Introduction
Gastric cancer (GC) is the fourth leading cause of
cancer death worldwide (1).
Although surgical resection with D2 lymphadenectomy is currently a
standard strategy for advanced GC, the survival outcome of patients
with stage III GC remains poor, with a 5-year overall survival (OS)
rate of 40 to 70% (2). Neoadjuvant
chemotherapy (NAC) for advanced GC has been used in clinical
practice since the 1990s (3). NAC
aims to control microscopic metastasis and decrease the tumor
volume. For patients with clinically resectable disease at high
risk of recurrence, such as patients with extensive lymph node (LN)
metastasis or large type 3 or 4 tumors, NAC is more commonly used
than adjuvant chemotherapy. Although no randomized trials have been
performed to compare these approaches, NAC is more likely to
provide maximum response to systemic therapy. NAC is a promising
strategy that is associated with significantly higher curative
resection and downstaging rates than provided by surgery alone,
highlighting its potential to improve the OS of patients with GC
(4). The MAGIC trial was a
landmark study that evaluated the survival benefit of perioperative
chemotherapy plus surgery vs. surgery alone in patients with
curative GC (4). The study was
concluded by a phase III trial comparing surgery with vs. without
perioperative chemotherapy, and the results showed a similar 5-year
OS rate in favor of perioperative chemotherapy (5). In the randomized phase II PETRARCA
trial, the addition of trastuzumab and pertuzumab in patients with
human epidermal growth factor 2-positive resectable esophagogastric
adenocarcinoma resulted in a high pathologic complete response rate
and significantly improved LN-negative rates (6). Because accumulating reports including
the above-mentioned clinical trials have shown that the response to
NAC is considered an important factor related to survival outcomes
in patients with GC, NAC followed by surgery is more likely to
improve disease-free survival (DFS) and OS (7–9).
However, optimal methods to predict the therapeutic effect in
patients with GC have not been developed, and a novel approach to
evaluate the response to NAC for prediction of survival outcomes is
required.
Tumor markers and imaging modalities, such as
endoscopy and computed tomography (CT), have been used to evaluate
the response to NAC. However, these evaluation methods involve
measurement of the tumor volume at the primary tumor site (PT),
which is sometimes difficult because of the luminal structure
(10). In contrast, LNs have a
clearer border and are included in the Response Evaluation Criteria
in Solid Tumors (RECIST) criteria. Thus, NAC response evaluations
that target LNs instead of the PT might provide a useful
alternative for predicting survival outcomes in patients with GC.
The presence of LN metastasis (LNM) is considered one of the most
important prognostic factors after curative resection. More than
half of patients with GC have LNM at the time of diagnosis,
resulting in a 5-year OS rate of <30% (11). Because LNM is considered to be more
closely associated with micrometastases than is the PT, evaluating
the LN response to NAC might be more accurate than assessing the PT
for predicting treatment benefits and survival outcomes and
managing treatment strategies.
In the present study, by analyzing PT and LN
responses to NAC using CT images, we evaluated the clinical use of
NAC responses for predicting survival outcomes. Our simple approach
may be used in clinical decision-making for patients with GC before
and after NAC.
Materials and methods
Patient cohorts
This study involved 1220 patients with GC who
underwent standard surgical procedures (gastrectomy and regional
lymphadenectomy) at Tokushima University from 1994 to 2020. All
patients were diagnosed with GC by pathologists according to the
8th edition of the American Joint Committee on Cancer/Union for
International Cancer TNM staging system. Of these 1220 patients, we
retrospectively reviewed 160 patients with clinical stage III GC
who underwent NAC followed by surgery (n=14) and upfront surgery
(n=146). The exclusion criteria were the inability to detect the
size of the PT or LNs by CT images, the presence of
non-adenocarcinoma, and lack of available clinical information. The
pathological findings were evaluated by pathologists of Tokushima
University, and the histological grade was determined according to
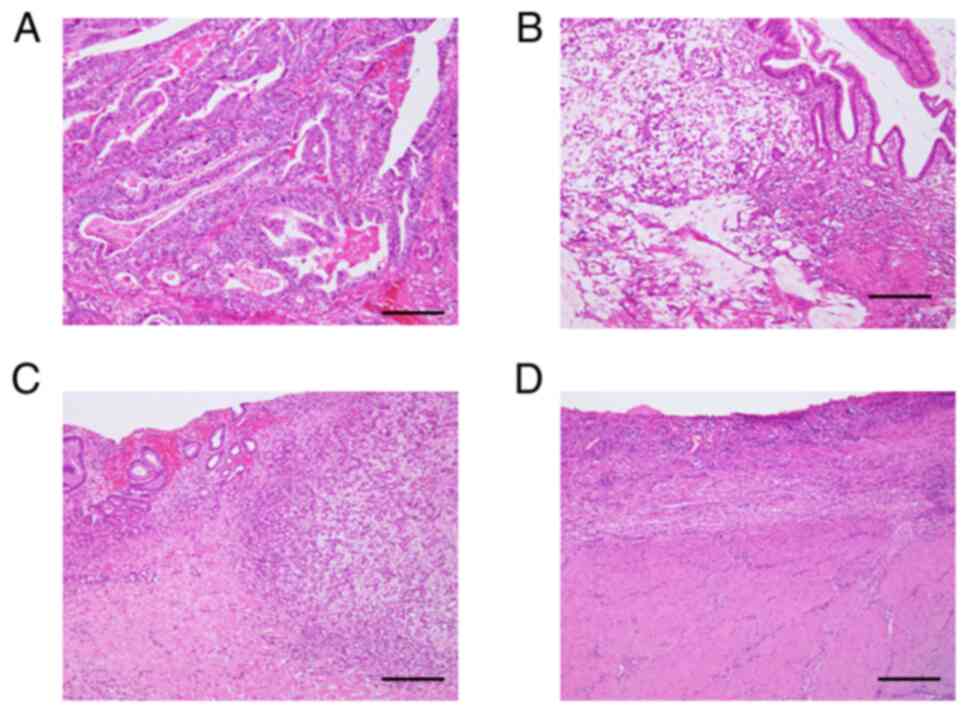
the Japanese classification of GC; the evaluation criteria are
provided in Fig. 1 (12). The patients' immunonutritional
status was assessed using the neutrophil-lymphocyte ratio (NLR),
lymphocyte-C-reactive protein ratio (LCR), and prognostic
nutritional index (PNI) (13–15).
