Introduction
Colorectal cancer (CRC) exhibits the third highest
incidence rate and the second highest cancer-related mortality rate
worldwide (1). The global disease
burden of CRC is expected to increase by 60% by 2035, with new
cases rising to 2.5 million (2,3). The
increasing incidence of CRC will be accompanied by an increase in
mortality (4), particularly for
patients experiencing recurrence and metastasis following curative
surgery (5). At present, clinical
settings and prognostic indicators, including pathological
indicators, are used to guide the clinical management of patients
with CRC. Notably, pathological indicators include classic TNM
stage, differentiation, invasion, and blood and stool proteins,
such as carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9,
and CA-195. Pathological indicators also include molecular markers,
such as microsatellite instability, chromosome 18q loss of
heterozygosity, P53, KRAS, BRAF, epidermal growth factor receptor,
and vascular endothelial growth factor (6,7).
However, the effect of these indicators on the accurate prediction
of patient prognosis remains unsatisfactory (8,9).
Therefore, it is necessary to explore new biomarkers for the
improved prediction of patient prognosis (9).
In 1983, Gerdes et al (10) discovered the Ki67 antigen and
determined that Ki67 was associated with the active proliferation
of cells (10). Ki67 is only
expressed in the interphase and mitotic phases of mitosis and is
not expressed in the resting phase (G0). During mitosis, Ki67
expression gradually increases until expression reaches a peak
(11). Thus, Ki67 expression may
reflect the growth fraction of cell populations, and results of
numerous previous studies have demonstrated that Ki67 may serve as
a prognostic or predictive biomarker for different types of tumors
(12,13). Moreover, numerous previous studies
and meta-analyses demonstrated that high expression levels of Ki67
are associated with adverse overall survival and disease-free
survival of patients with CRC, and may therefore be used as a
valuable marker of CRC prognosis (14–16).
Investigating Ki67 expression in tumor tissues using
immunohistochemistry is a routine and reliable examination strategy
for the determining the proliferative activity of tumor cells
following therapeutic surgery (17,18).
However, the results of testing have not yet been used to guide the
clinical management of patients with CRC, as the optimal cut-off
value remains undetermined (14,19).
Miller et al (20)
demonstrated that Ki67 expression plays a key role in the cell
cycle following the tracking of Ki67 expression in a single cell
over time (20). Based on the
different expression levels of Ki67 throughout the cell cycle, and
the results obtained by Miller et al (20), it was hypothesized that Ki67 may
possess a non-linear association with CRC prognosis, rather than a
linear one, as previously suggested (21). Thus, the present
immunohistochemical study aimed to use a cohort of patients with
non-metastatic CRC and the Restricted Cubic Spline (RCS) model to
analyze the association between Ki67 levels and the risk of patient
death and metastasis. The present study aimed to obtain an
evidence-based cut-off value of Ki67, to guide the clinical
management of patients with CRC.
Materials and methods
Patients
A retrospective cohort study was employed for the
present analysis. Data were collected from patient medical records
stored in the Electronic Health Information System of the First
Affiliated Hospital of Kunming Medical University. Patients with
CRC included in the present study underwent therapeutic surgery at
the aforementioned hospital between January 2014 and December 2020.
The inclusion criteria for patients were as follows: i) Patients
with stage I–III CRC who received therapeutic surgery, but did not
receive preoperative chemoradiation, and ii) patients with complete
demographic, clinical treatment, associated laboratory test, and
follow-up data, whose tumor tissue was tested for Ki67. Patients
with CRC who did not meet the aforementioned criteria were excluded
from the present analysis. The covariates included in the present
analysis consisted of demographic features, including patient sex,
age at diagnosis, race, body mass index (BMI), CEA and Ki67, and
tumor pathological features, including type, location,
differentiation, stage, vascular invasion, and perineural invasion
of CRC.
