Introduction
Hepatocellular carcinoma (HCC) is a major health
problem, which represents the sixth most commonly diagnosed cancer
and third most common cause of cancer death worldwide (1). The mortality from HCC is primarily
due to its metastasis, complex pathogenesis and postsurgical
recurrence (2). Early HCC onset is
usually undetected and most patients are in the late stage at
diagnosis, which is also an important reason for the poor long-term
prognosis (2). Molecularly
targeted agents and immune checkpoint inhibitors have been
developed; however, the therapeutic effect of such treatments on
HCC is still unsatisfactory (3).
Drug resistance and limited therapeutic targets are problems to be
solved. Therefore, it is critical to study the mechanisms
underlying the development and progression of HCC to develop novel
therapeutics that may improve the overall survival of patients with
HCC across its stages.
Post-transcriptional RNA modifications are crucial
regulators of gene expression and are involved in the pathogenesis
of numerous diseases, including different types of cancer (4). Notably, 5-methylcytosine (m5C) is an
abundant and conserved modification in numerous types of RNA,
including transfer RNA, mRNA, ribosomal (r)RNA and other types of
noncoding RNA (4). The roles of
the RNA m5C modification and its modifying enzymes, defined as
writers (proteins upregulating modification level), readers
(proteins recognizing modification) and erasers (proteins
downregulating modification level), in gene regulation and
chromatin organization remain largely unclear (5). NOP2/Sun RNA methyltransferase 5
(NSUN5) is one of the eight evolutionarily conserved m5C RNA
methylases (NSUN1-7 and DNA methyltransferase 2) that have been
recognized as ‘writers’ (4,6,7).
Previous studies have reported the role of NSUN5 in
human cancer. For example, DNA methylation-associated epigenetic
silencing of NSUN5 has been reported to be a hallmark of the
long-term survival of patients with glioma (8). In colorectal cancer, NSUN5 has been
reported to act as a promotor of tumor development via cell cycle
regulation (9). However, to the
best of our knowledge, the role of NSUN5 in HCC remains
unknown.
In the present study, the clinical significance of
NSUN5 expression in patients with HCC was evaluated using The
Cancer Genome Atlas (TCGA) database. In addition, the role of NSUN5
in HCC was assessed using in vitro and in vivo
experiments to evaluate whether NSUN5 represented a novel
therapeutic target for HCC.
Materials and methods
Patients and samples
Written informed consent was obtained from patients
and approval for the present study was obtained from the Ethics
Committee of The Eastern Hepatobiliary Surgery Hospital (Shanghai,
China; approval no. EHBHKY2018-02-014). A total of 120 paired HCC
and corresponding adjacent noncancerous liver (ANL) tissues were
collected from patients undergoing hepatectomy (without
preoperative treatment) at The Eastern Hepatobiliary Surgery
Hospital. Detailed clinical characteristics are presented in
Table SI. Among the samples, 40
pairs (Cohort 1) were collected from April 2021 to July 2021 and
assessed using reverse transcription-quantitative (RT-q)PCR and
western blotting, and the other 80 pairs (Cohort 2) were collected
from February 2014 to November 2015- and assessed using
immunohistochemistry (IHC).
RT-qPCR
Total RNA was isolated from human HCC tissues, ANL
tissues or HCC cell lines using TRIzol® reagent (cat.
no. 1559602. Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized (15 min at 37°C; 5 sec at 85°C and 4°C) using a cDNA
Synthesis Kit (cat. no. RR036A; Takara Biotechnology Co., Ltd.) and
qPCR was then performed using a StepOnePlus™ Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and TB Green
Fast qPCR Mix (cat. no. RR430A; Takara Biotechnology Co., Ltd.).
The quantification of NSUN5 was performed using the
2−ΔΔCq method (10)
with normalization to β-actin (ACTB). The thermocycling conditions
were as follows: Holding stage (95°C, 3 min), cycling stage (35
cycles; 10 sec at 95°C, 15 sec at 60°C and 20 sec at 72°C), melt
curve stage (15 sec at 95°C, 1 min at 60°C and 15 sec at 95°C). The
primer sequences used were as follows: NSUN5 forward (F),
5′-CGCTACCATGAGGTCCACTAC-3′ and reverse (R),
5′-GCATCTCGCACCACGTCTT-3′; and ACTB F, 5′-CCACCATGTACCCTGGCATTG-3′
and R, 5′-TCATCTTGTTTTCTGCGCAAGTTA-3′.
Western blotting
Whole-cell lysates were prepared from HCC tissues
and cells (Hep3B and SNU387) using RIPA solution (Beyotime
Institute of Biotechnology). The concentration of protein in the
lysates was determined by BCA protein assay kit (cat. no. P0012;
Beyotime Institute of Biotechnology) The lysates (50 µg protein per
lane) were then separated by SDS-PAGE on 4–20% gels (SurePAGE™;
Bis-Tris; 10×8; 4–20%; gradient, 12 wells; cat. no. M00656;
GenScript Biotechnology Co., Ltd.), transferred to a PVDF membrane
and blocked using 5% nonfat milk for 1 h at room temperature
(~25°C). Primary antibodies were added after blocking and the
membranes were incubated overnight at 4°C. The next day, the
membranes were incubated for 2 h with the appropriate secondary
antibody at room temperature. Finally, the protein bands were
visualized using enhanced chemiluminescent solution (Beyotime
Institute of Biotechnology) and detected using a chemiluminescence
system (Bio-Rad Laboratories, Inc.). The primary antibodies used in
the present study were NSUN5 (1:1,000; cat. no, 15449-1-AP;
Proteintech Group, Inc.) and ACTB (1:5,000; cat. no, 66009-1-Ig;
Proteintech Group, Inc.). The secondary antibodies used were goat
anti-mouse IgG H&L (HRP) (1:2,000; cat. no. ab6789; Abcam) and
goat anti-rabbit IgG H&L (HRP) (1:2,000; cat. no. ab6721;
Abcam). Image J (version 1.8.0; Bharti Airtel, Ltd) was used to
semi-quantify western blots.
IHC and tissue microarray (TMA)
analysis
A TMA was constructed as previously described
(11). Briefly, it consisted of
collection and selection of tissue blocks (formalin-fixed
paraffin-embedded HCC and ANL tissues), design and organization of
TMA, punching of TMA and technical cutting of TMA (sections should
be ≤5 µm). IHC was performed on the TMA using a two-step
immune-peroxidase technique (12)
(https://www.abcam.com/ps/pdf/protocols/ihc_p.pdf).
