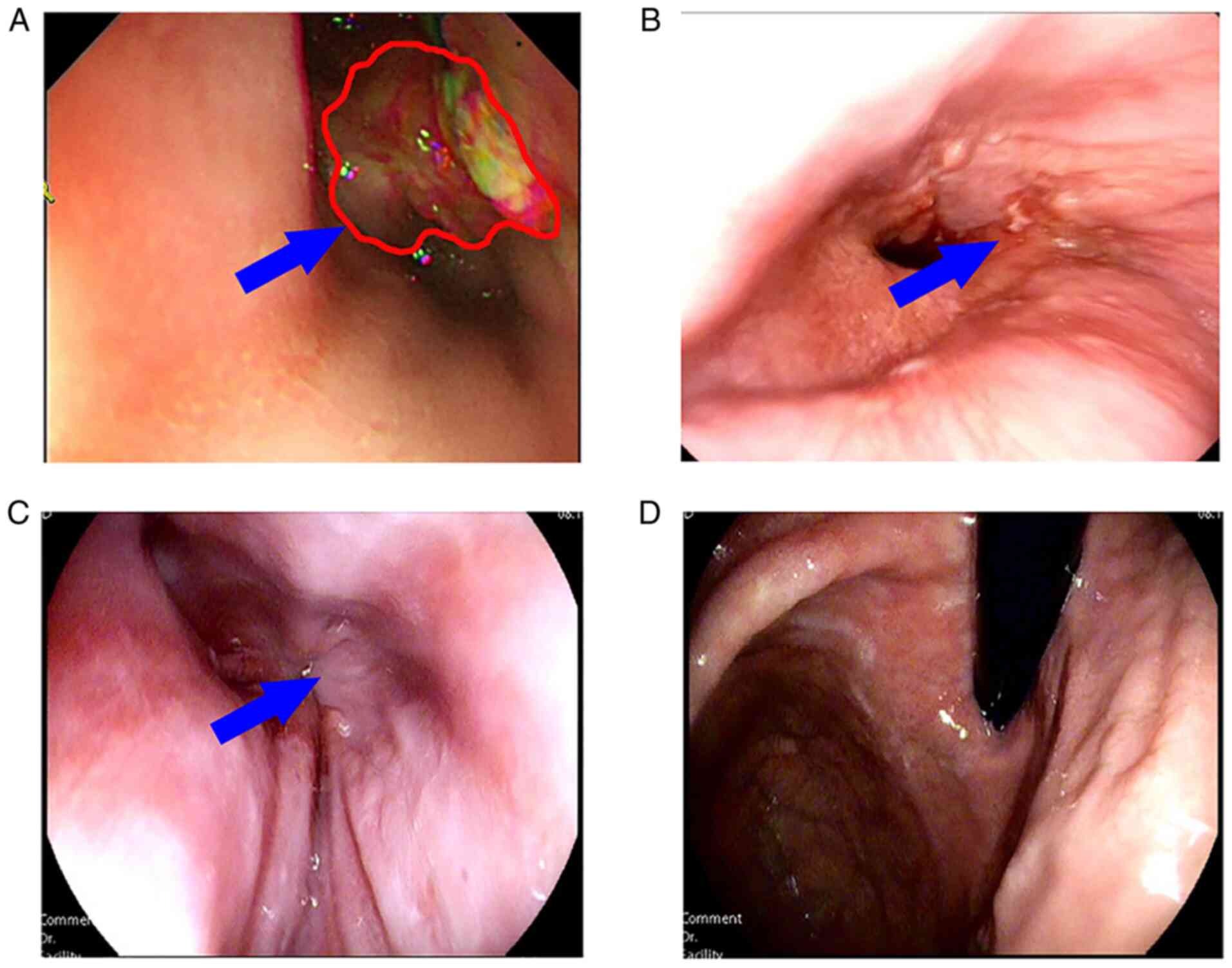
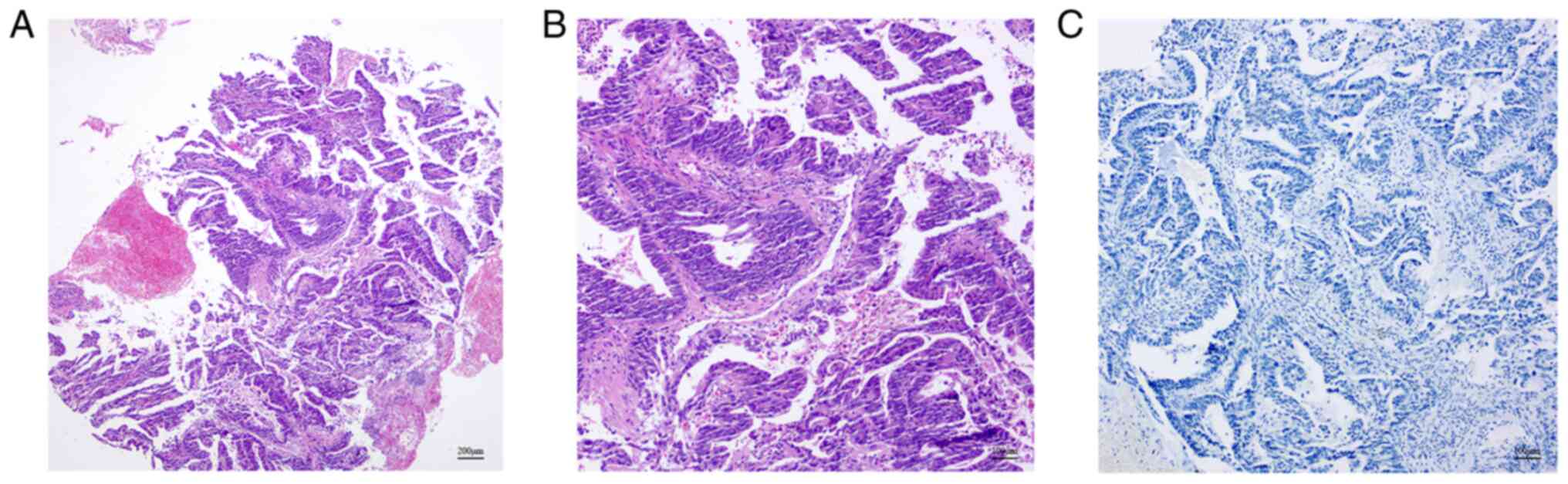
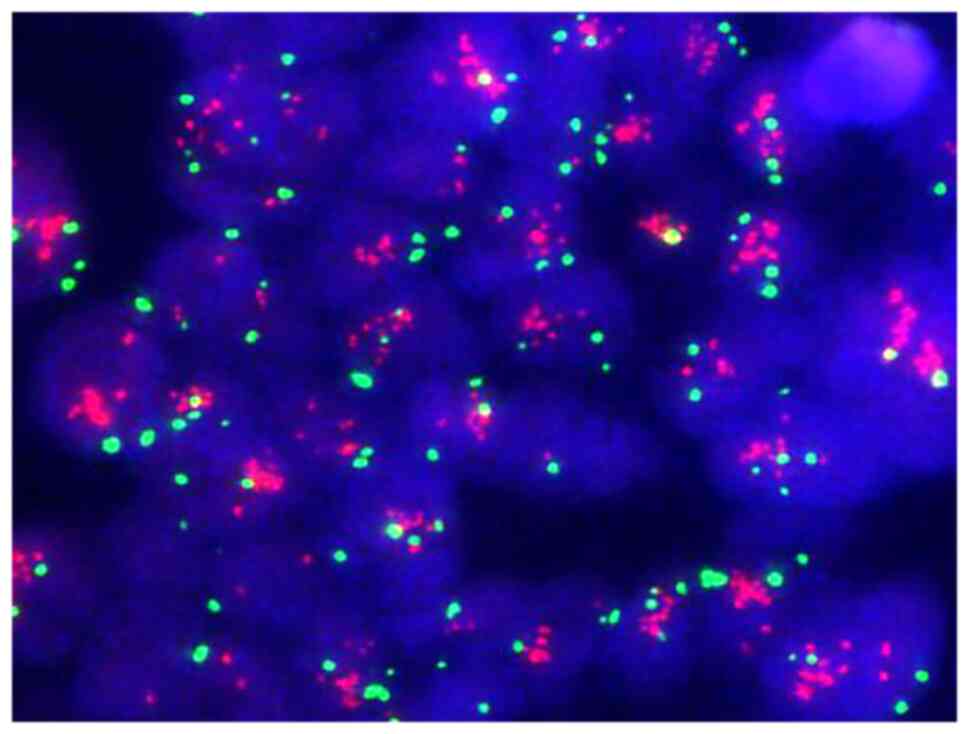
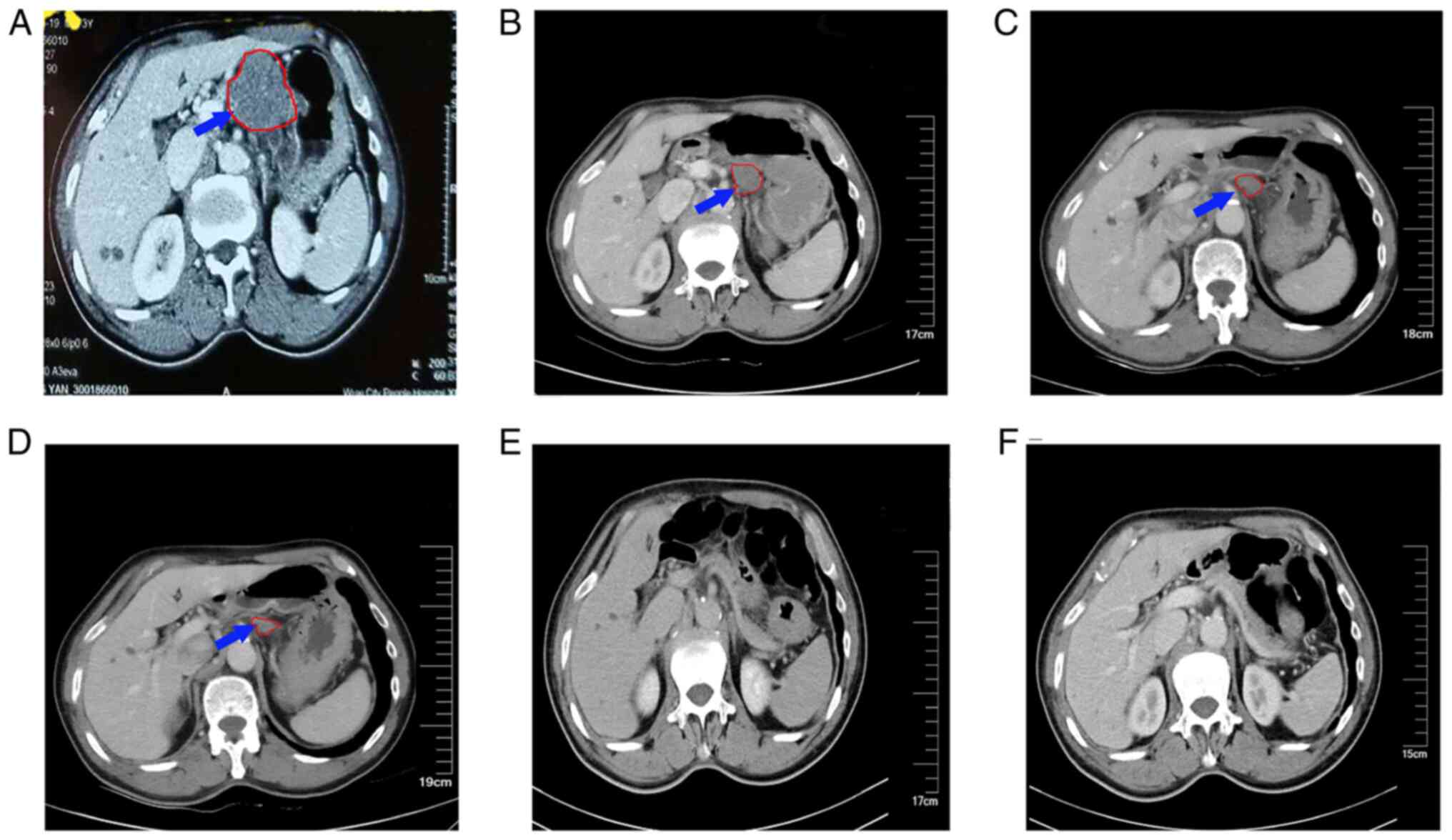
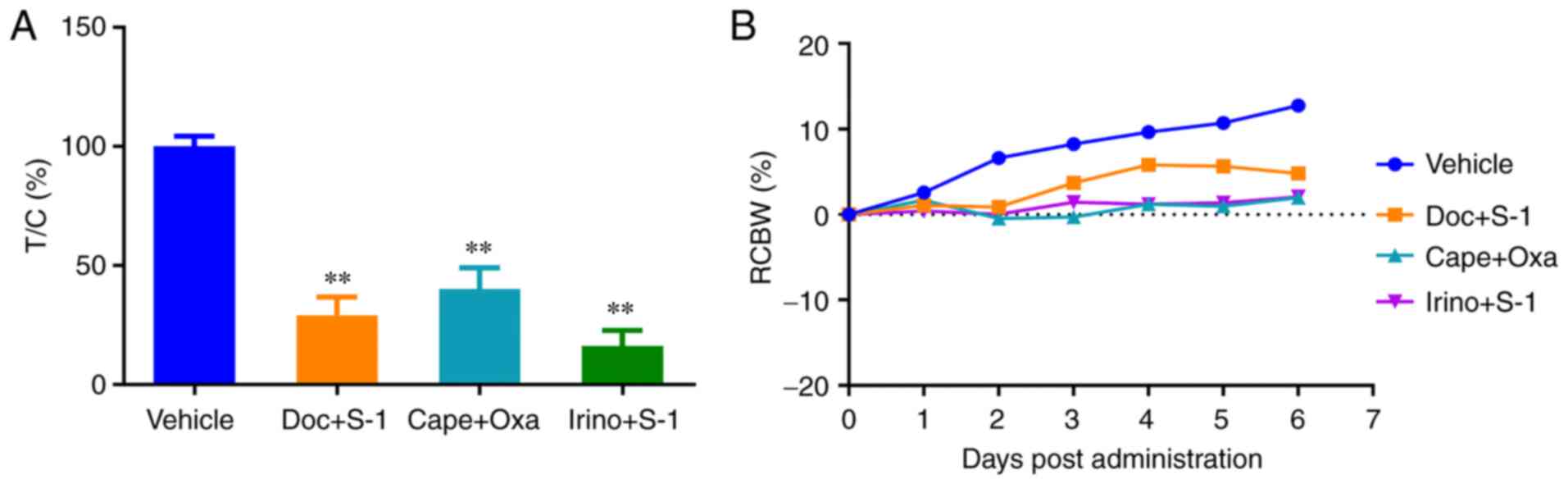
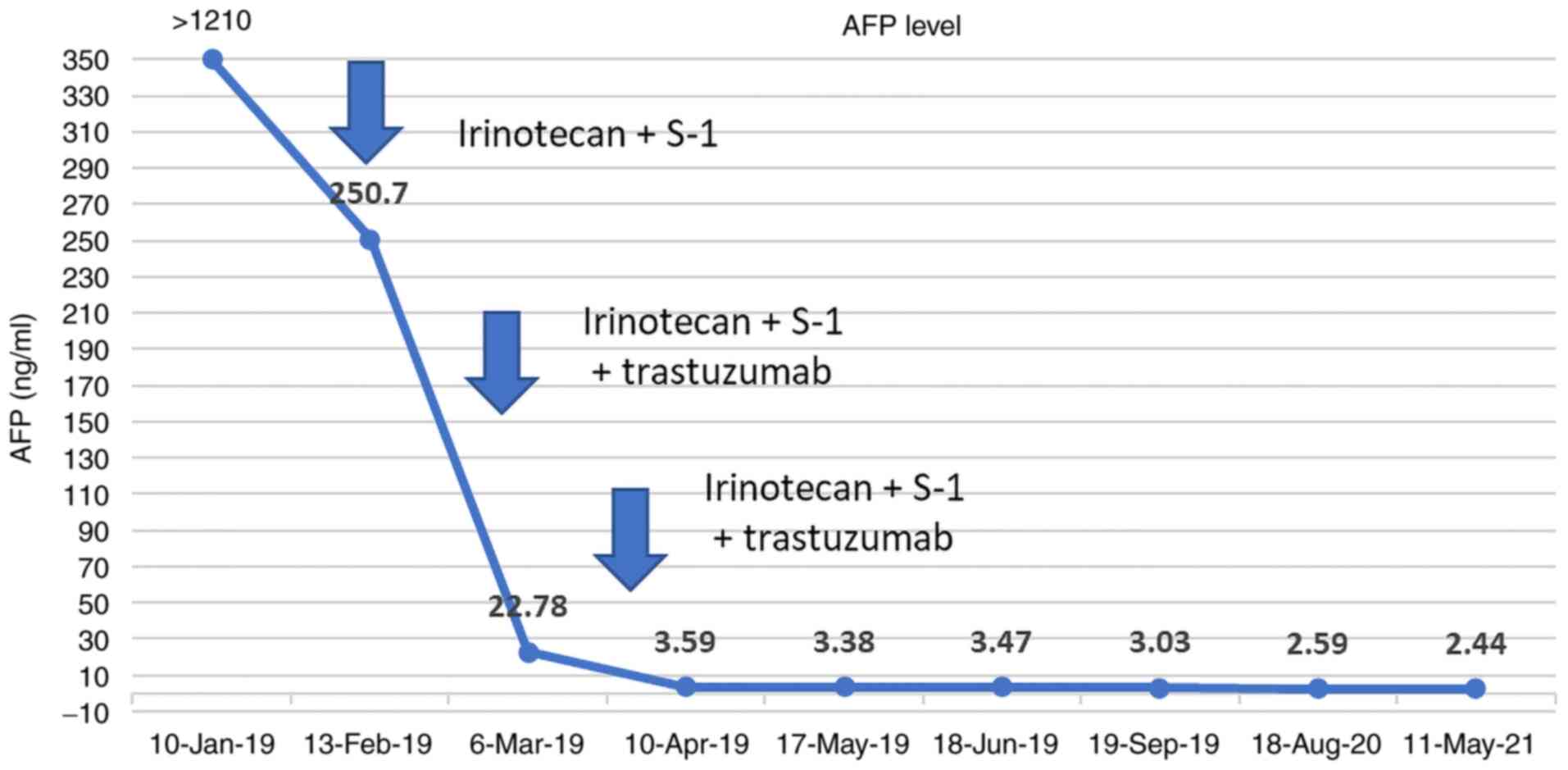
|
1
|
International Agency for Research on
Cancer (IARC), . World Cancer Day: Breast overcancer takes lung
cancer as leading cause of worldwidecancer. IARC showcases key
research projects to address breast cancer. IARC; Lyon: 2021,
https://www.iarc.who.int/wp-content/uploads/2021/02/pr294_e.pdfFebruary
