Introduction
Upper tract urothelial carcinoma (UTUC) derived from
the renal calyces, renal pelvis or ureters is a relatively rare
tumor type, accounting for 5–6% of all cases of UC (1). At the time of diagnosis, two-thirds
of UTUCs have developed local invasion (2). For patients with metastatic UTUC,
first-line platinum-based chemotherapy is prescribed, but they are
considered incurable and have demonstrated a poor prognosis so far.
Recently, programmed death 1 (PD-1)/programmed death ligand 1
(PD-L1) inhibitors, such as pembrolizumab, nivolumab, atezolizumab,
avelumab and durvalumab, were approved for second-line therapy
(3). Subsequently, atezolizumab
and pembrolizumab were approved in the first-line setting for
cisplatin-ineligible patients with PD-L1-positive tumors. Thus,
PD-1/PD-L1 inhibitors have been recognized as key drugs to control
the progression of malignant tumors (4). The treatment effects of immune
checkpoint inhibitors (ICIs) have been indicated to be limited
(objective response rate, 11–27%) (3) and the treatment effects of emerging
ICIs, such as anti-T-cell immunoreceptor with immunoglobulin and
ITIM domains (TIGIT) drugs, lymphocyte activation gene-3
inhibitors, and T-cell immunoglobulin and mucin-domain containing-3
inhibitors, are promising (4,5).
TIGIT was reported to be associated with the immune
checkpoint in NK cells and T cells in 2013 (6). CD155, which interacts with TIGIT, is
expressed in numerous types of tumor and is recognized to be a poor
prognostic factor (7–10). With regard to UC, high expression
of CD155, which is associated with poor prognosis, has been
confirmed in bladder cancer, but has not been demonstrated in UTUC
(8). Mechanistic analysis revealed
that binding of CD155 and TIGIT facilitates tumor invasion and
suppresses antitumor immunity (11,12).
Thus, CD155/TIGIT is recognized as a new treatment target and
clinical trials of anti-TIGIT drugs have been launched (4,13).
Genomic alternations in fibroblast growth factor
receptor 3 (FGFR3) have been well described in UC and have been
recognized as therapeutic targets (14). FGFR3 belongs to the super-family of
receptor tyrosine kinases and is involved in transmitting FGF
signals. FGFR3 signaling may be associated with UC development,
angiogenesis and lower T-cell infiltration (14–16).
Furthermore, FGFR inhibition may activate the immune environment
and is expected to benefit patients who do not respond to ICIs
(17). Although the association
between PD-L1 and FGFR3 has been investigated in UC (18), the association between CD155 and
FGFR3 has not been explored.
The present study aimed to evaluate the association
of CD155 expression with clinicopathological factors in UTUC and
examine whether CD155 is a prognostic factor when compared with
existing pathological factors. In addition, the correlation of
immunohistochemical expression was analyzed among CD155, PD-L1 and
FGFR3, all of which have been recognized as new therapeutic
targets.
Materials and methods
Case selection
After receiving institutional review board approval
(nos. 2018036 and 2019209), the medical records of 222 patients
underwent radical nephroureterectomy for UTUC at Kansai Medical
University Hospital (Hirakata, Japan) between January 2006 and
December 2017 were retrospectively reviewed. An opt-out approach
was used to obtain informed consent on the website of Kansai
Medical University Hospital (Hirakata, Japan). A total of 14
patients were excluded from this study for the following reasons:
Synchronous bilateral tumors (n=2), presence of metastasis (n=4),
simultaneous radical cystectomy (n=3) and insufficient pathological
material (n=5). Thus, the data of a total of 208 patients
(pTa-4Nx-2M0) who underwent radical nephroureterectomy for UTUC
were extracted from our institutional database for this study.
Clinicopathological characteristics, including grade, pathological
stage, lymphovascular invasion (LVI), surgical margin and divergent
differentiation/subtypes were reviewed. Slides stained with H&E
were re-evaluated by a urologic pathologist (CO) using the 2016
World Health Organization classification (19) and the 2017 Union for International
Cancer Control TNM staging system (20).
Histological evaluation and tissue
microarray (TMA) construction
Two representative tumor locations showing tumor
invasion (if a variant existed, that area was included as well)
were selected for TMA construction of radical nephroureterectomy
specimens. A total of 10 TMA blocks were built from representative
tumor areas, including normal urothelium samples from
formalin-fixed paraffin-embedded (FFPE) tumor material. Each FFPE
tissue block was sampled with 2.0-mm cores using a tissue arraying
instrument (Azumaya Corporation). To validate the expression of
CD155 in the preoperative biopsy specimens, other TMA sections from
14 biopsy cases which were included in the 208 patients that
underwent radical nephroureterectomy were evaluated. Biopsies were
performed on patients whose tumors were not detected on imaging or
urine cytology.