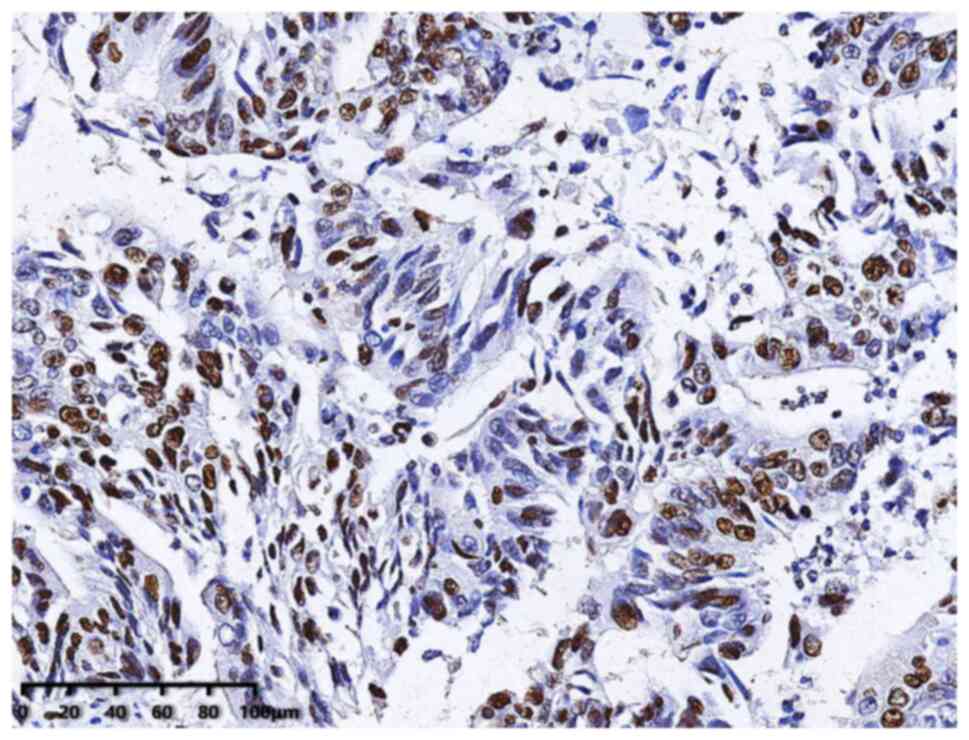
Immunohistochemistry
Immunohistochemistry was used to detect Ki67
expression in tumor tissue following therapeutic surgery, and this
was performed according to the manufacturer's instructions for the
antibody (ProteinTech Group, Inc.). Briefly, tumor tissue was
embedded in paraffin wax and subsequently dewaxed using xylene and
hydrated, and antigen retrieval was performed. Endogenous
peroxidase activity was blocked using 3% H2O2
and then the slides were blocked with 5% BSA blocking buffer at
room temperature for 1 h. Subsequently, tumor tissue was incubated
with Ki67 polyclonal antibody (ProteinTech Group, Inc.; cat. no.
27309-1-AP, RRID: AB_2756525, 1:2,000) at 37°C for 1 h followed by
incubation with anti-rabbit IgG (ProteinTech Group, Inc.; cat. no.
SA00004-2, RRID: AB_2890944; 1:200) at 37°C for 1.5 h.
Immunostaining was detected using DAB substrate solution and
samples were counterstained using hematoxylin at room temperature
for 10–30 sec. Immunohistochemistry was performed following
surgery. A score was assigned to the Ki67 testing result of each
patient to represent the Ki67 level, and the guidelines recommended
by the International Ki67 in Breast Cancer Working Group were
adopted as the scoring method (22). Immunohistochemical scoring was
performed independently by two specialists in pathology who were
blinded to the clinicopathological characteristics and prognosis of
the patients. A total of five non-overlapping high-power fields
(objective, ×40, Leica Microsystems GmbH) were randomly selected in
the chromatically homogeneous area, and Ki67 expression was
calculated as the proportion of tumor cells with positive nuclear
staining in all tumor cells.
Patient follow-up
Patient follow-up following therapeutic surgery
included death, distant metastasis, and survival without
metastasis. The outcome data were obtained from the Electronic
Health Information System, or by contacting patients or family
members via telephone. The survival time was calculated from the
time of diagnosis of CRC to the time of metastasis or death. The
date of the last follow-up was recorded if metastasis or death did
not occur. The last date of follow-up was 31st August 2021.
Statistical analysis
Quantitative data was presented as the mean ±
standard deviation or the median (interquartile range, IQR), and
comparisons between two groups was performed using an independent
Student's t-test or Mann-Whitney U test, based on the distribution
of data. Categorical data are presented as the frequency
(percentage), and a comparison between groups were performed using
χ2 or Fisher's exact test. The RCS model was used to
determine the non-linear association between Ki67 levels in the
tumor tissue and the risk ratio of metastasis and death of patients
with CRC. The association between Ki67 expression with distant
metastasis or death of patients was analyzed using univariate and
multivariate Cox proportional hazards regression models, using
hazard ratios (HR), 95% confidence intervals (CIs) to describe the
risk ratio, and a test level α ≥0.05. SPSS (version, 24.0; IBM
Corp) was used for statistical analysis, baseline data comparisons,
and Cox regression analysis. R (version, 4.1.2; R Project for
Statistical Computing) and R packages (‘rms’, ‘survival’, and
‘ggplot2’) were used to perform RCS analysis and visualization.
Results
A total of 293 patients with CRC who met the
inclusion criteria were first included in the present study;
however, a total of 83 patients were lost during the follow-up
period. A total of 210 (71.67%) patients with CRC with an age range
of 23–88 years old, median age of 62.5 years old, and a BMI at
diagnosis of 22.1 kg/m2 (IQR, 20.3-24.2
kg/m2) were included in the present research. These
included 107 male patients (51.0%) and 103 female patients (49.0%).
