Introduction
Oral cancer is a major public health concern in
India (1). According to the
GLOBOCAN 2020 data, oral and lip cancer accounts for ~177,757
related deaths and 377,713 new cases annually worldwide (2), and for 75,290 related deaths and
135,929 new cases yearly in India. Lip and oral cancer is the most
common malignant neoplasm among males in India (3). The age-standardised incidence of oral
cancer in Trivandrum (also known as Thiruvananthapuram; India)
constitutes 14.5/100,000 males and 5.6/100,000 females (4). At the Regional Cancer Centre in
Trivandrum, lip and oral cancer constitutes 21.7% of registered
male patients with cancer and 6.8% of female patients (5). Squamous cell carcinoma (SCC) is the
most common histological type, which develops from the stratified
squamous epithelium of the mucosa. Males are found to be more
commonly affected by SCC than females (6). Tobacco, alcohol consumption and the
habit of chewing betel nut leaves rolled with lime and tobacco
(termed pan), are the common aetiological causes for oral cancer in
India. Other factors include exposure to ultraviolet light, human
papillomavirus infection, orodental factors, dietary deficiencies,
syphilis and chronic candidiasis (6). HPV testing (P-16
immunohistochemistry) is not routinely recommended for oral and lip
tumours as the prevelance of HPV in these sites is said to be low,
unlike for oropharyngeal malignancies where the HPV incidence is
reported to be high.
The American Joint Committee on Cancer Staging
Manual (AJCC) is commonly used to stage oral cancer (7). Early stage lip tumours (AJCC stages I
and II) are treated with single modality treatment, either using
radiotherapy [external beam radiotherapy (EBRT) or brachytherapy]
or surgery. The cure and local control rates are high, irrespective
of treatment with radiotherapy or surgery for early stage lip
carcinoma (8). Locally advanced
tumours (AJCC stages III and IVa) are treated with surgery followed
by adjuvant treatment (9). The
advantage of radiation is an improvement in organ preservation with
a positive cosmetic outcome; thus, radiotherapy is often used as
the single modality treatment. When using brachytherapy, it is
possible to deliver a high localised dose of radiation to the
tumour with rapid dose fall-off when compared to EBRT (10). To date, to the best of our
knowledge, no randomised studies comparing these different
treatment strategies have been reported. In the clinic, the type of
treatment modality is selected based on the size and location of
the tumour, the expected functional outcome and the accessibility
of the treatment type (11). The
aims of the present study were to retrospectively evaluate the
clinical profile and treatment outcomes of patients with SCC of the
lip treated with radical intent at the Regional Cancer Centre.
Patients and methods
Patients and data collection
The present study retrospectively analysed the data
of all patients with biopsy-confirmed carcinoma of the lip treated
with radical radiotherapy (brachytherapy or EBRT) or surgery with
or without adjuvant treatment at the Regional Cancer Centre. Only
patients who were treated with palliative intent were excluded from
the study. A total of 120 patients treated between January 2010 and
December 2016 were eligible for the analysis. The case records of
each of these patients were reviewed and data on patient
demographics, clinical treatment and follow-up details were
captured in a structured proforma. The 7th edition of the American
Joint Committee on Cancer Staging Manual was used to stage the
patients included in the study (12). The follow-up information was
collected until November 20, 2021, and if information was not
available, the patients were contacted over the telephone and their
status was updated accordingly.
Statistical analysis
Survival estimates were generated using Kaplan-Meier
analysis using IBM SPSS for windows version 21.0 (13). Disease-free survival (DFS) was
defined as the period from the date of diagnosis to the date of
first documentation of any recurrence. Overall survival (OS) was
defined as the period from the date of diagnosis until death due to
any cause, or the date of the last follow-up. Data of all 120
patients were used for the final analysis. Univariate and
multivariate Cox regression analyses were performed to determine
the impact of the patient- and tumour-related factors and treatment
modality on patient outcomes (DFS and OS). Age, sex, performance
status, smoking, alcohol consumption, pan chewing, primary tumour
stage, nodal stage and composite stage were tested for statistical
significance in the univariate and multivariate analyses using the
Cox-proportional hazards regression model. P<0.05 was considered
to indicate a statistically significant difference.