Immunohistochemical analysis of
TMAs
Immunohistochemical staining was performed on TMA
sections (4-µm thick) using a Ventana Discovery Ultra Autostainer
(Roche Diagnostics) or Leica Bond-III (Leica Microsystems, Ltd.).
Primary antibodies against CD155 (#81254 rabbit monoclonal; 1:200
dilution; Cell Signaling Technology, Inc.) and FGFR3 (sc-13121;
mouse monoclonal; 1:50 dilution; Santa Cruz Biotechnology, Inc.)
were visualized using the OptiView DAB IHC Detection Kit (Ventana
Medical Systems) according to the manufacturer's instructions.
Anti-PD-L1 primary antibodies (PA0832; rabbit monoclonal;
prediluted; Leica Microsystems, Ltd.) were visualized using BOND
Polymer Refine Detection (Leica Microsystems, Ltd.) according to
the manufacturer's instructions. The cell membrane and cytoplasmic
expression patterns of CD155 in tumor cells were
semi-quantitatively assessed by using the H-score. The H-score was
determined by multiplying the staining intensity (0, none; 1, weak;
2, moderate; 3, strong) and the percentage of positive cells
(range, 0–300), as previously described (21). Representative CD155
immunohistochemical expression patterns in normal urothelium and
tumor cells are presented in Fig.
S1. The final scores (average H-score for the two cores) were
classified into two categories (low, H-score <20; high, H-score
≥20), with the cutoff determined by a receiver operating
characteristic curve for 5-year recurrence. PD-L1 and FGFR3 were
also evaluated by the H-score and divided into two categories (low,
H-score <20; high, H-score ≥20). Immunohistochemical evaluation
was independently performed by two pathologists (JI and CO) blinded
to clinical outcomes and discordant patterns were resolved by
consensus.
Statistical analysis
Continuous data were presented as the median and
interquartile range (IQR) and count data as n (%). Fisher's exact
test and the Mann-Whitney U-test were used for comparisons between
two groups. Pearson's product-moment correlation coefficient was
measured between two immunohistochemical expression patterns.
Recurrence-free survival (RFS), cancer-specific survival (CSS) and
overall survival (OS) were assessed using the Kaplan-Meier method
and a univariate Cox proportional hazards model. Bladder relapse
was not defined as recurrence in the present study. Logistic
regression analysis using the multivariate Cox proportional hazards
model was performed to determine the hazard ratio (HR). All
statistical analyses were performed using EZR version 1.55 (Saitama
Medical Center) (22). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Of the 208 patients included, 64 (30.8%) were female
and 144 (69.2%) were male, with a median age of 72 years (IQR,
68–78 years) (Table SI). A total
of 22 patients had divergent differentiation/subtypes including
squamous differentiation, glandular differentiation and sarcomatoid
subtypes. Furthermore, 72 patients (34.6%) experienced recurrence
and 57 patients (27.4%) died due to UTUC. The median follow-up time
was 72.2 months (IQR, 46.4-96.2 months).
Immunohistochemical expressions of
CD155, PD-L1 and FGFR3
Immunohistochemical analysis of CD155 indicated that
177 patients (85.1%) had high expression and 31 patients (14.9%)
had low expression (Table I). High
PD-L1 expression was noted in 63 patients (30.6%) and low
expression in 143 patients (69.4). High FGFR3 expression was noted
in 187 patients (90.8%) and low expression in 19 patients (9.2%)
(Table SI). To identify the
percentage of patients eligible for combination therapy, the
combined immunohistochemical expression profiles of CD155, PD-L1
and FGFR3 were reviewed, as presented in Table SII.
 | Table I.Association between CD155 expression
and clinicopathological factors (n=208). |
Table I.
Association between CD155 expression
and clinicopathological factors (n=208).
