Introduction
Pathological examination and immunohistochemical
analysis are the main methods to diagnose follicular dendritic cell
sarcoma (FDCS). The diagnosis of FDCS is challenging and even
oncologists frequently misdiagnose it. Surgical resection is the
first choice of treatment for confirmed patients. The best
chemotherapy scheme is still under investigation and there is
currently no unified standard. The effect of neoadjuvant therapy on
extranodal FDCS is not described in the literature (1). However, there is no evidence that
adjuvant therapy contributes to improved survival (2). Radical resection of tumors is the
standard treatment for patients with local tumors and radiotherapy
does not significantly improve the survival rate (3). Patients who cannot remove tumors or
patients whose multiple organs are infiltrated by tumors are more
suitable for chemotherapy (4). The
best chemotherapy regimen for this rare disease has not been
determined and some patients with FDCS have been treated with
cytotoxic drugs for malignant lymphoma or soft tissue sarcoma
(5–8). Therefore, in order to clarify the
pathophysiology of FDCS and formulate the best treatment strategy,
it is required to accumulate more clinical cases. The present study
reported a case of FDCS whose disease was not improved through a
variety of treatment schemes and the survival time was only 9
months.
Case report
In November 2020, a male patient came to the First
People's Hospital of Guangyuan (Guangyuan, China) for routine
physical examination and a spleen mass was identified. There were
no obvious symptoms at that time. Ultrasound and computed
tomography images of the patient are provided in Fig. 1. At the first presentation,
ultrasound examination with a Mindray M9 (Shenzhen Mindray
Biomedical Electronics Co., Ltd; two-dimensional; Colo Flow; model
of ultrasonic probe, C5-1S, 8 Hz) indicated that the dimensions of
the tumor were 4.5×7.5 cm (Fig.
1A) and the patient did not undergo any immediate surgical
treatment, as there was no discomfort. The patient was hospitalized
at the First People's Hospital of Guangyuan due to left abdominal
pain (Guangyuan, China) 74 days after the first presentation.
Ultrasound indicated a tumor mass in the spleen, which had grown,