The majority (206 patients, 98.1%) of the included patients with
CRC were Han ethnic group (Table
I). A total of 29 patients died and 32 patients experienced
metastases during the follow-up period; the median survival time
following surgery for those who died was 45.3 months. In addition,
the median follow-up time following surgery for patients with CRC
was 42.6 months. Patients in the Ki67 ≥60% group predominantly
exhibited a younger age, were male and of an ethnic minority,
exhibited a low BMI, a high CEA, non-adenocarcinoma, moderate
differentiation, stage III CRC, no vascular infiltration, and no
nerve infiltration; however, differences between groups were not
statistically significant (Table
I).
 | Table I.Characteristics of the 210 patients
with non-metastatic colorectal cancer stratified by Ki67
levels. |
Table I.
Characteristics of the 210 patients
with non-metastatic colorectal cancer stratified by Ki67
levels.
|
|
| Ki67 level |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n=210 | <60%, n=97,
46.2% | ≥60%, n=113,
53.8% | P-value |
|---|
| Age at diagnosis,
median (IQR), (y) | 62.5 (54.0,
71.3) | 64.0 (54.0,
71.5) | 62.0 (53.0,
71.5) | 0.708 |
| Age, n (%) |
|
|
| 0.524 |
|
<60 | 85 | 37 (38.1) | 48 (42.5) |
|
|
≥60 | 125 | 60 (61.9) | 65 (57.5) |
|
| Sex, n (%) |
|
|
| 0.343 |
|
Male | 107 | 46 (47.4) | 61 (54.0) |
|
|
Female | 103 | 51 (52.6) | 52 (46.0) |
|
| Race, n (%) |
|
|
| 0.725 |
|
Han | 206 | 96 (99.0) | 110 (97.3) |
|
| Ethnic
minority | 4 | 1 (1.0) | 3 (2.7) |
|
| BMI, median (IQR),
kg/m2 | 22.1 (20.3,
24.2) | 22.2 (20.8,
24.0) | 22.0 (20.2,
24.3) | 0.660 |
| BMI, n (%) |
|
|
| 0.389 |
|
≤18.4 | 25 | 10 (10.3) | 15 (13.3) |
|
|
18.5–23.9 | 124 | 61 (62.9) | 63 (55.8) |
|
|
24.0–27.9 | 49 | 23 (23.7) | 26 (23.0) |
|
|
≥28.0 | 12 | 3 (3.1) | 9 (8.0) |
|
| CEA, median (IQR),
ng/ml | 3.2 (1.7, 8.4) | 3.2 (1.7, 8.4) | 3.4 (1.7, 8.4) | 0.958 |
| Type, n (%) |
|
|
| 0.501 |
|
Adenocarcinoma | 208 | 97 (100.0) | 111 (98.2) |
|
|
Other | 2 | 0 (0.0) | 2 (1.8) |
|
| Location, n
(%) |
|
|
| 0.194 |
| Right
colon | 112 | 57(58.8) | 55 (48.7) |
|
| Left
colon | 53 | 19 (19.6) | 34 (30.1) |
|
|
Rectal | 45 | 21 (21.6) | 24 (21.2) |
|
| Differentiation, n
(%) |
|
|
| 0.176 |
|
High | 26 | 16 (16.5) | 10 (8.8) |
|
|
Middle | 163 | 70 (72.2) | 93 (82.3) |
|
|
Low | 21 | 11 (11.3) | 10 (8.8) |
|
| Stage, n (%) |
|
|
| 0.229 |
| I | 46 | 18 (18.6) | 28 (24.8) |
|
| II | 93 | 49 (50.5) | 44 (38.9) |
|
|
III | 71 | 30 (30.9) | 41 (36.3) |
|
| Vascular invasion,
n (%) |
|
|
| 0.054 |
| No | 166 | 71 (73.2) | 95 (84.1) |
|
|
Yes | 44 | 26 (26.8) | 18 (15.9) |
|
| Perineural
invasion, n (%) |
|
|
| 0.282 |
| No | 140 | 61 (62.9) | 79 (69.9) |
|
|
Yes | 70 | 36 (37.1) | 34 (30.1) |
|
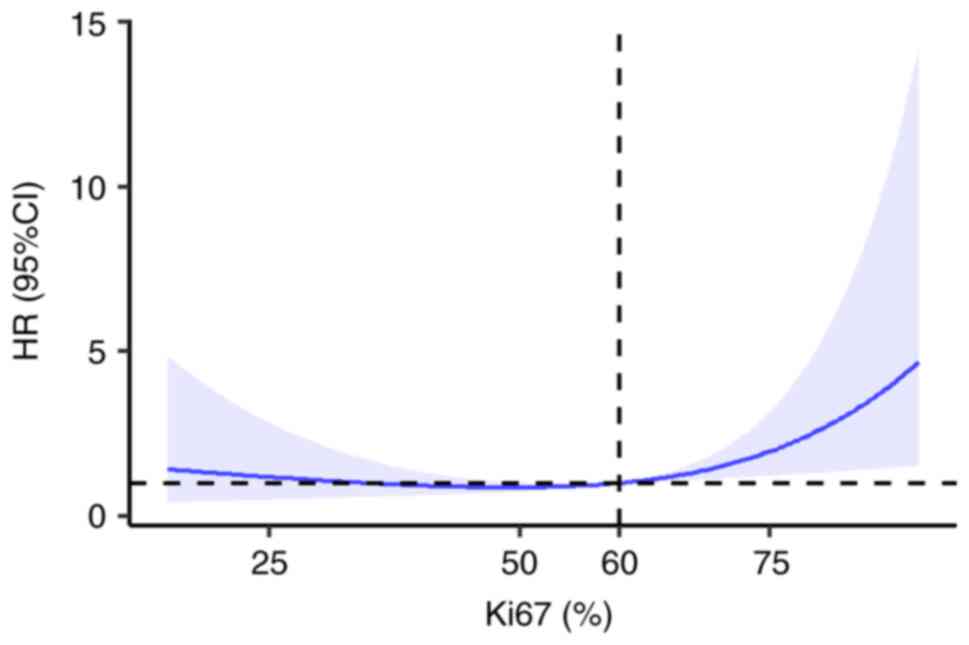
RCS model analysis demonstrated a non-linear
association between Ki67 expression levels and the HR of death in
patients with CRC (P=0.039). When Ki67 was <60%, the HR of death
in patients with CRC remained low. The HR of death increased as
Ki67 expression levels increased, and when Ki67 levels were ≥60%,
the HR of death was markedly increased (Figs. 1 and 2). However, the association between Ki67
expression and the HR of metastasis in patients with CRC was not
non-linear (P=0.068).
