Lung cancer remains the leading cause of cancer
incidence and mortality globally, with an estimated 2 million new
diagnoses and 1.8 million deaths in 2019 (1). Non-small cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases, with a 5-year survival
rate of only 16% (2,3) despite recent advancements in targeted
therapy and immune checkpoint inhibitors (4,5).
Metastasis is a major cause of death in patients with malignant
tumors, accounting for ~90% of cancer-associated deaths. Tumor
metastasis has been reported to depend on the formation of a
pre-metastatic niche (PMN) (6,7). The
PMN specifically refers to the microenvironment in which the
primary tumor foci are prepared for distant dissemination and
colonization of tumor cells. The features of this microenvironment
include inflammation, immunosuppression, angiogenesis/vascular
permeability, organophilicity, reprogramming and lymphangiogenesis
(8–10). An early study reported that lung
cancer metastasis was highly correlated with leukocytosis (11). It has been reported that
tumor-associated neutrophils (TANs), which are major components in
tumor-associated inflammation, support lung cancer cell growth,
invasion, angiogenesis and cancer cell metastasis, and are
associated with a poor prognosis (12–14).
Therefore, the present study reviewed the role and mechanisms of
TANs in lung cancer development and progression, as well as TANs as
a potential therapeutic target for lung cancer.
Neutrophils account for 50–70% of all white blood
cells in the circulation and serve an important role in the
inflammatory process, which is regarded as the first line of
defense against infection (15).
Neutrophils in the blood circulation are usually dormant and will
be activated once they migrate into the tissues (16). TANs are infiltrating neutrophils
within tumors. The chemokines produced by tumors attract the
neutrophils from the blood circulation, which enter the tumor
tissue through the blood vessel wall and then form TANs. The spleen
is a recently discovered reservoir of mononuclear cells, and a
large number of TAN precursor cells migrate from the spleen to the
tumor stroma (17). Furthermore,
splenectomy has been reported to decrease the number of primary
tumor infiltrating TANs, leading to a reduced number or size of
metastases (18). Once TANs are
activated in the tumor microenvironment, they appear to increase
the complexity of the inflammatory environment through a mechanism
that involves the attraction of other leukocytes. TANs secrete a
large amount of interleukin-8 (IL-8), which promotes neutrophil
survival and recruits more neutrophils (19). TANs serve a major role in numerous
aspects of tumor development, such as malignant transformation,
tumor progression, extracellular matrix modification, angiogenesis,
cell migration and immunosuppression (20–22).
Numerous studies have reported that neutrophils promote tumor
progression by degrading the stroma, immunosuppression, stimulating
tumor cell proliferation, increasing metastasis and promoting
angiogenesis (20,23). The enrichment of neutrophils in
tumors was also reported to be associated with lymph node
metastasis, tumor differentiation and grade, and tumor stage. These
findings indicated that tumors and the tumor microenvironment
regulated neutrophil recruitment and that TAN feedback regulated
tumor progression (24,25). Furthermore, clinical studies have
reported that patients with lung cancer had high levels of
neutrophils in both the tumor and the circulation (26–28).
The higher neutrophil/lymphocyte ratios were associated with higher
recurrence rates following operations, less effective treatment
responses and worse prognoses compared with the lower ratios
(29–32).
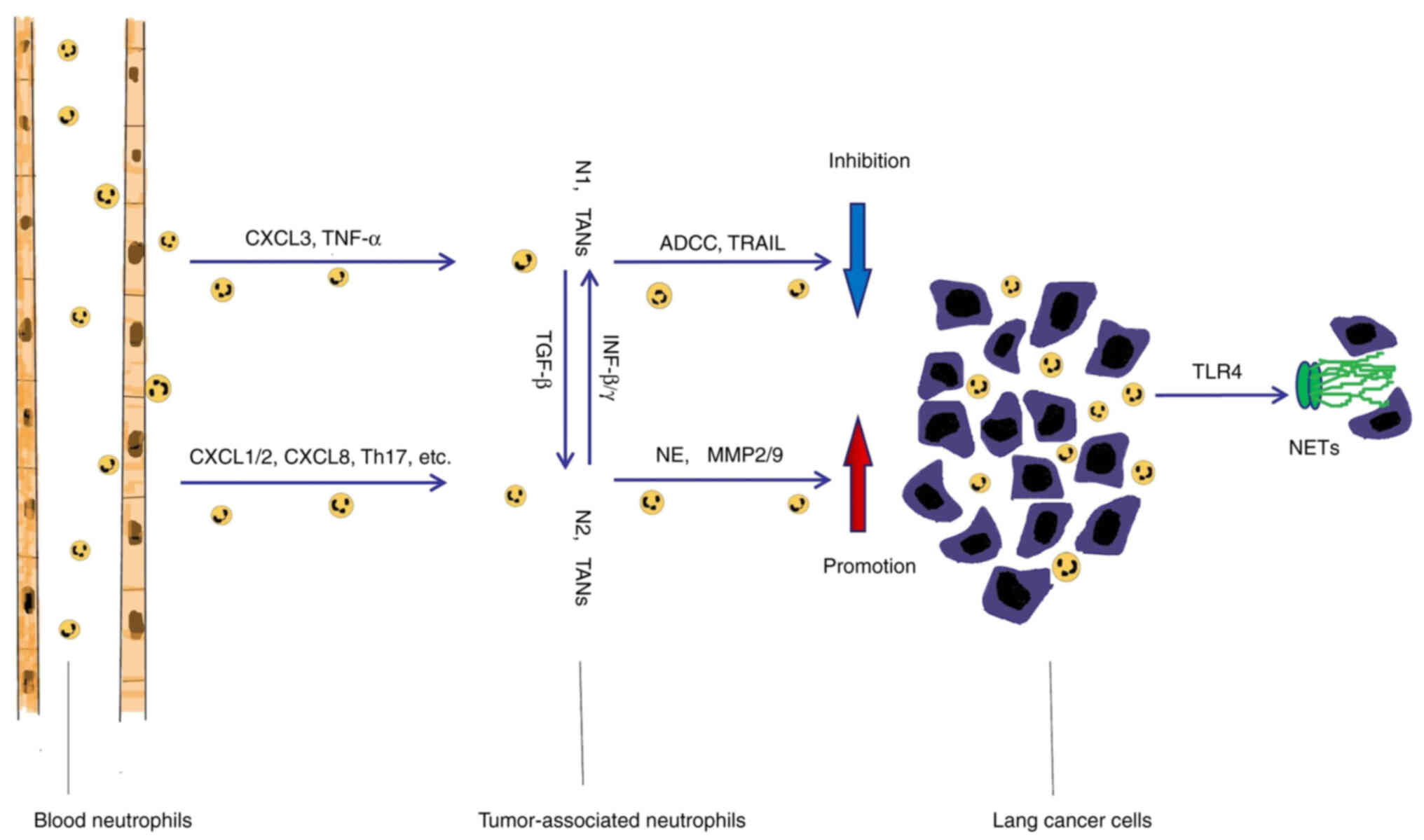
TANs have a high functional plasticity, which not
only inhibits cancer growth but also stimulates cancer progression.
