Introduction
Collisional tumors are primary tumors in which two
or more separate tissue origins coexist in the same anatomical site
and are tightly adherent or partially enveloped. However, their
diseased tissues are not intermingled (1). The ovaries are less commonly affected
by collisional tumors than other organs, such as the digestive and
urinary tract organs (2). Ovarian
collision tumors lack identifiable clinical and imaging signs and
are difficult to distinguish from other tumors, even with
intraoperative visualization, due to their complex pathological
composition (3). With the
development of pathological immunohistochemistry techniques, the
accuracy rate of correct postoperative pathological diagnoses has
been steadily increasing. However, knowledge about this type of
tumor remains limited, particularly with regard to unusual
pathological types. The ovary consists of three cell types:
Epithelial cells, germ cells and mesenchymal cells. Random
combinations of these three cell-derived tumors may generate a
variety of collision tumors, the most common of which are
combinations of epithelial and germ cell tumors, while the
incidence of other combination types is lower (4). To the best of our knowledge, the
collision tumor presented in the current study is the first of its
kind, comprising a sclerosing stromal tumor and a mature cystic
teratoma. The purpose of the present report is to analyze the
pathologic and imaging data of this rare ovarian collision tumor.
It is esteemed that sharing the experience of this rare tumor type
will assist clinicians in choosing the right treatment.
Case report
A 55-year-old female patient presented at the First
People's Hospital of Zunyi (Guizhou, China) in August 2021, where a