Briefly, it consisted of deparaffinization (xylene and ethanol),
antigen retrieval (vegetable steamer, 100°C) and
immunohistochemical staining (primary antibody incubated overnight
at 4°C; second antibody incubated for 1 h at room temperature;
chromogen, 10 min at room temperature). The primary antibody used
was NSUN5 (1:100; cat. no, 15449-1-AP; Proteintech Group, Inc.).
The blocking reagent was 0.3% H2O2 in TBS
(incubated for 15 min at room temperature). The secondary antibody
used was goat anti-rabbit IgG H&L (HRP) (1:1,000; cat. no.
ab6721; Abcam). TMA scanning was performed using PANNORAMIC DESK II
DW scanner (light microscope; 3DHISTECH, Ltd.). IHC scores were
assessed for the IHC stains of NSUN5 (13) by three separate observers who had
no knowledge of the patient characteristics. The intensity of
specific staining was characterized as follows: Not present, 0;
weak but detectable above control, 1+; distinct, 2+; and very
strong, 3+. For each observed tissue component, a summary value
referred to as the H-Score was calculated. This consisted of a sum
of the percentages of positively stained cells multiplied by a
weighted intensity of staining; H-Score=∑ Pi (i + 1), where ‘Pi’
was the percentage of stained cells in each intensity category and
‘i’ was the intensity.
Cell culture
The HCC Hep3B and SNU387 cell lines were purchased
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences and were authenticated using short tandem
repeat profiling. Cells were grown in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and maintained in a humidified 37°C incubator containing 5%
CO2. Using the sequence of NSUN5 mRNA (NM_148956)
acquired from the Nucleotide database of National Center for
Biotechnology Information (https://www.ncbi.nlm.nih.gov/nuccore/NM_148956) and a
lentiviral vector (GV492; Ubi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin;
Shanghai GeneChem Co., Ltd.), NSUN5 was overexpressed in Hep3B and
SNU387 cell lines using the methods previously described (14,15).
The viral titer of NSUN5-overexpression (NSUN5-oe) lentivirus and
negative control (NC) lentivirus was 4×108 and
3×108 TU/ml, respectively. A total of 48 h after
lentivirus transduction, puromycin (2 µg/ml) was added into the
culture medium to obtain stably transduced cells. After ~10 days of
puromycin selection, the stably transduced cells were collected and
subsequent experiments were performed.
In vitro cell behavior assays
For cell proliferation, a Cell Counting Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc.) was performed
according to the manufacturer's protocol. Briefly,
~2×103 cells/well were seeded on a 96-well plate for 24
h and 10 µl CCK-8 solution was added to wells at 0, 1, 2, 3 or 4
days. The 96-well plate was incubated at 37°C for 2 h after each
addition of CCK-8 solution before measuring the absorbance at 450
nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Cell migration was evaluated using a 12-µm pore
polycarbonate membrane Transwell chambers (MilliporeSigma). Cells
(105) were seeded into the upper Transwell chamber in
DEEM (cat no. 11995-065; Gibco; Thermo Fisher Scientific) without
FBS and 500 µl DMEM containing 10% FBS was added into the lower
chamber. The 24-well plate with Transwell chambers was incubated
for 48 h at 37°C. After incubation for 48 h, the cells were stained
using 0.1% crystal violet for 30 min at room temperature (~25°C).
Finally, the upper chambers were observed using a light microscope
(Olympus Corporation). These experiments were independently
repeated at least three times.
Animal studies
The animal experiments performed in the present
study conformed to the guidelines of Animal Research: Reporting of
In Vivo Experiments (http://www.nc3rs.org.uk/arrive-guidelines) and were
approved by the Institutional Animal Care and Use Committee of
Shanghai University of Traditional Chinese (Shanghai, China)
(approval no. PZSHUTCM210926007). BALB/c male nude mice (16 mice;
age, 5 weeks; weight, ~20 g) used in the present study were
purchased from the Laboratory Animal Resources, Chinese Academy of
Sciences. All mice were housed in laminar flow cabinets under
specific pathogen-free conditions at room temperature (humidity
40–60%) with a 12-h light/dark cycle, and ad libitum access
to food and water.
The subcutaneous tumor xenograft experiment was
performed as previously described (16). A total of eight BALB/C male nude
mice were used in the present study. Briefly, for each mouse,
NSUN5-oe SNU387 cells (1.5×106 cells in 150 µl 0.9%
sodium chloride) were injected subcutaneously into the armpit of
the right forelimb (site approved by the ethics committee) and the
same amount of NC SNU387 cells were injected subcutaneously into
the armpit of the left forelimb. Tumor development was assessed
weekly using a caliper and the tumor volume was calculated as
follows: Larger diameter × (smaller diameter)2/2. The
humane endpoints of the xenograft experiment conformed to the
Guidelines for the Welfare and Use of Animals in Cancer Research
(17). Briefly, immediate humane
termination was required when statistically significant effects
could be demonstrated, when the mean diameter of the xenograft
tumor exceeded ~1.2 cm or when the mice displayed severe symptoms
(persistent hypothermia, hind-limb paralysis or weakness). In the
present study, the mice were sacrificed by cervical dislocation 4
weeks after implantation, when NSUN5 showed statistically
significant growth promotion effects.
LinkedOmics
The ‘LinkFinder’ module in LinkedOmics (version 4)
(18) (http://linkedomics.org/login.php) was used to assess
NSUN5-correlated genes in HCC. The ‘RNA-seq’ platform was chosen
for both the ‘Search Dataset’ and ‘Target Dataset’, and the
‘Spearman Correlation test’ was selected as the statistical method.
The LinkFinder module of LinkedOmics was used to generate the
volcano plots.
NSUN5-correlated genes (‘Association Result’) were
then used to perform enrichment analyses in the ‘Link
Interpreter-GSEA (Gene Set Enrichment Analysis)’ module of
LinkedOmics. Biological process, cellular component, molecular
function and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathways analyses were performed individually. ‘Rank Criteria’ was
chosen as the ‘Statistic’ (‘Spearman correlation’ in this
situation), ‘Minimum Number of Genes’ was assigned a value of 3 and
‘Simulations’ was assigned a value of 500.
TCGA
The URL link for TCGA: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga.
