Introduction
Gastric cancer is the fifth most common cancer type
worldwide, following lung, breast, colorectal and prostate cancers.
Its incidence has declined since the mid-20th century, but it
remains the third leading cause of cancer-associated mortality,
with a 5-year survival rate of only 29% (1–4).
Multiple studies have demonstrated that surgery alone for gastric
cancer decreases the survival and increases the recurrence rate
compared with multimodal therapy (5–8).
Therefore, there is an urgent need for novel methods to predict the
efficacy of individual treatments (6–9).
The development of organoids is an important
challenge to overcome for establishing in vivo and in
vitro patient-derived personalized medicine platforms. While
cancer cell lines have been valuable in basic cancer research,
these models have the significant disadvantage of bearing little
resemblance to the patient tumor (7–11).
The development of high-throughput analytical methods now allows
addressing the clinical relevance of these human cancer-derived
cell lines. At the genomic level, driver mutations may be retained
in cancer cell lines. However, certain studies have revealed shifts
at the transcriptome level, suggesting that cancer cell lines are
more similar to each other than the clinical samples from which
they were originally derived. These shortcomings may be resolved by
establishing organoids (10–14).
The application of cancer organoids is of great
value in individualized therapy as an embodiment of the tumor of a
patient (15). Individual cancer
organoids may be used to predict the therapeutic response to
certain drugs, and the establishment of large patient-derived
organoid (PDO) biobanks with combined drug screening may help to
describe new therapeutic strategies for gastric cancer (16). Previously, research teams have
successively reported the establishment of gastric-related
organoids for research purposes (17–19).
In the present study, the establishment of gastric
cancer organoid models was explored using a new organoid culture
system to prolong the culture time of gastric cancer primary cells.
Furthermore, organoids were cultivated under three-dimensional (3D)
conditions to provide an experimental model for follow-up studies
of individualized treatment of patients.
Materials and methods
Tissue source
Gastric cancer tissues were collected from 3
patients with gastric cancer, who underwent primary surgery at The
First Hospital of Lanzhou University (Lanzhou, China) from July to
August 2021.
Inclusion criteria were preoperative pathologically
confirmed gastric cancer in patients over 18 years of age and
informed consent. The exclusion criteria were as follows: Patients
who received radiotherapy and/or chemotherapy prior to surgery or
did not agree to participate in the experiment.
The clinical and pathological data are as follows:
Patient 1 was a 75-year-old male, cardia cancer, moderately
differentiated; Patient 2 was a 58-year-old male, gastric antrum
cancer, poorly differentiated; Patient 3 was a 74-year-old male,
gastric antrum cancer, moderately-poorly differentiated. After
surgery, this patient received six cycles of systemic chemotherapy
(XELOX).
The present study was approved by the Ethics
Committee of the First Hospital of Lanzhou University (Lanzhou,
China; approval no. LDYYLL2022-292) and written informed consent
was obtained from the patients.
Main reagents and instruments
The 24-well culture plates were purchased from
Corning, Inc. DMEM/F12 medium, fetal bovine serum and
cryopreservation solution were purchased from Biological
Industries. GlutaMax, TrypLE, collagenase II and dispase II were
from Gibco (Thermo Fisher Scientific, Inc.). The light microscope
was from Nikon Corporation.
Preparation of the media
The complete medium consisted of DMEM/F12
supplemented with 1X GlutaMax, 10% fetal bovine serum and 1%
penicillin and streptomycin. The tissue transport medium comprised
DMEM/F12 with 1% penicillin and streptomycin. The mixed digestive
enzymes medium comprised DMEM/F12 with 1 mg/ml collagenase II and
1.5 mg/ml dispase II.
Organoid establishment
The principle of aseptic operation was strictly
followed. First, an appropriate amount of fresh tumor tissue (~5
mm2) was cut according to the size of the tumor and
stored in the transport solution. The retrieved cancer tissue
samples were placed in a 60-mm petri dish and washed with PBS to
remove blood clots, as well as necrotic and fibrous components. A
sterile surgical blade was then used to excise a part of the tumor
tissue and a portion of the sample was frozen in a −80°C
refrigerator for subsequent experiments. The remaining tissue (~0.3
cm3) was minced as much as possible, added to a 15-ml
centrifuge tube, mixed with 10 ml digestive enzymes solution and
placed in a shaker to be digested for ~40 min at 37°C. During this
period, the digestion of the tissue block was closely observed.
Once the tissue volume was reduced by half, the digestion solution
was filtered through a 100-mesh cell sieve, collected into a 15-ml
centrifuge tube and centrifuged at 400 × g for 4 min at 4°C, after
which the supernatant was discarded. The centrifugation step was
repeated twice, the cell pellet was collected and the cells were
resuspended in 300 µl complete culture medium. The cell suspensions
of 2 patients were individually mixed with 300 µl
Matrigel® (BD Pharmingen) at a ratio of 1:1 and then
inoculated into 24-well culture plates (cat. no. 3527; Corning,
Inc.) at 100 µl/well for organoid culture for 3 weeks. For the
third case, the cell suspension of 6 ml was directly inoculated
into a 24-well ultra-low adhesion plate (cat. no. 3473; Corning,
Inc.) at 1 ml/well and organoids were formed by suspension culture
at 37°C in a humidified atmosphere containing 5%
CO2.
Passaging, cryopreservation and
recovery of organoids
The organoids were passaged when they reached a size
of 100 µm under the microscope. The organoids and complete medium
to be passaged were transferred to a 15-ml centrifuge tube, an
appropriate amount of PBS was added, and the suspension was gently
mixed with a pipette tip and then centrifuged at 400 × g for 4 min
at 4°C, before the supernatant was removed and the pellet
collected. Subsequently, ~2 ml TrypLE was added to the pellet and
the suspension was gently mixed with a pipette to disperse the
clumps, followed by digestion at 37°C while maintaining microscopic
observation until the organoids were enzymatically digested to a
small cell clump state of 3–5 cells. When terminating the
digestion, 5 ml complete medium was added to the cell pellet, which
was gently mixed with a pipette tip and centrifuged at 400 × g for
4 min at 4°C before removing the supernatant and collecting the
pellet. The passage ratio was 1:2-1:3. A part of the organoids was
added to the freezing medium and frozen at a density of
~1×106 cells/ml; they were placed in a gradient freezing
box at −80°C overnight and then transferred to liquid nitrogen for
long-term storage. Prior to subsequent experiments, the
cryopreservation tube was placed in a 37°C water bath. After
thawing, the sample was quickly transferred to a 15-ml centrifuge
tube, 5 ml complete medium was added, and the cell suspension was
mixed by passing it through a pipette three times.