| Characteristic | CD155 low
(n=31) | CD155 high
(n=177) | P-value |
|---|
| Age, years | 76 (68.5-79) | 72 (67–78) | 0.20 |
| Sex |
|
| 0.84 |
|
Female | 10 (32.3) | 54 (30.5) |
|
|
Male | 21 (67.7) | 123 (69.5) |
|
| Grade |
|
| 0.40 |
|
Low | 6 (19.4) | 23 (13) |
|
|
High | 25 (80.6) | 154 (87) |
|
| pT stage |
|
| 0.04 |
|
pTa | 14 (45.2) | 33 (18.6) |
|
|
pTis | 0 (0.0) | 2 (1.1) |
|
|
pT1 | 5 (16.1) | 32 (18.1) |
|
|
pT2 | 3 (9.7) | 21 (11.9) |
|
|
pT3 | 9 (29.0) | 74 (41.8) |
|
|
pT4 | 0 (0.0) | 15 (8.5) |
|
| pN stage |
|
| 0.10 |
|
pNx | 0 (0.0) | 4 (2.3) |
|
|
pN0 | 30 (96.8) | 137 (77.4) |
|
|
pN1 | 0 (0.0) | 17 (9.6) |
|
|
pN2 | 1 (3.2) | 19 (10.7) |
|
| LVI |
|
| 0.001 |
|
Absent | 22 (71) | 68 (38.4) |
|
|
Present | 9 (29) | 109 (61.6) |
|
| Surgical
margin |
|
| 0.22 |
|
Absent | 31 (100) | 164 (92.7) |
|
|
Present | 0 (0) | 13 (7.3) |
|
| Divergent
differentiation/subtype |
|
| 0.54 |
|
Absent | 29 (93.5) | 157 (88.7) |
|
|
Present | 2 (6.5) | 20 (11.3) |
|
| Neoadjuvant
chemotherapy |
|
| 0.22 |
| No | 31 (100) | 165 (93.2) |
|
|
Yes | 0 (0) | 12 (6.8) |
|
| Adjuvant
chemotherapy |
|
| 0.007 |
| No | 30 (96.8) | 134 (75.7) |
|
|
Yes | 1 (3.2) | 43 (24.3) |
|
| PD-L1 |
|
| 0.03 |
|
Low | 26 (86.7) | 117 (66.5) |
|
|
High | 4 (13.3) | 59 (33.5) |
|
| FGFR3 |
|
| 0.32 |
|
Low | 1 (3.3) | 18 (10.2) |
|
|
High | 29 (96.7) | 158 (89.8) |
|
| Median follow-up,
months | 72.9
(51.1-94.9) | 70.3
(46.3-96.8) | 0.93 |
Association of CD155 expression with
clinicopathological factors
CD155 expression was significantly and positively
associated with the T stage (P=0.04), LVI (P=0.001), administration
of adjuvant chemotherapy (P=0.007) and PD-L1 expression (P=0.03)
(Table I).
Correlation among CD155, FGFR3 and
PD-L1
A weakly positive correlation was obtained between
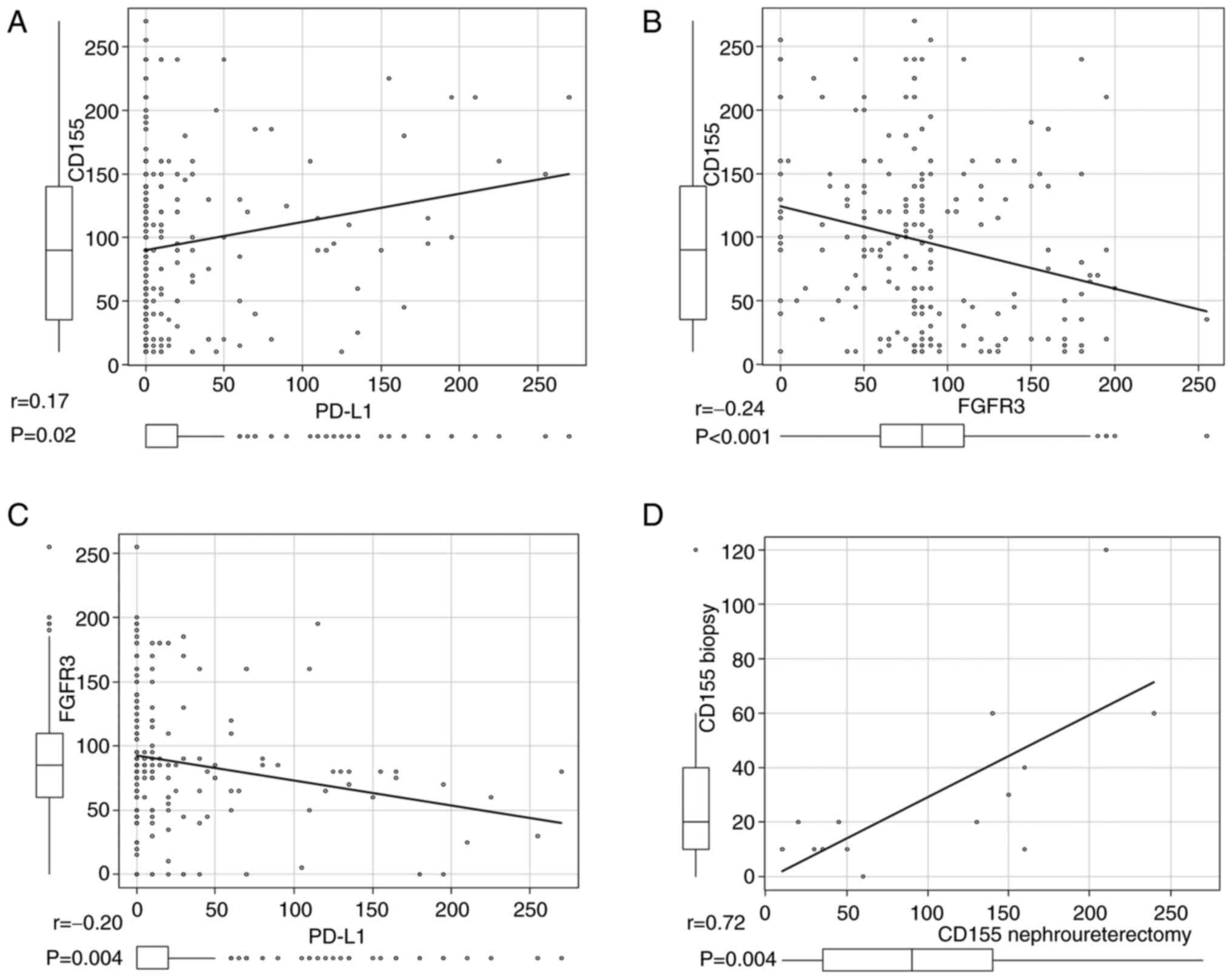
CD155 and PD-L1 [correlation coefficient (r)=0.17, P=0.02; Fig. 1A]. A weak negative correlation was
confirmed between CD155 and FGFR3, and between PD-L1 and FGFR3
(r=−0.24, P<0.001 and r=−0.20, P=0.004, respectively; Fig. 1B and C).