C-reactive protein levels were assessed by using LABOSPECT 008 α
(HITACHI, Tokyo, Japan). Neutrophils were calculated by using
SIEMENS ADVIA 2120i Hematology System (Siemens Health care Co.,
Tokyo, Japan). Present study was approved in advance by the
Institutional Review Board of the University of Tokushima Graduate
School of Medical Science (TOCMS: 3215-1).
NAC
Routine examinations before treatment included a
complete physical examination; assessment of medical and surgical
history; chest, abdominal, and pelvic CT scans; and endoscopy. NAC
consisted of a docetaxel, cisplatin, and S-1 (DCS) regimen or S-1
and oxaliplatin (SOX) regimen. DCS therapy involved two to six
cycles of docetaxel (60 mg/m2), cisplatin (60
mg/m2), and S-1 (40 mg/m2 twice daily on days
1–14) every 3 weeks. SOX therapy involved two to six cycles of S-1
(40 mg/m2 twice daily on days 1–14) and oxaliplatin (130
mg/m2) via rapid intravenous infusion every 3 weeks.
Radical resection with D2 lymphadenectomy was performed 2 to 4
weeks after NAC.
Evaluation of response to NAC
The response to NAC was evaluated by the volume of
the PT and the short axis of the LN lesion with the greatest
diameter on enhanced thin-slice (1-mm) CT scans before NAC and 2 to
4 weeks after NAC. The response to NAC in the PT and LNs was
evaluated as the percent reduction in the PT area and LN length,
respectively. We classified positive LNs as those of >8 mm in
size because a threshold of 8 mm is often arbitrarily used for
perigastric LNs (16). We
evaluated the diameter of each LN lesion and the median short axis
of the total LN lesions in patients with multiple LNM. Based on the
RECIST classification, the patients were classified into a
responder group (complete response and partial response) and a
non-responder group (stable disease and progressive disease)
(10). All patients underwent an
abdominal CT scan using a 320-slice Aquilion One scanner (Toshiba,
Tokyo, Japan). The CT device had auto exposure control and was
linked to a networked medical imaging system through which images
were electronically transferred to a centralized data system and
then retrieved at a workstation.
Statistical analysis
The cutoff thresholds of continuous variables were
divided by the median value among the total participants. The
nutrition indices (NLR, LCR, and PNI) were also divided by the
median value among the total participants. The patients'
clinicopathological characteristics were compared between
responders and non-responders using the chi-square test for
categorical data. DFS and OS were analyzed by the Kaplan-Meier
method using a weighted test. Univariate and multivariate Cox
proportional hazard regression models were used to identify
independent prognostic markers. A P-value of <0.05 was
considered statistically significant. Statistical analyses were
performed using MedCalc 16.2.0 statistical software (MedCalc
Software bvba, Ostend, Belgium) and JMP Pro 13 statistical software
(SAS Institute Japan, Tokyo, Japan).
Results
Patient characteristics and CT
measurements
We retrospectively reviewed 160 patients with
clinical stage III GC who had been diagnosed with one or more
positive LNs before treatment. Among these 160 patients, 14
underwent NAC followed by surgery and 146 underwent standard
radical surgery (gastrectomy and D2 lymphadenectomy). The patients'
detailed clinicopathological characteristics are provided in
Table I. There were no significant
differences in the clinicopathological characteristics between the
two groups. The median PT area before and after NAC was 924
mm2 (range: 600–2649 mm2) and 665
mm2 (range: 332–1956 mm2), respectively. The
median short axis of LN lesions before and after NAC was 11 mm
(range: 8–20 mm) and 7 mm (range: 5–12 mm), respectively. We
divided the patients into responder and non-responder groups based
on the RECIST criteria; six patients were PT responders and eight
were PT non-responders, and eight patients were LN responders and
six were LN non-responders. We then examined the difference in the
response to NAC between PTs and LNs (Table II).
 | Table I.Clinicopathological characteristics
of patients who underwent upfront surgery vs. NAC followed by
surgery. |
Table I.