Preparation of embedded organoid
sections
The organoids cultured in suspension were separated
by centrifugation at 400 × g for 4 min at 4°C, immersed in 4%
paraformaldehyde solution at 37°C for 24 h and then dehydrated with
an ethanol gradient (50, 70, 80, 95, 100% ×2; 30 min for each
gradient step). Subsequently, the dehydrated organoids were placed
into 50% xylene and 50% ethanol for 30 min and immersed in pure
xylene for 1 h twice at 37°C. A total of 200 organoids were
agar-embedded, cut into thin slices with a thickness of ~5 µm,
placed onto glass slides and dried.
Hematoxylin and eosin (H&E)
staining
The slices of the aforementioned steps were placed
in xylene I for 10 min, xylene II for 10 min, anhydrous ethanol I
for 5 min, anhydrous ethanol II for 5 min, 95% ethanol for 5 min,
90% ethanol for 5 min, 80% ethanol for 5 min, 70% ethanol for 5 min
and then distilled water for washing at 37°C. The sections were
then stained with Harris hematoxylin for 3–8 min at 37°C, washed
with tap water, differentiated with 1% hydrochloric acid ethanol
for several seconds, rinsed with tap water, incubated in 0.6%
ammonia water for nuclear staining, and rinsed with running water.
The sections were then stained in eosin staining solution at 37°C
for 1–3 min. Thereafter, the slices were placed for 5 min each into
95% ethanol I, 95% ethanol II, absolute ethanol I, absolute ethanol
II, xylene I and xylene II to dehydrate and generate transparent
slices at 37°C. After the last step, the slices were removed from
xylene and dried before being sealed with neutral gum. Finally, the
slides were examined under a light microscope (S40-Slider; Leica
Microsystems) and images were acquired.
Immunohistochemistry
A total of 500-1,000 PDO 3D spheres were centrifuged
at 500 × g for 5 min at 4°C, the supernatant was discarded, 4%
paraformaldehyde was added, and spheres were fixed overnight at
25°C. Subsequently, they were dehydrated with a graded ethanol
series, cleared with xylene for 1 h and soaked in paraffin
(temperature, <50°C) twice, each for 1 h. After embedding,
serial sections were made with a thickness of 3 µm. Another part of
the fresh gastric cancer tissue removed by surgery was immersed in
formaldehyde solution and then routinely fixed, dehydrated,
embedded and sliced. The PDO sections and primary tumor sections
were placed in a 65°C oven for 30 min, dewaxed with xylene,
hydrated with a gradient of ethanol, processed with 0.5 mol/l
sodium citrate antigen retrieval solution under high pressure at
98°C for 5 min and naturally cooled to 25°C. The sections were then
blocked with 3% bovine serum albumin (Wuhan Servicebio Technology
Co., Ltd.) at 25°C for 15 min and then incubated with primary
antibodies against human P53, Ki-67, cytokeratin low molecular
weight (CKL) and cytokeratin (CK)18 (Table I) at 4°C overnight. Subsequently,
the sections were incubated with 3% hydrogen peroxide at 25°C for
15 min, horseradish peroxidase-conjugated goat anti-mouse IgG (cat.
no. GB23303; 1:200 dilution; Wuhan Servicebio Technology Co., Ltd.)
at 25°C for 15 min and horseradish peroxidase-labeled streptavidin
(cat. no. A0303; 1:500 dilution; Beyotime Institute of
Biotechnology) at 25°C for 15 min, and the samples were then washed
three times with PBS for 3 min. After color development with
3,3′-diaminobenzidine, the nuclei were stained with hematoxylin at
37°C for 150 sec, then dehydrated with a gradient concentration of
ethanol, made transparent with xylene and observed under a light
microscope (IX73+DP74; Olympus Corporation).
 | Table I.Primary antibodies used. |
Table I.
Primary antibodies used.
| Antibody | Supplier | Catalogue
number | Dilution |
|---|
| P53 | Wuhan Servicebio
Technology Co., Ltd. | GB111740 | 1:200 |
| CK18 | Wuhan Servicebio
Technology Co., Ltd. | GB11232 | 1:600 |
| Ki-67 | Wuhan Servicebio
Technology Co., Ltd. | GB111499 | 1:400 |
| CKL | Fuzhou Maixin
Biotech Co., Ltd. | MAB-0051 | 1:300 |
Chemotherapy drug screening
The organoids cultured for 1 week were centrifuged
and the pellet was obtained and resuspended in complete medium as
previously mentioned. Subsequently, the cell suspension was seeded
into 24-well ultra-low adhesion plate (cat. no. 3473; Corning,
Inc.) at 0.5 ml/well (~500 organoids), and 0.5 ml complete medium
was added to top up the total liquid volume to 1 ml per well. The
experiment consisted of one control group and three experimental
groups. In the control group, the same concentration of solvent was
used. In the experimental groups, the commonly used chemotherapy
drugs for gastric cancer, paclitaxel (Jiangsu Osaikang
Pharmaceutical Co., Ltd.), oxaliplatin (Jiangsu Hengrui
Pharmaceutical Co., Ltd.) and fluorouracil (Tianjin Jinyao
Pharmaceutical Co., Ltd.), were used. At 24 h after inoculation,
chemotherapy drugs were added to each experimental group. According
to the relevant literature data (20,21),
the final concentrations were 20 ng/ml for paclitaxel, 20 µM for
oxaliplatin and 384 µM for fluorouracil. The morphological changes
of the organoids (morphology, structure, size and quantity) were
then observed under a light microscope (S40-Slider; Leica
Microsystems) and compared every 24 h for 96 h.
