Introduction
Breast cancer is the most common type of cancer in
the female population; based on the National Comprehensive Cancer
Network (NCCN) guidelines, the main treatment for breast cancer is
mastectomy (1). Although
mastectomy affords a favorable survival outcome in patients with
breast cancer, studies have indicated that distorted breast
appearance after mastectomy is associated with an increased risk of
mental health disorders, such as depression (2,3).
Breast reconstruction after mastectomy may improve the cosmetic
appearance and body image of patients with breast cancer, thereby
enhancing their mental health and quality of life (4,5).
Growing global interest in breast reconstruction
research and the growth of related studies have indicated that
breast reconstruction is becoming an increasingly common options
for both surgeons and patients, particularly for younger patients
with breast cancer (6,7). Postmastectomy breast reconstruction
may be performed immediately after the mastectomy or after systemic
treatment, local treatment (radiotherapy) or both. Immediate breast
reconstruction after mastectomy has provided favorable prognostic
outcomes, including simultaneous superior clinical and aesthetic
outcomes, in patients with breast cancer; hence, it has become a
preferred option among cancer surgeons (8–10).
In addition to mastectomy, systemic therapy
(including chemotherapy, hormone therapy and targeted therapy),
determined according to the breast cancer subtype, is a commonly
used breast cancer treatment (11–13).
The major concern related to postmastectomy breast reconstruction
is the delay in adjuvant treatment it may cause; a delayed adjuvant
treatment course is associated with worse breast cancer survival
outcomes (14,15). However, breast reconstruction has
been noted to increase the risk of surgical wound complications but
not necessarily delay chemotherapy initiation; as such, neoadjuvant
chemotherapy could be an alternative treatment option for patients
with early-stage breast cancer who undergo breast reconstruction
(16,17). Moreover, the reconstructive
complication rates between a neoadjuvant and adjuvant chemotherapy
cohort were similar in a Taiwanese breast cancer population with
immediate breast reconstruction (18).
Chemotherapy applied in both neoadjuvant and
adjuvant settings can provide further survival benefits for
patients with early-stage breast cancer who undergo immediate
breast reconstruction after mastectomy (19,20).
Moreover, additional radiotherapy, hormone therapy and targeted
therapy can be considered depending on the clinical indications of
the patient with breast cancer, such as the tumor size, lymph node
invasion and molecular subtype. Survival outcome, rather than
reconstruction outcome, after neoadjuvant and adjuvant chemotherapy
has not been compared sufficiently in patients with early-stage
breast cancer who have undergone immediate breast reconstruction
after mastectomy in Taiwan. Therefore, a comparison has been made
in this study.
Materials and methods
Data set
This retrospective case-control study was approved
by the Institutional Review Board (IRB no. CE21406B) of Taichung
Veterans General Hospital (TCVGH; Taichung, Taiwan); all data were
retrospectively collected from the health information system of
TCVGH under this approved protocol The inclusion criteria for the
study population were: i) Patients with early-stage breast cancer
treated with breast reconstruction immediately after mastectomy
between 1 January 2011 and 31 December 2018, and ii) completion of
the chemotherapy treatment course in an adjuvant or neoadjuvant
setting; breast cancer diagnosed at clinical stages I and IIIB was
considered early-stage. Patients who did not meet the inclusion
criteria were excluded. Data of 139 women with breast cancer met
the inclusion criteria. The clinical characteristics and treatment
characteristics of the study population were extracted from the
patients' medical records or the cancer registry database.
Post hoc power analysis
A post hoc power analysis for two independent groups
with dichotomous outcomes was used to evaluate the statistical
power of the population in the current study. It was assumed that
the probability of type I error (α) was 0.05, the population size
of the current study cohort was 136; the sample size of neoadjuvant
cases was 64, with a mortality rate of 23.4%, and the sample size
of adjuvant controls was 75, with a mortality rate of 1.3%. The
case-control sample size ratio was 1.172 and the absolute
difference between the two groups was 0.221. With the power
estimation formula, the estimated critical Z value for the
assumed α was ~2.122 (Φ), equal to the power of 0.983, indicating
that the assumed sample size may lead to results with 99.5%
statistical power.
For the matched case and control cohorts, there was
an assumed sample size of 37 for both the neoadjuvant cases and
adjuvant controls, with mortality rates of 14 and 0%, respectively;
the case-control sample size ratio was 1 and an absolute
between-group difference was 0.14. With the power estimation
formula, Φ was ~0.416, equal to the power of 0.661, indicating the
assumed sample size may lead to results with 66.1% statistical
power. In other words, the findings of the matched cohort may only
provide conservative inference.
Clinical characteristics
Several clinical characteristics were considered,
including age, clinical stage and molecular subtype at diagnosis.
The molecular subtypes of all patients were classified according to
the results of standard histological examinations, including
microscopic analysis with standard immunohistochemistry (IHC) for
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2), which was performed on
tissues from the primary tumor excised during mastectomy.
Pathohistological testing was conducted in the Department of
Pathology and Laboratory Medicine at TCVGH. Cases with + (mild), ++
(moderate) or +++ (strong) IHC results (according to the intensity
of staining) for ER and PR were defined as ER+ and
PR+ cases, respectively, whereas the remaining patients
were defined as ER− and PR−. Moreover, cases
with +++ IHC result for HER2 were defined as HER2+
cases, whereas those with - or + IHC results for HER2 were defined
as HER2−cases. In cases with ++ IHC results for HER2,
fluorescence in situ hybridization (FISH) was performed
using VENTANA HER2 Dual ISH DNA Probe Cocktail assay to analyze the
HER2-neu gene amplification status. Subsequently, cases with ++ IHC
and + FISH results for HER2 were defined as HER2+,
whereas the remaining cases were defined as HER2−. In
accordance with the classification system for molecular subtypes
presented in the St. Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2013 (21), breast cancer was classified as
luminal A (ER+/PR+ and HER2−),
luminal B (ER+/PR+ and HER2+),
HER2-enriched (ER−/PR− and HER2+)
or triple-negative (ER−/PR− and
HER2−).
Overall survival (OS) was considered a primary
endpoint; patients who succumbed during the within-study follow-up
duration were considered left-censored, whereas the remaining
patients were considered right-censored. The follow-up interval was
between the date of initial diagnosis and the date of
censoring.