Results
All 120 patients with SCC of the lip treated with
radical radiotherapy (brachytherapy or EBRT) or surgery with or
without adjuvant treatment between January 2010 and December 2016
were eligible for analysis. The mean age of the patients included
in the study was 62 years (range, 39–89 years). The majority of
patients (81.6%) were >50 years of age, and males comprised
52.5% of the study population. The stage-wise distribution of
patients was as follows: Stage I, 38 (31.7%); stage II, 26 (21.7%);
stage III, 26 (21.7%); and stage IVa, 30 (25.0%). The baseline
characteristics of these 120 patients are presented in Table I.
 | Table I.Baseline characteristics of the 120
patients included in the analysis. |
Table I.
Baseline characteristics of the 120
patients included in the analysis.
| Baseline
characteristics | Patients, n (%) |
|---|
| Age, years |
|
| ≤50 | 22 (18.3) |
|
>50 | 98 (81.7) |
| Sex |
|
| Male | 63 (52.5) |
|
Female | 57 (47.5) |
| Habits-Alcohol
use |
|
| Yes | 32 (26.7) |
| No | 88 (73.3) |
| Habits-Smoking |
|
| Yes | 28 (23.3) |
| No | 92 (76.7) |
| Habits-Pan
chewing |
|
| Yes | 93 (77.5) |
| No | 27 (22.5) |
| Tumour
stagea |
|
| 1 | 46 (38.3) |
| 2 | 45 (37.5) |
| 3 | 7 (5.8) |
| 4a | 22 (18.3) |
| Nodal
stagea |
|
| 0 | 68 (56.7) |
| 1 | 38 (31.7) |
| 2 | 14 (11.7) |
| Composite
stagea |
|
| I | 38 (31.7) |
| II | 26 (21.7) |
| III | 26 (21.7) |
| Iva | 30 (25.0) |
| Performance
statusb |
|
| 0 | 2 (1.7) |
| 1 | 104 (86.7) |
| 2 | 14 (11.7) |
| Treatment
modality |
|
|
Brachytherapy | 16 (13.3) |
| Surgery
with/without adjuvant treatment | 38 (31.7) |
| Radical
external beam radiotherapy | 66 (55.0) |
Of the 120 patients, 16 patients (13.3%) were
treated with brachytherapy, 38 (31.7%) with surgery with or without
adjuvant treatment and 66 (55.0%) with EBRT. Of the 16 patients
treated with brachytherapy, 15 patients (93.8%) were treated with a
dose schedule of 48 Gy in 12 fractions over a period of 6 days and
1patient (6.7%) was treated using a schedule of 44 Gy in 11
fractions over a period of 6 days. Of the 16 patients treated with
brachytherapy, 2 patients (12.5%) had residual disease at the first
follow-up and the remaining 14 patients (87.5%) went into clinical
remission after treatment. The most commonly used dose schedule for
patients treated with EBRT was 52.50 Gy in 15 fractions over 3
weeks in the majority of patients (75.8%) followed by 60 Gy in 26
fractions over 5 weeks (13.6%). At the Regional Cancer Centre,
accelerated radiotherapy treatment for oral cancer has been
practiced as per the Manchester schedule (52.50 Gy in 15 fractions
over 3 weeks) for several decades since 1980 (14). In total, 5 of the 66 patients
(7.6%) treated with EBRT received induction chemotherapy and 1
patient (1.5%) received concurrent chemotherapy (3 weekly cisplatin
administrations; two cycles) along with radical radiation. The
chemotherapy schedules used as induction therapy were single-agent
methotrexate (3 patients) and cisplatin + 5-flurouracil (2
patients). Of the 66 patients treated with EBRT, 11 patients
(16.7%) had residual disease at the first follow-up.
Of the 38 patients who were treated with surgery, 20
patients (52.6%) underwent wide local excision of the primary
tumour alone, 15 (39.5%) underwent wide excision of the primary
tumour with ipsilateral neck dissection and 3 (7.9%) underwent wide
excision of the primary tumour with bilateral neck dissection.