Results
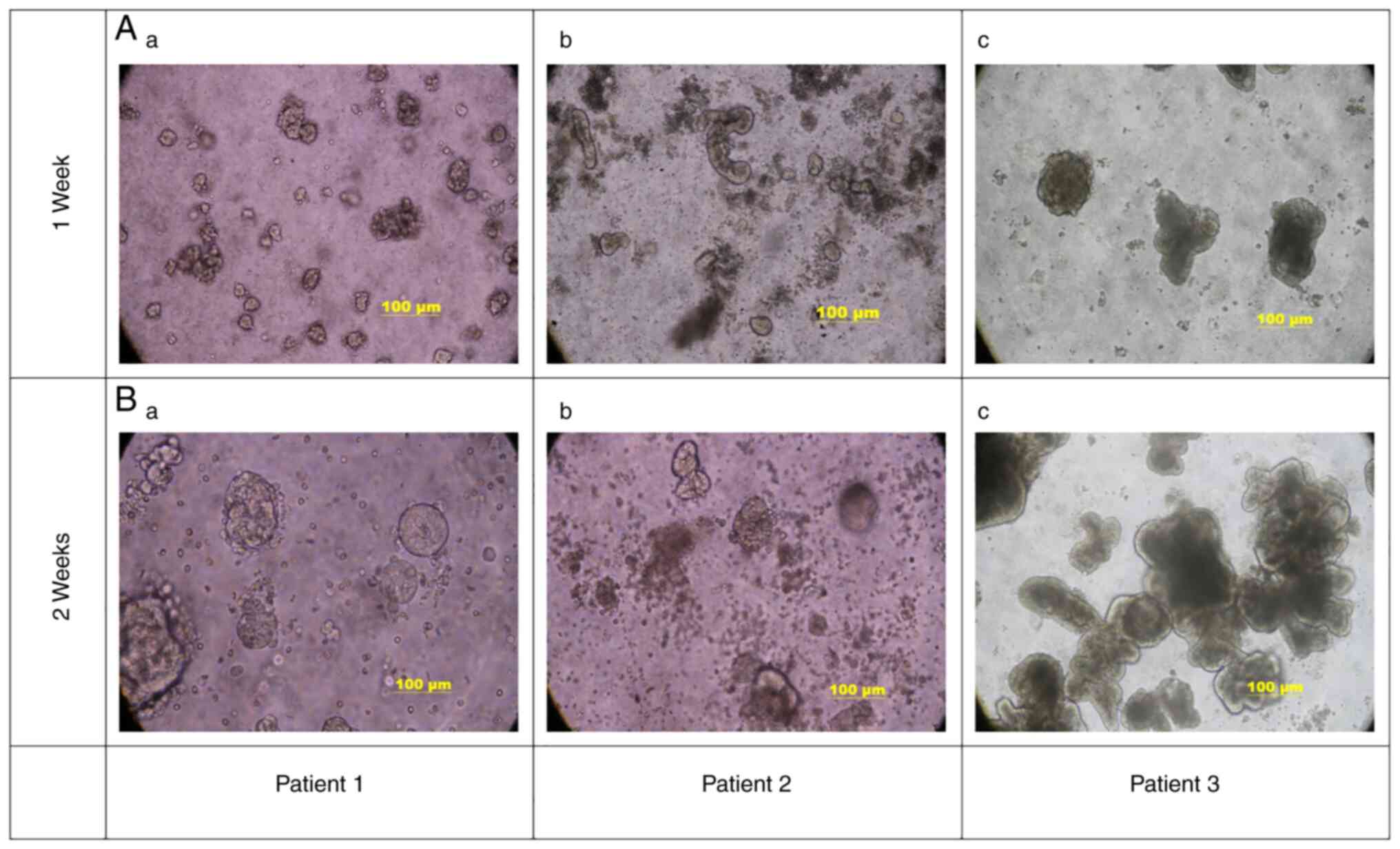
Establishment of gastric cancer
organoids
After the gastric cancer tissues from 3 patients
were individually digested with mixed enzymes, the organoids were
successfully established by gradually increasing from a small
granular type (Fig. 1Aa) to single
spherical or multiple spore-like structures of different sizes
(Fig. 1B). This culturing process
took between 7 and 21 days when organoids reached a size of 100 µm
under the microscope, depending on the characteristics of the tumor
itself.
Under 2D culture conditions, gastric cancer cells
exhibited a typical epithelioid cell morphology and
cobblestone-like adherent growth, whereas the cell morphology was
mainly of the short-spindle and round type (Fig. 2A and B). Under suspension culture
conditions, early tumor cells first formed spherical sac-like
structures (Fig. 2C), and with
time, the spherical sac-like structures continued to develop into
irregular clumps, and finally, gastric cancer organoids formed
typical irregular lobulated lobes (Fig. 2D).