Results of the univariate Cox regression analysis
demonstrated that increased age, stage III cancer, vascular
invasion, and Ki67 ≥60% (P<0.05) were risk factors for death in
patients with CRC. After adjusting for different confounding
factors, Ki67 ≥60% was considered a risk factor for death (HR,
2.640 and 95% CI, 1.066-6.539; P=0.036; Table II).
 | Table II.Relationship between Ki67 levels and
overall survival stratified based on a cutoff value of 60%. |
Table II.
Relationship between Ki67 levels and
overall survival stratified based on a cutoff value of 60%.
|
|
|
| Multivariate
models |
|---|
|
|
|
|
|
|---|
|
| Univariate
model | Model A | Model B | Model C |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
Continuously | 1.036
(1.002–1.072) | 0.038a | 1.040
(1.005–1.077) | 0.024a | 1.039
(1.003–1.075) | 0.031a | 1.038
(1.002–1.075) | 0.039a |
| Sex |
|
|
|
|
|
|
|
|
|
Male | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
|
Female | 1.004
(0.484–2.083) | 0.992 | 1.218
(0.577–2.571) | 0.606 | 1.133
(0.531–2.417) | 0.748 | 1.074
(0.492–2.345) | 0.858 |
| Ethnicity |
|
|
|
|
|
|
|
|
|
Han | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
| Ethnic
minority | 1.713
(0.233–12.608) | 0.597 | 1.558
(0.206–11.771) | 0.667 | 1.516
(0.199–11.545) | 0.688 | 1.884
(0.226–15.716) | 0.558 |
| Body mass index,
kg/m2 |
|
|
|
|
|
|
|
|
|
Continuously | 0.942
(0.841–1.055) | 0.301 | 0.935
(0.834–1.049) | 0.252 | 0.937
(0.833–1.054) | 0.278 | 0.928
(0.811–1.062) | 0.276 |
| CEA, ng/ml |
|
|
|
|
|
|
|
|
|
Continuously | 1.016
(0.999–1.033) | 0.065 |
|
| 1.018
(1.000–1.036) | 0.045a | 1.016
(0.997–1.036) | 0.1 |
| Type |
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 1 [Reference] |
|
|
| 1 [Reference] |
| 1 [Reference] |
|
|
Other | / | 0.766 |
|
| / | 0.979 | / | 0.978 |
| Location |
|
|
|
|
|
|
|
|
| Right
colon | 1 [Reference] |
|
|
| 1 [Reference] |
| 1 [Reference] |
|
| Left
colon | 0.835
(0.327–2.136) | 0.707 |
|
| 0.993
(0.371–2.662) | 0.989 | 0.738
(0.244–2.230) | 0.59 |
|
Rectal | 1.096
(0.450–2.670) | 0.84 |
|
| 1.122
(0.453–2.781) | 0.804 | 1.811
(0.615–5.330) | 0.281 |
|
Differentiation |
|
|
|
|
|
|
|
|
|
High | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Middle | 4.283
(0.556–31.616) | 0.154 |
|
|
|
| 2.397
(0.296–19.389) | 0.412 |
|
Low | 3.840
(0.399–36.965) | 0.244 |
|
|
|
| 2.545
(0.232–27.953) | 0.445 |
| Stage |
|
|
|
|
|
|
|
|
| I | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
| II | 0.763
(0.215–2.707) | 0.676 |
|
|
|
| 1.069
(0.251–4.559) | 0.928 |
|
III | 3.550
(1.203–10.476) | 0.022a |
|
|
|
| 4.714
(1.174–18.931) | 0.029a |
| Vascular
invasion |
|
|
|
|
|
|
|
|
| No | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Yes | 2.555
(1.220–5.354) | 0.013a |
|
|
|
| 2.062
(0.833–5.104) | 0.118 |
| Perineural
invasion |
|
|
|
|
|
|
|
|
| No | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Yes | 1.077
(0.508–2.284) | 0.846 |
|
|
|
| 0.641
(0.281–1.467) | 0.293 |
| Ki67 |
|
|
|
|
|
|
|
|
|
<60% | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
|
≥60% | 2.328
(1.061–5.109) | 0.035a | 2.428
(1.094–5.390) | 0.029a | 2.637
(1.163–5.977) | 0.02a | 2.640
(1.066–6.539) | 0.036a |
Moreover, results of the univariate Cox regression
analysis demonstrated that increased CEA, stage III cancer, neural