Clinicopathological characteristics
of patients who underwent upfront surgery vs. NAC followed by
surgery.
| Factors | Upfront surgery
(n=146) | NAC (n=14) | P-value |
|---|
| Age |
|
| 0.19 |
| ≥70
years | 79 | 5 |
|
| <70
years | 67 | 9 |
|
| Sex |
|
| 0.75 |
|
Male | 109 | 11 |
|
|
Female | 37 | 3 |
|
| Tumor location |
|
| 0.96 |
|
Upper/Middle | 84 | 8 |
|
|
Lower | 62 | 6 |
|
| Tumor size |
|
| 0.62 |
| ≥50
mm | 83 | 7 |
|
| <50
mm | 63 | 7 |
|
|
Differentiation |
|
| 0.08 |
|
Well/Moderate | 74 | 3 |
|
|
Poor | 58 | 7 |
|
|
Others | 14 | 4 |
|
| cT stage |
|
| 0.75 |
|
T1-3 | 76 | 6 |
|
| T4 | 70 | 8 |
|
| cN stage |
|
| 0.73 |
|
N0-1 | 80 | 7 |
|
|
N2-3 | 66 | 7 |
|
| Surgical
procedure |
|
| 0.62 |
| DG | 62 | 5 |
|
| TG | 84 | 9 |
|
| NAC regimen |
|
| - |
|
DCS | - | 9 |
|
|
SOX | - | 5 |
|
| Histological
grade |
|
| - |
| 0,
1 | - | 6 |
|
| 2,
3 | - | 8 |
|
 | Table II.Clinicopathological characteristics
according to PT and LN responses to NAC. |
Table II.
Clinicopathological characteristics
according to PT and LN responses to NAC.
|
| Primary tumor | Lymph node |
|---|
|
|
|
|
|---|
| Factors | Res (n=6) | Non-res (n=8) | P-value | Res (n=8) | Non-res (n=6) | P-value |
|---|
| Age |
|
|
|
|
| 0.33 |
| ≥70
years | 3 | 2 |
| 2 | 3 |
|
| <70
years | 3 | 6 | 0.33 | 6 | 3 |
|
| Sex |
|
|
|
|
| 0.35 |
|
Male | 6 | 5 |
| 7 | 4 |
|
|
Female | 0 | 3 | 0.10 | 1 | 2 |
|
| Tumor location |
|
|
|
|
| 0.12 |
|
Upper/Middle | 5 | 3 |
| 6 | 2 |
|
|
Lower | 1 | 5 | 0.09 | 2 | 4 |
|
| Tumor size |
|
|
|
|
| 0.12 |
| ≥50
mm | 2 | 5 |
| 2 | 4 |
|
| <50
mm | 4 | 3 | 0.28 | 6 | 2 |
|
|
Differentiation |
|
|
|
|
| 0.08 |
|
Well/Moderate | 1 | 2 |
| 2 | 1 |
|
|
Poor | 4 | 3 |
| 5 | 2 |
|
|
Others | 1 | 3 | 0.54 | 1 | 3 |
|
| cT stage |
|
|
|
|
| 0.53 |
|
T1-3 | 3 | 3 |
| 4 | 2 |
|