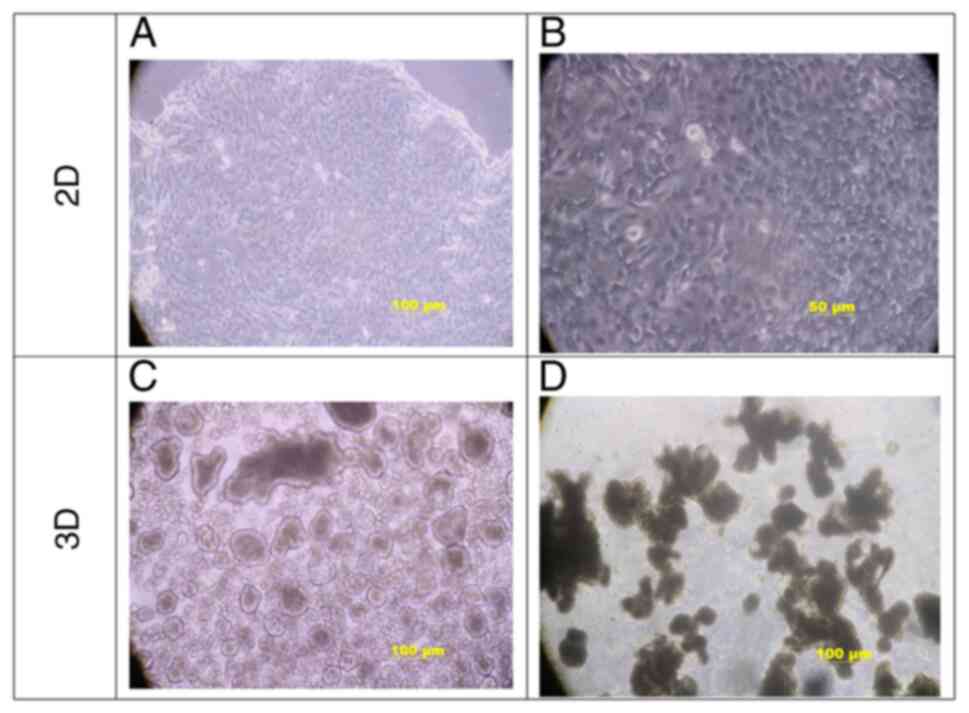 | Figure 2.Morphological comparison of gastric
cancer cells in 2D and 3D. (A and B) Under 2D culture conditions,
gastric cancer cells exhibited a typical epithelioid cell
morphology. The cell shape was mainly short, spindle-like and
round, with large nuclei and a small amount of cytoplasm. Tumor
cells grew in layers and lost contact inhibition, while they did
not detach [scale bars, (A) 100 µm; (B) 50 µm]. (C and D) 3D
morphology of gastric cancer organoids; when suspended in ultra-low
adsorption plates, (C) tumor cells first formed spherical sac-like
structures, and (D) over time the spherical sac-like structures
continued to develop into irregular clumps, and finally, gastric
cancer organoids formed typical irregular lobes (scale bars, 100
µm). D, dimensional. |
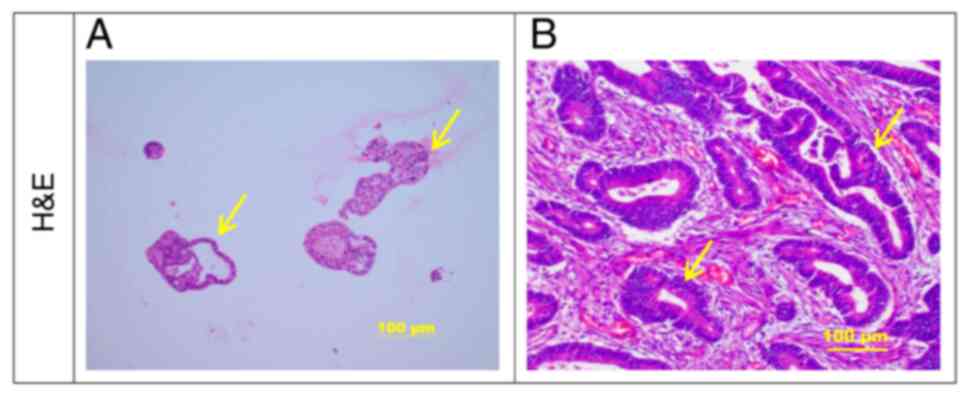
H&E staining of gastric cancer
organoids and original tumor tissue
After 2 weeks of culture, gastric cancer organoids
were embedded, sliced and analyzed by H&E staining. It was
observed that the gastric cancer organoids had cystic or irregular