Correlation between CD155 expression
in radical nephroureterectomy specimens and that in biopsy
specimens
A positive correlation was confirmed between CD155
expression in radical nephroureterectomy specimens and that in
biopsy specimens (r=0.72, P=0.004; Fig. 1D).
Association of CD155, PD-L1 and FGFR3
expression with patient prognosis
Kaplan-Meier survival analysis indicated that RFS
and CSS were significantly lower in patients with tumors having
high CD155 expression than in those with tumors exhibiting low
CD155 expression (HR=14.6, P=0.008; and HR=10.9, P=0.02; Fig. 2A and B, respectively). The OS rate
was not significantly different between the CD155 high and low
expression groups (HR=1.65, P=0.14; Fig. 2C). The median follow-up time was
72.9 months (IQR: 51.1-94.9) in the low CD155 expression group and
70.3 months (IQR: 46.3-96.8) in the high CD155 expression group,
and there were no significant differences between the two groups
(P=0.93; Table I). RFS, CSS and OS
were significantly worse in patients with tumors having high PD-L1
expression than in those with tumors having low PD-L1 expression
(HR=2.69, P<0.001; HR=2.79, P<0.001; and HR=1.78, P=0.007;
Fig. 2D-F, respectively). Although
RFS, CSS and OS were better in patients with high FGFR3 expression
than in those with low FGFR3 expression, the difference was not
statistically significant (Fig.
2G-I).
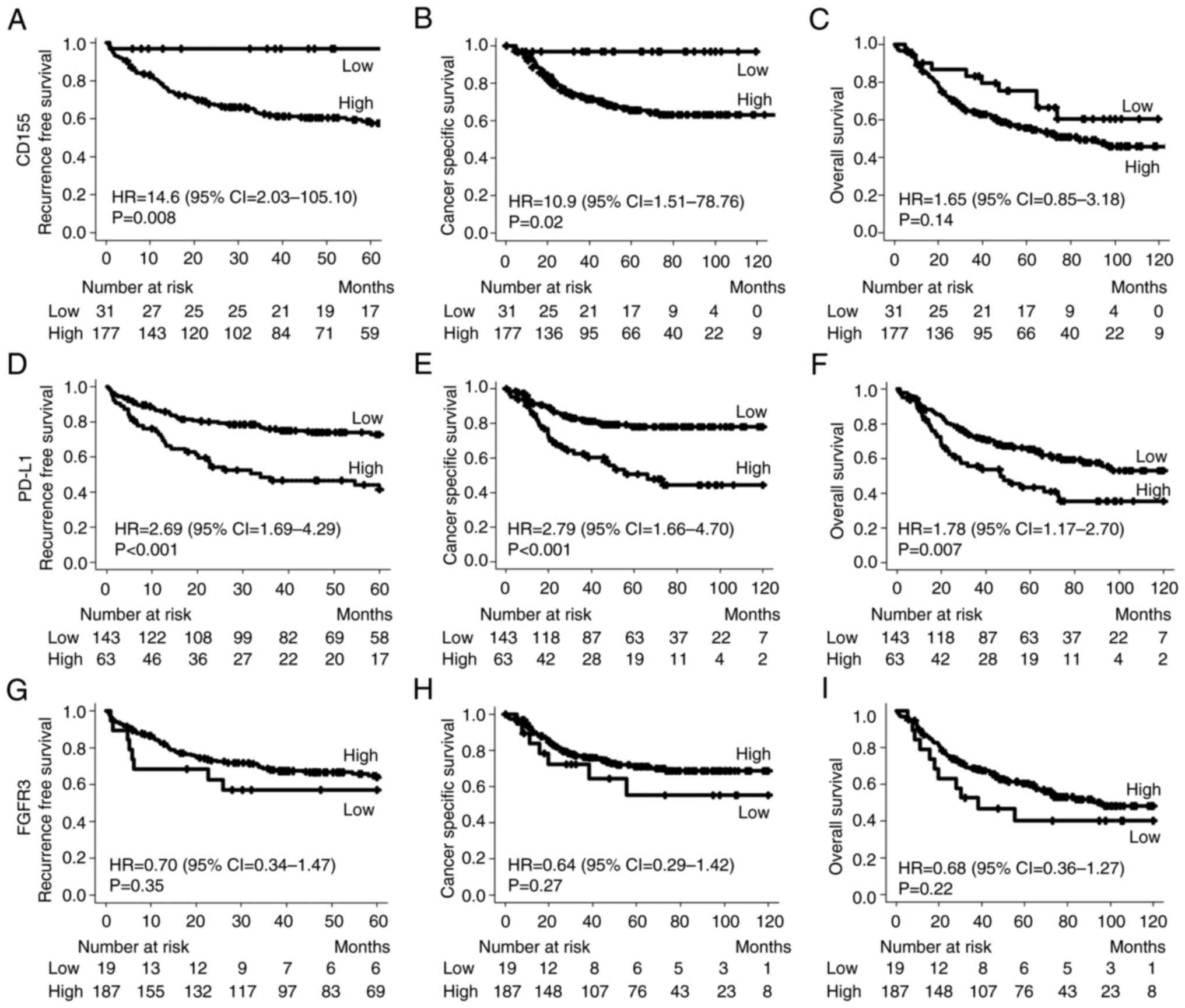 | Figure 2.Comparison of the survival curve and
HR for the immunohistochemical expression of CD155, PD-L1 and
FGFR3. Kaplan-Meier curves of (A) recurrence-free survival for
CD155 staining, (B) cancer-specific survival for CD155 staining,
(C) overall survival for CD155 staining, (D) recurrence-free
survival for PD-L1 staining, (E) cancer-specific survival for PD-L1
staining, (F) overall survival for PD-L1 staining, (G)
recurrence-free survival for FGFR3 staining, (H) cancer-specific
survival for FGFR3 staining and (I) overall survival for FGFR3
staining. PD-L1, programmed death-ligand 1; FGFR3, fibroblast