large mass in the right lower abdomen during an abdominal
ultrasound examination of a physical examination requested by the
patient after retirement. Physical examination indicated mild
tenderness in the right lower abdomen. Carbohydrate antigen 125
(CA125; 1,247.7 U/ml) was tens of times higher than the normal
reference value (0–35 U/ml), but CA19-9 (8.60 ku/l) and
α-fetoprotein (1.56 µg/l) remained within the normal reference
range (CA19-9: 0–40 ku/l and α-fetoprotein: 0–20 µg/l). The
patient's serum sex hormone levels were within normal ranges. The
patient became menopausal at the age of 52 years and denied having
any remarkable medical personal or family history.
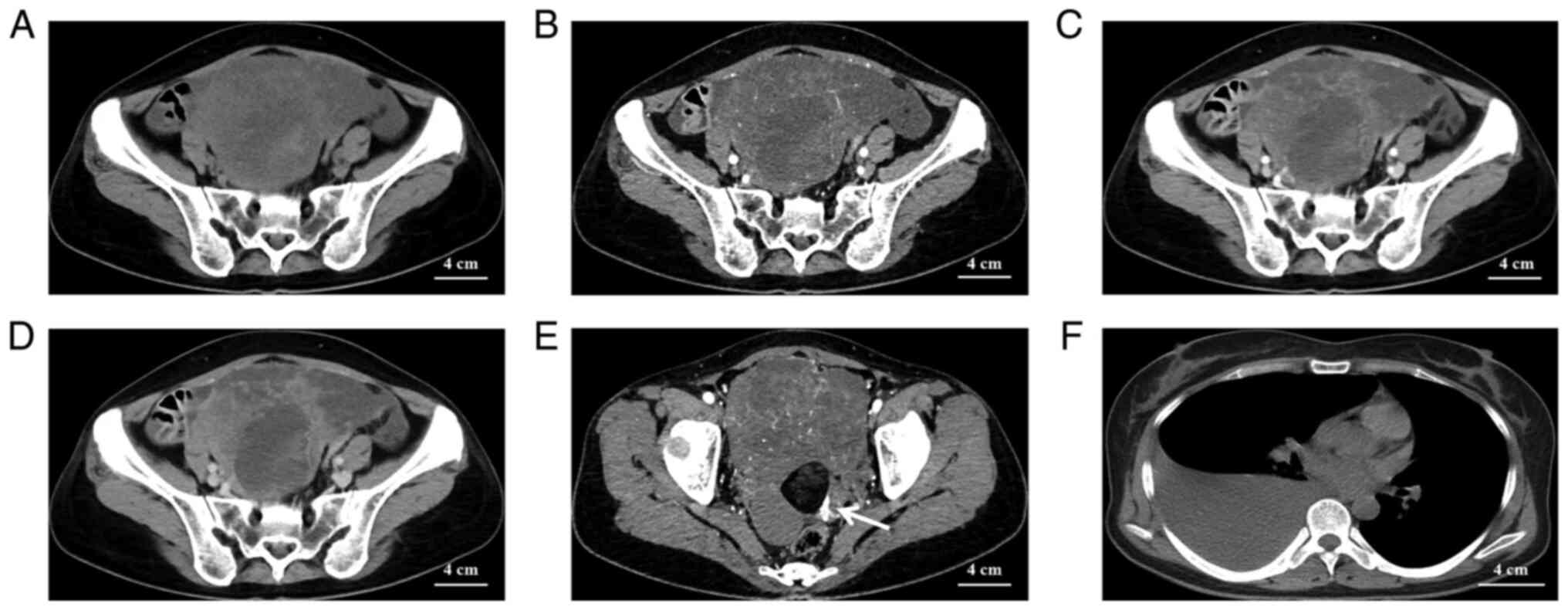
The CT scan of the pelvis revealed a large
mixed-density mass of 12×11×10 cm, with organs around the lesion
pushed away (Fig. 1A). The tumor
parenchymal section exhibited progressive enhancement (Fig. 1B-D). A thick left uterine artery
was seen supplying blood to the mass in the left posterior aspect
of the entire mass (Fig. 1E). In
the right posterior part of the whole mass, fluid-dense and
fat-dense masses were seen and no significant enhancement was
observed on enhancement CT (Fig.
1E). Finally, a large amount of pleural fluid was observed on
the right side of the patient's chest cavity on the CT plain
examination of the chest, while there was no fluid in the left
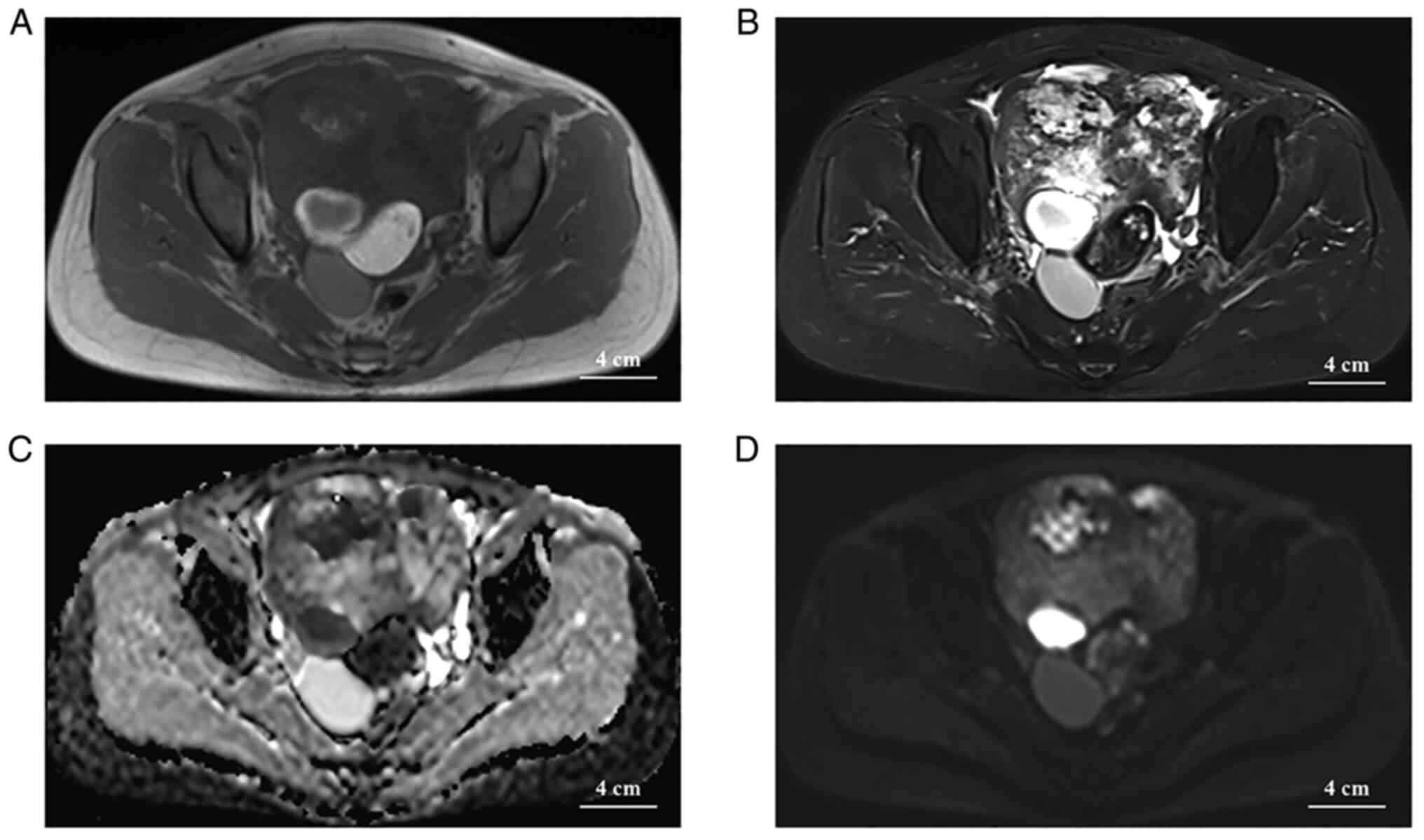
(Fig. 1F). On MRI, a vast
mixed-signal mass in the pelvis with an intact envelope was seen in
the pelvis, with a predominant hypersignal on T2-weighted imaging
(T2WI), a cystic necrotic area or edematous area was observed
within the mass and the parenchymal portion of the mass exhibited
an isosignal on T1WI and a slight hypersignal on T2WI (with the