glandular duct-like structures, an irregular cell arrangement,
large and hyperchromatic nuclei and irregular ring-shaped glandular
structures (Fig. 3A). This was
highly similar to the biological characteristics of the original
gastric cancer tissues (Fig.
3B).
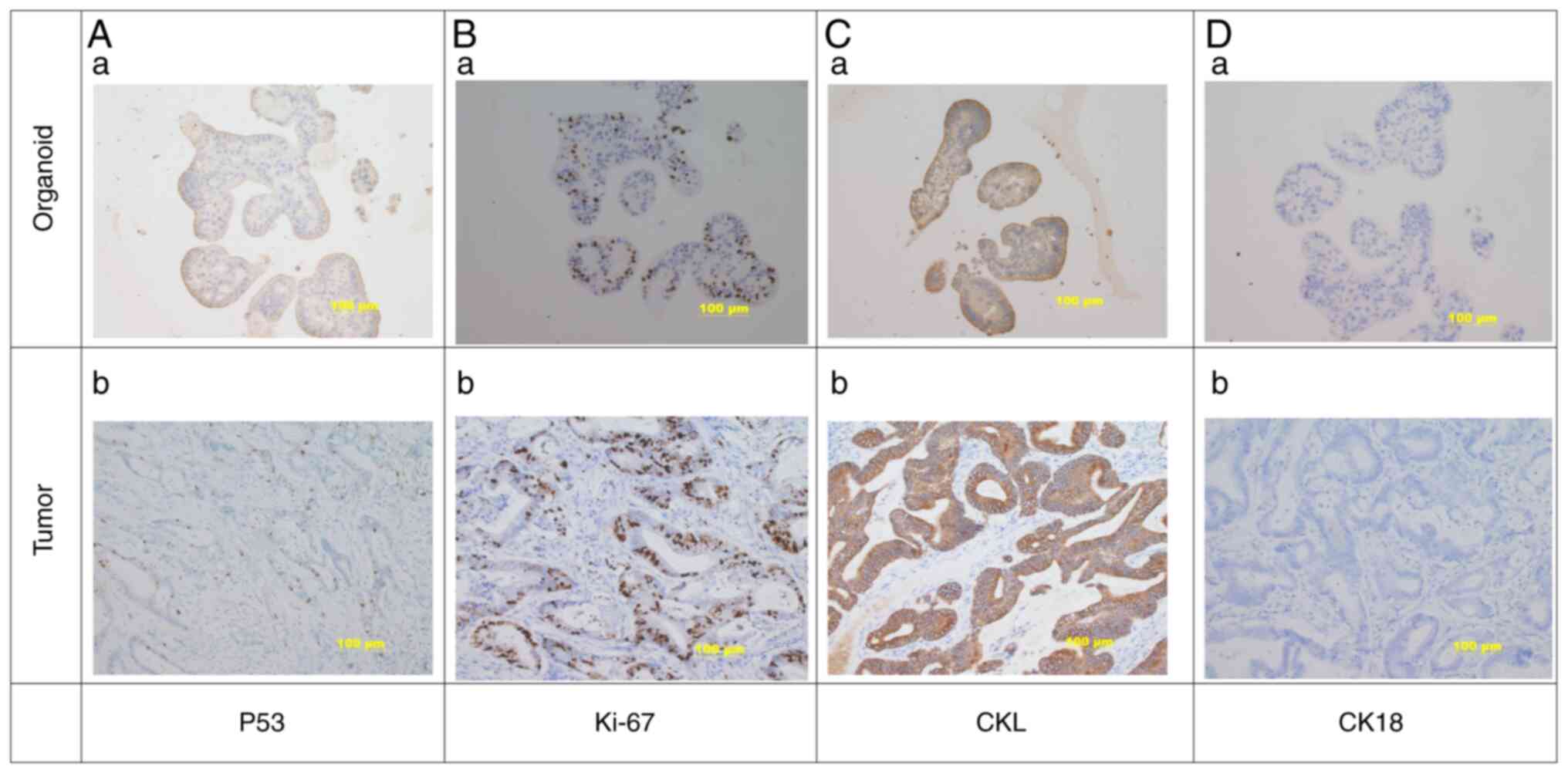
Immunohistochemistry of gastric cancer
organoids and original tumor tissue
Immunohistochemistry was used to detect the protein
levels of P53, Ki-67, cytokeratin low molecular weight (CKL) and
cytokeratin (CK)18 in gastric cancer organoids and original tumor
tissues (Fig. 4). The results
indicated that P53 (Fig. 4Aa),
Ki-67 (Fig. 4Ba) and CKL (Fig. 4Ca) were all expressed in the
cultured gastric cancer organoids, while staining for CK18 was
negative (Fig. 4Da). The results
were consistent with those for the original gastric cancer tissue
(Fig. 4Ab, Bb, Cb and Db).
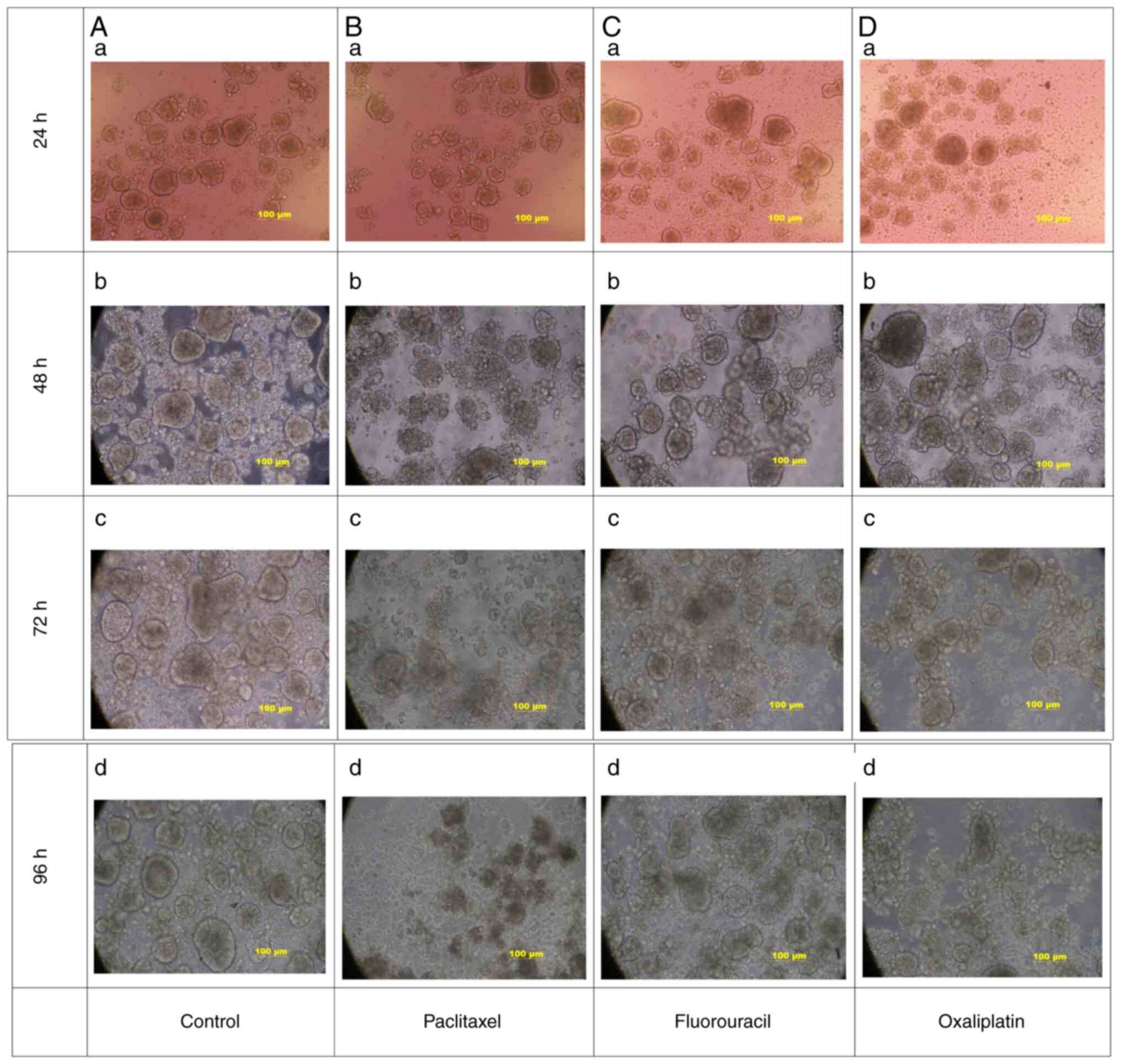
Chemotherapy drug screening
Paclitaxel, oxaliplatin and fluorouracil were added
to the gastric cancer organoids cultured to the 8th day. The
morphological changes of the organoids were observed every 24 h.