invasion, and Ki67 ≥60% were risk factors for distant metastasis in
patients with CRC (P<0.05). After adjusting for different
confounding factors, Ki67 ≥60% was considered a risk factor for
metastasis (HR, 2.558; 95% CI, 1.079-6.064; P=0.033; Table III).
 | Table III.Relationship between Ki67 levels and
metastasis stratified based on a cutoff value of 60%. |
Table III.
Relationship between Ki67 levels and
metastasis stratified based on a cutoff value of 60%.
|
|
|
| Multivariate
models |
|---|
|
|
|
|
|
|---|
|
| Univariate
model | Model A | Model B | Model C |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
Continuously | 0.999
(0.971–1.028) | 0.962 | 1.003
(0.975–1.032) | 0.826 | 1.005
(0.975–1.035) | 0.76 | 1.008
(0.976–1.040) | 0.642 |
| Sex |
|
|
|
|
|
|
|
|
|
Male | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
|
Female | 1.460
(0.720–2.959) | 0.294 | 1.559
(0.759–3.204) | 0.227 | 1.538
(0.747–3.168) | 0.243 | 1.116
(0.520–2.397) | 0.778 |
| Ethnicity |
|
|
|
|
|
|
|
|
|
Han | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
| Ethnic
minority | 1.743
(0.238–12.781) | 0.585 | 1.619
(0.214–12.254) | 0.641 | 1.603
(0.208–12.367) | 0.651 | 2.428
(0.298–19.779) | 0.407 |
| Body mass index,
kg/m2 |
|
|
|
|
|
|
|
|
|
Continuously | 0.958
(0.865–1.061) | 0.407 | 0.961
(0.868–1.065) | 0.452 | 0.960
(0.862–1.069) | 0.455 | 0.966
(0.863–1.081) | 0.544 |
| CEA, ng/ml |
|
|
|
|
|
|
|
|
|
Continuously | 1.018
(1.002–1.034) | 0.023a |
|
| 1.020
(1.004–1.037) | 0.013a | 1.025
(1.008–1.043) | 0.005b |
| Type |
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 1 [Reference] |
|
|
| 1 [Reference] |
| 1 [Reference] |
|
|
Other | 5.462
(0.737–40.494) | 0.097 |
|
| 4.608
(0.585–36.314) | 0.147 | 5.747
(0.628–52.622) | 0.122 |
| Location |
|
|
|
|
|
|
|
|
| Right
colon | 1 [Reference] |
|
|
| 1 [Reference] |
| 1 [Reference] |
|
| Left
colon | 1.092
(0.467–2.554) | 0.838 |
|
| 1.007
(0.401–2.528) | 0.988 | 0.673
(0.238–1.907) | 0.456 |
|
Rectal | 1.253
(0.536–2.929) | 0.603 |
|
| 1.308
(0.549–3.115) | 0.544 | 1.740
(0.664–4.559) | 0.26 |
|
Differentiation |
|
|
|
|
|
|
|
|
|
High | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Middle | 2.427
(0.578–10.191) | 0.226 |
|
|
|
| 1.526
(0.336–6.933) | 0.584 |
|
Low | 1.333
(0.188–9.471) | 0.774 |
|
|
|
| 0.945
(0.118–7.576) | 0.957 |
| Stage |
|
|
|
|
|
|
|
|
| I | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
| II | 2.211
(0.478–10.233) | 0.31 |
|
|
|
| 2.419
(0.468–12.518) | 0.292 |
|
III | 8.079
(1.893–34.471) | 0.005b |
|
|
|
| 7.778
(1.584–38.183) | 0.012a |
| Vascular
invasion |
|
|
|
|
|
|
|
|
| No | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Yes | 2.063
(0.994–4.281) | 0.052 |
|
|
|
| 1.569
(0.663–3.716) | 0.306 |
| Perineural
invasion |
|
|
|
|
|
|
|
|
| No | 1 [Reference] |
|
|
|
|
| 1 [Reference] |
|
|
Yes | 2.177
(1.086–4.364) | 0.028a |
|
|
|
| 1.672
(0.777–3.600) | 0.189 |
| Ki67 |
|
|
|
|
|
|
|
|
|
<60% | 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
| 1 [Reference] |
|
|
≥60% | 2.356
(1.104–5.029) | 0.027a | 2.404
(1.124–5.141) | 0.024a | 2.556
(1.164–5.610) | 0.019a | 2.558
(1.079–6.064) | 0.033a |
Discussion
Ki67 has been widely used as a marker of cell
proliferation in numerous types of tumors (22–24).