Previous studies have reported that the phenotypes of TANs are
similar to the phenotypes of tumor-associated macrophages, with
both the ‘N1’ type that inhibits tumor growth and the ‘N2’ type
that promotes tumor growth and malignant metastasis (33,34).
N1 neutrophils can produce and release cytotoxic mediators such as
reactive oxygen species (ROS) and myeloperoxidase (MPO), to kill
tumor cells directly or inhibit tumor cell proliferation. N1
neutrophils have been reported to inhibit tumor metastasis and
infiltration in breast cancer by releasing C-C motif chemokine
ligand 2 (CCL2) and promoting the production of ROS; in gastric
carcinoma, a high density of N1 type neutrophils also inhibits
lymph node metastasis (35,36).
However, N2 neutrophils participate in tumor proliferation,
invasion and metastasis through the synthesis and secretion of
proteases. Studies have reported that N2 type neutrophil elastase
(NE), matrix metallopeptidase-9 (MMP-9) and MMP-2 promote tumor
growth and angiogenesis, which results in distant metastasis
(37–41). It was also reported that TANs
maintained some functional plasticity and could be ‘alternately
activated’ when exposed to different tumor microenvironments
(21,42). For example, transforming growth
factor-β (TGF-β) induced a pro-tumor phenotype (N2-TAN) (42,43),
whereas interferon-β (IFN-β) or inhibition of TGF-β signaling led
to an antitumor (N1-TAN) phenotype (41,44).
Further studies reported that administration of TGF-β could convert
TANs from an antitumor (N1) phenotype into a more permissible (N2)
phenotype, whereas blocking TGF-β transformed TANs from the N2
phenotype to the N1 phenotype in mouse models of mesothelioma and
lung cancer (45,46). IFN-γ and granulocyte macrophage
colony-stimulating factor (GM-CSF) were reported to be key factors
in the differentiation process of TANs; IFN-γ had a dose-dependent
specific effect on neutrophil differentiation, inducing a hybrid
phenotype with immune-stimulating T cell characteristics at low
doses, whereas a hybrid phenotype induced by programmed
death-ligand 1 (PD-L1) expression with inhibition of T cell
response was stimulated at high doses (47). In the early stage of tumor
development, TANs mainly manifest as the N1 type and serve an
antitumor role. However, in the late stages of tumor development,
TANs primarily function as the N2 type and promote tumor
development, invasion and metastasis (19). Furthermore, the phenotype and
function of TANs are different in different locations and tumor
stages. Studies using animal experiments reported that mice
injected with circulating TANs (cTANs) demonstrated more pulmonary
metastatic nodules, which suggested that the increase in cTANs
enhanced tumor metastasis (48).
Compared with circulating peripheral blood neutrophils, neutrophils
absorbed into lung tumors exhibit an activated phenotype (19). It was reported that there was no
difference in the number of neutrophils entering tumors between the
early and late stage of lung cancer in a mouse model (49). However, neutrophils, in the early
stage of tumor development, are almost completely located at the
edge of the tumor. It was only during the later stages that
neutrophils were discovered scattered among the tumor cells.
Moreover, TANs in early stage tumors demonstrate greater
cytotoxicity to tumor cells by producing higher levels of tumor
necrosis factor (TNF)-α, nitric oxide (NO) and hydrogen peroxide
(49). These results provide a new
concept for tumor intervention, namely, the inhibition of cancer
progression by finding strategies to activate N1 neutrophils and/or
convert N2 to N1 neutrophils (Fig.
1).
NETs are released by neutrophils in response to
extracellular pathogens and are typically composed of densified
fibrous chromatin and polymerized histones, MPO and numerous
cytoplasmic proteins that promote disease progression and
transmission (50,51). Previous studies have reported that
NETs are not only involved in the inflammatory process, but also
promote the adhesion of circulating tumor cells and the occurrence
of micro-metastasis (33,52). Compared with that in healthy
control and early stage tumor groups, the Net levels were reported
to be significantly increased in patients with advanced cancer of
the esophagus, stomach, pancreas and lung, which was related to TNM
staging, supported metastasis and was negatively correlated with
prognosis (53–57). Furthermore, the molecules released
from Lewis lung cancer cells were found to activate toll-like
receptor 4 (TLR4), promote the formation of NETs, increase the
adhesion of cancer cells and promote lung cancer progression
(58–60). NETs also function as a cancer cell
adhesion matrix to promote tumor progression (61,62).
The capture of tumor cells by NETS has no cytotoxic effect on the
cells, rather it enhances tumor proliferation, invasion and
metastasis by triggering the expression of tumor IL-8 (63). NETs have also been reported to
trigger metastatic potential through the internalization of
captured tumor cells and the activation of TLR4/9-COX2 signaling,
mediating cell death resistance and enhancing invasion (64). Elevated COX2 expression levels are
associated with higher metastatic behavior, including protective
cell death, the induction of invasion, the stimulation of
angiogenesis and the suppression of immune surveillance (65–67).
Furthermore, NETs promote tumor micro-metastases by the activation
of cancer-related fibroblasts (68). A previous study reported that
tumor-secreted protease cathepsin C in breast cancer induced lung
metastasis by the regulation of neutrophil recruitment and the
formation of NETs (69) (Fig. 1).
TANs can be differentiated on the basis of their
activation and cytokine status, and their effect on the growth of
N1 and N2 TAN tumor cells. N1 TANs are characterized by high
expression levels of TNFα, CCL3 and intercellular adhesion molecule
(ICAM)-1, and low expression levels of argininase axis proteins,
whereas N2 neutrophils are typified by the upregulation of
chemokines, including CCL2, CCL3, CCL4, CCL8, CCL12, CCL17, C-X-C
motif chemokine ligand (CXCL)1, CXCL2, IL-8/CXCL8 and CXCL16
(33). A previous study reported
that the serum CXCL1 level was significantly upregulated in
tumor-bearing mice with Lewis lung carcinoma (3LL) cells and that
the depletion of CXCL1 in 3LL cells significantly inhibited
neutrophil infiltration, which led to decreased tumor growth in
vivo (70). These results
indicated that CXCL1 was involved in TAN infiltration of lung
cancer and promoted tumor growth. CXCR2 and its ligands (i.e.,
CXCL1-3, CXCL5, CXCL7 and CXCL8) were reported to be responsible
for the recruitment of neutrophils under normal physiological
conditions and to participate in the mobilization of TANs (71). Furthermore, oxidative sterols
derived from Lewis lung cancer cells serve a critical role in the
promotion of neutrophil recruitment to tumor tissues by CXCR2
(72). TANs participate in the
tumor microenvironment through secretion of PD-L1, CXCR4, CCR5,
Adam 17 and NOS2, and are reported to have an immunosuppressive
effect in T-cell proliferation assays. Tumors with overexpression
of CXCL5 have reduced frequencies of lung metastasis and neutrophil
depletion reverses this effect (73). Tumor suppressor chemokines CXCL8
and CXCL6 are also involved in neutrophil infiltration (74). Recently, the CXCL8-CXCR1/2 axis was
reported to serve an important role in the occurrence of solid
tumors, including cancer of the lung, colon and breast. CXCL8 was
reported to promote tumor growth and metastasis through increased
MMP-2 activity (75,76). Changes in cell adhesion molecules
(CD62L and CD54) and CXC chemokine receptors (CXCR1 and CXCR2) are
related to leukocyte activation, chemotaxis enhancement and trans
endothelial migration (77,78).