| T4 | 3 | 5 | 0.64 | 4 | 4 |
|
| cN stage |
|
|
|
|
| 0.28 |
| N1 | 2 | 5 |
| 3 | 4 |
|
|
T2-3 | 4 | 3 | 0.28 | 5 | 2 |
|
| Surgical
procedure |
|
|
|
|
| 0.33 |
| DG | 2 | 3 |
| 2 | 3 |
|
| TG | 4 | 5 | 0.87 | 6 | 3 |
|
| NAC regimen |
|
|
|
|
| 0.87 |
|
DCS | 4 | 5 |
| 5 | 4 |
|
|
SOX | 2 | 3 | 0.87 | 3 | 2 |
|
| Histological
grade |
|
|
|
|
| 0.12 |
| 0,
1 | 2 | 4 |
| 2 | 4 |
|
| 2,
3 | 4 | 4 | 0.53 | 6 | 2 |
|
| LCR |
|
|
|
|
| 0.53 |
|
High | 4 | 2 |
| 4 | 4 |
|
|
Low | 2 | 6 | 0.12 | 4 | 2 |
|
| NLR |
|
|
|
|
| 0.35 |
|
High | 5 | 2 |
| 7 | 2 |
|
|
Low | 1 | 6 | 0.71 | 1 | 4 |
|
| PNI |
|
|
|
|
| 0.20 |
|
High | 5 | 4 |
| 4 | 1 |
|
|
Low | 1 | 4 | 0.20 | 4 | 5 |
|
Prognostic potential of NAC response
for predicting survival outcomes in patients with GC
To evaluate the prognostic potential of NAC
responses, we performed a survival analysis to compare OS and DFS
between patients who underwent NAC and those who underwent upfront
surgery. There were no significant differences in survival outcomes
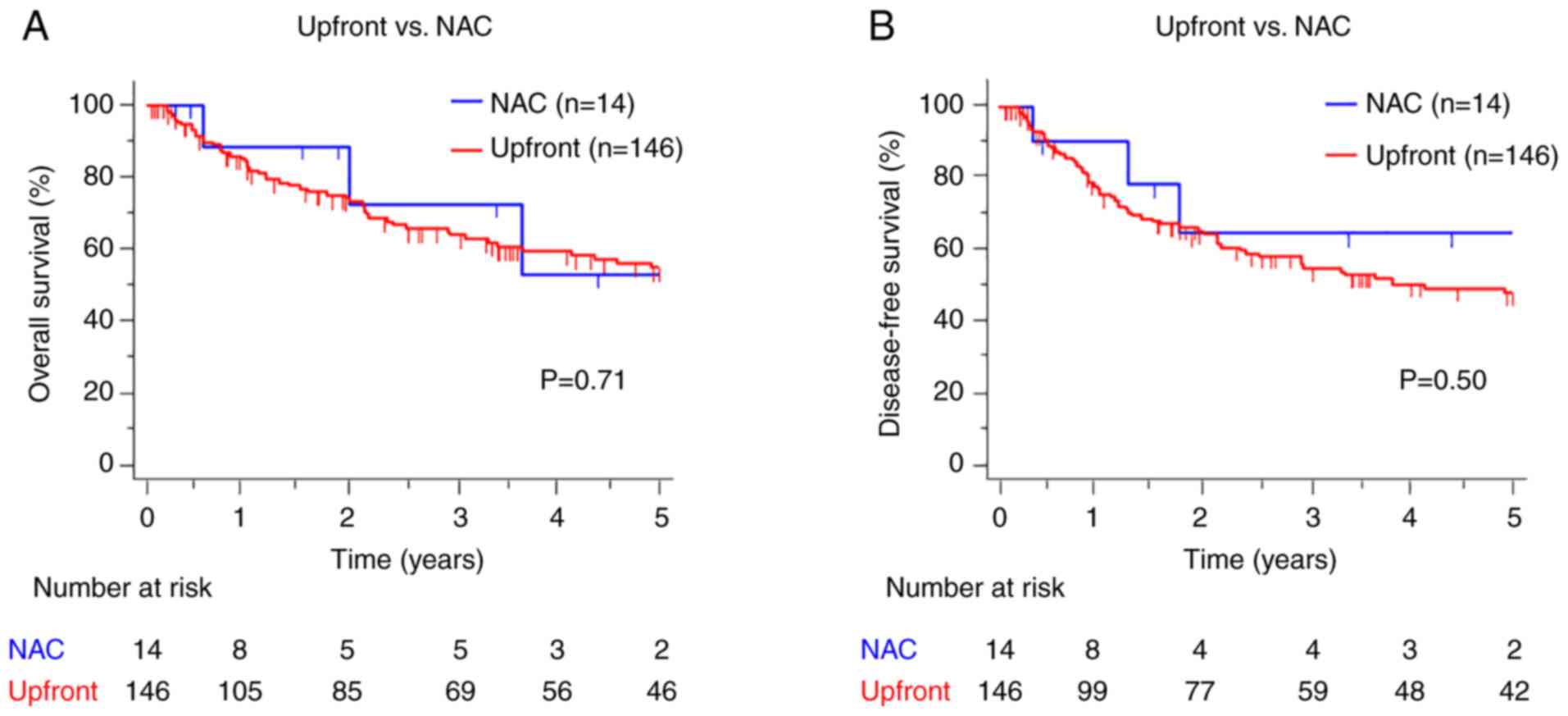
between the two groups (OS: P=0.71, DFS: P=0.50) (Fig. 2A and B). We then investigated the
prognostic potential of NAC responses between patients categorized
as responders and non-responders for PTs and LNs. The results
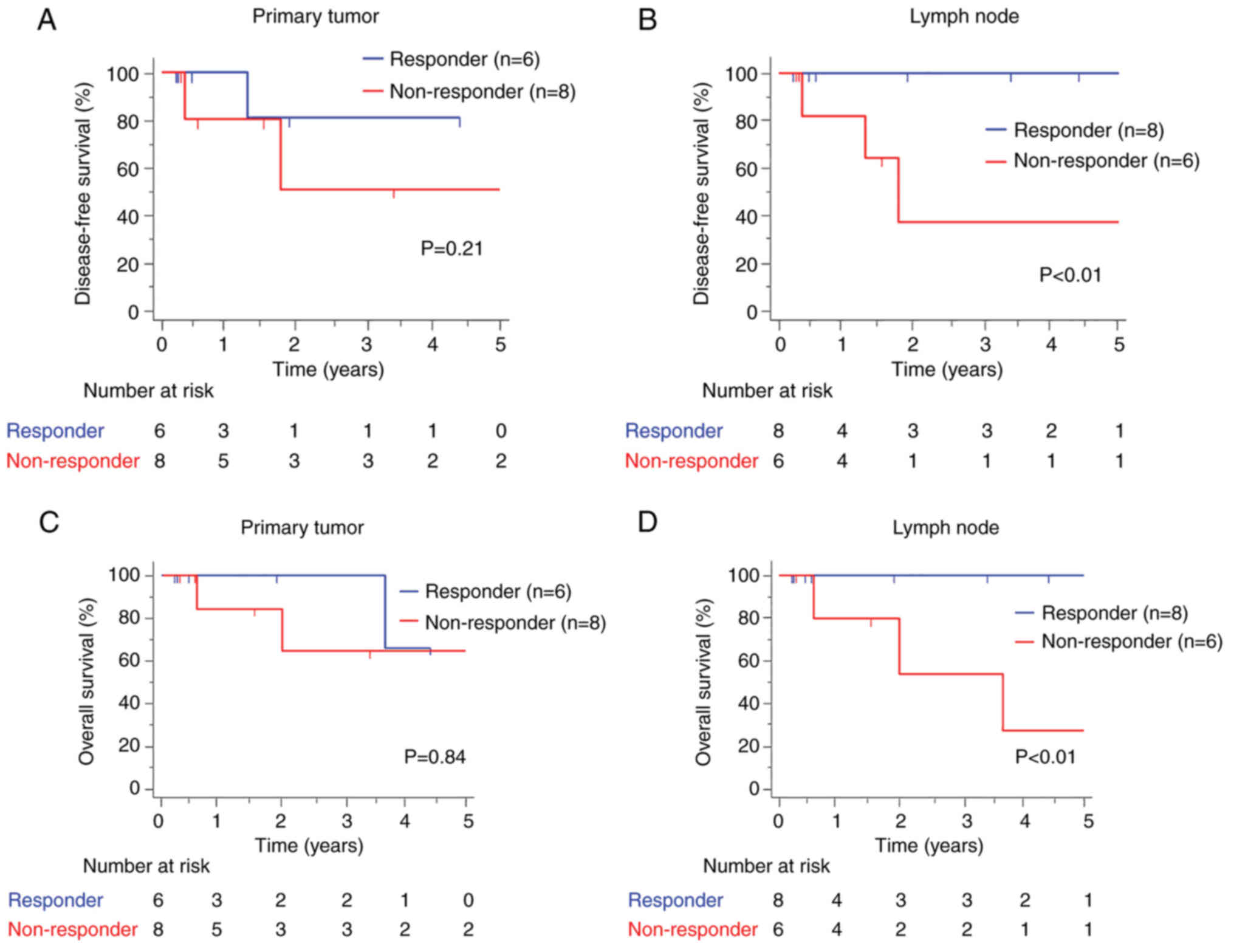
showed no significant differences in the 5-year DFS rate between
the PT non-responder and responder groups (64.3% vs. 66.7%, P=0.93)
(Fig. 3A). More importantly, we
observed that LN non-responders had significantly worse DFS than LN
responders (30.0% vs. 100%, P<0.01) (Fig. 3B). Moreover, LN non-responders had
significantly worse OS, whereas no significant difference in OS was
observed between PT responders and non-responders (PT: 62.5% vs.
50.0%, P=0.84; LN: 26.7% vs. 100%, P<0.01) (Fig. 3C and D). These findings highlight