growth factor receptor 3; HR, hazard ratio. |
Univariate and multivariate analyses
for predicting recurrence
The association between clinicopathological factors
and recurrence after radical nephroureterectomy is presented in
Table II. The univariate analysis
indicated that grade, pT stage, LVI, surgical margin, CD155
expression and PD-L1 expression were associated with recurrence
(all P<0.05). Multivariate analysis was performed on these
significantly different factors, suggesting that grade (HR=4.34,
P=0.04), pT stage (HR=2.28, P=0.01), LVI (HR=3.24, P=0.003),
surgical margin (HR=3.45, P<0.001) and CD155 expression
(HR=7.32, P=0.049) were significant factors affecting
recurrence.
 | Table II.Univariate and multivariate analysis
of clinicopathological factors for predicting recurrence. |
Table II.
Univariate and multivariate analysis
of clinicopathological factors for predicting recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (>65
vs. ≤65) | 1.82
(0.96-3.46) | 0.07 | - | - |
| Sex (male vs.
female) | 0.76
(0.47-1.22) | 0.26 | - | - |
| Grade (high vs.
low) | 7.87
(1.93-32.12) | 0.004 | 4.34
(1.05-18.03) | 0.04 |
| pT stage (>2 vs.
≤2) | 5.68
(3.29-9.82) | <0.001 | 2.28
(1.22-4.28) | 0.01 |
| LVI (present vs.
absent) | 7.83
(3.89-15.77) | <0.001 | 3.24
(1.48-7.09) | 0.003 |
| Surgical margin
(present vs. absent) | 5.17
(2.60-10.24) | <0.001 | 3.45
(1.69-7.05) | <0.001 |
| CD155 (present vs.
absent) | 14.6
(2.03-105.10) | 0.008 | 7.32
(1.01-53.35) | 0.049 |
| PD-L1 (high vs.
low) | 2.69
(1.69-4.29) | <0.001 | 1.24
(0.76-2.01) | 0.38 |
| FGFR3 (high vs.
low) | 0.70
(0.34-1.47) | 0.35 | - | - |
Discussion
In the present study, the association between CD155
expression and clinicopathological factors in UTUC was
investigated. It was indicated that high CD155 expression was
significantly associated with poor prognosis. Furthermore,
multivariate analysis suggested that CD155 was an independent
unfavorable prognostic factor. Therefore, confirming the
immunohistochemical expression of CD155 may be useful in predicting
prognosis.