Using light microscopy, it was indicated that, compared with that
in the control group (Fig. 5A),
the morphology of the experimental group was more disordered than
that of the control group with certain organoids exhibiting cell
death, among which the paclitaxel and oxaliplatin groups exhibited
the most obvious changes. Organoid death gradually increased as the
culture time progressed. After 96 h, it was observed that most of
the organoids in the paclitaxel and oxaliplatin groups had
disintegrated appearance and lost their morphological structure,
while only a few organoids in the fluorouracil group had
disintegrated (Fig. 5B-D). Patient
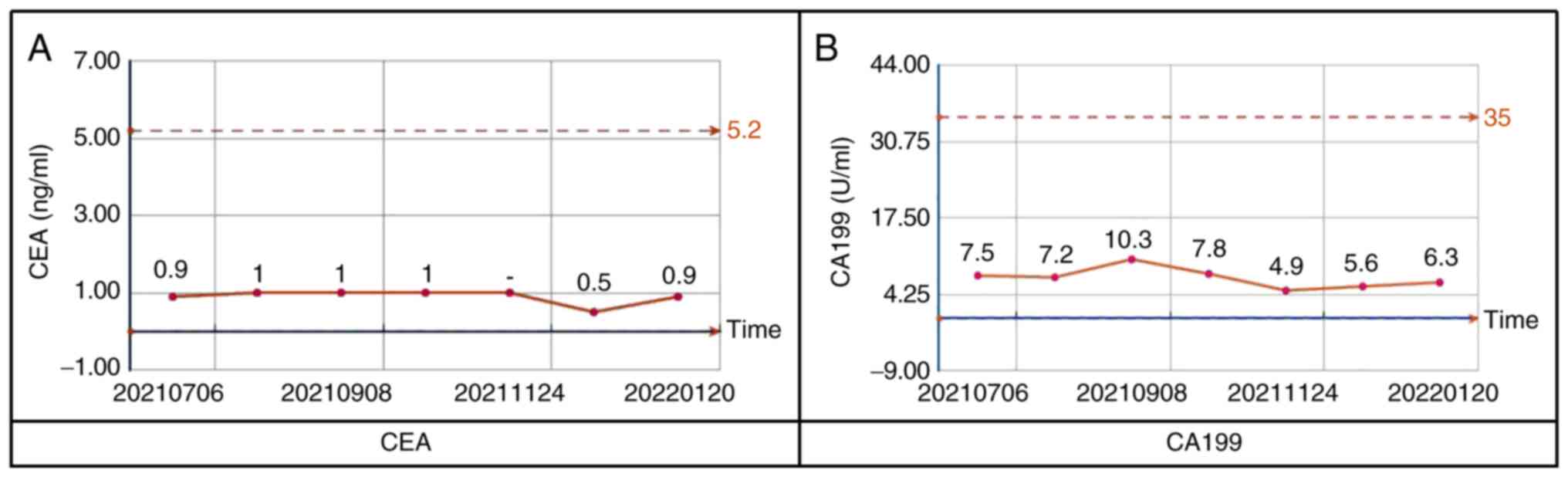
3 was treated with oxaliplatin and capecitabine combination
chemotherapy for six cycles after surgery; carcinoembryonic antigen
(Fig. 6A) and carbohydrate antigen
19-9 (Fig. 6B) have been stable,
and no signs of tumor recurrence were found for 7 months after the
operation. This verifies the feasibility and effectiveness of
organoid drug screening to a certain extent.
Discussion
In the present study, gastric cancer organoids were
successfully established by enzymatic digestion of surgically
resected gastric cancer tissue to obtain gastric cancer primary
cells for subsequent organoid culture. Histochemical staining
proved that the original tissue and the cultured PDOs had a high
degree of similarity. Finally, the efficacy of a variety of
traditional tumor chemotherapy drugs on the obtained patient tumor
tissue organoids was examined to test the relationship between
organoid drug screening and clinical treatment. In accordance, the
similarity of drug sensitivity between organoids and the patients
verified that the construction of the gastric cancer organoid model
and its application in individualized drug screening has clinical
translational significance.
In China, gastric cancer ranks third in terms of
incidence and mortality, accounting for >40% of global cases of
gastric cancer (22). Endoscopic
or surgical resection is the only potential cure; however, since
most patients are in the middle and late stages of the disease,
comprehensive treatments, such as surgery, radiotherapy and
chemotherapy, targeted therapy, immunotherapy and traditional
Chinese medicine, are required to achieve a therapeutic effect
(16,23,24).
Individualized chemotherapy is a method of using
specific and optimal chemotherapeutic drug regimens according to
the pharmacogenetic characteristics of patients with cancers.
Individualized chemotherapy may help patients receive appropriate
chemotherapy drugs, improve the pertinence of treatment and prolong
the overall survival to the greatest extent. In recent years, with
the development and maturation of tumor organoid models for
screening and selecting appropriate chemotherapy drugs,
individualized chemotherapy has become a reasonable choice to
improve efficacy and reduce ineffective treatments (16,25–27).