the clinical importance of LN responses to NAC.
Furthermore, we conducted univariate and
multivariate Cox proportional hazard regression analyses to
identify prognostic factors, and patients categorized as LN
non-responders to NAC had significantly worse DFS than patients
categorized as LN responders [univariate: hazard ratio (HR)=7.79,
95% confidence interval (CI)=1.16-63.51, P=0.02; multivariate:
HR=7.44, 95% CI=1.45-56.78, P=0.01] (Table III). In contrast, PT responders
and non-responders to NAC showed no significant differences in DFS
(univariate: HR=2.35, 95% CI=0.62-8.85, P=0.21). Among the
clinicopathological factors, an elevated NLR tended to be
associated with poor DFS (univariate: HR=4.23, 95% CI=0.57-31.42,
P=0.06; multivariate: HR=6.45, 95% CI=1.23-45.34, P=0.04). These
results indicate that the LN response to NAC and the NLR have
prognostic potential in predicting the survival outcomes of
patients with GC.
 | Table III.Univariate and multivariate Cox
proportional hazard regression analyses for disease-free
survival. |
Table III.
Univariate and multivariate Cox
proportional hazard regression analyses for disease-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| (≥70
vs. <70 years) | 2.10 | 0.59-7.43 | 0.25 |
|
|
|
| Gender |
|
|
|
|
|
|
| (Male
vs. Female) | 0.31 | 0.04-2.53 | 0.28 |
|
|
|
| Tumor location |
|
|
|
|
|
|
|
(Upper/Middle vs. Lower) | 1.45 | 0.37-5.77 | 0.59 |
|
|
|
| Tumor size |
|
|
|
|
|
|
| (≥50 mm
vs. <50 mm) | 0.64 | 0.16-2.56 | 0.53 |
|
|
|
|
Differentiation |
|
|
|
|
|
|
|
(Well/Moderate vs.
Poor/Others) | 1.13 | 0.28-4.41 | 0.88 |
|
|
|
| cT |
|
|
|
|
|
|
| (T4 vs.
<T3) | 2.61 | 0.73-9.40 | 0.14 |
|
|
|
| cN |
|
|
|
|
|
|
| (≥N2
vs. <N1) | 1.18 | 0.31-4.48 | 0.81 |
|
|
|
| Surgical
procedure |
|
|
|
|
|
|
| (TG vs.