Previously, the expression of CD155 has been
immunohistochemically evaluated in muscle-invasive bladder cancer
(8), while the expression in UTUC
has not been assessed, to the best of our knowledge. Thus, the
present study was the first to investigate the correlation between
CD155 immunohistochemical expression and clinicopathological
factors in UTUC. Furthermore, as the association between the
expression of CD155, PD-L1 and FGFR3 had not been previously
investigated, the correlation between these three markers was
investigated in the present study.
CD155, originally identified as a poliovirus
receptor, is a type I transmembrane glycoprotein that belongs to
the immunoglobulin superfamily, known as nectin-like 5 (NECL5)
(23–25). CD155 has been reported to be
overexpressed in numerous types of cancer (26) and to be associated with tumor
invasion and metastasis (11). The
binding of CD155 and TIGIT in NK and T cells leads to evasion of
tumor immunity (5,12). TIGIT blockade has been demonstrated
to enhance NK cell activity and numerous clinical trials of
anti-TIGIT monoclonal antibodies, which are applied in combination
with PD-1/PD-L1 inhibitors, have been performed (4,13).
The present results suggested that prognosis was
unfavorable in patients with tumors with high expression of CD155
than in those with tumors having low CD155 expression. High CD155
expression was associated with high T stage, LVI and administration
of adjuvant chemotherapy. As advanced pathological findings, such
as a high T stage and LVI, were detected in tumors having high
CD155 expression, adjuvant chemotherapy may be provided to avoid
cancer recurrence. Sloan et al (11) showed that CD155 promotes tumor cell
invasion and migration. The role of tumor angiogenesis and
proliferation has been drawing attention (26). High vascular endothelial growth
factor (VEGF) expression in bladder cancer was associated with
advanced pathological stage and lymph node metastasis (27). Furthermore, CD155 was associated
with VEGF receptor 2 and regulated VEGF-induced angiogenesis
(7,28). Chauvin and Zarour (12) reported that CD155/TIGIT was
associated with immune suppression. Greater infiltration of immune
cells in UC has been indicated to be associated with favorable
prognosis (21). The reason for
the poor prognosis in the high CD155 group may be that immune cell
infiltration was suppressed by the activation of the CD155/TIGIT
immune checkpoint. By investigating its association with
clinicopathological factors, the present study confirmed that CD155
may promote tumor progression. However, further studies are
necessary to reveal the molecular mechanism of tumor
development.
CD155 and PD-L1 are immune checkpoints expressed on
the tumor surface. The present study confirmed a weakly positive
correlation between the expression of CD155 and PD-L1 (r=0.17,
P=0.02). CD155 and PD-L1 may suppress the cytotoxicity of
tumor-infiltrating lymphocytes via interaction with ligands
expressed on the lymphocytes (9).
Since the two immune checkpoints of CD155 and PD-L1
may have a similar status, a positive correlation of expression
patterns was confirmed. Furthermore, the efficacy of combination
therapies of TIGIT inhibitors and PD-1/PD-L1 inhibitors is expected
(13). The association between
co-stimulatory molecules, co-suppressive molecules and their
ligands are complex (9) and
further investigation is required.
In the present study, a weak negative correlation
was found between the expression of CD155 and FGFR3 (r=−0.24,
P<0.001). Furthermore, a negative correlation between PD-L1 and
FGFR3 (r=−0.20, P=0.004) was obtained, which was in agreement with
a previous report (18). The
immune exclusion system is activated by the Wnt/β-catenin signal
(29), which is associated with
the non-T-cell-inflamed phenotype (30). An active Wnt/β-catenin signal is
associated with impaired T-cell infiltration and low PD-L1
expression (30,31). On the other hand, an active FGFR3
pathway may contribute to T-cell exclusion (32). Furthermore, a study indicated an
overlap of Wnt/β-catenin and FGF signaling (33). Therefore, a weak negative
correlation between CD155 and FGFR3 was to be expected. As the
effectiveness of the combination therapy of PD-1/PD-L1 inhibitors
and FGFR inhibitors in metastatic UC has been proven (17,34),
the combination therapy of TIGIT inhibitors and FGFR inhibitors may
also be useful.
The present study suggested that high CD155
expression was associated with significantly reduced RFS and CSS.