surrounding muscle tissue signal as an isosignal reference). In
addition, liquid and fat signals were observed in the right back of
the mass, with clear boundaries, and the fat portion of the mass
had significantly lower signals on T2WI (Fig. 2A and B). The necrotic or edematous
area of the cystic lesion had a hypersignal (b=1,000
sec/mm2) on diffusion-weighted imaging (DWI) and a
hyposignal in the apparent diffusion coefficient (ADC), and the
parenchymal portion displayed as an iso-/hypersignal on DWI and an
iso-/hypersignal in the ADC (Fig. 2C
and D). No enlarged lymph nodes were seen in the pelvis or
groin. At the time, radiologists initially considered ovarian
cystic adenocarcinoma with mature cystic teratoma and thickening of
the peritoneum in both the abdominal and thoracic cavity effusion,
suggesting metastasis based on the patient's age and imaging.
However, subsequent thoracentesis revealed that the patient's right
pleural effusion was a slightly turbid, yellowish exudative fluid
with a total protein concentration of 40.3 g/l and no cancer cells
were found. However, it was not possible to rule out the
possibility of other malignancies and teratomas combined. After a
multidisciplinary discussion, it was finally decided that the best
treatment strategy was to perform surgery with intraoperative
pathological frozen biopsy.
The patient then underwent laparoscopic hysterectomy
and bilateral oophorectomy. The surgical specimen had two
contiguous masses, the majority of which consisted of grayish
flesh-like tissue and the remainder of which consisted of fatty
fluid and hair. Intraoperative pathological frozen section findings
indicated tumors of the interstitial origin of the sex cords.