with a measured size of 10.4×8.5 cm (Fig. 1B). A contrast-enhanced computed
tomography scan (Ingenuity CT; Philips Medical Systems Inc.; slice
thickness, 1 mm; center, 45; width, 250) of the patient's abdomen
suggested that the spleen was slightly enlarged, there was a mass
in the spleen and multiple necrotic areas were visible in the mass.
The measured dimensions of the mass were 10×7.5 cm (Fig. 1C). Splenectomy with perisplenic
lymph nodes and pancreatic tail lymphadenectomy was performed using
laparoscopy 78 days after the first presentation. During the
operation, the measured value of the spleen was 15×12×8 cm, and
there was a mass at the lower end of the spleen, with the measured
dimensions of 8×7×5 cm. The mass protruded from the spleen capsule
and the mass adhered to the anterior fascia of the left kidney. The
pathological examination of frozen splenic masses during the
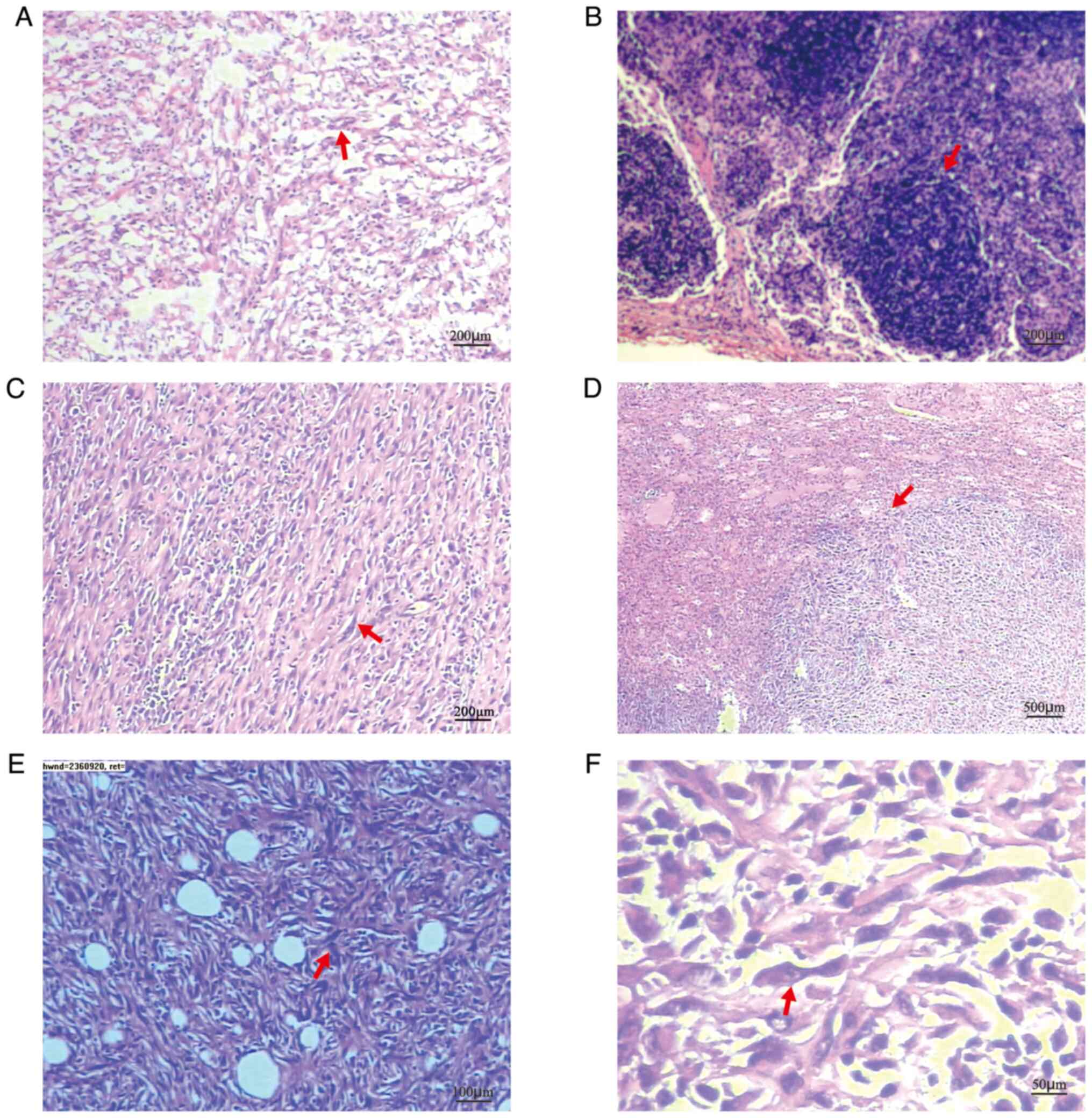
operation indicated sarcoma (Fig.
2A). The pathological examination results of perisplenic lymph
nodes were normal and no sarcoma cells were found (Fig. 2B). The spleen tumor tissue was
collected and fixed in 10% formalin solution, then dehydrated, cut
into 4-µm-thick sections, stained with hematoxylin and eosin, and
then observed under a microscope. Microscopic observation indicated
that the spindle-shaped tumor cells were arranged in bundles with a
large number of lymphocytes and plasma cells in the background
(Fig. 2C). The tumor boundary was
clear (Fig. 2D). The tumor cells
were spindle cells, focal coagulative necrosis was present and
tumor cells invaded the fat and vascular tissue on the concave
surface of the spleen (Fig. 2E and
F). The sections were analyzed with an immunohistochemical
(IHC) staining instrument (IHC pretreatment system: PT Link PT200;
Dako Denmark A/S. Pathological section tissue staining machine:
Autostainer Link 48; Dako North America, Inc.). First, the tumor
was immersed in a fixation solution containing 10% formalin for 24
h at 25°C. The tissue was then embedded in paraffin for 3 h at 56°C
and cut into 4-µm-thick sections. The mounted, formalin-fixed,
paraffin-embedded tissue sections were immersed in pre-heated
EnVision FLEX Target Retrieval Solution (working solution; cat. no.