In the present study, gastric cancer organoids were
successfully cultured by inoculation in Matrigel and suspension
culture. Differing from the organoid culture method including
inoculation in Matrigel (28–30),
the establishment of gastric cancer organoids by suspension culture
has the following advantages: i) In the absence of Matrigel, the
cost of the organoid culture may be markedly reduced; ii) in terms
of collection, passage, cryopreservation and drug screening, the
model is simpler and more convenient to handle; and iii) without
Matrigel in the culture process, the impact of the interaction
between mouse-derived components and human-derived tumors is
reduced. Regarding the acquisition of primary cells, based on the
literature and previous cell culture experience (28–32),
it is recommended that during the enzymatic digestion process,
tissue digestion is diligently observed and duly terminated in
time. In this regard, cell mass-like structures rather than single
cells may markedly improve the success rate of cell lines or
organoids.
In the present study, an ordinary complete medium
was used rather than a protocol of serum-free medium and high
concentrations of various growth factors recommended in the
literature (17,19,33).
The success of this model indicates that for the culture of tumor
organoids, the characteristics of the cell itself are most
important. Even in a simplified culture system, tumor organoids may
be successfully established and maintain the phenotypical and
molecular biological characteristics of the primary tumor, which
met the needs of subsequent experimental applications, such as drug
screening and functional verification.
Previous studies by our group have indicated how
fibroblast contamination may be reduced during organoid culture
(31,32). First, the epithelial layer should
be sampled as much as possible when the tumor tissue is obtained.
Furthermore, the digestion time in the process of tumor tissue
digestion should be controlled to avoid excessive digestion of the
tissue into single cells. In addition, a 100-mesh sieve should be
used to remove large pieces of undigested tissue. The rotation
speed of the centrifuge should be adjusted to settle the tumor cell
clusters and sub-duct structures. Through the above measures, most
of the fibroblasts may be removed. Of course, a small number of
fibroblasts may remain in the gastric cancer organoid culture.
However, this does not obviously conflict with the experimental
purpose of organoid culture. As fibroblasts actually exist in
gastric cancer tissue, in theory, the presence of fibroblasts in
organoids is closer to the actual situation in the human body and
the drug sensitivity results obtained are more accurate in terms of
the clinical application, which is also the reason for exploring
organoid co-culture at present.
The present study further validates the feasibility
and effectiveness of tumor organoids for personalized drug
screening. However, unlike other tumors, the success rate of
gastric cancer organoids is relatively low and the 50% success rate
reported in the literature for gastric cancer organoids is already
high (19,34). Therefore, the gastric cancer
organoid culture system of the present study requires further
optimization to continuously improve the success rate of gastric
cancer organoid culture. This model may serve as a guide for
individualized treatment of gastric cancer, which is important for
improving the long-term survival rate of patients with an advanced
stage of cancer. As a limitation of the present study, the number
of gastric cancer samples used was relatively small and not all
differentiated types of gastric cancer were included. In this
experiment, the organoids were not quantified. Instead, the
organoids were centrifuged and resuspended with culture medium, and
the suspension of the same volume was inoculated to each well. The
present experiments aim to avoid ineffective treatment through
rapid and economical drug selection methods for clinical
individualized treatment. The results of the present study are
qualitative, not quantitative results. The present experiments only
verified the feasibility of organoid screening for chemotherapeutic
drugs. In the next step, the number of samples and types of
differentiation will be increased, and it will be endeavored to
pursue the quantification of experimental results and refinement of
the models, including the co-culture of tumor organoids with
patient-derived stromal cells and immune cells for individualized
screening of targeted or immunotherapeutic drugs.
In summary, gastric cancer organoids with high
similarity to the original tissue may be successfully constructed
by the suspension growth culture method. The established organoids
may serve as an effective model for individualized drug
screening.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Gansu Provincial
Science and Technology Plan (grant no. 21JR1RA099) and the
Intra-Hospital Fund of the First Hospital of Lanzhou University
(grant nos. ldyyyn2015-10, ldyyyn2019-97, ldyyyn2021-61 and
ldyyyn2021-68).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization, HX and WCZ; methodology design,
HX, XM, CMW and KXZ; validation, HX, XM, CMW, CPC, HT; formal
analysis, HT and JJH; investigation, ZJZ and HZ; resources, HX,
CPC, HT, KXZ; collection of clinical data in the study, and the
collection and backup of records and photos generated during the
experiment, WL and KXZ; writing-original draft preparation, XM, CMW
and KXZ; writing-review and editing, HX and WCZ; visualization, XM,
CPC, HT, WL and KXZ; supervision, HX, KXZ and WCZ; project
administration, HX, WCZ; funding acquisition, HX, CPC, ZJZ and WL.
XM, CMW, KXZ, WCZ and HX checked and confirmed the authenticity of
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the institutional Ethics
Committee of the First Hospital of Lanzhou University (Lanzhou,
China; no. LDYYLL2022-292). The patients provided written informed
consent for the use of their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steele NG, Chakrabarti J, Wang J, Biesiada
J, Holokai L, Chang J, Nowacki LM, Hawkins J, Mahe M, Sundaram N,
et al: An organoid-based preclinical model of human gastric cancer.
Cell Mol Gastroenterol Hepatol. 7:161–184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malvezzi M, Bonifazi M, Bertuccio P, Levi
F, La Vecchia C, Decarli A and Negri E: An age-period-cohort
analysis of gastric cancer mortality from 1950 to 2007 in Europe.
Ann Epidemiol. 20:898–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmad SA, Xia BT, Bailey CE, Abbott DE,
Helmink BA, Daly MC, Thota R, Schlegal C, Winer LK, Ahmad SA, et
al: An update on gastric cancer. Curr Probl Surg. 53:449–490. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gunturu KS, Woo Y, Beaubier N, Remotti HE
and Saif MW: Gastric cancer and trastuzumab: First biologic therapy
in gastric cancer. Ther Adv Med Oncol. 5:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2 protein
expression assessed by immunohistochemistry in gastric cancer.
Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
8
|
Abrahão-Machado LF, Jácome AA, Wohnrath
DR, dos Santos JS, Carneseca EC, Fregnani JH and Scapulatempo-Neto
C: HER2 in gastric cancer: Comparative analysis of three different
antibodies using whole-tissue sections and tissue microarrays.