Postoperative immunohistochemical staining (5) using antibodies from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. revealed positivity for
Vimentin (cat. no. ZM-0260; prediluted by the manufacturer), smooth
muscle actin (cat. no. ZM-0003; prediluted by the manufacturer) and
Wilms' tumor protein (WT-1; cat. no. ZM-0269; prediluted by the
manufacturer), negativity for S-100 protein (cat. no. ZM-0224;
prediluted by the manufacturer) and epithelial markers and a low
Ki-67 (cat. no. ZM-0166; prediluted by the manufacturer)
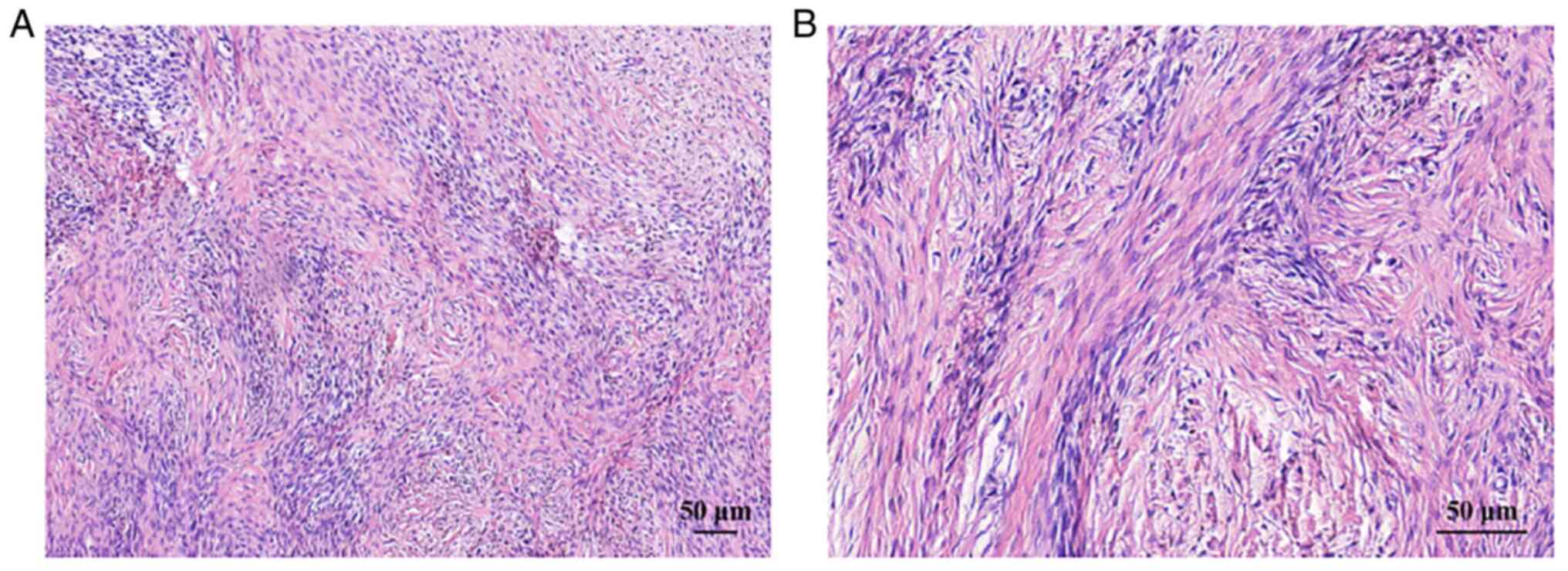
proliferation index (~3%). Histopathological analysis (6) of the mass revealed an alternating
pseudolobular pattern of a cellular zone composed of ovoid
vacuolated oocytes and a hypocellular zone consisting of dense
collagenous tissue (Fig. 3A and
B). The final pathological diagnosis was a sclerosing stromal
tumor of the ovary with a mature cystic teratoma. The patient
received postoperative anti-infection treatment, vital signs were
stable and the patient recovered well. The abdominal and pleural
effusion disappeared within 15 days after surgery and CA125 (cat.
no. ZM-0019; prediluted by the manufacturer) decreased
significantly (315.50 vs. 1,247.70 U/ml prior to surgery). The
patient was followed up for 12 months is now in a healthy condition
with good treatment results, and no recurrence or metastasis was
detected during an abdominal ultrasound examination in the last
month.