To evaluate the relationship between the expression of NSUN5 and
the clinical characteristics of patients with HCC, the clinical
information of 374 patients with HCC was downloaded from TCGA liver
HCC database (https://portal.gdc.cancer.gov/) (Table SII). NSUN5 mRNA sequencing data
for these 374 HCC tissues and 50 ANL tissues (paired with 50 of the
374 HCC tissues) were also downloaded (19) (https://portal.gdc.cancer.gov/) (columns C and D,
Table SII).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM, Corp.). Comparisons among continuous
variables were performed using paired Student's t-test, unpaired
Student's t-test, Mann-Whitney U test or Wilcoxon signed-rank test,
whereas comparisons among categorical variables were performed
using the Pearson's χ2 test or Fisher's exact test. The
survival curves were generated using the Kaplan-Meier method, and
the differences were assessed using a log-rank test. P<0.05
(two-tailed) was considered to indicate a statistically significant
difference.
Results
NSUN5 is upregulated and predicts poor
prognosis in HCC according to TCGA database
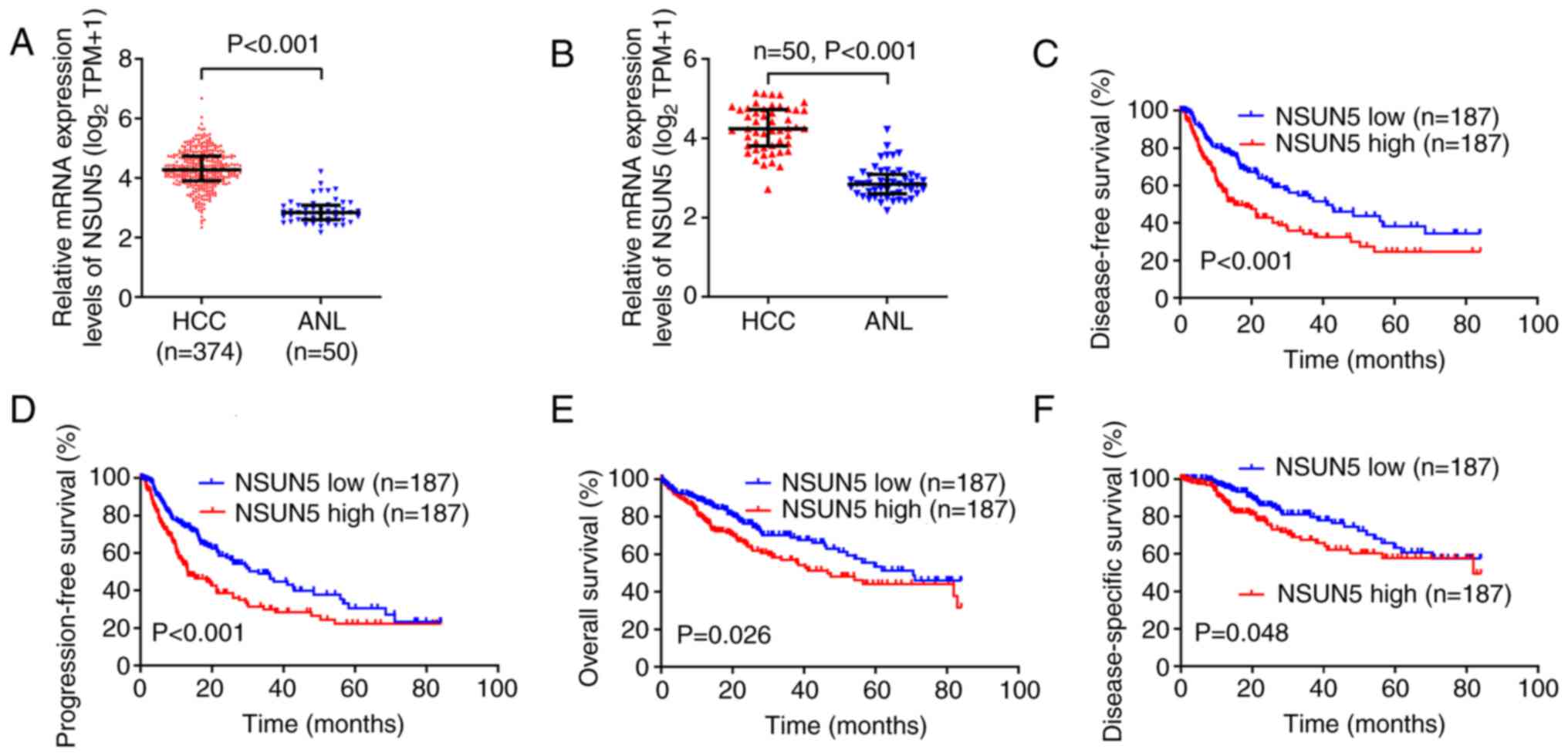
The present study demonstrated that the mRNA
expression levels of NSUN5 were significantly higher in the 374 HCC
tissues compared with those in the 50 ANL tissues (Fig. 1A; P<0.001). The mRNA expression
levels of NSUN5 in 50 HCC tissues were also significantly higher
than those in 50 paired ANL tissues (Fig. 1B; P<0.001). The 374 patients
with HCC were divided into NSUN5-high and NSUN5-low groups
according to the median mRNA expression (transcript per million,
18.497) levels of NSUN5 in the 374 HCC tissues. The longest
follow-up time assessed in the present study was 7 years (84
months). Kaplan-Meier survival curves demonstrated that higher
NSUN5 mRNA expression levels in HCC were associated with
significantly lower disease-free survival (Fig. 1C; P<0.001), lower
progression-free survival (Fig.
1D; P<0.001), lower overall survival (Fig. 1E; P=0.026) and lower
disease-specific survival (Fig.
1F; P=0.048) compared with the NSUN5-low group.
Furthermore, analysis of the association between
NSUN5 expression in HCC tissues and the clinical characteristics of
patients with HCC demonstrated that higher NSUN5 mRNA expression
levels were significantly positively associated with lower weight
(P=0.003), lower body mass index (P=0.001), higher serum
α-fetoprotein levels (P=0.003), worse tumor node metastasis (TNM)
stage (20) (P=0.043), worse
histological grade (P=0.004) and vascular invasion (P=0.002)
compared with the NSUN5-low group (Table I). These results indicated that
NSUN5 may be associated with HCC tumor growth and migration.
 | Table I.Clinical characteristics of 374
patients with hepatocellular carcinoma from The Cancer Genome Atlas
according to the mRNA expression levels of NSUN5. |
Table I.