Univariate and multivariate survival analyses revealed that high
CD155 expression was an independent risk factor. Thus, CD155 may be
a robust biomarker to predict recurrence in UTUC. According to
preliminary data by our group on the prediction of CD155 expression
with preoperative biopsy specimens (n=14), a positive correlation
was statistically confirmed between CD155 expression in radical
nephroureterectomy specimens and that in preoperative biopsy
specimens.
Confirming the immune checkpoint status in biopsy
samples may predict the efficacy of TIGIT inhibitors in cases
ineligible for surgery. Furthermore, the possibility of prognosis
prediction using preoperative biopsy specimens may be further
investigated.
The present study has several limitations. First, it
was a retrospective single-center study. Furthermore, CD155
expression was evaluated with TMAs constructed with two
representative cores. In addition, the present results should be
externally validated with other cohorts. As another limitation,
CD155 expression was evaluated using only TMAs and not the whole
section, which may have caused unidentified bias. Furthermore, it
was not possible to validate the multiplexed immunofluorescence
analysis to determine CD155 PD-L1 and FGFR3 co-expressed in a cell.
Further investigation by multicolor fluorescence methods and
spatial gene expression analysis is required to analyze the
association between cancer cells and immune cells in the tumor
microenvironment. Despite these limitations, the present results
add a new role to the immunohistochemical detection of CD155
expression in UTUC.
In conclusion, the present study indicated that
confirming the expression of CD155 by immunohistochemistry may be
useful for predicting recurrence of UTUC. In addition, FGFR3 and
immune checkpoint signaling molecules, such as CD155 and PD-L1, had
a weak negative correlation. The immunohistochemical expression
profiles of CD155, PD-L1 and FGFR3 may help us understand the roles
of FGFR-targeted therapies in immunotherapy for UTUC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors acknowledge the assistance provided by
Mr. Ryosuke Yamaka (Departments of Pathology, Kansai Medical
University, Hirakata, Japan) in tissue microarray construction.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JI, CO and KT designed the study. JI, CO and TY
performed data collection and pathological assessments. JI
performed the statistical analyses. JI, CO, TY, RS, KT and HK
interpreted the data. JI and CO drafted the manuscript. All authors
read and approved the final version of the manuscript. JI and CO
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at the Kansai Medical University Hospital (Hirakata
Japan; approval nos. 2018036 and 2019209). Informed consent was
obtained in the form of opt-out on the website of Kansai Medical
University Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TIGIT
|
T cell immunoreceptor with Ig and ITIM
domains
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
UC
|
urothelial carcinoma
|
|
FGFR
|
fibroblast growth factor receptor
|
|
ICI
|
immune checkpoint inhibitor
|
|
UTUC
|
upper tract urothelial carcinoma
|
References
|
1
|
Huben RP, Mounzer AM and Murphy GP: Tumor
grade and stage as prognostic variables in upper tract urothelial
tumors. Cancer. 62:2016–2020. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG;
Upper Tract Urothelial Carcinoma Collaboration The Upper Tract
Urothelial Carcinoma Collaboration, : Outcomes of radical
nephroureterectomy: A series from the upper tract urothelial
carcinoma collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Califano G, Ouzaid I, Verze P, Hermieu JF,
Mirone V and Xylinas E: Immune checkpoint inhibition in upper tract
urothelial carcinoma. World J Urol. 39:1357–1367. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin S, Xu L, Yi M, Yu S, Wu K and Luo S:
Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol
Cancer. 18:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stanietsky N, Rovis TL, Glasner A, Seidel
E, Tsukerman P, Yamin R, Enk J, Jonjic S and Mandelboim O: Mouse
TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur
J Immunol. 43:2138–2150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Clinical significance of CD155 expression in human pancreatic
cancer. Anticancer Res. 35:2287–2297. 2015.PubMed/NCBI
|
|
8
|
Zhang J, Zhu Y, Wang Q, Kong Y, Sheng H,
Guo J, Xu J and Dai B: Poliovirus receptor CD155 is up-regulated in
muscle-invasive bladder cancer and predicts poor prognosis. Urol
Oncol. 38:41.e11–41.e18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y, Cui G, Jiang Z, Li N and Zhang X:
Survival analysis with regard to PD-L1 and CD155 expression in
human small cell lung cancer and a comparison with associated
receptors. Oncol Lett. 17:2960–2968. 2019.PubMed/NCBI
|
|
10
|
Atsumi S, Matsumine A, Toyoda H, Niimi R,
Iino T and Sudo A: Prognostic significance of CD155 mRNA expression
in soft tissue sarcomas. Oncol Lett. 5:1771–1776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sloan KE, Eustace BK, Stewart JK,
Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag
LL and Jay DG: CD155/PVR plays a key role in cell motility during
tumor cell invasion and migration. BMC Cancer. 4:732004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chauvin JM and Zarour HM: TIGIT in cancer
immunotherapy. J Immunother Cancer. 8:e0009572020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanchez-Correa B, Valhondo I, Hassouneh F,
Lopez-Sejas N, Pera A, Bergua JM, Arcos MJ, Bañas H, Casas-Avilés
I, Durán E, et al: DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel
immune checkpoints for natural killer cell-based cancer
immunotherapy. Cancers (Basel). 11:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Kim K, Kwon GY, Lee SJ and Park
SH: Fibroblast growth factor receptor 3 (FGFR3) aberrations in
muscle-invasive urothelial carcinoma. BMC Urol. 18:682018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Gong Y, Saci A, Szabo PM, Martini
A, Necchi A, Siefker-Radtke A, Pal S, Plimack ER, Sfakianos JP, et
al: Fibroblast growth factor receptor 3 alterations and response to
PD-1/PD-L1 blockade in patients with metastatic urothelial cancer.