Circulating neutrophils were reported to be attracted to tumor
tissues from blood vessels by chemokines, which bind to the G
protein coupled receptors CXCR1 and CXCR2 on the surface of
neutrophils (34,79). Moreover, it has been reported that
neutrophils and T helper cell 17 (Th17) cells can stimulate each
other in a chemokine/cytokine-dependent manner. Th17-produced
cytokines, including IL-17A and IL-17F, can indirectly induce the
recruitment of neutrophils, and active neutrophils release CCL2 and
CCL20 chemokines to induce chemotaxis of Th17 cells. Furthermore,
activated Th17 cells can directly attract neutrophils by releasing
bioactive CXCL8 (80). IL-17C was
also reported to promote neutrophil recruitment, tumor-related
inflammation, and tumor proliferation and growth (81).
Angiogenesis is essential for tumor metastasis.
Previous studies have reported that TANs support tumor metastasis
by generating angiogenic factors and stromal degrading enzymes to
drive tumor angiogenesis (82,83).
TANs are an important source of MMP-9 in NSCLC (84,85).
Furthermore, in a tumor xenotransplantation model, the upregulation
of Bv8 (also known as activin-2) in neutrophils induced by
granulocyte colony-stimulating factor (G-CSF) was reported to
promote tumor angiogenesis (86).
It has also been reported that G-CSF promotes the recruitment of
neutrophils to tumors, which express Bv8 leading to induction of
angiogenesis and resistance to anti-vascular endothelial growth
factor (VEGF) therapy (87,88).
EMT serves an important role in tumor invasion and metastasis
(89,90). Several molecular pathways that
mediate EMT in cancer cells have been reported, such as the TGF-β,
Ras, Notch and Wnt/β-catenin cascades (91). In previous studies, the formation
of vascular mimicry (VM) was induced by cancer-associated
fibroblast (CAFs) in both in vitro and in vivo
experiments. Notch 2-Jagged 1 cell-cell contact between cancer
cells and CAFs contributes to VM network formation. Intercellular
adhesion molecule-2 contributes to VM-mediated neutrophil
infiltration. VM networks not only change the phenotype of
neutrophils from the tumor-suppressing N1 type to the
tumor-promoting N2 type, but also provide a vital channel for the
infiltration of neutrophils. VM networks serve a crucial role in
the promotion of lung cancer metastasis and chemoresistance to
angiostatic therapy (92–94). Tumor cells and mesenchymal cells
secrete chemokines, which bind to CXCR1 and CXCR2, the G-protein
coupled receptors expressed on the surface of neutrophils,
promoting tumor progression (34,79).
TANs were also reported to promote the EMT of lung adenocarcinoma
cells in vitro and enhance their migration activity
(91,95).
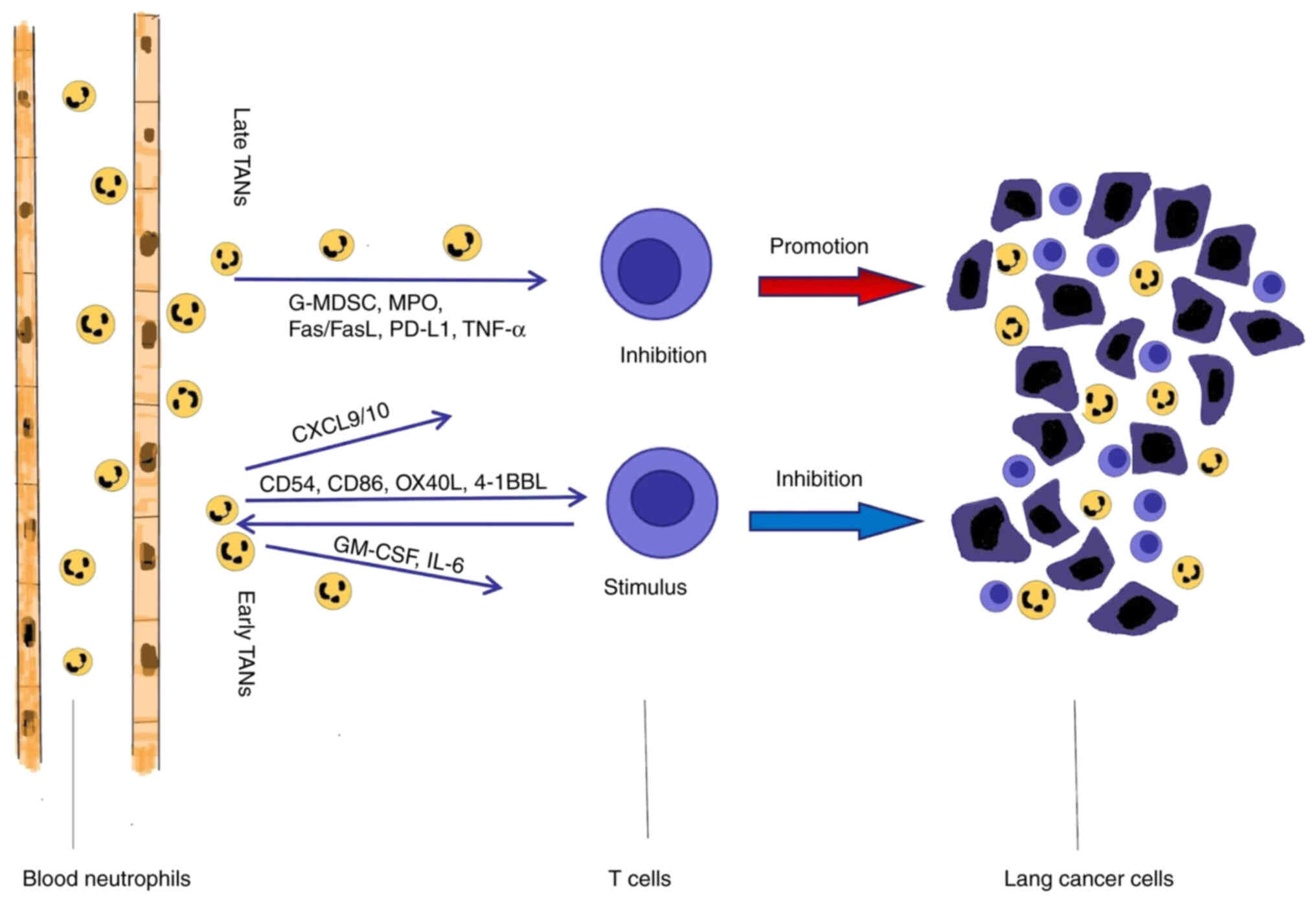
As well as acting directly on tumor cells, TANs
regulate T lymphocytes to control tumor growth. It has been
previously reported that granulocyte myeloid inhibitory cells are
able to inhibit CD8+ T cell proliferation and
activation. The infiltrating neutrophils in tumor tissues express
higher levels of MPO, Fas/Fas ligand and PD-L1, which are involved
in the TAN-mediated inhibition of CD4+ and
CD8+ T cells (70).