4–2021
|
|
2
|
Yang J, Wang R, Zhang W, Zhuang W, Wang M
and Tang C: Clinicopathological and prognostic characteristics of
hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract.
2014:1405872014. View Article : Google Scholar
|
|
3
|
Wang YK and Zhang XT: AFP-producing
gastric cancer and hepatoid gastric cancer. Zhonghua Zhong Liu Za
Zhi. 39:801–807. 2017.(In Chinese).
|
|
4
|
Jang Y, Peng Z, Wei B, et al: Lymph node
metastasis of early submucosal gastric cancer: A report of 290
cases. World Chin J Dig. 19:2970–2973. 2011. View Article : Google Scholar
|
|
5
|
Wang J, Tian SD and Chen XY: Advances in
the treatment of advanced gastric cancer. Chin Clin Oncol.
37:171–175. 2010.(In Chinese).
|
|
6
|
Aparicio S, Hidalgo M and Kung AL:
Examining the utility of patient-derived xenograft mouse models.
Nat Rev Cancer. 15:311–316. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izumchenko E, Meir J, Bedi A, Wysocki PT,
Hoque MO and Sidransky D: Patient-derived xenografts as tools in
pharmaceutical development. Clin Pharmacol Ther. 99:612–621. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Nomie K, Zhang H, Bell T, Pham L,
Kadri S, Segal J, Li S, Zhou S, Santos D, et al: B-cell lymphoma
patient-derived xenograft models enable drug discovery and are a
platform for personalized therapy. Clin Cancer Res. 23:4212–4223.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byrne AT, Alférez DG, Amant F, Annibali D,
Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, et
al: Interrogating open issues in cancer precision medicine with
patient-derived xenografts. Nat Rev Cancer. 17:254–268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xian Y, Xie Y, Song B, Ou Z, Ouyang S, Xie
Y, Yang Y, Xiong Z, Li H and Sun X: The safety and effectiveness of
genetically corrected iPSCs derived from β-thalassaemia patients in
nonmyeloablative β-thalassaemic mice. Stem Cell Res Ther.
11:2882020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Wang W, Long Y, Liu H, Cheng J,
Guo L, Li R, Meng C, Yu S, Zhao Q, et al: Characterization of drug
responses of mini patient-derived xenografts in mice for predicting
cancer patient clinical therapeutic response. Cancer Commun (Lond).