Eur Urol. 76:599–603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kacew A and Sweis RF: FGFR3 alterations in
the era of immunotherapy for urothelial bladder cancer. Front
Immunol. 11:5752582020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Zhang N, Shao J, Wang T and Wang
X: Multi-omics perspective on the tumor microenvironment based on
PD-L1 and CD8 T-Cell infiltration in urothelial cancer. J Cancer.
10:697–707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO classification of tumours of the urinary system and
male genital organs. WHO Classification of Tumours. 8. 4th edition.
International Agency for Research on Cancer; Lyon: 2016
|
|
20
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 8th edition. John Wiley
& Sons Inc.; Hoboken, NJ: 2017
|
|
21
|
Ikeda J, Ohe C, Yoshida T, Kuroda N, Saito
R, Kinoshita H, Tsuta K and Matsuda T: Comprehensive pathological
assessment of histological subtypes, molecular subtypes based on
immunohistochemistry, and tumor-associated immune cell status in
muscle-invasive bladder cancer. Pathol Int. 71:173–182. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Molfetta R, Zitti B, Lecce M, Milito ND,
Stabile H, Fionda C, Cippitelli M, Gismondi A, Santoni A and
Paolini R: CD155: A multi-functional molecule in tumor progression.
Int J Mol Sci. 21:9222020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bowers JR, Readler JM, Sharma P and
Excoffon KJDA: Poliovirus receptor: More than a simple viral
receptor. Virus Res. 242:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rikitake Y, Mandai K and Takai Y: The role
of nectins in different types of cell-cell adhesion. J Cell Sci.
125:3713–3722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Zheng Q, Xin N, Wang W and Zhao C:
CD155, an onco-immunologic molecule in human tumors. Cancer Sci.
108:1934–1938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shariat SF, Youssef RF, Gupta A, Chade DC,
Karakiewicz PI, Isbarn H, Jeldres C, Sagalowsky AI, Ashfaq R and
Lotan Y: Association of angiogenesis related markers with bladder
cancer outcomes and other molecular markers. J Urol. 183:1744–1750.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinugasa M, Amano H, Satomi-Kobayashi S,
Nakayama K, Miyata M, Kubo Y, Nagamatsu Y, Kurogane Y, Kureha F,
Yamana S, et al: Necl-5/poliovirus receptor interacts with VEGFR2
and regulates VEGF-induced angiogenesis. Circ Res. 110:716–726.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luke JJ, Bao R, Sweis RF, Spranger S and
Gajewski TF: WNT/β-catenin pathway activation correlates with
immune exclusion across human cancers. Clin Cancer Res.
25:3074–3083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xue G, Romano E, Massi D and Mandalà M:
Wnt/β-catenin signaling in melanoma: Preclinical rationale and
novel therapeutic insights. Cancer Treat Rev. 49:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sweis RF, Spranger S, Bao R, Paner GP,
Stadler WM, Steinberg G and Gajewski TF: Molecular drivers of the
non-T-cell-inflamed tumor microenvironment in urothelial bladder
cancer. Cancer Immunol Res. 4:563–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buchtova M, Oralova V, Aklian A, Masek J,
Vesela I, Ouyang Z, Obadalova T, Konecna Z, Spoustova T,
Pospisilova T, et al: Fibroblast growth factor and canonical
WNT/β-catenin signaling cooperate in suppression of chondrocyte
differentiation in experimental models of FGFR signaling in
cartilage. Biochim Biophys Acta. 1852:839–850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao JF, Caliri AW, Duex JE and
Theodorescu D: Targetable pathways in advanced bladder cancer: FGFR
signaling. Cancers (Basel). 13:48912021. View Article : Google Scholar : PubMed/NCBI
|