Bilateral neck dissection was performed in patients with bilateral
enlarged cervical nodes. Out of these 38 patients, 4 patients
(10.5%) with stage III and IVa disease received induction
chemotherapy to decrease the tumour bulk prior to surgery (2
patients received induction chemotherapy with cisplatin +
5-flurouracil and 2 patients received induction chemotherapy with
single-agent methotrexate). A total of 12 patients (31.6%) received
adjuvant radiation following primary surgery. Of these 12 patients,
4 patients (33.3%) had node-positive disease, 2 (16.7%) had T3 and
T4 disease and 2 (16.7%) exhibited perineural spread in the
pathological analysis following surgery. The remaining 4 patients
(33.3%) had received induction chemotherapy for stage III and IVa
disease prior to surgery. All 12 patients completed the planned
standard course of adjuvant radiation using 60 Gy in 30 fractions
over 6 weeks. Only 1 of the 12 patients (8.3%) received adjuvant
concurrent chemo-radiation with 3 weekly cisplatin (2 cycles), as
the patient had extracapsular spread in the nodes.
The median follow-up period was 67.6 months (range,
3.5-128.5 months). The follow-up information available to calculate
5-year survival figures was limited; therefore, 4-year survival
estimates were calculated. With 76% of the follow-up information
available at the time of the analysis, the OS and DFS rates at 4
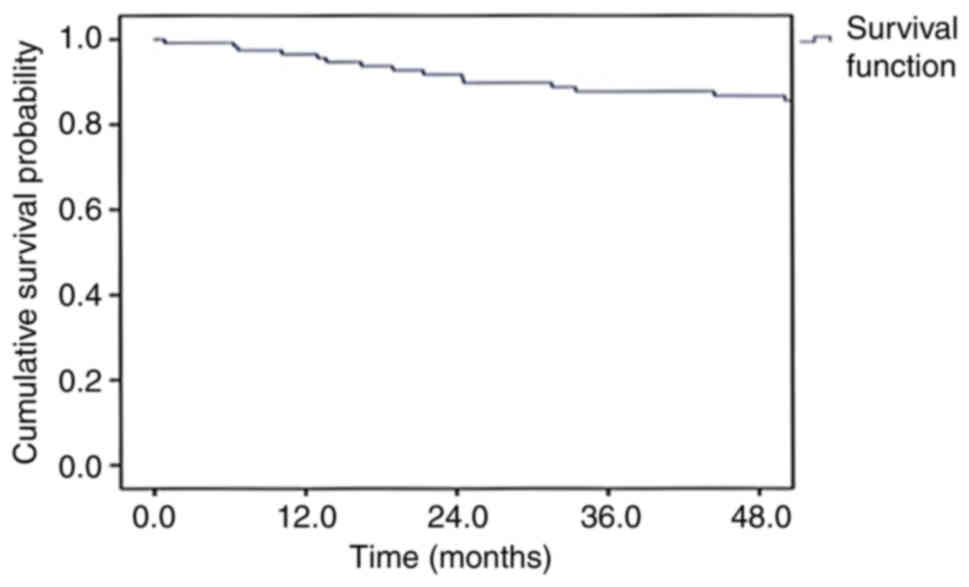
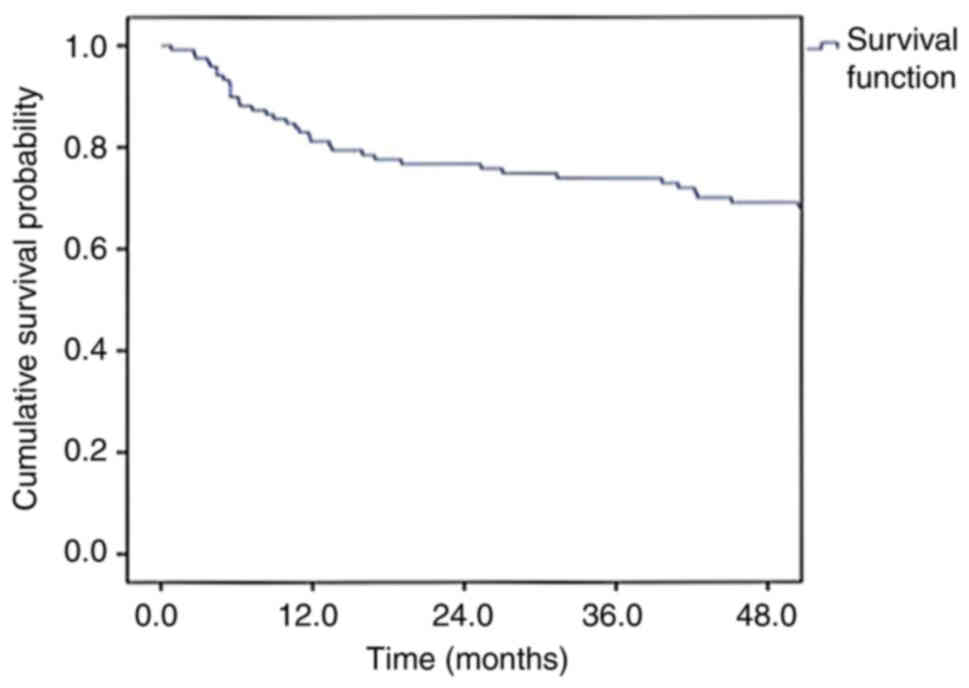
years for the entire cohort were 86.7 and 69.1%, respectively
(Figs. 1 and 2). The 4-year OS rates of patients with
stage I, II, III and IVa disease were 88.9, 95.2, 86.8 and 75.3%,
respectively, and the DFS rates were 83.6, 69.5, 78.8 and 42.9%,
respectively (Table II).