Treatment characteristics
The treatment characteristics of the study
population were classified according to the treatment type:
Chemotherapy, radiotherapy, hormone therapy or targeted therapy
(e.g. anti-HER2 therapy). In all patients, the treatment selection
was mainly dependent on the individual clinical indications (i.e.,
age, tumor size, lymph node invasion and molecular subtype at
diagnosis), as suggested by the NCCN guidelines for breast cancer
treatment (1). The chemotherapy
drugs used in the current settings included an anthracycline
(epirubicin) with an alkylating agent (cyclophosphamide) followed
by a taxane (paclitaxel or docetaxel). An antineoplastic agent
(carboplatin) was used for patients with triple-negative breast
cancer (TNBC) or HER2-enriched breast cancer. Most patients with
T3, T4 or lymph node metastasis received intensity-modulated
radiotherapy, whereas a few patients were treated with external
beam radiation therapy. The hormone therapy agents used in
premenopausal and postmenopausal patients were tamoxifen and an
aromatase inhibitor (letrozole or anastrozole), respectively.
Dual-blockade anti-HER2 agents (i.e. pertuzumab and trastuzumab)
were used for HER2-enriched patients. Here, pertuzumab was
administered at 840 mg in the first cycle and then at 420 mg in the
following cycles, whereas trastuzumab was administered at 8 mg/kg
in the first cycle and then at 6 mg/kg in the following cycles. All
HER2-enriched patients received complete dual-blockade or
single-blockade therapy for one year.
Unmatched and matched cohort
The overall study population that underwent
immediate breast reconstruction after mastectomy was considered an
unmatched cohort, comprising 75 patients who received adjuvant
chemotherapy and 64 control patients who received neoadjuvant
chemotherapy. The treatment responses after neoadjuvant
chemotherapy were reported. Furthermore, age group, clinical stage
and molecular subtype at diagnosis were used as matching criteria
to generate a well-balanced case-control subgroup matched at a 1:1
ratio, to further reduce the bias and clarify the feasibility role
of neoadjuvant and adjuvant chemotherapy in the study population.
In this study, 37 neoadjuvant cases were well matched with 37
adjuvant controls, which was used as a matched cohort (n=74).
Statistical analysis
The clinical characteristics, treatment
characteristics and OS rates of the study population are presented
the mean ± SD or as frequencies and percentages. All analyses were
performed using R (version 4.1.2; R core team, 2021). The
distribution difference of baseline characteristics between the
cases and controls was estimated using an independent two-sample
Student's t-test, χ2 test or Fisher's exact test. The
survival rates and the correspond 95% confidence interval (CI) of
different subgroups were computed using Kaplan-Meier estimators,
and OS was compared between the subgroups using the log-rank test.
A post-hoc pairwise log-rank test were performed for multiple
comparison correction. All P-values were two-sided, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient clinical characteristics
Table I summarizes
the clinicopathological characteristics, treatment and OS of the
unmatched cohort. The mean age at diagnosis of the unmatched cohort
was 43.0±8.6 years; 31 (22.3%) of the patients were aged ≥50 and
108 (77.7%) were <50 years. The mean ages at diagnosis of the
adjuvant controls and neoadjuvant cases were 44.1±9.2 and 41.8±7.8
years, respectively, and the differences between the adjuvant
controls and neoadjuvant cases were non-significant (P=0.117).
Similar ratios were obtained for the age group distribution between
the adjuvant controls and neoadjuvant cases. However, the clinical
stages at diagnosis differed significantly between the adjuvant
controls and neoadjuvant cases. For example, significantly more
neoadjuvant cases were at clinical stages II and III compare with
the adjuvant controls (79.7 vs. 45.3% and 15.6 vs. 1.3%,
respectively; both P<0.001). Moreover, more neoadjuvant cases
received radiotherapy compared with the adjuvant controls (53.1 vs.
32.0%; P=0.012). According to the clinical tumor staging, the tumor
sizes ranged between 2 and 5 cm; most patients were at stages T1
(n=51; 36.7%) or T2 (n=76; 54.7%), followed by those at T3 (n=8;
5.8%). One (0.7%) patient in the adjuvant group was at TisN1; the
remaining 3 (2.2%) patients were neoadjuvant cases at stage T4 at
diagnosis. However, their tumor size decreased after neoadjuvant
chemotherapy to a pathological tumor stage of T3, T4b or T1a. In
total, 19 (13.7%) HER2-enriched breast cancer, 87 (62.6%) luminal A
breast cancer, 26 (18.7%) luminal B breast cancer and 7 (5.0%) TNBC
cases were identified in the unmatched cohort; the adjuvant
controls and neoadjuvant cases demonstrated a similar molecular
subtype distribution (P=0.453). The proportions of neoadjuvant
cases and adjuvant controls receiving hormone and targeted therapy
demonstrated a non-significant difference. In addition, 10 (15.6%)
of the 64 neoadjuvant cases reached pathologic complete response
(pCR) after neoadjuvant chemotherapy. However, 16 (11.5%) patients
succumbed during follow-up; this proportion was higher among
neoadjuvant cases compared with adjuvant controls (23.4 vs. 1.3%;
P<0.001).
 | Table I.Clinicopathological characteristics,
treatment and overall survival status of patients treated with
adjuvant (n=75) and neoadjuvant (n=64) chemotherapy in the
unmatched cohort (n=139). |
Table I.
Clinicopathological characteristics,
treatment and overall survival status of patients treated with
adjuvant (n=75) and neoadjuvant (n=64) chemotherapy in the
unmatched cohort (n=139).