Clinical characteristics of 374
patients with hepatocellular carcinoma from The Cancer Genome Atlas
according to the mRNA expression levels of NSUN5.
|
| NSUN5 |
|
|---|
|
|
|
|
|---|
| Characteristic | Low | High | P-value |
|---|
| n | 187 | 187 |
|
| Median age, years
(IQR) | 61 (52–68) | 61 (51–69) | 0.956a |
| Median height, cm
(IQR) | 168 (161–175) | 167 (161–172) | 0.289a |
| Median weight, kg
(IQR) | 73 (61.5-85.5) | 68 (58.0-77.5) | 0.003a |
| Median BMI,
kg/m2 (IQR) | 25.65
(22.41-29.85) | 23.86
(20.80-27.44) | 0.001a |
| Median AFP, ng/ml
(IQR) | 9.5
(4.00-52.25) | 26
(4.00-1772.25) | 0.003a |
| Median albumin,
g/dl (IQR) | 4 (3.5-4.3) | 4 (3.5-4.3) | 0.895a |
| Median prothrombin
time, sec (IQR) | 1.1
(1.00-9.45) | 1.1
(1.00-8.75) | 0.302a |
| TNM stage, n
(%) |
|
| 0.043c |
| Stage
I | 100 (53.5%) | 73 (39.0%) |
|
| Stage
II | 37 (19.8%) | 50 (26.7%) |
|
| Stage
III | 37 (19.8%) | 48 (25.7%) |
|
| Stage
IV | 2 (1.1%) | 3 (1.6%) |
|
|
Missing | 11 (5.9%) | 13 (7.0%) |
|
| Sex, n (%) |
|
| 0.320b |
|
Female | 65 (34.8%) | 56 (29.9%) |
|
|
Male | 122 (65.2%) | 131 (70.1%) |
|
| Residual tumor, n
(%) |
|
| 0.713c |
| R0 | 166 (88.8%) | 161 (86.1%) |
|
| R1 | 8 (4.3%) | 9 (4.8%) |
|
| R2 | 0 (0.0%) | 1 (0.5%) |
|
|
Missing | 13 (7.0%) | 16 (8.6%) |
|
| Histologic grade, n
(%) |
|
| 0.004b |
| G1 | 34 (18.2%) | 21 (11.2%) |
|
| G2 | 98 (52.4%) | 80 (42.8%) |
|
| G3 | 46 (24.6%) | 78 (41.7%) |
|
| G4 | 6 (3.2%) | 6 (3.2%) |
|
|
Missing | 3 (1.6%) | 2 (1.1%) |
|
| Adjacent hepatic
tissue inflammation, n (%) |
|
| 0.835b |
|
None | 65 (34.8%) | 53 (28.3%) |
|
|
Mild | 52 (27.8%) | 49 (26.2%) |
|
|
Severe | 9 (4.8%) | 9 (4.8%) |
|
|
Missing | 61 (32.6%) | 76 (40.6%) |
|
| Child-Pugh grade, n
(%) |
|
| 1.000c |
| A | 115 (61.5%) | 104 (55.6%) |
|
| B | 11 (5.9%) | 10 (5.3%) |
|
| C | 1 (0.5%) | 0 (0.0%) |
|
|
Missing | 60 (32.1%) | 73 (39.0%) |
|
| Fibrosis Ishak
score, n (%) |
|
| 0.088b |
| 0 | 45 (24.1%) | 30 (16.0%) |
|
|
1/2 | 13 (7.0%) | 18 (9.6%) |
|
|
3/4 | 10 (5.3%) | 18 (9.6%) |
|
|
5/6 | 45 (24.1%) | 36 (19.3%) |
|
|
Missing | 74 (39.6%) | 85 (45.5%) |
|
| Vascular invasion,
n (%) |
|
| 0.002b |
| No | 122 (65.2%) | 86 (46.0%) |
|
|
Yes | 44 (23.5%) | 66 (35.3%) |
|
|
Missing | 21 (11.2%) | 35 (18.7%) |
|
Collectively, the bioinformatics analysis of TCGA
data demonstrated that NSUN5 was upregulated, associated with worse
clinical characteristics and predicted poor prognosis in HCC.
NSUN5 is upregulated and predicts poor
prognosis in HCC in cohorts of the present study
To further evaluate the relationship between the
expression of NSUN5 and the clinical characteristics of patients
with HCC, the mRNA and protein expression levels of NSUN5 were
evaluated in the cohorts of the present study (patients with HCC
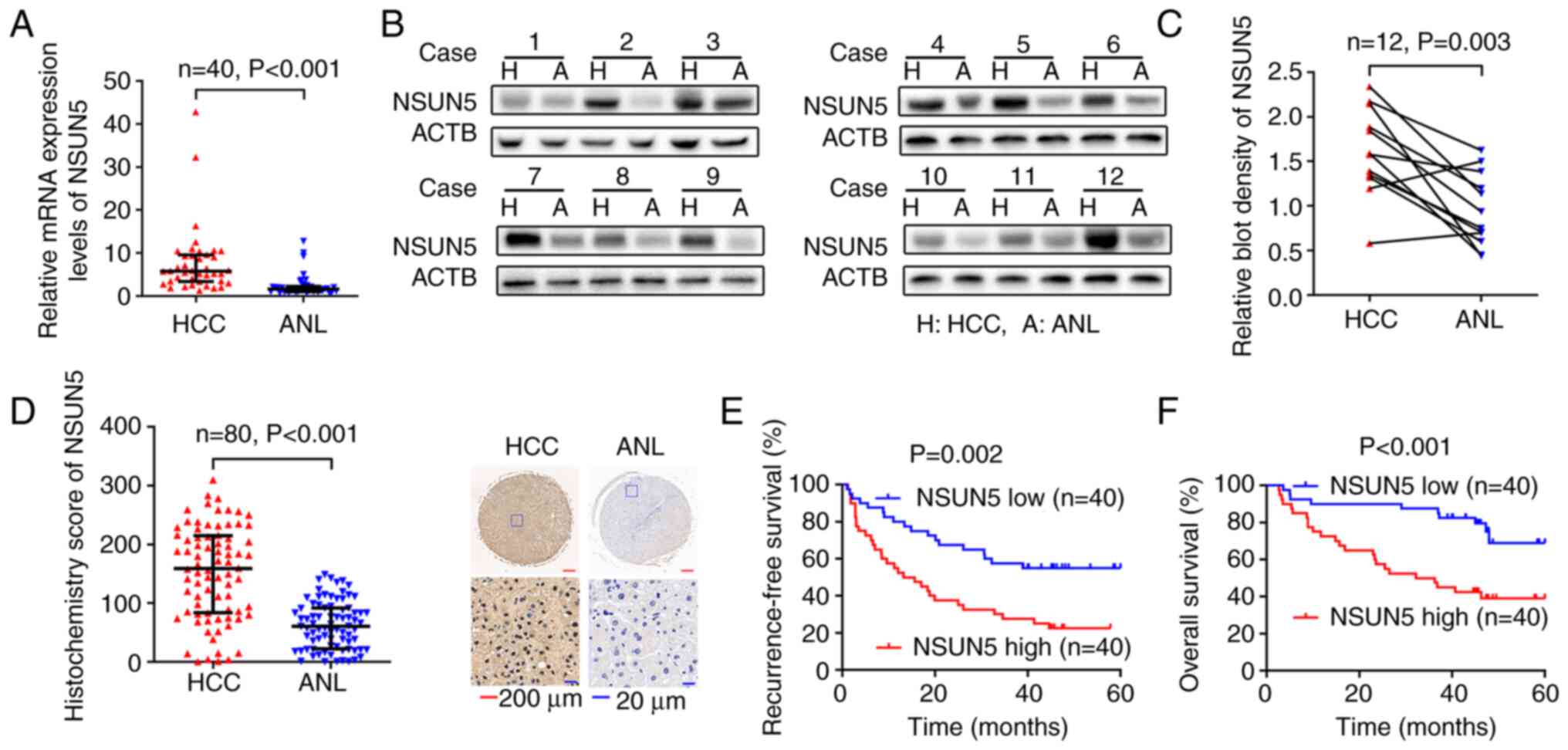
from The Eastern Hepatobiliary Surgery Hospital). RT-qPCR performed
in 40 paired HCC and ANL tissues (Cohort 1) demonstrated that the
mRNA expression levels of NSUN5 were significantly upregulated in