Michaeli et al (96)
reported that TANs promote immunosuppression by strongly inducing
CD8+ T cell apoptosis, which leads to tumor progression.
The mechanism by which TANs induce CD8+ T cell death
involves the TNF signaling pathway and NO production. In the early
stage of lung cancer, TANs are not immunosuppressive, rather they
stimulate the T-cell response and enhance the immune response of
cytotoxic T lymphocytes, which leads to the inhibition of tumor
development (19,97). Activated T cells lead to
significant upregulation of CD54, CD86, OX40L and 4-1BBL
costimulatory molecules on the surface of neutrophils, which
further support T-cell proliferation in a positive feedback loop.
In the case of lung cancer, a subset of TANs, at the early stage of
tumor development, stimulates T-cell activation by secreting
GM-CSF. Moreover, TANs produce classical pro-inflammatory
cytokines, such as GM-CSF and IL-6, which serve as positive
regulators of T cells, leading to tumor suppression (98). Neutrophils can also secrete
cytokines, including TNFR and cathepsin G, which act directly on T
cells and enhance the overall adaptive immune antitumor response
(99). Furthermore, TANs have been
reported to support the adaptive antitumor immune response by
secreting chemokines such as CXCL9 or CXCL10 to recruit T cells to
tumor sites (46) (Fig. 2).
NE is a neutrophil-derived protease with broad
substrate and neutrophil specificity (100). NE promotes lung cancer growth in
the LOX-Stop-Stop-K-ras mouse model. This effect of NE is not
through the degradation of the extracellular matrix, but rather by
direct action on the tumor cells (37). NE is not only involved in the
occurrence of lung cancer, but also induces distant metastasis.
Wislez et al (101)
studied the role of neutrophils in the metastasis of
bronchoalveolar carcinoma and reported that human lung
adenocarcinoma cells interact with neutrophils. The neutrophils
promoted the abduction of cancer cells through secretion of NE,
which suggested that NE is involved in tumor metastasis (101,102). NE also accelerates the lung tumor
growth through mediation of the degradation of IRS-1. In addition
to NE, matrix metalloproteinases (MMPs) are important proteolytic
enzymes released by neutrophils. MMPs degrade the extracellular
matrix to promote neovascularization and tumor metastasis (40,103).
Contrary to the aforementioned tumor-promoting
effect of TANs, a number of studies using animal tumor models have
reported the antitumor and anti-metastatic functions of
neutrophils. A subset of TANs exhibit antitumor presenting cell
(APC) characteristics in early stage human lung cancer (103). IFN-γ and GM-CSF partially promote
the APC characteristics of immature neutrophils by downregulation
of the transcription factor Ikaros (104). TANs directly inhibit tumor cell
proliferation through antibody-dependent cell-mediated
cytotoxicity. The antibodies recognize tumors through the Fc
receptor on the surface of TANs, releasing cytotoxic mediators,
which results in cytotoxicity and the killing of tumor cells. TANs
also directly produce cytotoxic mediators such as ROS and MPO, and
NADP oxidase, to eliminate tumor cells. TANs have also been
reported to release TNF-related apoptosis-inducing ligand, which
results in the apoptosis of tumor cells (105–107). Previous studies have reported
that type I IFN can change the phenotype of TANs to antitumor
characteristics and prolong the life span of neutrophils in both
mice and humans. In the absence of IFN-β, TANs exhibit pro-tumor
characteristics, such as the low expression of NETs, decreased
tumor cytotoxicity, and the low expression of ICAM-1 and TNF-α. In
both a mouse melanoma model and patients with melanoma, IFN-β has
been reported to induce the polarization of N1 neutrophils towards
the antitumor type N1 TAN (41).
IFN-β can inhibit the expression of angiogenic factors such as VEGF
and MMP-9 in neutrophils during tumor invasion (108). In the LLC lung cancer model, the
lung metastasis rate of IFFNAR1(−/-) mice with impaired type I
interferon signal was reported to be higher than that of the
control group. Formation of the pre-metastatic niche and reduced
neutrophil toxicity to tumor cells enhanced the metastatic process
in IFFNAR1(−/-) mice (109).
Furthermore, IFN-β serves a major role in regulating neutrophil
production and their longevity in the primary tumor (110,111). The antitumor effect of
neutrophils in the tumor microenvironment in solid tumors varies
with tumor type, tumor progression stage and treatment type.
Although numerous clinical data suggest that neutrophil
infiltration leads to poor prognoses in solid tumors, some tumor
therapies depend on functioning neutrophils to be effective.
Therefore, controlling neutrophil phenotype could be an effective
treatment option, even though the factors that mediate neutrophil
polarization are still not entirely clear.
Chronic inflammation is not only related to the
onset and progression of lung cancer, but also negatively affects
the chemotherapeutic response and prognosis of patients. Previous
studies reported that the density of tumor-associated CD66b
neutrophils in NSCLC was an adverse prognostic factor and a marker
of systemic blood inflammation (112). Inflammation was also reported to
interfere with the effect of immunotherapy. In patients with NSCLC,
low neutrophil absolute value and high lymphocyte and eosinophil
counts were reported to be positively correlated with nivolumab
therapeutic outcome. Furthermore, higher neutrophil-to-lymphocyte
ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are negatively
correlated with overall survival (OS) and progression-free survival
(PFS) times, as well as with a poor response to nivolumab in
patients with metastatic NSCLC. However, low NLR and PLR are
associated with longer PFS times (30,113,114). Furthermore, elevated white blood
cell counts are associated with survival in those patients with
NSCLC suitable for resection. In a study involving patients with
early stage (stage I to III) NSCLC, high CD66b neutrophil density
was reported to have a small effect on OS, but was associated with
the incidence of recurrence after surgical resection (32). CD66b positive neutrophils were
reported to be elevated in 50% of NSCLCs and the increase in
CD66b-positive cells was associated with a higher cumulative
incidence of relapse (CIR) (median CIR, 51 months in patients with
low CD66b-positive cell density vs. 36 months in patients with high
CD66b-positive cell density) and tended to have worse OS time
(median OS, 57 months for patients with low CD66b-positive cell
density vs. 54 months for high CD66b-positive cell density group).
Furthermore, Rakaee et al (115) reported the relative
subtype-specific prognostic significance of TANs in patients with
early NSCLC; the presence of CD66b TANs in squamous cell carcinoma
was described as a positive prognostic factor, whereas in
adenocarcinoma it was reported as a negative prognostic factor.