DG) | 1.29 | 0.33-5.08 | 0.71 |
|
|
|
| NAC regimen |
|
|
|
|
|
|
| (DCS
vs. SOX) | 3.27 | 0.78-13.73 | 0.09 | 2.37 | 0.67-8.98 | 0.45 |
| Histological
grade |
|
|
|
|
|
|
| (0, 1
vs. 2, 3) | 1.71 | 0.32-9.00 | 0.53 |
|
|
|
| LCR |
|
|
|
|
|
|
| (Low
vs. High) | 2.53 | 0.67-9.60 | 0.17 |
|
|
|
| NLR |
|
|
|
|
|
|
| (High
vs. Low) | 4.23 | 0.57-31.42 | 0.06 | 6.45 | 1.23-45.34 | 0.04a |
| PNI |
|
|
|
|
|
|
| (Low
vs. High) | 1.90 | 0.45-8.07 | 0.39 |
|
|
|
| PT response |
|
|
|
|
|
|
|
(Non-res vs. Res) | 2.35 | 0.62-8.85 | 0.21 |
|
|
|
| LN response |
|
|
|
|
|
|
|
(Non-res vs. Res) | 7.79 | 1.16-63.51 | 0.02a | 7.44 | 1.45-56.78 | 0.01a |
Discussion
Radical resection with D2 lymphadenectomy is
currently the gold standard for advanced GC worldwide (12,17–19).
However, GC is one of the most aggressive malignancies; it has high
metastatic potential, and the 5-year OS rates of patients after D2
lymphadenectomy remain poor (18).
Therefore, a multi-treatment strategy consisting of NAC has been
suggested for GC because NAC reportedly reduces the tumor volume
and micrometastases and improves the R0 resection rate (4). Since the 1990s, NAC has been used in
clinical practice for advanced GC (3). Furthermore, several clinical studies
on the efficacy of NAC or different chemotherapeutic regimens have
recently demonstrated survival benefits compared with surgery alone
in patients with advanced GC (4,5,20),
emphasizing the clinical significance of predicting the response to
NAC.
Imaging modalities, including fluoroscopy, CT, and
endoscopy, are standard tools for confirming the efficacy of NAC
(21–23). Although the most accurate approach
for evaluating the response to NAC is pathological classification,
some studies have shown that the pathological grading scores of PTs
have no predictive value for survival outcomes, and the predictive
effect of the pathological complete response rate is not associated
with that of the LNM rate (24,25).
In the present study, we investigated the clinicopathological
factors and PT/LN responses to NAC by CT imaging. The results
showed that the PT response to NAC had no predictive significance,
whereas patients classified as LN responders to NAC had a
significantly higher 5-year DFS rate (30.0% vs. 100%, P<0.01)
and 5-year OS rate (26.7% vs. 100%, P<0.01) than non-responders.
Accumulating reports have shown that the rate of LNM predicts
survival outcomes and local recurrence in patients with GC
(26–28), emphasizing that the LN response to
NAC has prognostic potential.
Additionally, we evaluated the LCR, PNI, and NLR,
which have been suggested to reflect the balance between the
pro-tumor inflammatory status and anti-tumor immune status. In
previous studies, patients with several cancers (including GC) who
had an increased NLR showed neutrophilic leukocytosis and
lymphocytopenia (29–33). In the present study, an elevated
NLR was associated with recurrence in patients with GC before
treatment and with the LN response to NAC, emphasizing the clinical
importance of this nutritional index. Several reports have also
shown that cancer-related systemic inflammatory responses are
associated with increased numbers of circulating neutrophils.
Neutrophils secrete cytokines and chemokines, which play an
important role in cancer progression (34), and the NLR is one of the most
robust biomarkers for predicting the prognosis of various types of
cancer (35,36). In the present study, an elevated
NLR tended to be associated with poor DFS. However, other
nutritional indices (lower PNI and LCR) were not associated with
poor survival. A lower PNI and LCR have also been reported to be
independently associated with poor survival (33,37–40).
Therefore, additional studies involving more patients are needed to
address the potential prognostic value of nutritional indices in
GC.
This study has several limitations. First, its
retrospective and single-institute design may have resulted in
selection bias. Thus, a prospective clinical trial is required to
confirm our findings. However, the patient population was uniform,
and all patients received the same treatment strategy [i.e., NAC
(DCS or SOX regimen) followed by distal or total gastrectomy with
D2 lymphadenectomy]. Second, we analyzed a small number of patients
who underwent gastrectomy with NAC. As a result, the findings of
the present study are only applicable to patients with positive LNM
who undergo NAC followed by surgery. We must confirm the accuracy
of our findings in larger numbers of patients, and future studies
should evaluate the feasibility of targeting distant metastatic
lesions in addition to LNs to predict the response to chemotherapy.
Third, we validated the predictive potential of LN responses to NAC
by analyzing CT images and clinicopathological factors. Future
experiments investigating other previously reported prognostic
factors, such as the microsatellite instability status and GC
subtypes (41–44), are needed to increase the
convenience and prognostic accuracy of our approach. Finally,
diagnosing clinical stage III GC by imaging modalities is
difficult. However, our study provides important evidence for
predicting survival outcomes in patients with GC, and these
findings may be a significant step toward the management of lethal
malignancies.