| Clinicopathological
characteristics | Unmatched cohort, n
(%) | Adjuvant, n
(%) | Neoadjuvant, n
(%) |
P-valuea |
|---|
| Age, years (mean ±
SD) | 43.0±8.6 | 44.1±9.2 | 41.8±7.8 | 0.117 |
| Age group |
|
|
| 0.603 |
| <50
years | 108 (77.7) | 57 (76.0) | 51 (79.7) |
|
| ≥50
years | 31 (22.3) | 18 (24.0) | 13 (20.3) |
|
| Clinical tumor
staging |
|
|
| <0.001 |
| is | 1 (0.7) | 1 (1.3) | 0 (0.0) |
|
| 1 | 51 (36.7) | 41 (54.7) | 10 (15.6) |
|
| 2 | 76 (54.7) | 32 (42.7) | 44 (68.8) |
|
| 3 | 8 (5.8) | 1 (1.3) | 7 (10.9) |
|
| 4 | 3 (2.2) | 0 (0.0) | 3 (4.7) |
|
| Clinical node
staging |
|
|
| <0.001 |
| 0 | 92 (66.2) | 68 (90.7) | 24 (37.5) |
|
| 1 | 42 (30.2) | 7 (9.3) | 35 (54.7) |
|
| 2 | 5 (3.6) | 0 (0.0) | 5 (7.8) |
|
| Clinical stage |
|
|
| <0.001 |
| I | 43 (30.9) | 40 (53.3) | 3 (4.7) |
|
| II | 85 (61.2) | 34 (45.3) | 51 (79.7) |
|
|
III | 11 (7.9) | 1 (1.3) | 10 (15.6) |
|
| Molecular
subtype |
|
|
| 0.453 |
|
HER2-enriched | 19 (13.7) | 10 (13.3) | 9 (14.1) |
|
| Luminal
A | 87 (62.6) | 51 (68.0) | 36 (56.3) |
|
| Luminal
B | 26 (18.7) | 11 (14.7) | 15 (23.4) |
|
|
TNBC | 7 (5.0) | 3 (4.0) | 4 (6.3) |
|
| Treatment response,
pCR | n/a | n/a | 10 (15.6) | n/a |
| Treatment |
|
|
|
|
|
Radiotherapy | 58 (41.7) | 24 (32.0) | 34 (53.1) | 0.012 |
| Hormone
therapy | 105 (75.5) | 59 (78.7) | 46 (71.9) | 0.353 |
|
Targeted therapy | 41 (29.5) | 17 (22.7) | 24 (37.5) | 0.056 |
|
Mortality | 16 (11.5) | 1 (1.3) | 15 (23.4) | <0.001 |
OS comparison in the unmatched
cohort
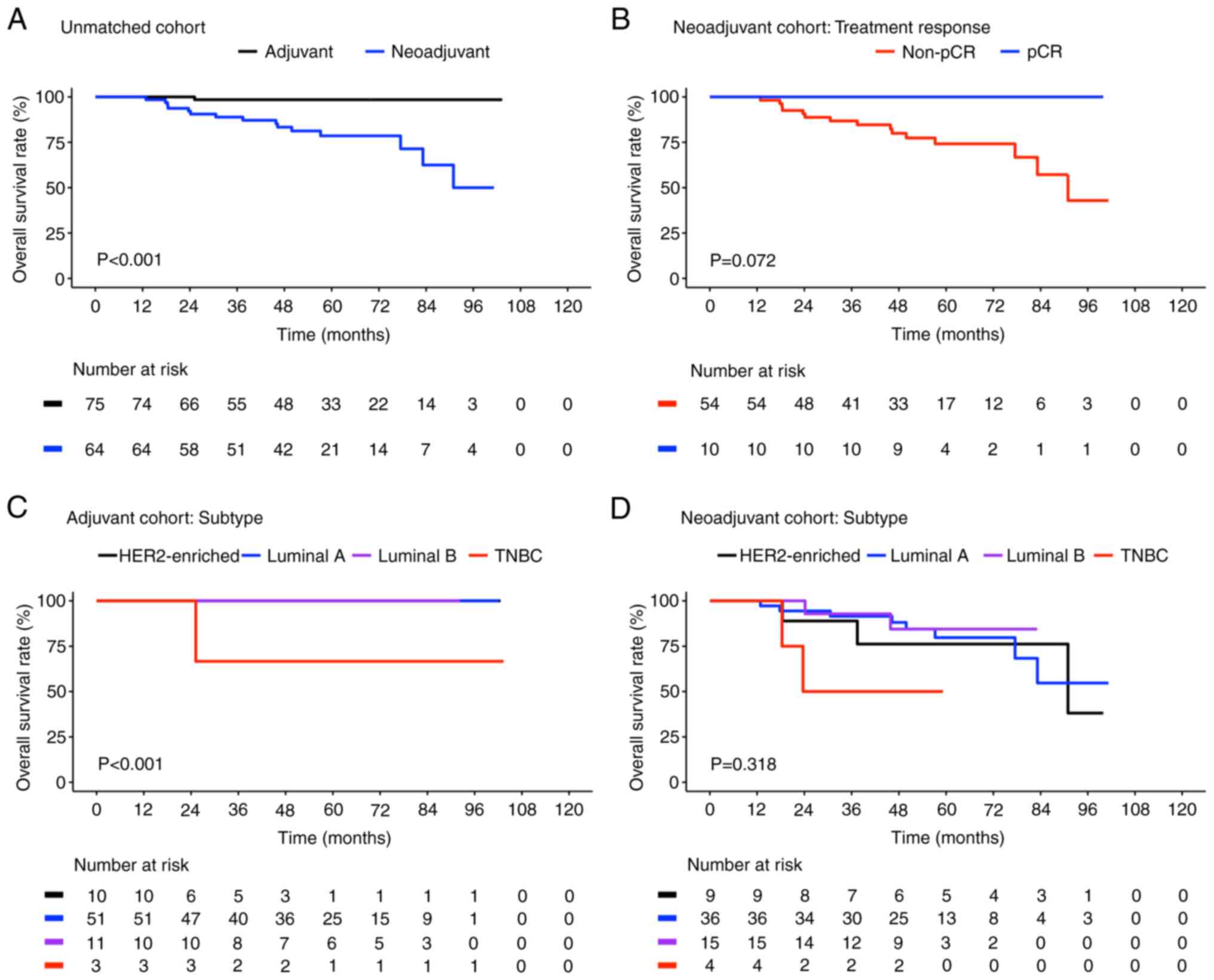
Fig. 1 presents the
Kaplan-Meier plots for OS of the different subgroups in the
unmatched cohort. The OS rates (95% CI) of the neoadjuvant cases
and adjuvant controls were 50 (28.5-87.7) and 98.5% (95.5-100),
respectively (Fig. 1A), with the
neoadjuvant cases exhibiting significantly poorer OS compared with
the adjuvant controls (P<0.001). Among the neoadjuvant cases,
the OS rate was higher in patients who achieved pCR compared with
those who did not [100 vs. 42.9% (95% CI, 21.4-86.1); P=0.072;
Fig. 1B].
OS comparison based on molecular
subtypes in the unmatched cohort
Fig. 1C and D
present a comparison of the OS according to different molecular
subtypes among the adjuvant controls and neoadjuvant cases,
respectively. The difference in OS between the adjuvant controls
with different molecular subtypes was significant (P<0.001;
Fig. 1C). The survival curves of
individuals with HER2-enriched, luminal A and luminal B breast
cancer overlapped because they had similar survival outcomes; their
OS rates were all 100%, whereas the OS rate in individuals with
TNBC was 66.7% (95% CI, 30–100). However, the post-hoc pairwise
comparison results revealed that individuals with TNBC had
significantly poorer OS only when compared with those with luminal
A breast cancer (P<0.001). By contrast, the differences in the
neoadjuvant cases with different molecular subtypes were not
significant (P=0.029; Fig. 1D).