However, a different study reported that high neutrophil counts
were associated with a poor prognosis in patients with squamous
cell carcinoma, but not adenocarcinoma (26). Moreover, high neutrophil counts
were reported to be associated with a poor prognosis in patients
with NSCLC who were treated with immune checkpoint inhibitors
(116).
Neutrophils have recently emerged as an important
factor in the occurrence and development of lung cancer. However, a
number of the current studies on the role of neutrophils in lung
cancer are based on animal experiments and most of the clinical
data reported were obtained from the separation of neutrophils from
peripheral blood. Data on the phenotype and function of TANs in
patients with lung cancer remain limited and mostly come from
patients with early disease. The potential of TANs as a therapeutic
target for cancer still requires further study. The change in the
phenotype and function of TANs should be one of key research topics
in lung cancer, which could lead to the development of a new
immunotherapeutic strategy. As TGF-β induces the N2 phenotype and
inhibits the N1 antitumor phenotype of TANs, blocking TGF-β could
be a potential therapeutic approach. Furthermore, neutralization of
chemokines to impair recruitment of neutrophils to the tumor could
be another effective therapeutic strategy. For instance, blockage
of the CXCL-8/CXCR-1/CXCR-2 axis using neutralizing antibodies
could inhibit the function of N2 TANs. Therefore, further studies
are required to gain a detailed understanding of TANs and lung
cancer, and to evaluate the modulation of TANs as a novel antitumor
therapy in lung cancer.
There is increasing evidence demonstrating that TANs
infiltrate lung cancer tissue. TANs exhibit plasticity between the
antitumor N1 and pro-tumor N2 phenotypes, which is determined by
the levels of related signaling factors in the tumor
microenvironment. The present review evaluated the role of TANs in
the onset and progression of lung cancer and the underlying
mechanisms, including the direct and indirect effect of TANs on
tumor cells, with emphasis on the role of TANs in lung cancer
progression. The literature strongly indicated that TANs are
crucial factors in lung cancer and could be a new immunotherapeutic
target in this disease.
Not applicable.
The present study was supported by the Shandong Province
Medicine and Health Project (grant no. 202003020509).
Not applicable.
JZ wrote the manuscript and reviewed the literature.
HL assisted in collection and review of the literature. WW and SJ
provided guidance, and revised and corrected the manuscript. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosen R and Karachaliou N: Large-scale
screening for somatic mutations in lung cancer. Lancet.
387:1354–1356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Wang X, Ji Z, Wang J, Bi N, Hui Z,
Lyu J, Liang J, Zhou Z, Feng Q, et al: Outcome of concurrent
chemoradiotherapy in locally advanced non-small-cell lung cancer
patients. Zhonghua Zhong Liu Za Zhi. 37:863–867. 2015.(In Chinese).
PubMed/NCBI
|
|
4
|
Moro-Sibilot D, Smit E, de Castro Carpeño
J, Lesniewski-Kmak K, Aerts J, Villatoro R, Kraaij K, Nacerddine K,
Dyachkova Y, Smith KT, et al: Outcomes and resource use of
non-small cell lung cancer (NSCLC) patients treated with first-line
platinum-based chemotherapy across Europe: FRAME prospective
observational study. Lung Cancer. 88:215–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu H and Jiang Z: Advances in antibody
therapeutics targeting small-cell lung cancer. Adv Clin Exp Med.
27:1317–1323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bielenberg DR and Zetter BR: The
contribution of angiogenesis to the process of metastasis. Cancer
J. 21:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nemeth JA, Cher ML, Zhou Z, Mullins C,
Bhagat S and Trikha M: Inhibition of alpha(v)beta3 integrin reduces
angiogenesis, bone turnover, and tumor cell proliferation in
experimental prostate cancer bone metastases. Clin Exp Metastasis.
20:413–420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Cao X: Characteristics and
significance of the pre-metastatic niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoenfeld Y, Tal A, Berliner S and Pinkhas
J: Leukocytosis in non hematological malignancies-a possible
tumor-associated marker. J Cancer Res Clin Oncol. 111:54–58. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tecchio C, Scapini P, Pizzolo G and
Cassatella MA: On the cytokines produced by human neutrophils in
tumors. Semin Cancer Biol. 23:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houghton AM: The paradox of
tumor-associated neutrophils. Cell Cycle. 9:1732–1737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gregory A and Houghton AM:
Tumor-associated neutrophils: New targets for cancer therapy.
Cancer Research. 71:2411–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J and Bae JS: Tumor-Associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borregaard N: Neutrophils, from marrow to
microbes. Immunity. 33:657–670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B,
Gorbatov R, et al: Origins of tumor associated macrophages and
neutrophils. Proc Natl Acad Sci USA. 109:2491–2496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stöth M, Freire Valls A, Chen M, Hidding
S, Knipper K, Shen Y, Klose J, Ulrich A, Ruiz de Almodovar C,
Schneider M and Schmidt T: Splenectomy reduces lung metastases and
tumoral and metastatic niche inflammation. Int J Cancer.
145:2509–2520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eruslanov EB, Bhojnagarwala PS, Quatromoni
JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A,
Litzky L, Hancock WW, et al: Tumor-associated neutrophils stimulate
T cell responses in early-stage human lung cancer. J Clin Invest.
124:5466–5480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piccard H, Muschel RJ and Opdenakker G: On
the dual roles and polarized phenotypes of neutrophils in tumor
development and progression. Crit Rev Oncol Hematol. 82:296–309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sionov RV, Fridlender ZG and Granot Z: The
multifaceted roles neutrophils play in the tumor microenvironment.
Cancer Microenviron. 8:125–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:443–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Houghton AM: The paradox of
tumor-associated neutrophils: Fueling tumor growth with cytotoxic
substances. Cell Cycle. 9:1732–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen M, Hu P, Donskov F, Wang G, Liu Q and
Du J: Tumorassociated neutrophils as a new prognostic factor in
cancer: A systematic review and meta-analysis. PLoS One.
9:e982592014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uribe-Querol E and Rosales C: Neutrophils
in cancer: Two sides of the same coin. J Immunol Res.
2015:9836982015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Wu S, Yang Y, Zhao M, Zhu G and Hou
Z: The prognostic landscape of tumor-infiltrating immune cell and
immunomodulators in lung cancer. Biomed Pharmacother. 95:55–61.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shaul ME, Eyal O, Guglietta S, Aloni P,
Zlotnik A, Forkosh E, Levy L, Weber LM, Levin Y, Pomerantz A, et
al: Circulating neutrophil subsets in advanced lung cancer patients
exhibit unique immune signature and relate to prognosis. FASEB J.
34:4204–4218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Chen H, Mao B, Zhou Y, Shi X, Tang
L, Jiang H, Wang G and Zhuang W: Transcriptional characterization
of the tumor immune microenvironment and its prognostic value for
locally advanced lung adenocarcinoma in a Chinese population.