In conclusion, we have revealed the clinical
importance of the LN response to NAC with CT imaging for prediction
of survival outcomes in patients with GC. Our findings highlight
the potential clinical impact of optimizing treatment strategies
and improving the selection and management of patients with this
malignancy.
Acknowledgements
The authors would like to thank Dr Melissa Crawford
and Dr Angela Morben for editing a draft of this manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, MN and MS designed the study. YW, MN, KY, CT, TT
and TN performed the data analyses. YW, MN, MS, HK and TY acquired
the clinical data and drafted the manuscript. MS revised the
manuscript. All authors read and approved the final manuscript. YW
and MN confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and approved by the Institutional Review
Board of the University of Tokushima Graduate School (approval no.
3215). Consent to participate was not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
CT
|
computed tomography
|
|
DCS
|
docetaxel, cisplatin, and S-1
|
|
DFS
|
disease-free survival
|
|
GC
|
gastric cancer
|
|
HR
|
hazard ratio
|
|
LCR
|
lymphocyte-C-reactive protein
ratio
|
|
LN
|
lymph node
|
|
LNM
|
lymph node metastasis
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NLR
|
neutrophil-lymphocyte ratio
|
|
OS
|
overall survival
|
|
PNI
|
prognostic nutritional index
|
|
PT
|
primary tumor site
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
SOX
|
S-1 and oxaliplatin
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Songun I, Keizer HJ, Hermans J,
Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van
Krieken JH and van de Velde CJ: Chemotherapy for operable gastric
cancer: Results of the Dutch randomised FAMTX trial. The Dutch
gastric cancer group (DGCG). Eur J Cancer. 35:558–562. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ychou M, Boige V, Pignon JP, Conroy T,
Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM,
Saint-Aubert B, et al: Perioperative chemotherapy compared with
surgery alone for resectable gastroesophageal adenocarcinoma: An
FNCLCC and FFCD multicenter phase III trial. J Clin Oncol.
29:1715–1721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hofheinz RD, Merx K, Haag GM, Springfeld
C, Ettrich T, Borchert K, Kretzschmar A, Teschendorf C, Siegler G,
Ebert MP, et al: FLOT versus FLOT/trastuzumab/pertuzumab
perioperative therapy of human epidermal growth factor receptor
2-positive resectable esophagogastric adenocarcinoma: A randomized
phase II Trial of the AIO EGA study group. J Clin Oncol.
JCO2200380. 2022.(epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng L, Yang W, Zhang Z, Liu H and Hua Y:
Clinical features and prognosis analysis of 21 gastric cancer
patients with pathological complete response after neoadjuvant
chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi. 20:1168–1173.
2017.(In Chinese). PubMed/NCBI
|
|
8
|
Cho H, Nakamura J, Asaumi Y, Yabusaki H,
Sakon M, Takasu N, Kobayashi T, Aoki T, Shiraishi O, Kishimoto H,
et al: Long-term survival outcomes of advanced gastric cancer
patients who achieved a pathological complete response with
neoadjuvant chemotherapy: A systematic review of the literature.
Ann Surg Oncol. 22:787–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Peng Z and Chen L: Survival
analysis of gastric cancer cases with pathological complete
response received neoadjuvant chemotherapy. Zhonghua Yi Xue Za Zhi.
96:1582–1584. 2016.(In Chinese). PubMed/NCBI
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY
and Shen KH: Peritoneal carcinomatosis and lymph node metastasis
are prognostic indicators in patients with Borrmann type IV gastric
carcinoma. Hepatogastroenterology. 49:874–877. 2002.PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
13
|
Grenader T, Waddell T, Peckitt C, Oates J,
Starling N, Cunningham D and Bridgewater J: Prognostic value of
neutrophil-to-lymphocyte ratio in advanced oesophago-gastric
cancer: Exploratory analysis of the REAL-2 trial. Ann Oncol.