HCC tissues compared with those in the ANL tissues (Fig. 2A; P<0.001). Western blotting in
12 paired HCC and ANL tissues randomly selected from Cohort 1
demonstrated that the protein expression levels of NSUN5 were
significantly upregulated in HCC tissues compared with those in the
ANL tissues (Fig. 2B and C;
P=0.003). Moreover, IHC in Cohort 2 demonstrated that NSUN5 was
mainly expressed in the nucleus and indicated that the protein
expression levels [indicated by the IHC score (21)] of NSUN5 in 80 HCC tissues was
significantly higher than those in the paired ANL tissues (Fig. 2D; P<0.001). The 80 patients with
HCC from Cohort 2 were then divided into NSUN5-high and NSUN5-low
groups according to the median IHC score (159.029) of NSUN5.
Kaplan-Meier survival curves demonstrated that higher NSUN5
expression in HCC was associated with significantly lower
recurrence-free survival (Fig. 2E;
P=0.002) and lower overall survival (Fig. 2F; P<0.001) compared with the
NSUN5-low group.
Furthermore, analysis of the association between the
expression of NSUN5 in HCC tissues and the clinical characteristics
of patients with HCC demonstrated that higher NSUN5 protein
expression levels were positively associated with multiple tumor
number (P<0.001), large tumor size (P=0.003), presence of
microvascular invasion (P=0.001), worse TNM stage (P<0.001) and
worse Barcelona Clinic Liver Cancer stage (22) (P=0.001) compared with the NSUN5-low
group (Table II).
 | Table II.Clinical characteristics of 80
patients with hepatocellular carcinoma according to the protein
expression levels of NSUN5. |
Table II.
Clinical characteristics of 80
patients with hepatocellular carcinoma according to the protein
expression levels of NSUN5.
|
| NSUN5 |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| n | 40 | 40 |
|
| Age, years |
|
| 0.653a |
|
≤50 | 23 | 21 |
|
|
>50 | 17 | 19 |
|
| Sex |
|
| 0.793a |
|
Female | 9 | 10 |
|
|
Male | 31 | 30 |
|
| HBsAg |
|
| 1.000b |
|
Negative | 3 | 2 |
|
|
Positive | 37 | 38 |
|
| Liver
cirrhosis |
|
| 0.633a |
|
Without | 12 | 14 |
|
|
With | 28 | 26 |
|
| AFP, µg/l |
|
| 0.143a |
|
≤20 | 15 | 9 |
|
|
>20 | 25 | 31 |
|
| Pathological
satellite |
|
| 0.179a |
|
Absent | 24 | 18 |
|
|
Present | 16 | 22 |
|
| Tumor number |
|
|
<0.001a |
|
Solitary | 39 | 27 |
|
|
Multiple | 1 | 13 |
|
| Edmondson's
grade |
|
| 0.363a |
| I +
II | 8 | 5 |
|
| III +
IV | 32 | 35 |
|
| Tumor size, cm |
|
| 0.003a |
| ≤5 | 24 | 11 |
|
|
>5 | 16 | 29 |
|
| Microvascular
invasion |
|
| 0.001a |
|
Absent | 31 | 16 |
|
|
Present | 9 | 24 |
|
| Encapsulation |
|
| 1.000a |
|
Complete | 14 | 14 |
|
|
Incomplete | 26 | 26 |
|
| TNM stage |
|
|
<0.001a |
| I | 39 | 25 |
|
| I +
II | 1 | 15 |
|
| BCLC stage |
|
| 0.001a |
| 0 +
A | 39 | 28 |
|
| B +
C | 1 | 12 |
|
In summary, NSUN5 mRNA and protein expression levels
were significantly upregulated in HCC, associated with worse
clinical characteristics and predictive of poor prognosis in the
present study cohorts.