World J Gastroenterol. 19:6438–6446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manion E, Hornick JL, Lester SC and Brock
JE: A comparison of equivocal immunohistochemical results with
anti-HER2/neu antibodies A0485 and SP3 with corresponding FISH
results in routine clinical practice. Am J Clin Pathol.
135:845–851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domcke S, Sinha R, Levine DA, Sander C and
Schultz N: Evaluating cell lines as tumour models by comparison of
genomic profifiles. Nat Commun. 4:21262013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ertel A, Verghese A, Byers SW, Ochs M and
Tozeren A: Pathway-specifific differences between tumor cell lines
and normal and tumor tissue cells. Mol Cancer. 5:552006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gillet JP, Calcagno AM, Varma S, Marino M,
Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al:
Redefifining the relevance of established cancer cell lines to the
study of mechanisms of clinical anti-cancer drug resistance. Proc
Natl Acad Sci USA. 108:18708–18713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sandberg R and Ernberg I: Assessment of
tumor characteristic gene expression in cell lines using a tissue
similarity index (TSI). Proc Natl Acad Sci USA. 102:2052–2057.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stein WD, Bates SE and Fojo T: Intractable
cancers: The many faces of multidrug resistance and the many
targets it presents for therapeutic attack. Curr Drug Targets.
5:333–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vlachogiannis G, Hedayat S, Vatsiou A,
Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford
I, Burke R, et al: Patient-derived organoids model treatment
response of metastatic gastrointestinal cancers. Science.
359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seidlitz T, Koo BK and Stange DE: Gastric
organoids-an in vitro model system for the study of gastric
development and road to personalized medicine. Cell Death Differ.
28:68–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seidlitz T, Merker SR, Rothe A, Zakrzewski
F, von Neubeck C, Grützmann K, Sommer U, Schweitzer C, Schölch S,
Uhlemann H, et al: Human gastric cancer modelling using organoids.
Gut. 68:207–217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nanki K, Toshimitsu K, Takano A, Fujii M,
Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et
al: Divergent Routes toward Wnt and R-spondin Niche Independency
during Human Gastric Carcinogenesis. Cell. 174:856–869.e17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan HHN, Siu HC, Law S, Ho SL, Yue SSK,
Tsui WY, Chan D, Chan AS, Ma S, Lam KO, et al: A comprehensive
human gastric cancer organoid biobank captures tumor subtype
heterogeneity and enables therapeutic screening. Cell Stem Cell.
23:882–897.e11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peraldo-Neia C, Massa A, Vita F, Basiricò
M, Raggi C, Bernabei P, Ostano P, Casorzo L, Panero M, Leone F, et
al: A novel multidrug-resistant cell line from an Italian
intrahepatic cholangiocarcinoma patient. Cancers (Basel).
13:20512021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varamo C, Peraldo-Neia C, Ostano P,
Basiricò M, Raggi C, Bernabei P, Venesio T, Berrino E, Aglietta M,
Leone F and Cavalloni G: Establishment and characterization of a
new intrahepatic cholangiocarcinoma cell line resistant to
gemcitabine. Cancers (Basel). 11:5192019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang FH, Shen L, Li J, Zhou ZW, Liang H,
Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al: The Chinese
Society of Clinical Oncology (CSCO): Clinical guidelines for the
diagnosis and treatment of gastric cancer. Cancer Commun (Lond).
39:102019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunt RH, Camilleri M, Crowe SE, El-Omar
EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM,
Rugge M, et al: The stomach in health and disease. Gut.
64:1650–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp
HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg
H, et al: Histopathological regression after neoadjuvant docetaxel,
oxaliplatin, fluorouracil, and leucovorin versus epirubicin,
cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): Results from the phase 2 part of a multicentre,
open-label, randomised phase 2/3 trial. Lancet Oncol. 17:1697–1708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seidlitz T and Stange DE: Gastrointestinal
cancer organoids-applications in basic and translational cancer
research. Exp Mol Med. 53:1459–1470. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lau HCH, Kranenburg O, Xiao H and Yu J:
Organoid models of gastrointestinal cancers in basic and
translational research. Nat Rev Gastroenterol Hepatol. 17:203–222.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu H, Jiao D, Liu A and Wu K: Tumor
organoids: Applications in cancer modeling and potentials in
precision medicine. J Hematol Oncol. 15:582022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Günther C, Winner B, Neurath MF and
Stappenbeck TS: Organoids in gastrointestinal diseases: From
experimental models to clinical translation. Gut. 71:1892–1908.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun L, Wang Y, Cen J, Ma X, Cui L, Qiu Z,
Zhang Z, Li H, Yang RZ, Wang C, et al: Modelling liver cancer
initiation with organoids derived from directly reprogrammed human
hepatocytes. Nat Cell Biol. 21:1015–1026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stroulios G, Stahl M, Elstone F, Chang W,
Louis S, Eaves A, Simmini S and Conder RK: Culture Methods to Study
Apical-Specific Interactions using Intestinal Organoid Models. J
Vis Exp. 23((169))2021.PubMed/NCBI
|
|
31
|
Peng J, Xu H and Cai J: Establishment and
characterization of a new gastric cancer cell line, XGC-1. Cancer
Cell Int. 20:4372020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu H, Peng JG, Zhuang YF, Chen JJ, Luo QC,
Huang WF, Lin CD and Cai JC: Establishment and characterization of
an expanding-type gastric cancer cell line by Ming's
classification. Oncol Rep. 36:3030–3036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tiriac H, Belleau P, Engle DD, Plenker D,
Deschênes A, Somerville TDD, Froeling FEM, Burkhart RA, Denroche
RE, Jang GH, et al: Organoid profiling identifies common responders
to chemotherapy in pancreatic cancer. Cancer Discov. 8:1112–1129.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li G, Ma S, Wu Q, Kong D, Yang Z, Gu Z,
Feng L, Zhang K, Cheng S, Tian Y, et al: Establishment of gastric
signet ring cell carcinoma organoid for the therapeutic drug
testing. Cell Death Discov. 8:62022. View Article : Google Scholar : PubMed/NCBI
|