 | Table II.Overall survival and disease-free
survival rates based on the clinical staging and treatment modality
of 120 patients. |
Table II.
Overall survival and disease-free
survival rates based on the clinical staging and treatment modality
of 120 patients.
| Parameter | Patients, n | 4-year overall
survival, % | 4-year disease-free
survival, % |
|---|
| Clinical
stagea |
|
|
|
| I | 38 | 88.9 | 83.6 |
| II | 26 | 95.2 | 69.5 |
|
III | 26 | 86.8 | 78.8 |
|
Iva | 30 | 75.3 | 42.9 |
| Treatment
modality |
|
|
|
|
Brachytherapy | 16 | 92.9 | 68.2 |
| Surgery
with/without adjuvant treatment | 38 | 85.6 | 75.4 |
| Radical
external beam radiotherapy | 66 | 85.4 | 65.8 |
Out of the 120 patients treated, 36 patients (30.0%)
developed a relapse, and the most common site of failure was at the
primary tumour site [in 33 of the 36 (91.7%) patients]. The median
time until relapse was 15.9 months (range, 1–113 months). A total
of 2 patients (5.6%) developed an isolated nodal relapse and 1
patient (2.8%) developed distant metastasis to the bone.
The 4-year OS rates of patients treated with
surgery, EBRT and brachytherapy were 85.6, 85.4 and 92.9%,
respectively, and the 4-year DFS rates of patients treated with
surgery, EBRT and brachytherapy were 75.4, 65.8 and 68.2%,
respectively. Of the 16 patients treated with brachytherapy, 2
patients (12.5%) who had residual disease at the first follow-up
later developed progression of the residual disease and another 3
patients (18.8%) developed disease recurrence during follow-up. All
of these 5 patients had disease at the primary site. In total, 3 of
the 5 patients (60.0%) with disease were later treated with salvage
surgery. There were no nodal relapses reported among the patients
treated with brachytherapy.
Of the 66 patients treated with radical EBRT, 11
patients (16.7%) who had residual disease at the first follow-up
later developed progression of the residual disease and another 13
patients (19.7%) developed disease recurrence during their
follow-up period. All of the aforementioned 24 patients had disease
at the primary site. Out of the 24 patients with disease, only 6
patients (25.0%) underwent salvage surgery later. There were no
nodal relapses reported among the patients treated with radical
EBRT.
Of the 38 patients treated with surgery with or
without adjuvant therapy, 7 patients (18.4%) developed a relapse.
The most common site of relapse following surgery with or without
adjuvant therapy was at the primary site [in 4 of the 7 (57.1%)
patients]. In addition, 2 patients (5.3%) developed an isolated
nodal relapse and 1 patient (2.6%) developed distant metastasis to
the bone. All 3 patients who developed isolated nodal relapse and
bone metastasis had stage IVa disease at presentation.
A total of 22 (18.3%) patients developed a secondary
malignancy during their follow-up period. The most common site for
the development of a secondary malignancy was another subsite in
the oral cavity [in 17 of the 22 (77.3%) patients].