NSUN5 promotes the proliferation and
migration of HCC cells
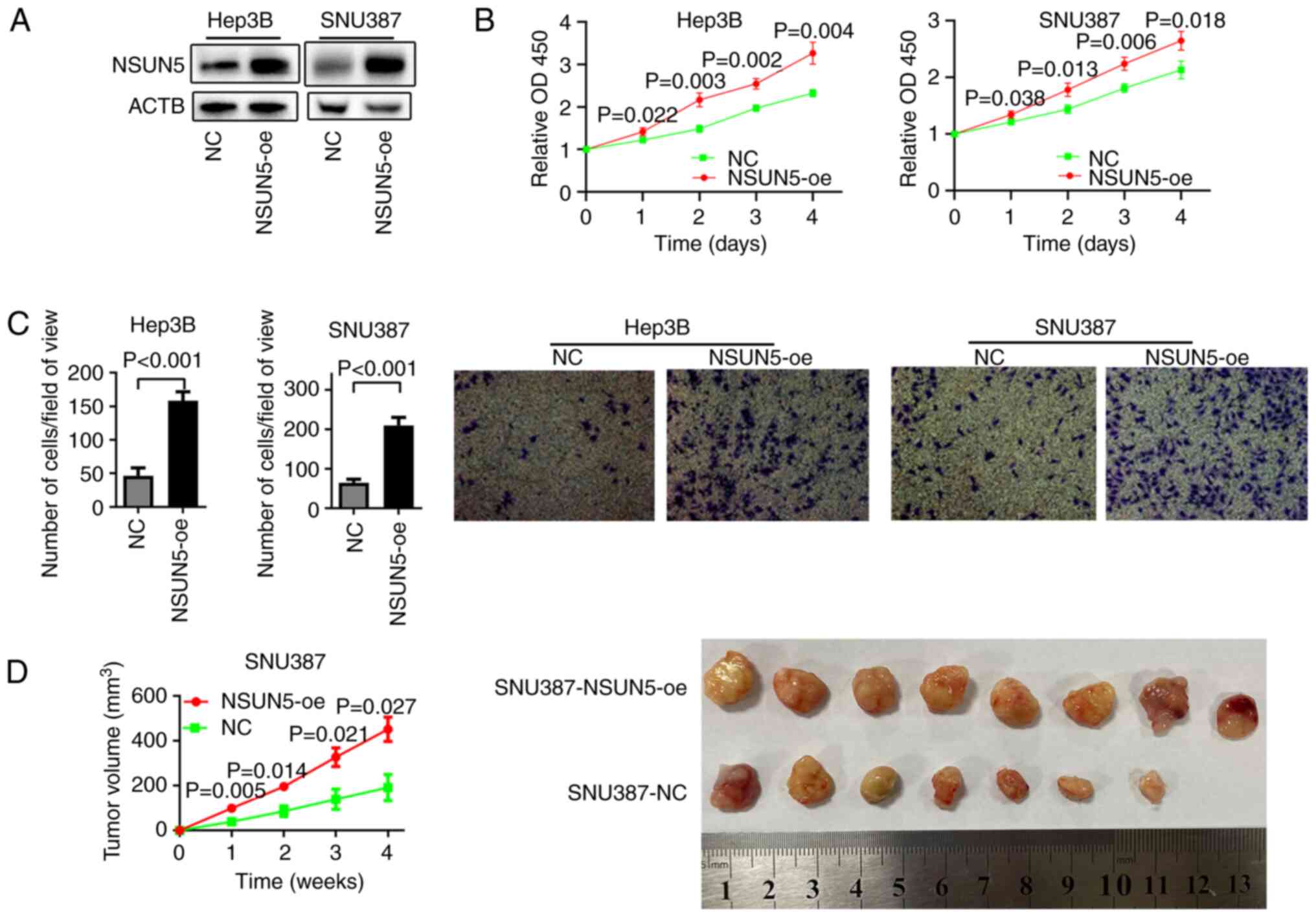
To assess the role of NSUN5 in HCC progression,
NSUN5 was overexpressed in Hep3B and SNU387 HCC cells (Fig. 3A). CCK-8 and Transwell migration
assay results demonstrated that overexpression of NSUN5
significantly promoted the proliferation (Fig. 3B) and migration (Fig. 3C) of HCC cells compared with in the
NC group. To assess the function of NSUN5 in vivo, a
subcutaneous tumor xenograft experiment was performed, which
demonstrated that the NSUN5-oe SNU387 ×enograft grew significantly
faster than the negative control (Fig.
3D). Notably, the NSUN5-NC cells injected into one of the mice
did not develop into a tumor.
These results indicated that NSUN5 could promote the
proliferation and migration of HCC in vitro, and induce the
tumor growth of HCC in vivo.
NSUN5 is positively correlated with
translation in HCC
To evaluate the mechanism by which NSUN5 promoted
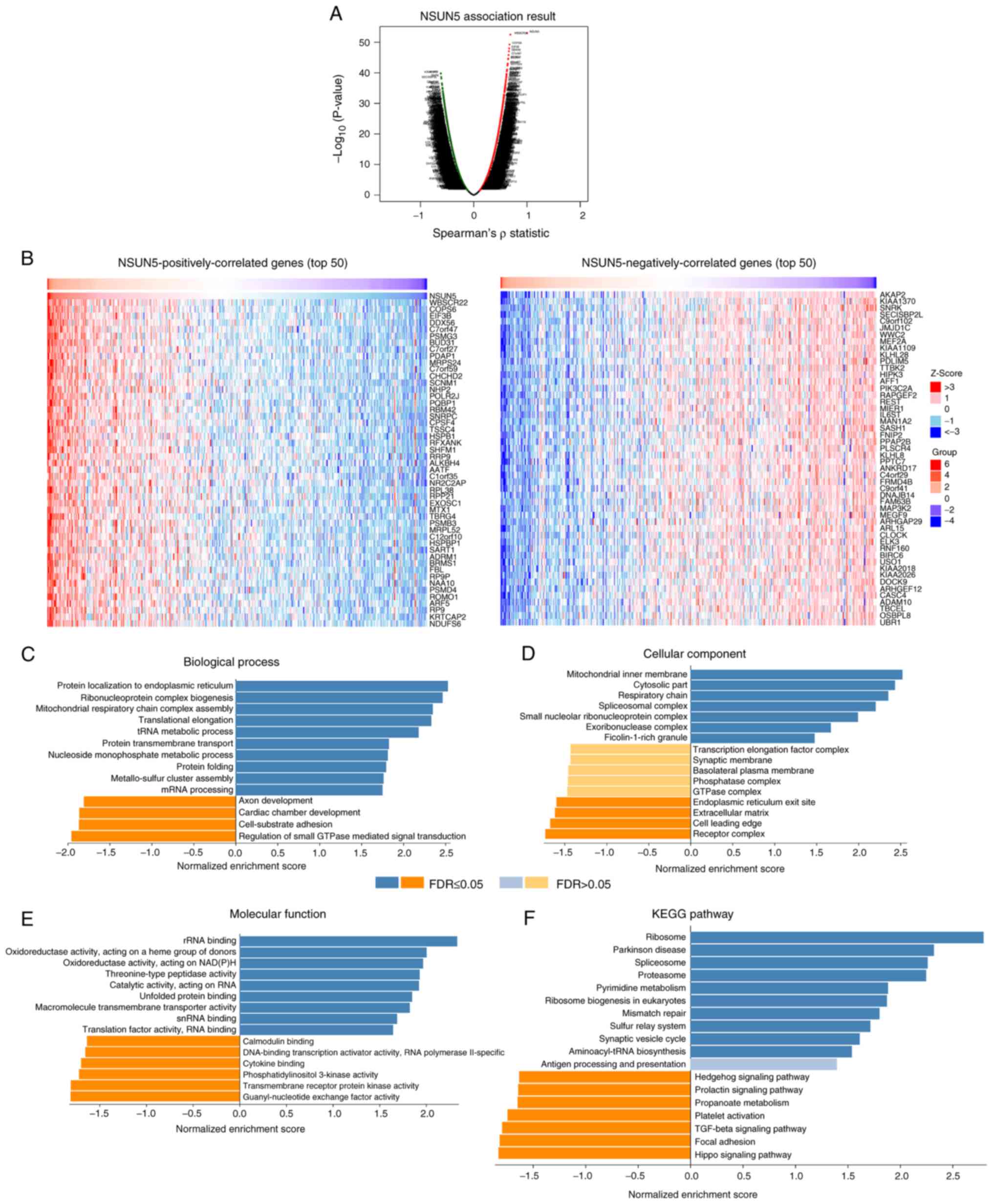
the progression of HCC, a bioinformatics analysis of
NSUN5-correlated genes was performed using LinkedOmics. Among the
19,922 genes analyzed in Table
SIII, 4,212 genes were positively correlated and 5,981 genes
were negatively correlated with NSUN5 in HCC (P<0.01; Table SIII). The volcano plot showing the
correlated genes is presented in Fig.