The discrepancy between neoadjuvant cases with TNBC and those with
other subtypes was large in the post-hoc pairwise comparison: The
differences between neoadjuvant cases with TNBC (40.0% OS; 95% CI,
13.7-100) and those with HER2-enriched (83.3% OS; 95% CI, 58.3-100;
P=0.560), luminal A (44.0% OS; 95% CI, 21.0-92.3; P=0.300) and
luminal B (84.4% OS; 95% CI, 66.6-100; P=0.300) breast cancer were
not significant. Taken together, both the neoadjuvant cases and
adjuvant controls with TNBC demonstrated poor OS, whereas
neoadjuvant therapy led to no OS benefits in the patients with
luminal A breast cancer (blue lines in Fig. 1C and D; the adjuvant controls
achieved improved OS outcome compared to neoadjuvant cases).
Moreover, the patients with HER2-enriched and luminal B breast
cancer in both the neoadjuvant and adjuvant cohorts demonstrated
similar OS improvement.
OS comparison based on treatment
subgroups in the unmatched cohort
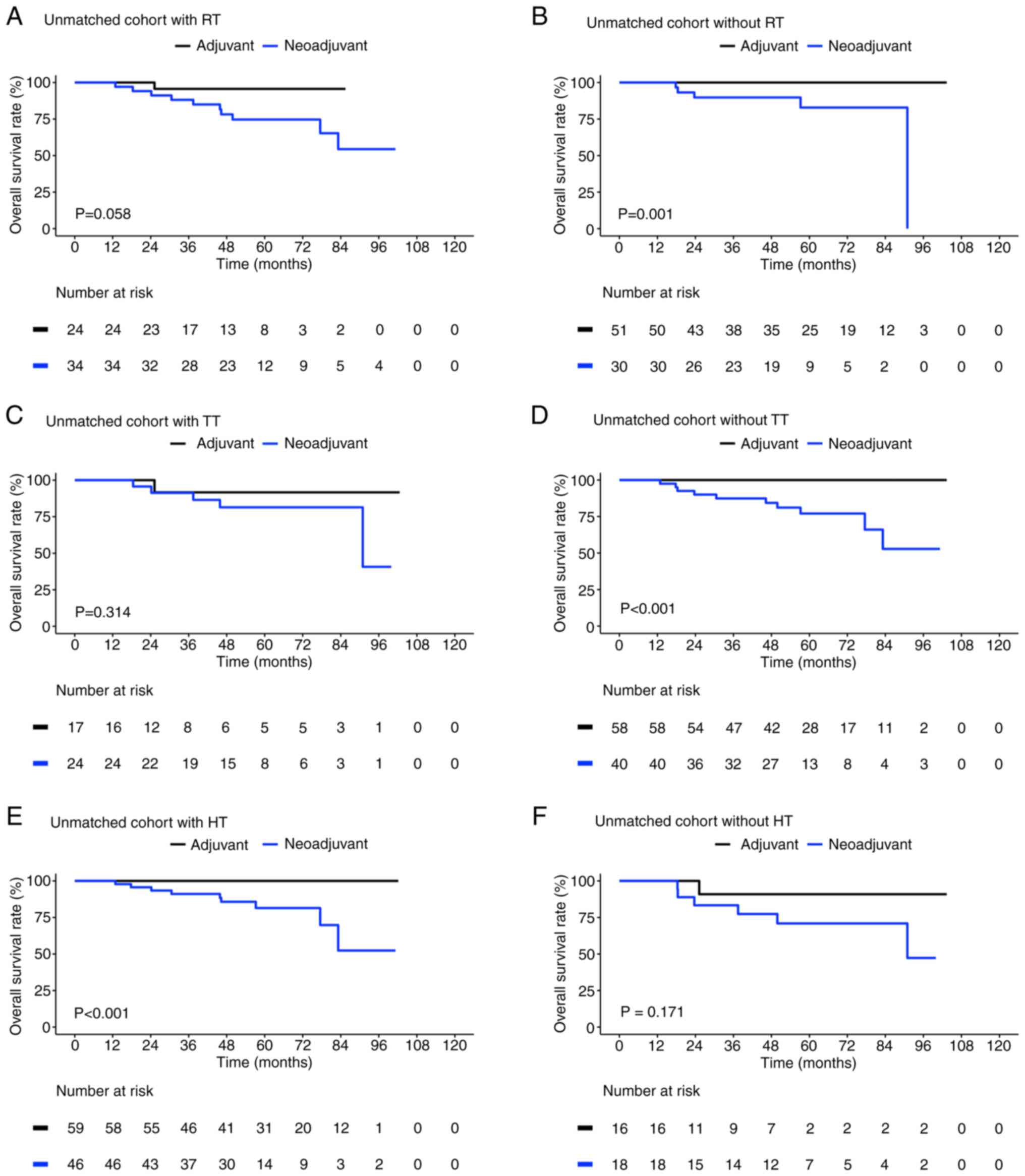
Fig. 2 presents a
comparison of the OS between the adjuvant controls and neoadjuvant
cases in the unmatched cohort according to their treatment
characteristics. In both the adjuvant and neoadjuvant settings, the
combined use of chemotherapy either with radiotherapy [95.7% (95%
CI, 87.7-100) vs. 54.4%, (95% CI, 33.4-88.7), respectively;
P=0.058; Fig. 2A] or with targeted
therapy [91.7% (95% CI, 77.3-100) vs. 40.7% (95% CI, 10.0-88.7),
respectively; P=0.314; Fig. 2C]
led to different OS outcomes which were not significant. The
neoadjuvant cases demonstrated significantly poorer OS than did the
adjuvant controls in the subgroups without radiotherapy (100 vs.
0%, respectively; P=0.001; Fig.
2B) and without therapy [91.7%, (95% CI, 77.3–100%) vs. 40.7%
(95% CI, 29.1–100%); P<0.001; Fig.
2D].
The patients who received hormone therapy combined
with neoadjuvant chemotherapy demonstrated significantly poorer OS
than those who received hormone therapy combined with adjuvant
chemotherapy [100 vs. 52.4%, respectively; 95% CI, 27–100%,
P<0.001; Fig. 2E]. By contrast,
no significant difference was identified between the patients who
did not receive hormone therapy in the adjuvant control [90.9% (95%
CI, 75.4–100%)] and neoadjuvant case [47.3% (95% CI, 20.1–100%)
groups (P=0.171; Fig. 2F)].
Clinical characteristics of the
matched cohort
To further understand the survival differences
between the matched neoadjuvant cases and adjuvant controls, an
age-matched, clinical stage-matched and molecular subtype-matched
cohort was generated comprising 74 patients (n=37 patients/group).