The treatment modality used and the patient- and
tumour-related factors with potential prognostic value with regard
to OS and DFS were recorded and analysed. Primary tumour (P=0.025),
nodal (P=0.005) and composite clinical (P=0.006) stage were found
to significantly affect DFS rates in the univariate analysis
(Tables SI and SII). In the multivariate analysis, only
nodal stage (P=0.005; Table SIII)
was found to be a significant factor affecting DFS rates. The
modality of treatment used was not found to be a determinant of DFS
or OS.
Discussion
Lip cancer is the most common malignancy arising in
the head and neck region, and the majority of cases present at an
early stage (8). Early stage lip
SCC is associated with high cure rates compared with other head and
neck tumours (15). Carcinomas of
the lip can be successfully treated by surgery, EBRT or
brachytherapy alone or in combination. Different combinations of
the aforementioned modalities are used, depending on the stage of
the disease and the histopathological findings. The selection of
treatment modality is based on various factors that include the
resectability of the tumour, disease control probability, the
expected functional and cosmetic outcomes, the patient's preference
and general condition, and the availability of resources and
expertise (9).
When using brachytherapy, it is possible to deliver
a high localised dose of radiation to the tumour with rapid dose
fall-off, without the need for additional planning margins to be
taken into account. This conformity cannot be achieved with any
EBRT technique. A study has reported that brachytherapy, when used
alone, is an appropriate treatment option for patients with early
stage lip tumours (stages T1 and T2-N0) with similar survival and
local control rates as surgery (16). The cosmetic and functional outcomes
reported are good with brachytherapy, with no severe complications
reported thus far (17).
EBRT and surgical treatment with wide local excision
of the primary lesion and negative margins appear to be equally
effective in the treatment of early stage lip cancer (8). The histopathological assessment of
the primary tumour that can predict the biological behaviour of the
tumour and, thereby influence prognosis, is possible following
surgical treatment (18). In
addition, a surgical procedure may cause a functional and/or
cosmetic deficit due to the need to obtain wide margins. By
contrast, radiotherapy has been considered to offer better cosmetic
and functional results compared with surgery (19). A final histological report is also
lacking in patients who receive radiotherapy. Retrospective series
[e.g., Ashley et al (20)]
have reported no differences in local failure and survival rates
between surgery and EBRT. To the best of our knowledge, no
randomised studies comparing these different treatment strategies
have been reported to date. The present study was performed to
analyse the clinical profile and treatment outcomes of patients
with SCC of the lip treated with radical radiotherapy
(brachytherapy or EBRT) or surgery with or without adjuvant
treatment at the Regional Cancer Centre.
With a median follow-up period of 67.6 months, the
DFS and OS rates at 4 years for the entire cohort of 120 patients
were 69.1 and 86.7%, respectively. These survival results are
similar to those of previous studies published in the literature
(21,22). The 4-year OS rates of patients with
stage I, II, III and IVa disease were 88.9, 95.2, 86.8 and 75.3%,
respectively, and the DFS rates were 83.6, 69.5, 78.8 and 42.9%,
respectively. These results suggest that the cure rate for lip
cancer is high, especially when treated at the early stages.
The 4-year OS rates of patients treated with
surgery, EBRT and brachytherapy were 85.6, 85.4 and 92.9%,
respectively, and the 4-year DFS rates of the patients treated with
surgery, EBRT and brachytherapy were 75.4, 65.8 and 68.2%,
respectively. These results suggest that the cure rates of patients
with early stages of carcinoma of the lip are favourable, whether
they were treated with EBRT, brachytherapy or surgery. The 5-year
survival rate of patients with lip cancer treated with high
dose-rate brachytherapy using moulds was 68.8% in the study by
Unetsubo et al (23). This
survival value is comparable to those of the present study.
In the present study, 30% of patients developed
recurrence following treatment and the majority had local
recurrence only. In the literature, the recurrence rates following
treatment range from 15 to 30% (21), and the most common sites of
recurrence reported are local recurrences (24), similar to those observed in the
present study. The number of patients undergoing salvage surgery
for recurrences following radiotherapy was low in the present
study.
The retrospective study published by Ben Arie et
al (25) reported the rates of
secondary malignancy to be ~17% in patients with head and neck
cancer, which is comparable to the findings of the present study.