Discussion
Ovarian collision tumors are rare tumors in which
there is no mixture of tumor cells or tissues and they are
separated by their respective stroma (1,2). It
is important to note that ovarian collision tumors must be primary
tumors of ovarian origin and not secondary tumors; for instance,
ovarian cancer secondary to lymphoma and mature cystic teratoma
secondary to squamous cell carcinoma are not considered to be
ovarian collision tumors (7,8). To
date, the pathogenesis of collisional tumors has remained elusive;
however, there are several hypotheses: i) A mere chance encounter
of two different types of tumors at the same anatomical site
(9); ii) the occurrence of other
different types of tumors due to changes in the surrounding
microenvironment caused by the presence of the first type of tumor
(10); iii) genetically
homogeneous clonal cells that differentiate into two different
histological types of tumor cells (11).
Ovarian collision tumors most frequently occur in
middle-aged females. Clinical manifestations of ovarian collision
tumors are nonspecific and certain patients do not have any
clinical manifestations. Certain patients may complain of
intermittent abdominal pain, bloating and frequent urination due to
large lumps compressing the bladder (1,12),
while others may experience ascites and dysfunctional uterine
bleeding, although this is not common (13). The biological behavior of ovarian
collision tumors depends on the type of tumor. If the composed
tumor contains a malignant tumor, it will exhibit that tumor's
biological behavior, such as infiltration into adjacent tissues and
organs and distant metastasis. However, in the present case, the
collision tumor consisted of an ovarian sclerosing stromal tumor
(OSST) and a mature cystic teratoma, both of which belonged to the
category of benign tumors, and lacked the biological
characteristics frequently observed in malignant tumors. OSST is a
rare benign tumor of the ovary that was first reported in 1973
(14). It belongs to the category
of ovarian gonadal mesenchymal tumors and has an incidence of
<5%, with the highest incidence in females aged 25–29 years
(15). By contrast, the patient of
the present study was a 53-year-old menopausal female. The
diagnosis of OSST is rare in postmenopausal women. This may be
because symptoms associated with the menstrual cycle in older women
may be masked by the patient's menopause, and various common
conditions may cause nonspecific abdominal pain in the older
population, making the diagnosis of OSST in older women more
difficult (16). OSST has an
abundant blood supply but is a benign tumor that may be combined
with elevated CA125 and Meigs syndrome (pleural and ascites fluid
disappeared soon after tumor resection). Mature cystic teratoma is
one of the most prevalent benign ovarian tumors. It grows slowly,
usually has an intact envelope, rarely invades adjacent structures
and may be treated surgically (17).
OSST has a weak sex hormone secretion function and
its serum sex hormone indicators are usually in the normal range.
Rarely, it may cause elevated levels of estrogen, testosterone and
luteinizing hormone, which may result in corresponding clinical
symptoms, such as menstrual disorders, infertility and abnormal
uterine bleeding, but not all OSSTs have increased hormones
(14). The patient of the present
study displayed no obvious clinical symptoms. In addition, the
patient was detected as having ascites and a right pleural
effusion. According to the initial hypothesis of Meigs, ascites and
pleural fluid may arise because the benign ovarian tumor irritates
the peritoneal surface, causing increased peritoneal exudation, and
the increased ascites flow into the thoracic cavity through the
orifice on the diaphragm because the channels are more developed on
the right side and there is a greater chance of pleural effusion on
the right side than on the left side (18); the case of the present study
supports this hypothesis. However, there is no conclusive evidence
as to the cause of Meigs syndrome. Furthermore, the patient's CA125
levels were also substantially higher than normal. Elevated CA125
is caused by peritoneal and omental expression, not benign tumor
expression (19). In addition,
Liou et al (20) discovered
a substantial positive association between abdominal water volume
and CA125 levels in patients with benign ovarian tumors combined
with Meigs syndrome and increased CA125, regardless of tumor size.