4A. The top 50 NSUN5 positively and negatively correlated genes
are presented in Fig. 4B.
Furthermore, Gene Ontology (biological process, cellular component,
molecular function) and KEGG pathway analyses of the
NSUN5-correlated genes were performed (Fig. 4C-F). Among the 10 biological
processes in which NSUN5-positively correlated genes were enriched,
five (‘protein localization to endoplasmic reticulum’,
‘ribonucleoprotein complex biogenesis’, ‘translational elongation’,
‘protein transmembrane transport’ and ‘protein folding’) were
associated with translation (Fig.
4C). Among the seven cellular components in which
NSUN5-positively correlated genes were enriched, two (‘spliceosomal
complex’ and ‘small nucleolar ribonucleoprotein complex’) were
associated with translation (Fig.
4D). Among the nine molecular functions in which
NSUN5-positively correlated genes were enriched, three (‘rRNA
binding’, ‘unfolded protein binding’ and ‘translation factor
activity, RNA binding’) were associated with translation (Fig. 4E). Among the 10 KEGG pathways in
which NSUN5-positively correlated genes were enriched, three
(‘ribosome’, ‘spliceosome’ and ‘ribosome biogenesis in eukaryotes’)
were associated with translation (Fig.
4F).
Taken together, these results indicated that NSUN5
was positively correlated with translation in HCC.
Discussion
HCC has a high mortality-to-incidence ratio
(23) and the treatment options
are severely limited (24);
therefore, new treatment approaches are urgently needed. Although
emerging immunotherapies (such as immune checkpoint inhibitors) can
prolong the survival time of certain patients, the therapeutic
effect is limited (25,26). Identifying novel mechanisms may aid
the design of new targeted therapies. To the best of our knowledge,
the present study is the first to report NSUN5 as a promising
molecular target for HCC treatment.
Previous studies on NSUN5 have mostly focused on
nervous system diseases. For example, NSUN5 has been reported to
contribute to the pathology of Williams-Beuren syndrome (WBS)
(27), a neurodevelopmental
disorder caused by microdeletions of 28 genes, and characterized by
cognitive disorders and hypertrophy of the corpus callosum
(28,29). The NSUN5 gene is deleted in ~95% of
patients with WBS (28).
NSUN5-knockout mice have been reported to exhibit spatial cognition
deficits, impaired cerebral cortex development and corpus callosum
agenesis (5,28,29).
In recent years, the role of NSUN5 in human cancer
has been reported. For example, Jiang et al (9) reported that NSUN5 promoted the
proliferation of colorectal cancer via regulation of the cell
cycle. In addition, Janin et al (8) reported that NSUN5 inhibited the
growth of glioma tumors in vivo. In the present study, it
was demonstrated that NSUN5, which is mainly expressed in the
nucleus, was upregulated in HCC, and this was associated with worse
clinical characteristics and was predictive of poor prognosis in
HCC, according to TCGA database and the present study cohorts. The
results of the present study also indicated that NSUN5 could
promote the proliferation and migration of HCC in vitro and
induce the growth of HCC tumors in vivo. As NSUN5 may serve
contradictory roles in different organs, potential drugs targeting
NSUN5 in HCC should be hepatocyte specific. It has been reported
that N-acetylgalactosamine or apolipoprotein-modified nanoparticles
can selectively deliver small interfering (si)RNAs to HCC cells
(30). Therefore, siRNAs against
NSUN5 could be developed as a drug to treat HCC using this method
in the future.
Heissenberger et al (27) reported that NSUN5 is an rRNA
methyltransferase that can mediate the m5C modification of the
C3782 position of 28S rRNA in humans and the C3438 position of 28S
rRNA in mice. Notably, in this previous study, NSUN5 knockout
inhibited the proliferation of human cervical carcinoma HeLa cells
and knockout of Nsun5 in the whole mouse body reduced mouse body
weight (27). Mechanistically, the
loss of NSUN5 was reported to have generated a lower m5C level of
28S rRNA, which impaired the functions of ribosomes and translation
of global proteins, thereby decreasing cell proliferation and size
(27). In the present study, the
bioinformatics analysis demonstrated that NSUN5 was significantly
positively correlated with genes associated with ribosomes and
translation in HCC. Therefore, it could be hypothesized that
overexpression of NSUN5 in HCC would upregulate the m5C level of
28S rRNA; therefore, strengthening the functions of ribosomes and
translation of global proteins, and promoting the growth and
migration of HCC. Further experiments need to be performed to
evaluate this hypothesis in the future.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that NSUN5 promoted the
development of HCC. Therefore, it could be hypothesized that NSUN5
may represent a potential therapeutic target in HCC.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82002458 and
82102482), the Shanghai Sailing Program (grant no. 19YF1459600) and
the Shanghai Clinical Research Center for Acupuncture and
Moxibustion (grant no. 20MC1920500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XWZ, HRL, YH and LYW performed the experiments. LYW,
HRL and CFG designed the experiments. QQ, CFG and LiZ collected the
clinical data. LuZ and RZ performed the statistical analysis. LYW
and JY wrote the manuscript. JY, LiZ and HGW conceived the project.