Han et al (26) reported
that the primary tumour and nodal stages were considered as
determinants of survival for lip SCC, which is also similar to the
results observed in the present study. The composite clinical stage
is another important prognostic factor associated with survival
(15), as was also observed in the
present study.
There are only a few studies available to date that
have focussed on the treatment outcomes of patients with lip
cancer. The present retrospective study on the outcomes of patients
with SCC of the lip treated with radical intent highlights the fact
that high cure rates can be obtained for lip cancer when treatment
is administered at the early stages. The cure and local control
rates reported in the present study were high, irrespective of
whether patients were treated with radiotherapy or surgery for
early stage SCC of the lip, as was also reported in the study by
Gooris et al (27).
The main limitation of the present study was the
bias associated with a retrospective study design. Data on the
exact site of the tumour (whether it was a tumour of the upper or
lower lip) and late morbidity data could not be obtained in the
present study. In addition, a number of patients were lost to
follow-up, and the follow-up information of only 76% of patients
could be obtained for estimating the 4-year OS and DFS times, and
rates at the time of analysis.
In conclusion, in the present retrospective study on
120 patients with carcinoma of the lip treated with radical
radiotherapy (brachytherapy or EBRT) or surgery with or without
adjuvant treatment, the 4-year DFS and OS rates were 69.1 and
86.7%, respectively. The findings of the present study indicate
that the outcomes of patients with carcinoma of the lip are
favourable when treatment is administered at the early stages. The
salvage rates were poor following relapse after radiotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GB performed the literature search, designed the
study, collected and analysed the data, wrote the manuscript,
prepared the tables and edited the manuscript. RR was involved in
the design of the study and assisted with data collection and
analysis. MR assisted with data collection, the preparation of the
tables and in the editing of the manuscript. LMN assisted with the
intepretation of the data, updating the patient follow-up
information, and in the drafting of the manuscript and preparing
tables. FN assisted with the analysis of the data and in the
writing of the discussion. ST assisted with the study design and in
the overall preparation of the manuscript. PSG assisted with the
statistical analysis, and in the preparation of the figures and
tables. CTK performed the literature search, designed the study and
assisted with the writing and editing of the manuscript. GB and CTK
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of the Regional Cancer Centre (Thiruvananthapuram, India) and
no separate ethics committee approval was obtained, as this is a
retrospective analysis and all data that were analysed were
collected as part of routine diagnosis and treatment. Patients were
diagnosed and treated according to standard treatment guidelines;
the study does not report the use of experimental or new
protocols.
Patient consent for publication
Patient consent for the standard treatment had been
taken prior to commencing treatment. No separate consent was taken
for publication, as this is a retrospective analysis and all data
that were analysed were collected as part of routine diagnosis and
treatment. Patients were diagnosed and treated according to
standard treatment guidelines.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Laprise C, Shahul HP, Madathil SA,
Thekkepurakkal AS, Castonguay G, Varghese I, Shiraz S, Allison P,
Schlecht NF, Rousseau MC, et al: Periodontal diseases and risk of
oral cancer in Southern India: Results from the HeNCe Life study.
Int J Cancer. 139:1512–1519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
International Agency for Research on
Cancer (IARC), . India: Source: Globocan 2020. IARC; Lyon: 2020,
https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdfFebruary
17–2022
|
|
4
|
Mathew A, Sara George P, MC K, G P, KM JK
and Sebastian P: Cancer incidence and mortality: District cancer
registry, Trivandrum, South India. Asian Pac J Cancer Prev.