This may be because the peritoneum was mechanically stimulated,
causing ascites and CA125 secretion. Finally, the patient of the
present study was positive for WT-1, which is encoded by the WT1
gene on chromosome 11 and has an important role in the development
of the normal urogenital tract and is highly expressed in 77% of
stromal tumors (21). Currently,
WT-1 is frequently used as an adjuvant marker to detect ovarian sex
cord stromal tumors; therefore, immunohistochemical staining
indicating positive WT-1 may also support the tumor being
mesenchymal in origin.
The imaging appearance of ovarian collision tumors
is dependent not only on the imaging appearance of the individual
tumors of which they are composed but also on the pattern of
collision. In the present case, a substantial portion of the OSST
and the necrotic cystic and edematous areas were iso-intense and
slightly hypo-intense on CT, respectively. The tumor signal is more
uniform on T1WI because the fibrous tissue, cystic lesion and
edematous part inside the tumor are hyposignal or isosignal on
T1WI. By contrast, the tumor signal on T2WI is mixed and uneven,
and areas of hypersignal within the tumor do not exclusively
represent areas of necrotic cystic lesions but may also be sparse
edematous connective tissue. The parenchymal portion of the tumor
exhibited a hypersignal on DWI (b=1,000 sec/mm2),
suggesting a high cell density, which is consistent with the
abundance of tumor cells in the pseudolobules seen on pathology.
The ADC signal of the tumor is predominantly hypersignal,
suggesting to a certain extent that the tumor is benign, while the
ADC value of malignant tumors is usually low, which helps
differentiate it from malignant ovarian tumors. Enhanced scans
indicated a marked enhancement in the solid portion in the arterial
phase because of the rich vascularity of the tumor and delayed
enhancement in the venous phase due to the rich collagen fiber
component inside, showing an enhancement pattern similar to that of
cavernous hemangioma of the liver, while the cystic region was not
enhanced. A thick and tortuous uterine artery was seen on the left
side of the tumor to supply it, revealing the blood-rich properties
of OSST. A cystic fluid-dense and fat-dense mass was seen within
the whole mass on the right posterior side, which was surrounded by
a massive tumor in front. After contrast enhancement, no
significant enhancement was seen in this part of the mass. The
fatty part of these masses exhibited a significant hyposignal on
the T2 fat suppression sequence image. In addition, ascites were
observed in the periphery of the tumor, but no enlarged lymph nodes
were present, which was consistent with the biological behavior of
this tumor.
The differential diagnosis requires to be discussed
concerning different ovarian collision tumors among themselves and
with nonovarian collision tumors. The imaging appearance of ovarian
collision tumors relies on the compositional origin of the tumor.
The most frequent combination is an epithelial tumor with a germ
cell tumor, and the majority of reported epithelial tumors are
mucinous or serous cystadenoma (4). Mucinous cystadenoma usually present
as a multilocular cystic mass. The tumor displays with a
hypersignal on T1WI and T2WI due to the presence of mucin. Serous
cystadenomas are smaller than mucinous cystadenomas, but they
frequently have calcifications and papillary soft tissue
projections on the cyst wall and are more malignant than mucinous
cystadenomas. Imaging may easily diagnose both. Ovarian serous
cystadenoma usually deteriorates to form serous cystadenocarcinoma,
which has distinct papillary protrusions on the cyst wall and
uneven wall thickness compared to serous cystadenoma, with
significant enhancement of the septum, cyst wall and the
parenchymal portion of the mass enhancement. It is difficult to
identify specific masses on imaging when their imaging presentation
is atypical. The combination of an epithelial tumor with a sex-cord
stromal tumor is rare. Granulosa cell tumors in the interstitial
cell tumors of the sex cords produce sex hormones and may cause a
range of endocrine-related clinical symptoms. The imaging
presentation is varied and may be large multilocular cystic, cystic
solid or solid masses that require hormone levels and clinical
presentation for diagnosis. Other types of stromal cell tumors,
such as ovarian fibrous tumors and Sertoli-Leydig cell tumors, are
challenging to diagnose by imaging alone and ultimately require
pathology to confirm the diagnosis.