Table II presents the
distribution of the clinicopathological characteristics, treatment
characteristics and OS rates in the matched cohort. The mean age at
diagnosis in the matched cohort was 42.0±8.4 years; 60 (81.1%)
patients in this cohort were <50 years old. Because one adjuvant
control at clinical stage III did not match with any of the
neoadjuvant cases based on the aforementioned criteria, the matched
cohort did not include patients at clinical stage III. The matched
cohort contained 6 (8.1%) clinical stage I and 68 (91.9%) clinical
stage II cases. The distribution of molecular subtypes was similar
to that of the unmatched cohort; thus, the treatment
characteristics also demonstrated a similar distribution between
the matched neoadjuvant cases and adjuvant controls. In total, 5
(13.5%) of the 37 matched cases achieved pCR after neoadjuvant
chemotherapy. However, 5 (13.5%) patients died during the follow-up
duration; all of these patients were matched neoadjuvant cases
(P=0.054) not in receipt of pCR.
 | Table II.Clinicopathological characteristics,
treatment characteristics, and overall survival status of patients
treated with adjuvant (n=37) and neoadjuvant (n=37) chemotherapy,
in the matched cohort (n=74). |
Table II.
Clinicopathological characteristics,
treatment characteristics, and overall survival status of patients
treated with adjuvant (n=37) and neoadjuvant (n=37) chemotherapy,
in the matched cohort (n=74).
| Clinicopathological
characteristics | Matched cohort, n
(%) | Adjuvant, n
(%) | Neoadjuvant, n
(%) |
P-valuea |
|---|
| Age, years (mean ±
SD) | 42.0±8.4 | 42.7±9.3 | 41.2±7.4 | 0.458 |
| Age
groupb |
|
|
| 1.000 |
| <50
years | 60 (81.1) | 30 (81.1) | 30 (81.1) |
|
| ≥50
years | 14 (18.9) | 7 (18.9) | 7 (18.9) |
|
| Clinical
stageb |
|
|
| 1.000 |
| I | 6 (8.1) | 3 (8.1) | 3 (8.1) |
|
| II | 68 (91.9) | 34 (91.9) | 34 (91.9) |
|
|
III | - | - | - |
|
| Molecular
subtypeb |
|
|
| 1.000 |
|
HER2-enriched | 6 (8.1) | 3 (8.1) | 3 (8.1) |
|
| Luminal
A | 56 (75.7) | 28 (75.7) | 28 (75.7) |
|
| Luminal
B | 10 (13.5) | 5 (13.5) | 5 (13.5) |
|
|
TNBC | 2 (2.7) | 1 (2.7) | 1 (2.7) |
|
| Treatment response,
pCR | - | - | 5 (13.5) | - |
| Treatment |
|
|
|
|
|
Radiotherapy | 30 (40.5) | 12 (32.4) | 18 (48.6) | 0.155 |
| Hormone
therapy | 60 (81.1) | 31 (83.8) | 29 (78.4) | 0.553 |
|
Targeted therapy | 13 (17.6) | 5 (13.5) | 8 (21.6) | 0.359 |
|
Mortality | 5 (6.8) | - | 5 (13.5) | 0.054 |
OS comparison in the matched
cohort
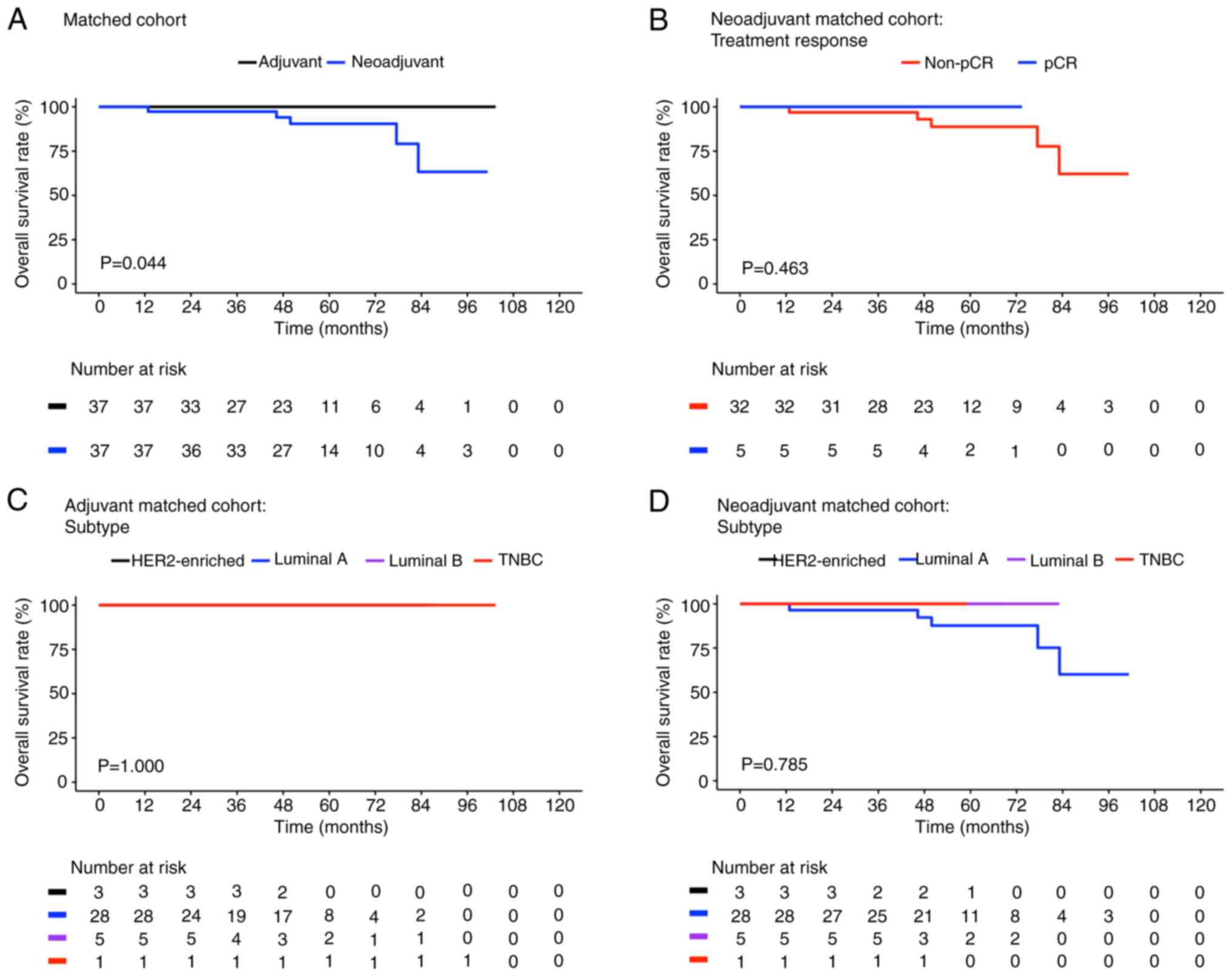
Fig. 3 presents the
Kaplan-Meier plots for the OS of the different subgroups in the
matched cohort. The neoadjuvant cases demonstrated significantly
poorer OS compared with the adjuvant controls [63.3% (95% CI,
37.5-100) vs. 100%, respectively; P=0.044; Fig. 3A]. However, in terms of treatment
characteristics and molecular subtypes, the neoadjuvant cases and
adjuvant controls demonstrated differences which were not
significant (Fig. 3B-D). In
addition, similar survival curves were noted in the population with
poor OS, as shown in Fig. 3A (blue
line, matched neoadjuvant cases), B (red line, non-pCR matched
neoadjuvant cases) and D (blue line, luminal A matched neoadjuvant
cases). Mortality events were also only noted in the neoadjuvant
cases with luminal A breast cancer [OS rate=71.8% (95% CI,
49.3–100%); Fig. 3D, blue
line].