However, there is still heterogeneity in the use of Ki67 as a
biomarker in CRC (25,26), which is comparable to its use in
breast cancer (22). Numerous
previous studies have demonstrated that increased Ki67 expression
is unfavorable in the progression and prognosis of patients with
CRC (19,27–32);
however, these results differ from other previous studies (14,15,33).
Notably, patients included in different studies may possess
different characteristics, such as undergoing preoperative
chemoradiotherapy or different tumor stages. In addition, results
of previous studies demonstrated the use of different cut-off
values of Ki67, varying from 5 to 62% (16,34,35).
A previous meta-analysis including 34 studies and 6,180 patients
confirmed that increased Ki67 expression was associated with
unfavorable disease-free survival and overall survival in patients
with CRC (16). Notably, the
present study included patients with stage I–III CRC who did not
receive preoperative chemoradiotherapy, and the results of the
present study demonstrated that increased Ki67 expression was
independently associated with distant metastasis and death in
patients with CRC.
Notably, numerous cut-off values were reported in
previous studies, and these studies selected a median or
alternative value to allocate patients into different groups
(34,36–39).
However, these values may obscure important clinical features or
prognostic outcomes. The optimal cut-off value should be derived
from maximizing the difference in HRs between groups (34). Results of the present study
demonstrated a bell-shaped association between Ki67 levels and
prognostic outcomes of CRC, which may be attributed to Ki67
expression only occurring in interphase and mitotic phases of
mitosis (G0). During mitosis, Ki67 expression is low in interphase
(G1, S, and G2) and gradually increases in the pre-mitotic phase
and metaphase. Ki67 expression reaches a peak and is markedly
decreased in anaphase and telophase (40), exhibiting a graded longitudinal
change (20). The RCS model is a
powerful tool in the analysis of non-linear dose-effect
associations between continuous exposure and outcome (41). In the present study, the RCS model
was used to analyze the association between Ki67 levels and the HR
of patient death, and the results of the present study determined
the optimal cut-off value for Ki67 was 60%. Moreover, this level
was verified using regression models, and the results demonstrated
that Ki67 ≥60% is an independent risk factor for distant metastasis
and death in patients with CRC (P<0.05). Notably, these results
are comparable to those obtained by Weber et al (27). The Ki67 cut-off value may enable
medical staff to accurately identify the risk of patient prognosis.
However, potential confounding factors may affect the association
between Ki67 and mortality, including comorbidities, patients
receiving R0 resection, and frailty.
However, there are limitations to the present study.
Notably, the statistically significant association between Ki67
>60% and mortality determined using multivariate Cox regression
analysis may only be a result of the small sample size used in the
present study. Thus, future studies will address this issue. In
addition, immunohistochemical scoring is subject to an individual's
experience. The objective evaluation of immunohistochemical
analysis is key for future research, and artificial intelligence
may be a viable option (42,43).
External verification was carried out using the limited samples in
the present study; however, further verification of the accuracy of
the cut-off value is required in future research. Moreover,
selection biases may have occurred due to the samples being
obtained from only one hospital, and some patients were lost in
follow-up. The study design was also retrospective, meaning the
integrity and authenticity of data records may affect the
reliability of the results. In addition, certain prognostic factors
could not be collected, such as comorbidities, history of R0
resection, and frailty. The detection of Ki67 expression in the
tumor tissue of each patient was performed at different times, and
detection conditions may have been inconsistent.
In conclusion, the results of the present study
demonstrated that Ki67 expression may be used to predict the
prognosis of patients with CRC, and the optimal cut-off value of
Ki67 is 60%. This cut-off value may be used as a classification
tool to guide the clinical management of patients with CRC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are not publicly available due to individual participants'
privacy but are available from the corresponding author on
reasonable request.