Ovarian collision tumors are easily confused with
common mixed and compound tumors of the ovary, which are defined
pathologically in completely different ways (22). Mixed tumors have various histologic
components from the same stem cell that are mixed at the tissue
level, such as mixed germ cell tumors, which appear as a single
mass with no separation (23).
Complex tumors have a mixture of two cell types of different tissue
origins within them, with no clear separation between them, and the
two distinct components are indistinguishable. Imaging alone is not
adequate to make a conclusive diagnosis.
As ovarian collision tumors have multiple origins,
benign tumors may be removed surgically, but malignant tumors may
require surgery, radiation and chemotherapy, and different tumors
require different treatment options. Once diagnosed, surgical
excision is sufficient for OSST and mature cystic teratoma. Since
benign and malignant tumors are treated differently, preoperative
diagnosis is crucial to avoid causing unnecessary damage to the
patient by rashly broadening the scope of surgery. When clinicians
encounter patients with ovarian cystic solid masses combined with
ascites and elevated CA125, they should not limit their diagnosis
to malignancy, particularly when the masse's enhancing pattern on
imaging is similar to cavernous hepatic hemangioma, and should also
consider OSST combined with Meigs sign and elevated CA125. Both
mature cystic teratoma and OSST are benign tumors. No local or
distant metastases or recurrences have been reported. The patient
had a regular follow-up examination 1 year after surgery and no
recurrence or distant metastasis was detected in this patient.
In summary, the present study reported the first
case of an ovarian collision tumor consisting of a sclerosing
stromal tumor combined with a mature cystic teratoma and its
imaging presentation. It is esteemed that this report will enhance
the knowledge of this disease among radiologists and further assist
clinicians in developing optimal treatment plans.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JRZ designed the study and wrote the manuscript. LH,
LY, JW and LW performed all of the experiments. LJ and XJM were
responsible for the collection and analysis of case data and
literature and confirmed the authenticity of all the raw data. All
authors agreed to the journal to which the article was submitted
and agreed to take responsibility for all aspects of the work. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First People's Hospital of Zunyi (Zunyi, China; approval no.
202112021). The patient provided written informed consent.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim SH, Kim YJ, Park BK, Cho JY, Kim BH
and Byun JY: Collision tumors of the ovary associated with
teratoma: Clues to the correct preoperative diagnosis. J Comput
Assist Tomogr. 23:929–933. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bige O, Demir A, Koyuncuoglu M, Secil M,
Ulukus C and Saygili U: Collision tumor: Serous cystadenocarcinoma
and dermoid cyst in the same ovary. Arch Gynecol Obstet.
279:767–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weigel J, Neher M, Schrey M, Wünsch PH and
Steiner HH: Collision tumor composed of meningioma and cavernoma. J
Korean Neurosurg Soc. 60:102–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng Y, Lin J, Guan J, Chen L, Zhang X, Li
S, Wang H, Liu M and Guo Y: Ovarian collision tumors: Imaging
findings, pathological characteristics, diagnosis, and differential
diagnosis. Abdom Radiol (NY). 43:2156–2168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Serpooshan V, Wu S, Demirci U, Chen
P and Güven S: Tissue engineering of 3D organotypic microtissues by
acoustic assembly. Methods Mol Biol. 1576:301–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roskell DE and Buley ID: Histopathological
assessment of metastasis. Methods Mol Biol. 878:51–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singhal N, Quilty S, George M, Davy M and
Selva Nayagam S: A tale of two cancers: Collision presentation of
ovarian carcinoma and lymphoma. Aust N Z J Obstet Gynaecol.