38:602018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Overmyer KA, Thonusin C, Qi NR, Burant CF
and Evans CR: Impact of anesthesia and euthanasia on metabolomics
of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS One.
10:e01172322015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aaron MK, Matthew H, Eric B, Sivachenko A,
Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly
M and DePristo MA: The genome analysis toolkit: A MapReduce
framework for analyzing next-generation DNA sequencing data. Genome
Res. 20:1297–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES
and Getz G: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–219. 2013. View
Article : Google Scholar
|
|
17
|
Wang D, Li C, Xu Y, Xing Y, Qu L, Guo Y,
Zhang Y, Sun X and Suo J: Clinicopathological characteristics and
prognosis of alpha-fetoprotein positive gastric cancer in Chinese
patients. Int J Clin Exp Pathol. 8:6345–6355. 2015.
|
|
18
|
Lin CY, Yeh HC, Hsu CM, Lin WR and Chiu
CT: Clinicopathologial features of gastric hepatoid adenocarcinoma.
Biomed J. 38:65–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao C, Wu F, Jiang H, Teng L, Song F,
Wang Q and Yang H: Hepatoid adenocarcinoma of the stomach: Nine
case reports and treatment outcomes. Oncol Lett. 10:1605–1609.
2015. View Article : Google Scholar
|
|
20
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar
|
|
21
|
Motoyama T, Aizawa K, Watanabe H, Fukase M
and Saito K: alpha-Fetoprotein producing gastric carcinomas: A
comparative study of three different subtypes. Acta Pathol Jpn.
43:654–661. 1993.PubMed/NCBI
|
|
22
|
Chen Y, Qu H, Jian M, Sun G and He Q: High
level of serum AFP is an independent negative prognostic factor in
gastric cancer. Int J Biol Markers. 30:e387–e393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garber K: From human to mouse and back:
‘Tumorgraft’ models surge in popularity. J Natl Cancer Inst.
101:6–8. 2009. View Article : Google Scholar
|
|
24
|
Hwang CI, Boj SF, Clevers H and Tuveson
DA: Preclinical models of pancreatic ductal adenocarcinoma. J
Pathol. 238:197–204. 2016. View Article : Google Scholar
|
|
25
|
Kochi M, Fujii M, Kaiga T, Takahashi T,
Morishita Y, Kobayashi M, Kasakura Y and Takayama T: FLEP
chemotherapy for alpha-fetoprotein-producing gastric cancer.
Oncology. 66:445–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Back SK, Han SW, Oh DY, Im SA, Kim TY and
Bang YJ: Clinicopathologic characteristics and treatment outcomes
of hepatoid adenocarcinoma of the stomach, a rare but unique
subtype of gastric cancer. BMC Gastroentero. 11:562011. View Article : Google Scholar
|
|
27
|
Giuffrè G, Ieni A, Barresi V, Caruso RA
and Tuccari G: HER2 status in unusual histological variants of
gastric adenocarcinomas. J Clin Pathol. 65:237–241. 2012.
View Article : Google Scholar
|
|
28
|
Fang Y, Wang L, Li GM, et al: Expression
of c-Met, VEGF, EGFR and Her-2 in AFP positive gastric cancer. Chin
J Cancer. 26:662–669. 2016.(In Chinese).
|
|
29
|
Amemiya H, Kono K, Mori Y, Takahashi A,
Ichihara F, Iizuka H, Sekikawa T and Matsumoto Y: High frequency of
c-Met expression in gastric cancers producing alpha-fetoprotein.
Oncology. 59:145–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin S, Ji J, Xu RH, Wang W, Tang Y, Bi F,
Li J, Wang K, Xu JM, Fan Q, et al: Treatment patterns and outcomes
in chinese patients with gastric cancer by HER2 status: A
noninterventional registry study (EVIDENCE). Oncologist.
26:e1567–e1580. 2021. View Article : Google Scholar
|
|
31
|
Chung HC, Bang YJ, S Fuchs C, Qin SK,
Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, et
al: First-line pembrolizumab/placebo plus trastuzumab and
chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811.
Future Oncol. 17:491–501. 2021. View Article : Google Scholar
|
|
32
|
Russell R, Perkhofer L, Liebau S, Lin Q,
Lechel A, Feld FM, Hessmann E, Gaedcke J, Güthle M, Zenke M, et al:
Loss of ATM accelerates pancreatic cancer formation and
epithelial-mesenchymal transition. Nat Commun. 6:76772015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|