OS comparison based on treatment
subgroups in the matched cohort
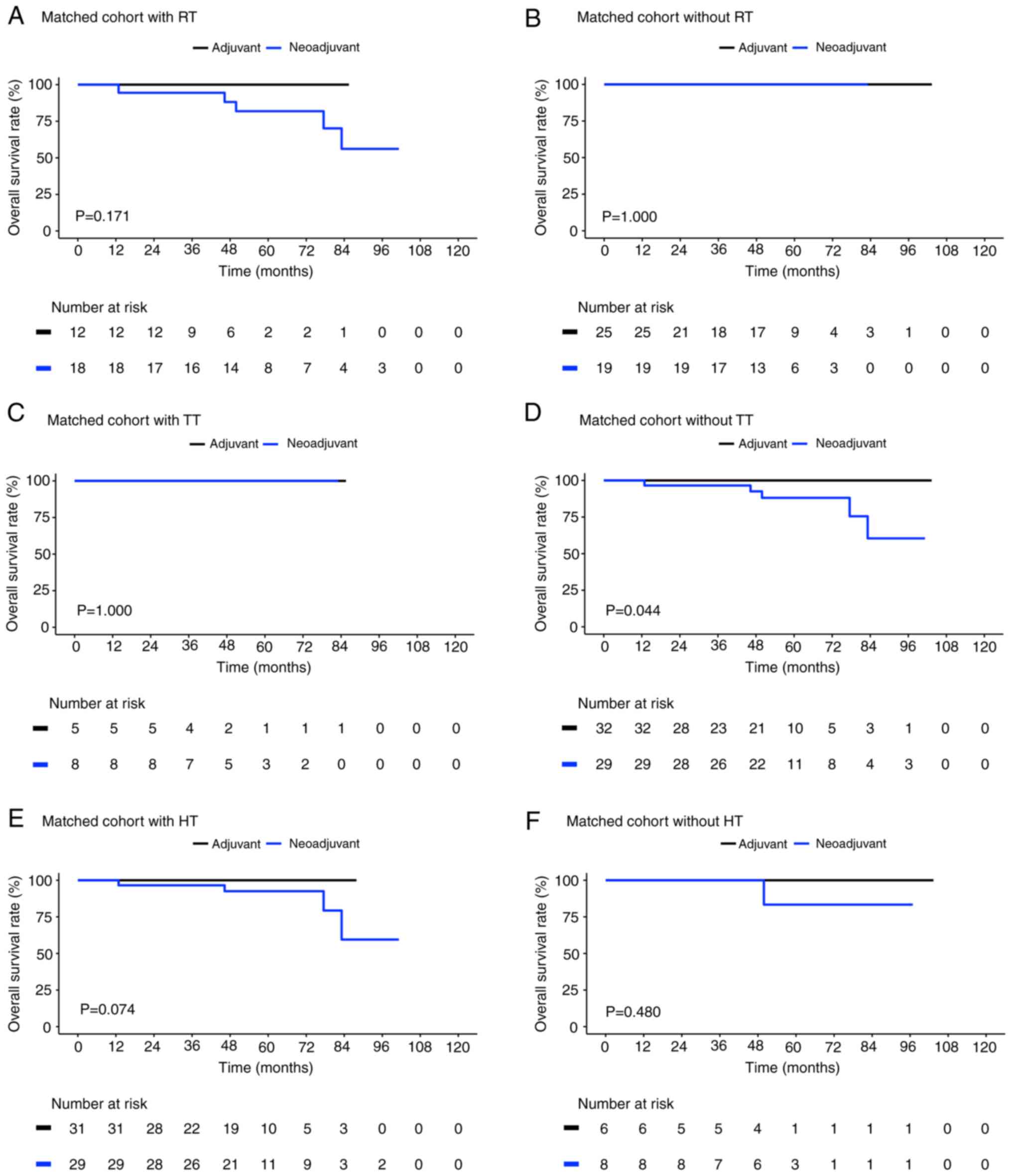
As shown in Fig. 4,
the matched neoadjuvant cases and adjuvant controls demonstrated
similar survival outcomes for those without radiotherapy (P=1.000;
Fig. 4B) and hormone therapy
(P=0.480; Fig. 4F). While the
matched adjuvant controls with radiotherapy (P=0.171; Fig. 4A) and hormone therapy (P=0.074;
Fig. 4E) showed improved survival
outcomes compared with matched neoadjuvant cases. However, the
matched neoadjuvant cases who did not receive targeted therapy [OS
rate=60.4%; 95% CI, 34.8-100] demonstrated significantly poorer OS
than the matched adjuvant controls who did not receive targeted
therapy (OS rate=100%; 95% CI; P=0.044; Fig. 4D). However, the combined use of
targeted therapy and chemotherapy led to the same favorable OS in
both the matched adjuvant controls and neoadjuvant cases (P=1.000;
Fig. 4C).
Discussion
In this study, a comparison was made on the
long-term OS outcomes after neoadjuvant and adjuvant chemotherapy
in Taiwanese women with breast cancer who had received immediate
breast reconstruction after mastectomy, by using a retrospective
matched case-control design. The OS outcome was similar between the
matched neoadjuvant cases and adjuvant controls who also received
targeted therapy combined with chemotherapy. Moreover, the patients
with luminal A breast cancer who received neoadjuvant chemotherapy,
including those who received the therapy in combination with
hormone therapy or radiotherapy, demonstrated poorer OS.