Authors' contributions
HTL, SY, YHH, NX, MZ, CJY, HLL, SK, ZHC and JF
contributed to study conception and design. SY, YHH, and MZ were
responsible for designing the methodology. SK and ZHC were
responsible for material preparation. CJY and HLL were responsible
for data collection. YHH and NX were responsible for data analysis.
HTL and SY were responsible for the original draft preparation. JF
was responsible for review and editing. All authors have read and
approved the final manuscript. HTL and YHH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Ethical approval was waived by the Ethics Committee
of the First Affiliated Hospital of Kunming Medical University in
view of the retrospective nature of the study and all the
procedures being performed were part of the routine care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lortet-Tieulent J, Georges D, Bray F and
Vaccarella S: Profiling global cancer incidence and mortality by
socioeconomic development. Int J Cancer. 147:3029–3036. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto T, Kawada K and Obama K:
Inflammation-related biomarkers for the prediction of prognosis in
colorectal cancer patients. Int J Mol Sci. 22:80022021. View Article : Google Scholar
|
|
6
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar
|
|
7
|
Lech G, Słotwiński R, Słodkowski M and
Krasnodębski IW: Colorectal cancer tumour markers and biomarkers:
Recent therapeutic advances. World J Gastroenterol. 22:1745–1755.
2016. View Article : Google Scholar
|
|
8
|
Walker J and Quirke P: Prognosis and
response to therapy in colorectal cancer. Eur J Cancer. 38:880–886.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahar AL, Compton C, Halabi S, Hess KR,
Weiser MR and Groome PA: Personalizing prognosis in colorectal
cancer: A systematic review of the quality and nature of clinical
prognostic tools for survival outcomes. J Surg Oncol. 116:969–982.
2017. View Article : Google Scholar
|
|
10
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715. 1984.
|
|
12
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019. View Article : Google Scholar
|
|
14
|
Fodor IK, Hutchins GG, Espiritu C, Quirke
P and Jubb AM: Prognostic and predictive significance of
proliferation in 867 colorectal cancers. J Clin Pathol. 65:989–995.
2012. View Article : Google Scholar
|
|
15
|
Li P, Xiao ZT, Braciak TA, Ou QJ, Chen G
and Oduncu FS: Association between Ki67 index and
clinicopathological features in colorectal cancer. Oncol Res Treat.
39:696–702. 2016. View Article : Google Scholar
|
|
16
|
Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, Huang WH
and Luo XZ: Increased expression of Ki-67 is a poor prognostic
marker for colorectal cancer patients: A meta analysis. BMC Cancer.
19:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma YL, Peng JY, Zhang P, Liu WJ, Huang L
and Qin HL: Immunohistochemical analysis revealed CD34 and Ki67
protein expression as significant prognostic factors in colorectal
cancer. Med Oncol. 27:304–309. 2010. View Article : Google Scholar
|
|
18
|
Iatropoulos MJ and Williams GM:
Proliferation markers. Exp Toxicol Pathol. 48:175–181. 1996.
View Article : Google Scholar
|
|
19
|
Tong G, Zhang G, Liu J, Zheng Z, Chen Y,
Niu P and Xu X: Cut-off of 25% for Ki67 expression is a good
classification tool for prognosis in colorectal cancer in the AJCC8
stratification. Oncol Rep. 43:1187–1198. 2020.PubMed/NCBI
|
|
20
|
Miller I, Min M, Yang C, Tian C, Gookin S,
Carter D and Spencer SL: Ki67 is a graded rather than a binary
marker of proliferation versus quiescence. Cell Rep.
24:1105–1112.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salminen E, Palmu S, Vahlberg T, Roberts
PJ and Söderström KO: Increased proliferation activity measured by
immunoreactive Ki67 is associated with survival improvement in
rectal/recto sigmoid cancer. World J Gastroenterol. 11:3245–3249.
2005. View Article : Google Scholar
|
|
22
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
international Ki67 in breast cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar
|
|
23
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar
|
|
24
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar
|
|
25
|
Graziano F and Cascinu S: Prognostic
molecular markers for planning adjuvant chemotherapy trials in
Dukes' B colorectal cancer patients: How much evidence is enough?
Ann Oncol. 14:1026–1038. 2003. View Article : Google Scholar
|
|
26
|
Torén W, Ansari D and Andersson R:
Immunohistochemical investigation of prognostic biomarkers in
resected colorectal liver metastases: A systematic review and
meta-analysis. Cancer Cell Int. 18:2172018. View Article : Google Scholar
|
|
27
|
Weber JC, Nakano H, Bachellier P,
Oussoultzoglou E, Inoue K, Shimura H, Wolf P, Chenard-Neu MP and
Jaeck D: Is a proliferation index of cancer cells a reliable
prognostic factor after hepatectomy in patients with colorectal
liver metastases? Am J Surg. 182:81–88. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Liu Z, Fisher KW, Ren F, Lv J,
Davidson DD, Baldridge LA, Du X and Cheng L: Prognostic value of
programmed death ligand 1, p53, and Ki-67 in patients with
advanced-stage colorectal cancer. Hum Pathol. 71:20–29. 2018.
