is overexpressed in laryngeal squamous cell carcinoma to
promote the tumour's progression via miR-7 signals. Cell Prolif.
51:e125212018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu X and Li Z: The role of microRNAs
expression in laryngeal cancer. Oncotarget. 6:23297–23305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Zhao X, Liu Y, An J, Xiao H and Wang
C: Aberrant expression of CDK8 regulates the malignant phenotype
and associated with poor prognosis in human laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 274:2205–2213. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Markowski J, Oczko-Wojciechowska M, Gierek
T, Jarzab M, Paluch J, Kowalska M, Wygoda Z, Pfeifer A, Tyszkiewicz
T, Jarzab B, et al: Gene expression profile analysis in laryngeal
cancer by high-density oligonucleotide microarrays. J Physiol
Pharmacol. 60 (Suppl 1):S57–S63. 2009.PubMed/NCBI
|
|
38
|
Shen Z, Li Q, Deng H, Lu D, Song H and Guo
J: Long non-coding RNA profiling in laryngeal squamous cell
carcinoma and its clinical significance: Potential biomarkers for
LSCC. PLoS One. 9:e1082372014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu CZ, Shi RJ, Chen D, Sun YY, Wu QW, Wang
T and Wang PH: Potential biomarkers for paclitaxel sensitivity in
hypopharynx cancer cell. Int J Clin Exp Pathol. 6:2745–2756.
2013.PubMed/NCBI
|
|
40
|
Chung CH, Parker JS, Karaca G, Wu J,
Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X,
et al: Molecular classification of head and neck squamous cell
carcinomas using patterns of gene expression. Cancer Cell.
5:489–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klamt F, Zdanov S, Levine RL, Pariser A,
Zhang Y, Zhang B, Yu LR, Veenstra TD and Shacter E: Oxidant-induced
apoptosis is mediated by oxidation of the actin-regulatory protein
cofilin. Nat Cell Biol. 11:1241–1246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu LI, Fu NI, Luo XU, Li XY and Li XP:
Overexpression of cofilin 1 in prostate cancer and the
corresponding clinical implications. Oncol Lett. 9:2757–2761. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Polachini GM, Sobral LM, Mercante AM,
Paes-Leme AF, Xavier FCA, Henrique T, Guimarães DM, Vidotto A,
Fukuyama EE, Góis-Filho JF, et al: Proteomic approaches identify
members of cofilin pathway involved in oral tumorigenesis. PLoS
One. 7:e505172012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Madak-Erdogan Z, Ventrella R, Petry L and
Katzenellenbogen BS: Novel roles for ERK5 and cofilin as critical
mediators linking ERalpha-driven transcription, actin
reorganization, and invasiveness in breast cancer. Mol Cancer Res.
12:714–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Liao R, Li H, Liu L, Chen X and
Chen H: Expression of Cofilin-1 and transgelin in esophageal
squamous cell carcinoma. Med Sci Monit. 21:2659–2665. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Camoretti-Mercado B, Forsythe SM, LeBeau
MM, Espinosa R III, Vieira JE, Halayko AJ, Willadsen S, Kurtz B,
Ober C, Evans GA, et al: Expression and cytogenetic localization of
the human SM22 gene (TAGLN). Genomics. 49:452–457. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou H, Zhang Y, Chen Q and Lin Y: AKT and
JNK signaling pathways increase the metastatic potential of
colorectal cancer cells by altering transgelin expression. Dig Dis
Sci. 61:1091–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou HM, Fang YY, Weinberger PM, Ding LL,
Cowell JK, Hudson FZ, Ren M, Lee JR, Chen QK, Su H, et al:
Transgelin increases metastatic potential of colorectal cancer
cells in vivo and alters expression of genes involved in cell
motility. BMC Cancer. 16:552016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu L, Gao Y, Chen Y, Xiao Y, He Q, Qiu H
and Ge W: Quantitative proteomics reveals that distant
recurrence-associated protein R-Ras and Transgelin predict
post-surgical survival in patients with stage III colorectal
cancer. Oncotarget. 7:43868–43893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dvorakova M, Jerabkova J, Prochazkova I,
Lenco J, Nenutil R and Bouchal P: Transgelin is upregulated in
stromal cells of lymph node positive breast cancer. J Proteomics.
132:103–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu X, Dong L, Zhang R, Ying K and Shen H:
Transgelin overexpression in lung adenocarcinoma is associated with
tumor progression. Int J Mol Med. 34:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bu J, Bu X, Liu B, Chen F and Chen P:
Increased expression of tissue/salivary transgelin mRNA predicts
poor prognosis in patients with oral squamous cell carcinoma
(OSCC). Med Sci Monit. 21:2275–2281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Roche J: Erratum: Roche, J. The
epithelial-to-mesenchymal transition in cancer. Cancers (Basel).
10:792018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin Y, Buckhaults PJ, Lee JR, Xiong H,
Farrell C, Podolsky RH, Schade RR and Dynan WS: Association of the
actin-binding protein transgelin with lymph node metastasis in
human colorectal cancer. Neoplasia. 11:864–873. 2009. View Article : Google Scholar : PubMed/NCBI
|