18:1485–1491. 2017.PubMed/NCBI
|
|
5
|
Regional Cancer Centre (RCC), . Over Three
Decades of Changing Lives. RCC; Thiruvananthapuram: 2022,
https://www.rcctvm.gov.in/pdf/annualreports/RCC%20AR%202018-19.pdfFebruary
17–2022
|
|
6
|
Ram H, Sarkar J, Kumar H, Konwar R, Bhatt
ML and Mohammad S: Oral cancer: Risk factors and molecular
pathogenesis. J Maxillofac Oral Surg. 10:132–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Visscher JG, Botke G, Schakenraad JA
and van der Waal I: A comparison of results after radiotherapy and
surgery for stage I squamous cell carcinoma of the lower lip. Head
Neck. 21:526–530. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang SH and O´Sullivan B: Oral cancer:
Current role of radiotherapy and chemotherapy. Med Oral Patol Oral
Cir Bucal. 18:e233–e240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chargari C, Deutsch E, Blanchard P, Gouy
S, Martelli H, Guérin F, Dumas I, Bossi A, Morice P, Viswanathan AN
and Haie-Meder C: Brachytherapy: An overview for clinicians. CA
Cancer J Clin. 69:386–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Ayachy R, Sun R, Ka K, Laville A,
Duhamel AS, Tailleur A, Dumas I, Bockel S, Espenel S, Blanchard P,
et al: Pulsed dose rate brachytherapy of lip carcinoma: Clinical
outcome and quality of life analysis. Cancers (Basel). 13:13872021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th Edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goel MK, Khanna P and Kishore J:
Understanding survival analysis: Kaplan-Meier estimate. Int J
Ayurveda Res. 1:274–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nair MK, Sankaranarayanan R, Padmanabhan
TK and Madhu CS: Oral verrucous carcinoma: Treatment with
radiotherapy. Cancer. 61:458–461. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biasoli ÉR, Valente VB, Mantovan B,
Collado FU, Neto SC, Sundefeld ML, Miyahara GI and Bernabé DG: Lip
Cancer: A clinicopathological study and treatment outcomes in a
25-year experience. J Oral Maxillofac Surg. 74:1360–1367. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayerra AQ, Mena EP, Fabregas JP, Miguelez
CG and Guedea F: HDR and LDR Brachytherapy in the treatment of lip
cancer: The experience of the catalan institute of oncology. J
Contemp Brachytherapy. 2:9–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guibert M, David I, Vergez S, Rives M,
Filleron T, Bonnet J and Delannes M: Brachytherapy in lip
carcinoma: Long-term results. Int J Radiat Oncol Biol Phys.
81:e839–e843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein AL and Tahan SR: Histologic
correlates of metastasis in primary invasive squamous cell
carcinoma of the lip. J Cutan Pathol. 21:16–21. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stranc MF, Fogel M and Dische S:
Comparison of lip function: Surgery vs radiotherapy. Br J Plast
Surg. 40:598–604. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashley FL, McConnell DV, Machida R,
Sterling HE, Galloway D and Grazer F: Carcinoma of the lip. A
comparison of five year results after irradiation and surgical
therapy. Am J Surg. 110:549–551. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zitsch RP III, Park CW, Renner GJ and Rea
JL: Outcome analysis for lip carcinoma. Otolaryngol Head Neck Surg.
113:589–596. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Listl S, Jansen L, Stenzinger A, Freier K,
Emrich K, Holleczek B, Katalinic A, Gondos A and Brenner H; GEKID
Cancer Survival Working Group, : Survival of patients with oral
cavity cancer in Germany. PLoS One. 8:e534152013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Unetsubo T, Matsuzaki H, Takemoto M,
Katsui K, Hara M, Katayama N, Waki T, Kanazawa S and Asaumi J:
High-dose-rate brachytherapy using molds for lip and oral cavity
tumors. Radiat Oncol. 10:812015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heller KS and Shah JP: Carcinoma of the
lip. Am J Surg. 138:600–603. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ben Arie G, Shafat T, Belochitski O,
El-Saied S and Joshua BZ: Treatment modality and second primary
tumors of the head and neck. ORL J Otorhinolaryngol Relat Spec.
83:420–427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han AY, Kuan EC, Mallen-St Clair J, Alonso
JE, Arshi A and St John MA: Epidemiology of squamous cell carcinoma
of the lip in the United States: A population-based cohort
analysis. JAMA Otolaryngol Head Neck Surg. 142:1216–1223. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gooris PJ, Maat B, Vermey A, Roukema JA
and Roodenburg JL: Radiotherapy for cancer of the lip. A long-term
evaluation of 85 treated cases. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 86:325–330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
OncologyPRO, . Performance Scales:
Karnofsky & ECOG Scores. ESMO; Lugano: 2022, https://oncologypro.esmo.org/oncology-in-practice/practice-tools/performance-scalesOctober
31–2022
|