27:687–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruixola G, Caballero J, Papaccio F,
Petrillo A, Iranzo A, Civera M, Moriana M, Bosch N, Maroñas M,
González I, et al: Prognostic nutritional index as an independent
prognostic factor in locoregionally advanced squamous cell head and
neck cancer. ESMO Open. 3:e0004252018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okugawa Y, Toiyama Y, Yamamoto A,
Shigemori T, Ide S, Kitajima T, Fujikawa H, Yasuda H, Hiro J,
Yoshiyama S, et al: Lymphocyte-C-reactive protein ratio as
promising new marker for predicting surgical and oncological
outcomes in colorectal cancer. Ann Surg. 272:342–351. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwee RM and Kwee TC: Imaging in assessing
lymph node status in gastric cancer. Gastric Cancer. 12:6–22. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 11:531–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okines A, Verheij M, Allum W, Cunningham D
and Cervantes A; ESMO Guidelines Working Group, : Gastric cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21 (Suppl 5):v50–v54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; European Society for Medical Oncology
(ESMO); European Society of Surgical Oncology (ESSO); European
Society of Radiotherapy and Oncology (ESTRO), : Gastric cancer:
ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 24 (Suppl 6):vi57–vi63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuhmacher C, Gretschel S, Lordick F,
Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan
B, Welch J, et al: Neoadjuvant chemotherapy compared with surgery
alone for locally advanced cancer of the stomach and cardia:
European organisation for research and treatment of cancer
randomized trial 40954. J Clin Oncol. 28:5210–5218. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SR, Lee JS, Kim CG, Kim HK, Kook MC,
Kim YW, Ryu KW, Lee JH, Bae JM and Choi IJ: Endoscopic ultrasound
and computed tomography in restaging and predicting prognosis after
neoadjuvant chemotherapy in patients with locally advanced gastric
cancer. Cancer. 112:2368–2376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Redondo-Cerezo E, Martínez-Cara JG,
Jiménez-Rosales R, Valverde-López F, Caballero-Mateos A,
Jérvez-Puente P, Ariza-Fernández JL, Úbeda-Muñoz M, López-de-Hierro
M and de Teresa J: Endoscopic ultrasound in gastric cancer staging
before and after neoadjuvant chemotherapy. A comparison with PET-CT
in a clinical series. United European Gastroenterol J. 5:641–647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smyth EC, Fassan M, Cunningham D, Allum
WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M,
et al: Effect of pathologic tumor response and nodal status on
survival in the medical research council adjuvant gastric
infusional chemotherapy trial. J Clin Oncol. 34:2721–2727. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomasello G, Petrelli F, Ghidini M,
Pezzica E, Passalacqua R, Steccanella F, Turati L, Sgroi G and
Barni S: Tumor regression grade and survival after neoadjuvant
treatment in gastro-esophageal cancer: A meta-analysis of 17
published studies. Eur J Surg Oncol. 43:1607–1616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takagane A, Terashima M, Abe K, Araya M,
Irinoda T, Yonezawa H, Nakaya T, Inaba T, Oyama K, Fujiwara H and
Saito K: Evaluation of the ratio of lymph node metastasis as a
prognostic factor in patients with gastric cancer. Gastric Cancer.
2:122–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kodera Y, Yamamura Y, Shimizu Y, Torii A,
Hirai T, Yasui K, Morimoto T, Kato T and Kito T: Metastatic gastric
lymph node rate is a significant prognostic factor for resectable
stage IV stomach cancer. J Am Coll Surg. 185:65–69. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komatsu S, Ichikawa D, Miyamae M, Kosuga
T, Okamoto K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki
A, et al: Positive lymph node ratio as an indicator of prognosis
and local tumor clearance in N3 gastric cancer. J Gastrointest
Surg. 20:1565–1571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
multinational trial organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L and Lv Y: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Int J Cancer. 134:2403–2413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim
KH and Kim HJ: Prognostic significance of neutrophil lymphocyte
ratio and platelet lymphocyte ratio in advanced gastric cancer
patients treated with FOLFOX chemotherapy. BMC Cancer. 13:3502013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng CB, Zhang QX, Zhuang LP and Sun JW:
Prognostic value of lymphocyte-to-C-reactive protein ratio in
patients with gastric cancer after surgery: A multicentre study.
Jpn J Clin Oncol. 50:1141–1149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Guo X, Wang M, Ma X, Ye X and Lin
P: Pre-treatment inflammatory indexes as predictors of survival and
cetuximab efficacy in metastatic colorectal cancer patients with
wild-type RAS. Sci Rep. 7:171662017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Jia H, Yu W, Xu Y, Li X, Li Q and
Cai S: Nomograms for predicting prognostic value of inflammatory
biomarkers in colorectal cancer patients after radical resection.
Int J Cancer. 139:220–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow inflammation outcome study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okugawa Y, Toiyama Y, Yamamoto A,
Shigemori T, Ichikawa T, Yin C, Suzuki A, Fujikawa H, Yasuda H,
Hiro J, et al: Lymphocyte-to-C-reactive protein ratio and score are
clinically feasible nutrition-inflammation markers of outcome in
patients with gastric cancer. Clin Nutr. 39:1209–1217. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirahara N, Tajima Y, Fujii Y, Kaji S,
Yamamoto T, Hyakudomi R, Taniura T and Kawabata Y: Prognostic
nutritional index as a predictor of survival in resectable gastric
cancer patients with normal preoperative serum carcinoembryonic
antigen levels: A propensity score matching analysis. BMC Cancer.
18:2852018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Migita K, Takayama T, Saeki K, Matsumoto
S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N and
Nakajima Y: The prognostic nutritional index predicts long-term
outcomes of gastric cancer patients independent of tumor stage. Ann
Surg Oncol. 20:2647–2654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xishan Z, Ye Z, Feiyan M, Liang X and
Shikai W: The role of prognostic nutritional index for clinical
outcomes of gastric cancer after total gastrectomy. Sci Rep.
10:173732020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC,
Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, et al: Clinical
significance of four molecular subtypes of gastric cancer
identified by the cancer genome atlas project. Clin Cancer Res.
23:4441–4449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L and Wang X: Identification of gastric
cancer subtypes based on pathway clustering. NPJ Precis Oncol.
5:462021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodriquenz MG, Roviello G, D'Angelo A,
Lavacchi D, Roviello F and Polom K: MSI and EBV positive gastric
cancer's subgroups and their link with novel immunotherapy. J Clin
Med. 9:14272020. View Article : Google Scholar : PubMed/NCBI
|