Cancer Manag Res. 11:9165–9173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sebastian NT, Raj R, Prasad R, Barney C,
Brownstein J, Grecula J, Haglund K, Xu-Welliver M, Williams TM and
Bazan JG: Association of Pre- and Posttreatment
neutrophil-lymphocyte ratio with recurrence and mortality in
locally advanced non-small cell lung cancer. Front Oncol.
10:5988732020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diem S, Schmid S, Krapf M, Flatz L, Born
D, Jochum W, Templeton AJ and Früh M: Neutrophil-to-Lymphocyte
ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic
markers in patients with non-small cell lung cancer (NSCLC) treated
with nivolumab. Lung Cancer. 111:176–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao K, Wang C, Shi F, Huang Y, Ma L, Li M
and Song Y: Combined prognostic value of the SUVmax derived from
FDG-PET and the lymphocyte-monocyte ratio in patients with stage
IIIB-IV non-small cell lung cancer receiving chemotherapy. BMC
Cancer. 21:662021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ilie M, Hofman V, Ortholan C, Bonnetaud C,
Coëlle C, Mouroux J and Hofman P: Predictive clinical outcome of
the intratumoralCD66b-positive neutrophil-to-CD8-positive T-cell
ratio in patients with resectable nonsmall cell lung cancer.
Cancer. 118:1726–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dumitru CA, Lang S and Brandau S:
Modulation of neutrophil granulocytes in the tumor
microenvironment: Mechanisms and consequences for tumor
progression. Semin Cancer Biol. 23:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abe H, Morikawa T, Saito R, Yamashita H,
Seto Y and Fukayama M: In Epstein-Barr virus-associated gastric
carcinoma a high density ofCD66b-positive tumor-associated
neutrophils is associated with intestinal-type histology and low
frequency of lymph node metastasis. Virchows Arch. 468:539–548.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
López-Lago MA, Posner S, Thodima VJ,
Molina AM, Motzer RJ and Chaganti RS: Neutrophil chemokines
secreted by tumor cells mount a lung antimetastatic response during
renal cell carcinoma progression. Oncogene. 32:1752–1760. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Houghton AM, Rzymkiewicz DM, Ji H, Gregory
AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR,
et al: Neutrophil Elastase-mediated degradation of IRS-1
accelerates lung tumor growth. Nat Med. 16:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deryugina EI, Zajac E, Juncker-Jensen A,
Kupriyanova TA, Welter L and Quigley JP: Tissue-infiltrating
neutrophils constitute the major in vivo source of
angiogenesis-inducing MMP-9 in the tumor microenvironment.
Neoplasia. 16:771–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46. 94–112.
2015.PubMed/NCBI
|
|
40
|
Grunwald B, Vandooren J, Locatelli E,
Fiten P, Opdenakker G, Proost P, Krüger A, Lellouche JP, Israel LL,
Shenkman L and Comes Franchini M: Matrix metalloproteinase-9
(MMP-9) as an activator of nanosystems for targeted drug delivery
in pancreatic cancer. J Control Release. 239:39–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Andzinski L, Kasnitz N, Stahnke S, Wu CF,
Gereke M, von Köckritz-Blickwede M, Schilling B, Brandau S, Weiss S
and Jablonska J: Type I IFNs induce anti-tumor polarization of
tumor associated neutrophils in mice and human. Int J Cancer.
138:1982–1993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang N, Wang Q, Chi J, Xiang F, Lin M,
Wang W, Wei F and Feng Y: Carcinoembryonic antigen cell adhesion
molecule 1 inhibits the antitumor effect of neutrophils in tongue
squamous cell carcinoma. Cancer Sci. 110:519–529. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krstic J and Santibanez JF: Transforming
growth factor-beta and matrix metalloproteinases: Functional
interactions in tumor Stroma-infiltrating myeloid cells.
ScientificWorldJournal. 2014:5217542014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim Y, Lee D, Lee J, Lee S and Lawler S:
Role of tumor-associated neutrophils in regulation of tumor growth
in lung cancer development: A mathematical model. PLoS One.
14:e02110412019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shaul ME, Levy L, Sun J, Mishalian I,
Singhal S, Kapoor V, Horng W, Fridlender G, Albelda SM and
Fridlender ZG: Tumor-associated neutrophils display a distinct N1
profile following TGF-β modulation: A transcriptomics analysis of
pro-vs. Antitumor TANs. Oncoimmunology. 5:e12322212016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Saha S and Biswas SK: Tumor-Associated
neutrophils show phenotypic and functional divergence in human lung
cancer. Cancer Cell. 30:11–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Qiao X, Shi H, Han X, Liu W, Tian
X and Zeng X: Circulating tumor-associated neutrophils (cTAN)
contribute to circulating tumor cell survival by suppressing
peripheral leukocyte activation. Tumour Biol. 37:5397–5404. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mishalian I, Bayuh R, Levy L, Zolotarov L,
Michaeli J and Fridlender ZG: Tumor-associated neutrophils (TAN)
develop pro-tumorigenic properties during tumor progression. Cancer
Immunol Immunother. 62:1745–1756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McDonald B, Urrutia R, Yipp BG, Jenne CN
and Kubes P: Intravascular neutrophil extracellular traps capture
bacteria from the bloodstream during sepsis. Cell Host Microbe.
12:324–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Urban CF, Ermert D, Schmid M, Abu-Abed U,
Goosmann C, Nacken W, Brinkmann V, Jungblut PR and Zychlinsky A:
Neutrophil extracellular traps contain calprotectin, a cytosolic
protein complex involved in host defense against Candida albicans.
PLoS Pathog. 5:e10006392009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spicer JD, McDonald B, Cools-Lartigue JJ,
Chow SC, Giannias B, Kubes P and Ferri LE: Neutrophils promote
liver metastasis via Mac-1-mediated interactions with circulating
tumor cells. Cancer Res. 72:3919–3927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rayes RF, Mouhanna JG, Nicolau I, Bourdeau
F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N,
et al: Primary tumors induce neutrophil extracellular traps with
targetable metastasis promoting effects. JCI Insight.
5:e1280082019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Erpenbeck L and Schön MP: Neutrophil
extracellular traps: Protagonists of cancer progression? Oncogene.
36:2483–2490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Richardson JJR, Hendrickse C, Gao-Smith F
and Thickett DR: Neutrophil extracellular trap production in
patients with colorectal cancer in vitro. Int J Inflam.