49:232–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allam-Nandyala P, Bui MM, Caracciolo JT
and Hakam A: Squamous cell carcinoma and osteosarcoma arising from
a dermoid cyst-a case report and review of literature. Int J Clin
Exp Pathol. 3:313–318. 2010.PubMed/NCBI
|
|
9
|
Brandwein-Gensler M, Urken M and Wang B:
Collision tumor of the thyroid: A case report of metastatic
liposarcoma plus papillary thyroid carcinoma. Head Neck.
26:637–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez FJ, Scheithauer BW, Fourney DR
and Robinson CA: Ependymoma and intraparenchymal calcifying
pseudoneoplasm of the neural axis: Incidental collision or unique
reactive phenomenon? Acta Neuropathol. 115:363–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii H, Zhu XG, Matsumoto T, Inagaki M,
Tokusashi Y, Miyokawa N, Fukusato T, Uekusa T, Takagaki T, Kadowaki
N and Shirai T: Genetic classification of combined
hepatocellular-cholangiocarcinoma. Hum Pathol. 31:1011–1017. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo EJ, Kwon HJ and Shim SI: Ovarian
serous cystadenoma associated with Sertoli-Leydig cell tumor-a case
report. J Korean Med Sci. 11:84–87. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy S, Mukhopadhayay S, Gupta M and
Chandramohan A: Mature cystic teratoma with co-existent mucinous
cystadenocarcinoma in the same ovary-a diagnostic dilemma. J Clin
Diagn Res. 10:ED11–ED13. 2016.PubMed/NCBI
|
|
14
|
Chalvardjian A and Scully RE: Sclerosing
stromal tumors of the ovary. Cancer. 31:664–670. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Li H, Li L, Zhang H, Zhang T and
Liu X: Sclerosing stromal tumor with marked atypia that mimics an
undifferentiated sarcoma. Pathol Int. 70:53–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim TH, Lee HH, Hong JA, Park J, Jeon DS,
Lee A and Koh ES: Sclerosing stromal tumor in an elderly
postmenopausal woman. J Menopausal Med. 20:80–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel IJ, Hsiao E, Ahmad AH, Schroeder C
and Gilkeson RC: AIRP best cases in radiologic-pathologic
correlation: Mediastinal mature cystic teratoma. Radiographics.
33:797–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meigs JV: Fibroma of the ovary with
ascites and hydrothorax; Meigs' syndrome. Am J Obstet Gynecol.
67:962–985. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buttin BM, Cohn DE and Herzog TJ: Meigs'
syndrome with an elevated CA 125 from benign Brenner tumors. Obstet
Gynecol. 98:980–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liou JH, Su TC and Hsu JC: Meigs' syndrome
with elevated serum cancer antigen 125 levels in a case of ovarian
sclerosing stromal tumor. Taiwan J Obstet Gynecol. 50:196–200.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marchevsky AM: Application of
immunohistochemistry to the diagnosis of malignant mesothelioma.
Arch Pathol Lab Med. 132:397–401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SB, Kim JK, Kim KR and Cho KS:
Imaging findings of complications and unusual manifestations of
ovarian teratomas. Radiographics. 28:969–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rha SE, Byun JY, Jung SE, Kim HL, Oh SN,
Kim H, Lee H, Kim BK and Lee JM: Atypical CT and MRI manifestations
of mature ovarian cystic teratomas. AJR Am J Roentgenol.
183:743–750. 2004. View Article : Google Scholar : PubMed/NCBI
|