View Article : Google Scholar
|
|
29
|
Ishida H, Miwa H, Tatsuta M, Masutani S,
Imamura H, Shimizu J, Ezumi K, Kato H, Kawasaki T, Furukawa H and
Kawakami H: Ki-67 and CEA expression as prognostic markers in
Dukes' C colorectal cancer. Cancer Lett. 207:109–115. 2004.
View Article : Google Scholar
|
|
30
|
Dawson H, Koelzer VH, Karamitopoulou E,
Economou M, Hammer C, Muller DE, Lugli A and Zlobec I: The
apoptotic and proliferation rate of tumour budding cells in
colorectal cancer outlines a heterogeneous population of cells with
various impacts on clinical outcome. Histopathology. 64:577–584.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernández-Cebrián JM, Santos MN, Kuborn
PV, de Lama MP, Martín-Cavanna J, Martínez PP, Escudero BF and
Fernández MR: Can the clinical outcome in stage II colon carcinomas
be predicted by determination of molecular marker expression? Clin
Transl Oncol. 9:663–670. 2007. View Article : Google Scholar
|
|
32
|
Wu XS, Xi HQ and Chen L: Lgr5 is a
potential marker of colorectal carcinoma stem cells that correlates
with patient survival. World J Surg Oncol. 10:2442012. View Article : Google Scholar
|
|
33
|
Melling N, Kowitz CM, Simon R, Bokemeyer
C, Terracciano L, Sauter G, Izbicki JR and Marx AH: High Ki67
expression is an independent good prognostic marker in colorectal
cancer. J Clin Pathol. 69:209–214. 2016. View Article : Google Scholar
|
|
34
|
Wei DM, Chen WJ, Meng RM, Zhao N, Zhang
XY, Liao DY and Chen G: Augmented expression of Ki-67 is correlated
with clinicopathological characteristics and prognosis for lung
cancer patients: An up-dated systematic review and meta-analysis
with 108 studies and 14,732 patients. Respir Res. 19:1502018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nielsen TO, Leung SCY, Rimm DL, Dodson A,
Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M, et al:
Assessment of Ki67 in breast cancer: Updated recommendations from
the international Ki67 in breast cancer working group. J Natl
Cancer Inst. 113:808–819. 2021. View Article : Google Scholar
|
|
36
|
Ajani JA, Wang X, Izzo JG, Crane CH, Eng
C, Skibber JM, Das P and Rashid A: Molecular biomarkers correlate
with disease-free survival in patients with anal canal carcinoma
treated with chemoradiation. Dig Dis Sci. 55:1098–1105. 2010.
View Article : Google Scholar
|
|
37
|
Nash GM, Gimbel M, Shia J, Nathanson DR,
Ndubuisi MI, Zeng ZS, Kemeny N and Paty PB: KRAS mutation
correlates with accelerated metastatic progression in patients with
colorectal liver metastases. Ann Surg Oncol. 17:572–578. 2010.
View Article : Google Scholar
|
|
38
|
Shin IY, Sung NY, Lee YS, Kwon TS, Si Y,
Lee YS, Oh ST and Lee IK: The expression of multiple proteins as
prognostic factors in colorectal cancer: Cathepsin D, p53, COX-2,
epidermal growth factor receptor, C-erbB-2, and Ki-67. Gut Liver.
8:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ivanecz A, Kavalar R, Palfy M, Pivec V,
Sremec M, Horvat M and Potrč S: Can we improve the clinical risk
score? The prognostic value of p53, Ki-67 and thymidylate synthase
in patients undergoing radical resection of colorectal liver
metastases. HPB (Oxford). 16:235–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seigneurin D and Guillaud P: Ki-67
antigen, a cell cycle and tumor growth marker. Pathol Biol (Paris).
39:1020–1028. 1991.(In French). PubMed/NCBI
|
|
41
|
Desquilbet L and Mariotti F: Dose-response
analyses using restricted cubic spline functions in public health
research. Stat Med. 29:1037–1057. 2010.PubMed/NCBI
|
|
42
|
Ramesh AN, Kambhampati C, Monson JR and
Drew PJ: Artificial intelligence in medicine. Ann R Coll Surg Engl.
86:334–338. 2004. View Article : Google Scholar
|
|
43
|
Hamet P and Tremblay J: Artificial
intelligence in medicine. Metabolism. 69s:S36–S40. 2017. View Article : Google Scholar : PubMed/NCBI
|