2017:49150622017.PubMed/NCBI
|
|
56
|
Jin W, Xu HX, Zhang SR, Li H, Wang WQ, Gao
HL, Wu CT, Xu JZ, Qi ZH, Li S, et al: Tumor-Infiltrating nets
predict postsurgical survival in patients with pancreatic ductal
adenocarcinoma. Ann Surg Oncol. 26:635–643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Thålin C, Lundström S, Seignez C, Daleskog
M, Lundström A, Henriksson P, Helleday T, Phillipson M, Wallén H
and Demers M: Citrullinated histone H3 as a novel prognostic blood
marker in patients with advanced cancer. PLoS One. 13:e01912312018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou J, Yang Y, Gan T, Li Y, Hu F, Hao N,
Yuan B, Chen Y and Zhang M: lung cancer cells release high mobility
group box 1 and promote the formation of neutrophil extracellular
traps. Oncol Lett. 18:181–188. 2019.PubMed/NCBI
|
|
59
|
Najmeh S, Cools-Lartigue J, Giannias B,
Spicer J and Ferri LE: Simplified human neutrophil extracellular
traps (NETs) isolation and handling. J Vis Exp. 526872015.doi:
10.3791/52687. PubMed/NCBI
|
|
60
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Monti M, De Rosa V, Iommelli F, Carriero
MV, Terlizzi C, Camerlingo R, Belli S, Fonti R, Di Minno G and Del
Vecchio S: Neutrophil extracellular traps as an adhesion substrate
for difffferent tumor cells expressing RGD-binding integrins. Int J
Mol Sci. 19:23502018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kanamaru R, Ohzawa H, Miyato H, Yamaguchi
H, Hosoya Y, Lefor AK, Sata N and Kitayama J: Neutrophil
extracellular traps generated by low density neutrophils obtained
from peritoneal lavage fluid mediate tumor cell growth and
attachment. J Vis Exp. 138:582012018.PubMed/NCBI
|
|
63
|
Yang L, Liu L, Zhang R, Hong J, Wang Y,
Wang J, Zuo J, Zhang J, Chen J and Hao H: IL-8 mediates a positive
loop connecting increased neutrophil extracellular traps (Nets) and
colorectal cancer liver metastasis. J Cancer. 11:4384–4396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei
R, Lin ZF, Wang XY, Wang CQ, Lu M, et al: Increased neutrophil
extracellular traps promote metastasis potential of hepatocellular
carcinoma via provoking tumorous inflammatory response. J Hematol
Oncol. 13:32020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kern MA, Haugg AM, Koch AF, Schilling T,
Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C,
Schulze-Bergkamen H, et al: yclooxygenase-2 inhibition induces
apoptosis signaling via death receptors and mitochondria in
hepatocellular carcinoma. Cancer Res. 66:7059–7066. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: Evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu L, Stevens J, Hilton MB, Seaman S,
Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL,
et al: COX-2 inhibition potentiates antiangiogenic cancer therapy
and prevents metastasis in preclinical models. Sci Transl Med.
6:242ra842014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Takesue S, Ohuchida K, Shinkawa T, Otsubo
Y, Matsumoto S, Sagara A, Yonenaga A, Ando Y, Kibe S, Nakayama H,
et al: Neutrophil extracellular traps promote liver micrometastasis
in pancreatic ductal adenocarcinoma via the activation of
cancer-associated fibroblasts. Int J Oncol. 56:596–605.
2020.PubMed/NCBI
|
|
69
|
Xiao Y, Cong M, Li J, He D, Wu Q, Tian P,
Wang Y, Yang S, Liang C, Liang Y, et al: Cathepsin C promotes
breast cancer lung metastasis by modulating neutrophil infiltration
and neutrophil extracellular trap formation. Cancer Cell.
39:423–437.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yuan M, Zhu H, Xu J, Zheng Y, Cao X and
Liu Q: Tumor-Derived CXCL1 promotes lung cancer growth via
recruitment of tumor-associated neutrophils. J Immunol Res.
2016:65304102016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eash KJ, Greenbaum AM, Gopalan PK and Link
DC: CXCR2 and CXCR4 antagonistically regulate neutrophil
traffificking from murine bone marrow. J Clin Investig.
120:2423–2431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Raccosta L, Fontana R, Maggioni D,
Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E,
Trincavelli ML, Daniele S, et al: The oxysterol-CXCR2 axis plays a
key role in the recruitment of tumor-promoting neutrophils. J Exp
Med. 210:1711–1728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Forsthuber A, Lipp K, Andersen L,
Ebersberger S, Graña-Castro, Ellmeier W, Petzelbauer P,
Lichtenberger BM and Loewe R: CXCL5 as regulator of neutrophil
function in cutaneous melanoma. J Invest Dermatol. 139:186–194.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Viola A, Sarukhan A, Bronte V and Molon B:
The pros and cons of chemokines in tumor immunology. Trends
Immunol. 33:496–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang SY, Mills L, Mian B, Tellez C,
McCarty M, Yang XD, Gudas JM and Bar-Eli M: Fully humanized
neutralizing antibodies to interleukin-8 (ABX–IL8) inhibit
angiogenesis, tumor growth, and metastasis of human melanoma. Am J
Pathol. 161:125–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Luca M, Huang S, Gershenwald JE, Singh RK,
Reich R and Bar-Eli M: Expression of interleukin-8 by human
melanoma cells up-regulates MMP-2 activity and increases tumor
growth and metastasis. Am J Pathol. 151:1105–1113. 1997.PubMed/NCBI
|
|
77
|
Pignatti P, Moscato G, Casarini S,
Delmastro M, Poppa M, Brunetti G, Pisati P and Balbi B:
Downmodulation of CXCL8/IL-8 receptors on neutrophils after
recruitment in the airways. J Allergy Clin Immunol. 115:88–94.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fortunati E, Kazemier KM, Grutters JC,
Koenderman L and Van den Bosch VJ: Human neutrophils switch to an
activated phenotype after homing to the lung irrespective of
inflammatory disease. Clin Exp Immunol. 155:559–566. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tazzyman S, Niaz H and Murdoch C:
Neutrophil-mediated tumour angiogenesis: Subversion of immune
responses to promote tumour growth. Semin Cancer Biol. 23:149–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pelletier M, Maggi L, Micheletti A,
Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato
F, Romagnani S and Cassatella MA: Evidence for a cross-talk between
human neutrophils and Th17 cells. Blood. 115:335–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jungnickel C, Schmidt LH, Bittigkoffer L,
Wolf L, Wolf A, Ritzmann F, Kamyschnikow A, Herr C, Menger MD,
Spieker T, et al: IL-17C mediates the recruitment of
tumor-associated neutrophils and lung tumor growth. Oncogene.
36:4182–4190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shojaei F, Singh M, Thompson JD and
Ferrara N: Role of Bv8 in neutrophildependent angiogenesis in a
transgenic model of cancer progression. Proc Natl Acad Sci.