The benefits of undergoing adjuvant chemotherapy
after immediate breast reconstruction have been well studied over
the last ten years (22,23). Neoadjuvant chemotherapy was
introduced to facilitate tumor shrinkage and thus improve the
outcomes of mastectomy or other relevant treatment modalities
(24). In the findings of present
study, relatively more neoadjuvant cases were at clinical stage II
or III because of the beneficial effects of neoadjuvant
chemotherapy, which were particularly notable in the patients with
breast cancer with advanced tumor sizes or aggressive tumor
characteristics. Although the application of neoadjuvant
chemotherapy after breast reconstruction has been controversial
(i.e. the accuracy of lymph nodes status following neoadjuvant
chemotherapy), studies have suggested that neoadjuvant chemotherapy
is a safe therapeutic option for patients with breast cancer who
undergo immediate breast reconstruction after mastectomy (25). Studies involving Taiwanese
populations have also indicated that neoadjuvant chemotherapy leads
to further advancement of tumor characteristics and that local
recurrence rates over a limited follow-up interval are comparable,
which is consisted with the present findings (18).
In the current study, the OS benefits were similar
in both the matched neoadjuvant cases and adjuvant controls who
also underwent targeted therapy. A previous study suggested that
neoadjuvant chemotherapy is the preferred treatment strategy for
patients with TNBC and HER2-enriched breast cancer (26), and the addition of an anti-HER2
regimen in both adjuvant and neoadjuvant settings can lead to
favorable survival outcomes (27–29).
However, in the present study, the treatment benefits of
chemotherapy in both adjuvant and neoadjuvant settings were not
observed in the patients with luminal A breast cancer following
matching, which contrasts with the findings of a previous study
(30). This may be due to the
small sample size used in the current study. Therefore, the present
findings should be interpreted with caution.
The current results reveal that 77.7% of the
unmatched and 81.8% of the matched patients were <50 years old,
indicating that the preference for immediate breast reconstruction
after mastectomy decreases with age. Studies have demonstrated that
compared with older patients, younger patients with breast cancer
are physiologically healthier, which makes them better candidates
for breast reconstruction (7,31).
Breast cancer in younger patients is likely to be more aggressive,
leading to poorer quality of life and more mental health issues;
however, the younger breast cancer cohort is also preferred for
reconstructive options (7,31,32).
Moreover, the recent increase in the awareness and knowledge of,
the increase in communication with professionals on and the
development of decision support tools related to breast
reconstruction may have also led to the increase in the preference
for breast reconstruction among the younger breast cancer cohort
(33,34). Nevertheless, breast reconstruction
outcomes have demonstrated no notable differences among different
age groups (31,35).
The retrospective nature of this study limited the
inclusion of additional OS-related characteristics. In addition,
all data were collected from a single institution, potentially
limiting the generalizability of the current findings; the small
sample size possibly also limited the practical analysis.
Furthermore, because of the imbalance in the distribution of the OS
rates between the cases and controls, Cox regression analysis could
not be applied to our data. Therefore, the survival analysis was
performed using only non-parametric methods, including the
Kaplan-Meier estimator and log-rank test. Despite these
limitations, the present study, using both unmatched and matched
case-control cohorts, provides reasonable evidence for OS
differences after neoadjuvant and adjuvant chemotherapy in patients
with early-stage breast cancer who undergo immediate breast
reconstruction after mastectomy.
In conclusion, the present study demonstrated that
the long-term OS outcomes between neoadjuvant and adjuvant
chemotherapy combined with targeted therapy groups were comparable
in patients with early-stage breast cancer with immediate breast
reconstruction after mastectomy. This supports the feasibility of
neoadjuvant chemotherapy combined with targeted therapy in
Taiwanese female patients with early-stage breast cancer with
immediate breast reconstruction after mastectomy. However, the low
power of our matched cohort restricts the generalizability of this
finding. Therefore, in a future study, a multicenter prospective
design to increase the sample size and statistical power to verify
the current findings will be implemented. Moreover, additional
relevant clinical characteristics and outcomes, such as drug
information, treatment responses to specific drugs, laboratory
measurements and disease progression status will be included.
Finally, the future study will use a longitudinal model to
investigate the time-varying or dose-varying effects of
chemotherapeutic and targeted therapeutic agents.
Acknowledgements
The authors would like to thank the Clinical
Informatics Research & Development Center and Cancer Registry
database of Taichung Veterans General Hospital (Taichung, Taiwan)
for their support with clinical data management.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHL and CCH conceptualized the study, reviewed the
literature and wrote and revised the manuscript. JRY and ICT
collected the data and revised the manuscript. CYH and LCY analyzed
the data and interpreted the results. All authors have read and
approved the final manuscript. CCH and CYH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The data protocol of this study was approved by the
Institutional Review Board Taichung Veterans General Hospital (IRB
no. CE21406B) with a waiver of informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, et
al: NCCN guidelines® insights: Breast cancer, version
4.2021. J Natxl Compr Canc Netw. 19:484–493. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Lv X, Xu X, Gao X and Wang B:
Meta-analysis for psychological impact of breast reconstruction in
patients with breast cancer. Breast Cancer. 25:464–469. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maass SW, Roorda C, Berendsen AJ, Verhaak
PF and de Bock GH: The prevalence of long-term symptoms of
depression and anxiety after breast cancer treatment: A systematic
review. Maturitas. 82:100–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Padmalatha S, Tsai YT, Ku HC, Wu YL, Yu T,
Fang SY and Ko NY: Higher risk of depression after total mastectomy
versus breast reconstruction among adult women with breast cancer:
A systematic review and metaregression. Clin Breast Cancer.
21:e526–e538. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chopra D and Robb G: Body image: Integral
aspect of psychosocial evaluation for patients undergoing breast
reconstruction. Breast J. 26:1929–1930. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wang X, Thomsen JB, Nahabedian MY,
Ishii N, Rozen WM, Long X and Ho YS: Research trends and
performances of breast reconstruction: A bibliometric analysis. Ann
Transl Med. 8:15292020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodby KA, Robinson E, Danielson KK, Quinn
KP and Antony AK: Age-dependent characteristics in women with
breast cancer: Mastectomy and reconstructive trends at an urban
academic institution. Am Surg. 82:227–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
See MH, Sinnadurai S, Lai LL, Tan KL, The
MS, Teoh LY, Jamaris S, Abdul Malik R and Bhoo-Pathy N: Outcomes
after mastectomy with immediate breast reconstruction for breast
cancer in a multiethnic, middle-income Asian setting. Surgery.