XWZ and JY confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study using human tissues was approved
by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital
(approval no. EHBHKY2018-02-014). Written informed consent was
obtained from each patient according to the policies of the
committee. The animal experiments in this study conformed to the
Animal Research: Reporting of In Vivo Experiments guidelines
(http://www.nc3rs.org.uk/arrive-guidelines) and were
approved by the Institutional Animal Care and Use Committee of
Shanghai University of Traditional Chinese Medicine (approval no.
PZSHUTCM210926007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puisieux MF, Pellat A, Assaf A, Ginestet
C, Brezault C, Dhooge M, Soyer P and Coriat R: Therapeutic
management of advanced hepatocellular carcinoma: An updated review.
Cancers (Basel). 14:23572022. View Article : Google Scholar
|
|
4
|
Gao Y and Fang J: RNA 5-methylcytosine
modification and its emerging role as an epitranscriptomic mark.
RNA Biol. 18:117–127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng JX, Chen L, Li Y, Cloe A, Yue M, Wei
J, Watanabe KA, Shammo JM, Anastasi J, Shen QJ, et al: RNA cytosine
methylation and methyltransferases mediate chromatin organization
and 5-azacytidine response and resistance in leukaemia. Nat Commun.
9:1163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Selmi T, Hussain S, Dietmann S, Heiß M,
Borland K, Flad S, Carter JM, Dennison R, Huang YL, Kellner S, et
al: Sequence- and structure-specific cytosine-5 mRNA methylation by
NSUN6. Nucleic Acids Res. 49:1006–1022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frye M, Harada BT, Behm M and He C: RNA
modifications modulate gene expression during development. Science.
361:1346–1349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janin M, Ortiz-Barahona V, de Moura MC,
Martínez-Cardús A, Llinàs-Arias P, Soler M, Nachmani D, Pelletier
J, Schumann U, Calleja-Cervantes ME, et al: Epigenetic loss of
RNA-methyltransferase NSUN5 in glioma targets ribosomes to drive a
stress adaptive translational program. Acta Neuropathol.
138:1053–1074. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Z, Li S, Han MJ, Hu GM and Cheng P:
High expression of NSUN5 promotes cell proliferation via cell cycle
regulation in colorectal cancer. Am J Transl Res. 12:3858–3870.
2020.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu L, Lau SH, Tzang CH, Wen JM, Wang W,
Xie D, Huang M, Wang Y, Wu MC, Huang JF, et al: Association of
Vimentin overexpression and hepatocellular carcinoma metastasis.
Oncogene. 23:298–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao QF, Yuan SX, Yang F, Yang Y, Yuan JH,
Wang ZG, Xu QG, Lin KY, Cai J, Yu J, et al: Aldolase B inhibits
metastasis through Ten-Eleven Translocation 1 and serves as a
prognostic biomarker in hepatocellular carcinoma. Mol Cancer.
14:1702015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
14
|
Hou G, Chen L, Liu G, Li L, Yang Y, Yan
HX, Zhang HL, Tang J, Yang YC, Lin X, et al: Aldehyde
dehydrogenase-2 (ALDH2) opposes hepatocellular carcinoma
progression by regulating AMP-activated protein kinase signaling in
mice. Hepatology. 65:1628–1644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tandon N, Thakkar KN, LaGory EL, Liu Y and
Giaccia AJ: Generation of stable expression mammalian cell lines
using lentivirus. BIO Protoc. 8:e30732018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Yuan JH, Huang JF, Yang F, Wang TT,
Ma JZ, Zhang L, Zhou CC, Wang F, Yu J, et al: Long noncoding RNA
FTX inhibits hepatocellular carcinoma proliferation and metastasis
by binding MCM2 and miR-374a. Oncogene. 35:5422–5434. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Workman P, Aboagye EO, Balkwill F, Bruder
G, Chaplin DJ, Double JA, Everitt J, Farningham DAH, Glennie MJ,
Kelland LR, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancer Genome Atlas Research Network.
Electronic address, . simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169:1327–1341.e23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao X and Zhang D: The 8th edition
american joint committee on cancer staging for
hepato-pancreato-biliary cancer: A review and update. Arch Pathol
Lab Med. 145:543–553. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma
JZ, Sun SH, Yang F and Zhou WP: Circular RNA cSMARCA5 inhibits
growth and metastasis in hepatocellular carcinoma. J Hepatol.
68:1214–1227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado A, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nelson ME, Lahiri S, Chow JDY, Byrne FL,
Hargett SR, Breen DS, Olzomer EM, Wu LE, Cooney GJ, Turner N, et
al: Inhibition of hepatic lipogenesis enhances liver tumorigenesis
by increasing antioxidant defence and promoting cell survival. Nat
Commun. 8:14689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson CL, Mann DA and Borthwick LA:
Epigenetic reprogramming in liver fibrosis and cancer. Adv Drug
Deliv Rev. 121:124–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas MB, Morris JS, Chadha R, Iwasaki M,
Kaur H, Lin E, Kaseb A, Glover K, Davila M and Abbruzzese J: Phase
II trial of the combination of bevacizumab and erlotinib in
patients who have advanced hepatocellular carcinoma. J Clin Oncol.
27:843–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun HC, Zhu XD, Huang C, Shen YH and Fan
J: Initially unresectable hepatocellular carcinoma treated by
combination therapy of tyrosine kinase inhibitor and anti-PD-1
antibody followed by resection. J Clin Oncol. 38:e16690. 2020.
View Article : Google Scholar
|
|
27
|
Heissenberger C, Liendl L, Nagelreiter F,
Gonskikh Y, Yang G, Stelzer EM, Krammer TL, Micutkova L, Vogt S,
Kreil DP, et al: Loss of the ribosomal RNA methyltransferase NSUN5
impairs global protein synthesis and normal growth. Nucleic Acids
Res. 47:11807–11825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen P, Zhang T, Yuan Z, Shen B and Chen
L: Expression of the RNA methyltransferase Nsun5 is essential for
developing cerebral cortex. Mol Brain. 12:742019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan Z, Chen P, Zhang T, Shen B and Chen
L: Agenesis and hypomyelination of corpus callosum in mice lacking
Nsun5, an RNA methyltransferase. Cells. 8:5522019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hajiasgharzadeh K, Somi MH, Shanehbandi D,
Mokhtarzadeh A and Baradaran B: Small interfering RNA-mediated gene
suppression as a therapeutic intervention in hepatocellular
carcinoma. J Cell Physiol. 234:3263–3276. 2019. View Article : Google Scholar : PubMed/NCBI
|