105:2640–2645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tazawa H, Okada F, Kobayashi T, Tada M,
Mori Y, Une Y, Sendo F, Kobayashi M and Hosokawa M: Infiltration of
neutrophils is required for acquisition of metastatic phenotype of
benign murine fibrosarcoma cells: Implication of
inflammation-associated carcinogenesis and tumor progression. Am J
Pathol. 163:2221–2232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Vannitamby A, Seow HJ, Anderson G, Vlahos
R, Thompson M, Steinfort D, Irving LB and Bozinovski S:
Tumour-associated neutrophils and loss of epithelial PTEN can
promote corticosteroid-insensitive MMP-9 expression in the
chronically inflamed lung microenvironment. Thorax. 72:1140–1143.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Coussens LM, Tinkle CL, Hanahan D and Werb
Z: MMP-9 supplied by bone marrow-derived cells contributes to skin
carcinogenesis. Cell. 103:481–490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qu X, Zhuang G, Yu L, Meng G and Ferrara
N: Induction of Bv8 expression by granulocyte colony-stimulating
factor in CD11b+Gr1+ cells: Key role of Stat3 signaling. J Biol
Chem. 287:19574–19584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek
T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al:
Granulocyte-colony stimulating factor promotes lung metastasis
through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci
USA. 107:21248–21255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hu P, Shen M, Zhang P, Zheng C, Pang Z,
Zhu L and Du J: Intratumoral neutrophil granulocytes contribute to
epithelial-mesenchymal transition in lung adenocarcinoma cells.
Tumor Boil. 36:7789–7796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tsai YM, Wu KL, Liu YW, Chang AW, Huang
YC, Chang CY, Tsai PH, Liao SH, Hung JY and Hsu YL: Cooperation
between cancer and fibroblasts in vascular mimicry and N2-type
neutrophil recruitment via Notch2-Jagged1 interaction in lung
cancer. Front Oncol. 11:6969312021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Patel S, Fu S, Mastio J, Dominguez GA,
Purohit A, Kossenkov A, Lin C, Alicea-Torres K, Sehgal M, Nefedova
Y, et al: Unique pattern of neutrophil migration and function
during tumor progression. Nat Immunol. 19:1236–1247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shao B, Zhao X, Liu T, Zhang Y, Sun R,
Dong X, Liu F, Zhao N, Zhang D, Wu L, et al: LOXL2 promotes
vasculogenic mimicry and tumour aggressiveness in hepatocellular
carcinoma. J Cell Mol Med. 23:1363–1374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mayer C, Darb-Esfahani S, Meyer AS, Hübner
K, Rom J, Sohn C, Braicu I, Sehouli J, Hänsch GM and Gaida MM:
Neutrophil granulocytes in ovarian cancer-induction of
epithelial-to-mesenchymal-transition and tumor cell migration. J
Cancer. 7:546–554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Michaeli J, Shaul ME, Mishalian I, Hovav
AH, Levy L, Zolotriov L, Granot Z and Fridlender ZG:
Tumor-associated neutrophils induce apoptosis of non-activated CD8
T-cells in a TNFα and NO-dependent mechanism, promoting a
tumor-supportive environment. Oncoimmunology. 6:e13569652017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Singhal S, Bhojnagarwala PS, O'Brien S,
Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L,
Deshpande C, et al: Origin and role of a subset of tumor-associated
neutrophils with antigen-presenting cell features in early-stage
human lung cancer. Cancer Cell. 30:120–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sica A, Porta C, Morlacchi S, Banfi S,
Strauss L, Rimoldi M, Totaro MG and Riboldi E: Origin and functions
of tumor-associated myeloid cells (TAMCs). Cancer Microenviron.
5:133–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lee WL and Downey GP: Leukocyte elastase:
Physiological functions and role in acute lung injury. Am J Respir
Crit Care Med. 164:896–904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wislez M, Antoine M, Rabbe N, Gounant V,
Poulot V, Lavolé A, Fleury-Feith J and Cadranel J: Neutrophils
promote aerogenous spread of lung adenocarcinoma with bronchiolo
alveolar carcinoma features. Clin Cancer Res. 13:3518–3527. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gong L, Cumpian AM, Caetano MS, Ochoa CE,
De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q
and Moghaddam SJ: Promoting effect of neutrophils on lung
tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol
Cancer. 12:1542013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liang W, Li Q and Ferrara N: Metastatic
growth instructed by neutrophil-derived transferrin. Proc Natl Acad
Sci USA. 115:11060–11065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
O'Brien S, Thomas RM, Wertheim GB, Zhang
F, Shen H and Wells AD: Ikaros imposes a barrier to CD8+ T cell
differentiation by restricting autocrine IL-2 production. J
Immunol. 11:5118–5129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang W, Erbe AK, Hank JA, Morris ZS and
Sondel PM: NK cell-mediated antibody dependent cellular
cytotoxicity in cancer immunotherapy. Front Immunol. 6:3682015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Amarante-Mendes GP and Gfiffith TS:
Therapeutic applications of TRAIL receptor agonists in cancer and
beyond. Pharmacol Ther. 155:117–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Granot Z, Henke E, Comen EA, King TA,
Norton L and Benezra R: Tumor entrained neutrophils inhibit seeding
in the premetastatic lung. Cancer Cell. 20:300–314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jablonska S, Leschner K and Westphal S:
Neutrophils responsive to endogenous IFN-β regulate tumor
angiogenesis and growth in a mouse tumor model. J Clin Invest.
120:1151–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu CF, Andzinski L, Kasnitz N, Kröger A,
Klawonn F, Lienenklaus S, Weiss S and Jablonska J: The lack of type
I interferon induces neutrophil-mediated pre-metastatic niche
formation in the mouse lung. Int J Cancer. 137:837–847. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Andzinski L, Wu CF, Lienenklaus S, Kröger
A, Weiss S and Jablonska J: Delayed apoptosis of tumor associated
neutrophils in the absence of endogenous IFN-β. Int J Cancer.
136:572–583. 2015.PubMed/NCBI
|
|
111
|
Jablonska J, Wu CF, Andzinski L, Leschner
S and Weiss S: CXCR2-mediated tumor associated neutrophil
recruitment is regulated by IFN-beta. Int J Cancer. 134:1346–1358.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Carus A, Ladekarl M, Hager H, Pilegaard H,
Nielsen PS and Donskov F: Tumor-associated neutrophils and
macrophages in non-small cell lung cancer: No immediate impact on
patient outcome. Lung Cancer. 81:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tanizaki J, Haratani K, Hayashi H, Chiba
Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K,
et al: Peripheral blood biomarkers associated with clinical outcome
in non-small cell lung cancer patients treated with nivolumab. J
Thorac Oncol. 13:97–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Taniguchi Y, Tamiya A, Isa SI, Nakahama K,
Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T,
et al: Predictive factors for poor progression-free survival in
patients with non-small cell lung cancer treated with nivolumab.
Anticancer Res. 37:857–5862. 2017.
|
|
115
|
Rakaee M, Busund LT, Paulsen EE,
Richardsen E, Al-Saad S, Andersen S, Donnem T, Bremnes RM and
Kilvaer TK: Prognostic effect of intratumoral neutrophils across
histological subtypes of non-small cell lung cancer. Oncotarget.
7:72184–72196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kubo S, Kobayashi N, Somekawa K, Hirata M,
Kamimaki C, Aiko H, Katakura S, Teranishi S, Watanabe K, Hara YU,
et al: Identification of biomarkers for non-small-cell lung cancer
patients treated with an immune checkpoint inhibitor. Anticancer
Res. 40:3889–3896. 2020. View Article : Google Scholar : PubMed/NCBI
|