170:1604–1609. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal J, Agarwal S, Pappas L and
Neumayer L: A population-based study of breast cancer-specific
survival following mastectomy and immediate or early-delayed breast
reconstruction. Breast J. 18:226–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou KW, Yu JC, Ho MH, Chiu WK, Ou KL, Chen
TM and Chen SG: Oncological safety and outcomes of nipple-sparing
mastectomy with breast reconstruction: A single-centered experience
in Taiwan. Ann Plast Surg. 74 (Suppl 2):S127–S131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali AM, Provenzano E, Bartlett JM, Abraham
J, Driver K, Munro AF, Twelves C, Poole CJ, Hiller L, Dunn JA, et
al: Prognosis of early breast cancer by immunohistochemistry
defined intrinsic sub-types in patients treated with adjuvant
chemotherapy in the NEAT/BR9601 trial. Int J Cancer. 133:1470–1478.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu F, Hu CH, Jiang SA, Lu FG, Lin MH and
Deng XG: Herceptin plus adjuvant chemotherapy for the prognosis of
patients with human epithelial growth factor receptor 2 positive
early-stage breast cancer: A meta-analysis. Zhong Nan Da Xue Xue
Bao Yi Xue Ban. 32:684–689. 2007.(In Chinese). PubMed/NCBI
|
|
13
|
Bonneterre J, Roché H, Kerbrat P, Brémond
A, Fumoleau P, Namer M, Goudier MJ, Schraub S, Fargeot P and
Chapelle-Marcillac I: Epirubicin increases long-term survival in
adjuvant chemotherapy of patients with poor-prognosis,
node-positive, early breast cancer: 10-Year follow-up results of
the French adjuvant study Group 05 randomized trial. J Clin Oncol.
23:2686–2693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brazda A, Estroff J, Euhus D, Leitch AM,
Huth J, Andrews V, Moldrem A and Rao R: Delays in time to treatment
and survival impact in breast cancer. Ann Surg Oncol. 17 (Suppl
3):S291–S296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gagliato Dde M, Gonzalez-Angulo AM, Lei X,
Theriault RL, Giordano SH, Valero V, Hortobagyi GN and
Chavez-Macgregor M: Clinical impact of delaying initiation of
adjuvant chemotherapy in patients with breast cancer. J Clin Oncol.
32:735–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mortenson MM, Schneider PD, Khatri VP,
Stevenson TR, Whetzel TP, Sommerhaug EJ, Goodnight JE Jr and Bold
RJ: Immediate breast reconstruction after mastectomy increases
wound complications: However, initiation of adjuvant chemotherapy
is not delayed. Arch Surg. 139:988–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baek SH, Bae SJ, Yoon CI, Park SE, Cha CH,
Ahn SG, Kim YS, Roh TS and Jeong J: Immediate breast reconstruction
does not have a clinically significant impact on adjuvant treatment
delay and subsequent survival outcomes. J Breast Cancer.
22:109–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang JR, Kuo WL, Yu CC, Chen SC and Huang
JJ: Reconstructive outcome analysis of the impact of neoadjuvant
chemotherapy on immediate breast reconstruction: A retrospective
cross-sectional study. BMC Cancer. 21:5222021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varghese J, Gohari SS, Rizki H, Faheem M,
Langridge B, Kümmel S, Johnson L and Schmid P: A systematic review
and meta-analysis on the effect of neoadjuvant chemotherapy on
complications following immediate breast reconstruction. Breast.
55:55–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connell RL, Rattay T, Dave RV, Trickey
A, Skillman J, Barnes NLP, Gardiner M, Harnett A, Potter S,
Holcombe C, et al: The impact of immediate breast reconstruction on
the time to delivery of adjuvant therapy: The iBRA-2 study. Br J
Cancer. 120:883–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen International expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong T, Hofer SO, McCready DR, Jacks LM,
Cook FE and Baxter N: A comparison of surgical complications
between immediate breast reconstruction and mastectomy: The impact
on delivery of chemotherapy-an analysis of 391 procedures. Ann Surg
Oncol. 19:560–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamahata A, Kubo K, Takei H, Saitou T,
Hayashi Y, Matsumoto H, Nagai S, Inoue K, Kurosumi M, Yamaki T and
Sakurai H: Impact of immediate breast reconstruction on
postoperative adjuvant chemotherapy: A single center study. Breast
Cancer. 22:287–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Halloran N, Lowery A, Curran C,
McLaughlin R, Malone C, Sweeney K, Keane M and Kerin M: A review of
the impact of neoadjuvant chemotherapy on breast surgery practice
and outcomes. Clin Breast Cancer. 19:377–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riba J, de Romani SE and Masia J:
Neoadjuvant chemotherapy for breast cancer treatment and the
evidence-based interaction with immediate autologous and
implant-based breast reconstruction. Clin Plast Surg. 45:25–31.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montemurro F, Nuzzolese I and Ponzone R:
Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert
Opin Pharmacother. 21:1071–1082. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
González-Santiago S, Saura C, Ciruelos E,
Alonso JL, de la Morena P, Santisteban Eslava M, Gallegos Sancho
MI, de Luna A, Dalmau E, Servitja S, et al: Real-world
effectiveness of dual HER2 blockade with pertuzumab and trastuzumab
for neoadjuvant treatment of HER2-positive early breast cancer (The
NEOPETRA Study). Breast Cancer Res Treat. 184:469–479. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hassett MJ, Li H, Burstein HJ and Punglia
RS: Neoadjuvant treatment strategies for HER2-positive breast
cancer: Cost-effectiveness and quality of life outcomes. Breast
Cancer Res Treat. 181:43–51. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okamoto M, Tajiri W, Ueo H, Masuda T,
Ijichi H, Koga C, Nakamura Y, Taguchi K, Ohno S and Tokunaga E:
Efficacy of Adjuvant combination therapy with trastuzumab and
chemotherapy in HER2-positive early breast cancer: A single
institutional cohort study from clinical practice. Anticancer Res.
40:3315–3323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandberg LJ, Clemens MW, Symmans WF,
Valero V, Caudle AS, Smith B, Kuerer HM, Hsu L and Kronowitz SJ:
Molecular profiling using breast cancer subtype to plan for breast
reconstruction. Plast Reconstr Surg. 139:586e–596e. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee CN and Foster RD: Breast
reconstruction after mastectomy in young women. Breast Dis.
23:47–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang YC, Hu WY, Chang YM and Chiu SC:
Changes in sexual life experienced by women in Taiwan after
receiving treatment for breast cancer. Int J Qual Stud Health
Well-being. 14:16543432019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin PJ, Fang SY and Kuo YL: Development
and usability testing of a decision support app for women
considering breast reconstruction surgery. J Cancer Educ.
36:160–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Momoh AO, Griffith KA, Hawley ST, Morrow
M, Ward KC, Hamilton AS, Shumway D, Katz SJ and Jagsi R: Patterns
and correlates of knowledge, communication, and receipt of breast
reconstruction in a modern population-based cohort of patients with
breast cancer. Plast Reconstr Surg. 144:303–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
August DA, Wilkins E and Rea T: Breast
reconstruction in older women. Surgery. 115:663–668.
1994.PubMed/NCBI
|