K8004; Agilent Technologies, Inc.) in tanks and incubated for 20
min at 97°C. The sections were left to cool in PT Link to 65°C.
Each Autostainer slide rack with the slides from the tank was
removed and slides were soaked in diluted EnVision FLEX Wash Buffer
(20X) (cat. no. K8007; Agilent Technologies, Inc.) for 5 min at
25°C. Slides were placed on an Autostainer Link instrument and
further processed. Each section was blown to remove excess buffer
and 100 µl EnVision FLEX Peroxidase-Blocking Reagent (cat. no.
K8002; Agilent Technologies, Inc.) was applied with incubation for
10 min at 25°C. The tissue sections were rinsed with EnVision FLEX
Wash Buffer (20X) (cat. no. K8007; Agilent Technologies, Inc.) for
10 min at 25°C. Each section was blown again and 100 µl
Ready-to-Use primary antibody was applied with incubation for 30
min at 25°C. The following antibodies were used (all for IHC): CD21
(cat. no. AR0038), discovered on gastrointestinal tumor-1 (DOG1;
cat. no. Kit-0029), proliferating cell nuclear antigen Ki-67 (cat.
no. AM0383), the common acute lymphoblastic leukemia antigen CD10
(cat. no. AM0032), myeloperoxidase (cat. no. AP0239), cytokeratin-7
(CK7; cat. no. AM0098), Vimentin (cat. no. AM0234),
tumor-associated macrophage marker CD68 (cat. no. AM0059), Desmin
(cat. no. AM0104); vascular endothelial cell-related factor CD34
(cat. no. AM0045; all from Xiamen Tongling Biomedical Technology
Co., Ltd.); calcium binding protein S100 (mouse; cat. no.
Kit-0007), activin receptor like kinase 1 (ALK1; mouse; cat. no.
MAB-0281), IgE receptor CD23 (rabbit; cat. no. RMA-0504), smooth
muscle actin (SMA; cat. no. Kit-0006), mast/stem cell growth factor
receptor CD117 (cat. no. Kit-0029), biomarker for macrophages CD163
(cat. no. MAB-0869), CD31 (PECAM-1; cat. no. MAB-0720),
transmembrane glycoprotein CD4 (cat. no. RMA-0620), Lysozyme (cat.
no. RAB-0115), transfer membrane glycoprotein CD1a (cat. no.
MAB-0336), Langerin (cat. no. MAB-0633), CD15 (cat. no. MAB-0779),
Myogenin (cat. no. MAB-0362), myogenic differentiation 1 (MyoD1;
cat. no. MAB-0822), murine double minute 2 (MDM2; cat. no.
MAB-0774), cyclin- dependent kinase 4 (CDK4; cat. no. MAB-0771),
transmembrane protein CD30 (cat. no. MAB-0023), SRY-related HMG-box
10 (SOX-10; cat. no. RMA-0726), melanoma antigen recognized by T
cells-1 (Melan-A; cat. no. MAB-1033), signal transducer and
activator of transcription 6 (STAT6; cat. no. RMA-0845) and
complement receptor 1 (CD35; mouse monoclonal antibody; cat. no.
MAB-0340; all from Fuzhou Maixin Biotechnology Development Co.,
Ltd.); transmembrane protease CD13 (cat. no. APC100; Shenzhen
Dakewei Bioengineering Co., Ltd); and Epstein-BarrVirus
(EBV)-encoded RNA (cat. no. ISH-7001; Wuxi Aorui Dongyuan
Biotechnology Co., Ltd). The tissue sections were rinsed with
EnVision FLEX Wash Buffer (20X) (cat. no. K8007; Agilent
Technologies, Inc.) for 10 min at 25°C. Each section was blown and
100 µl Ready-to-Use secondary antibody EnVision FLEX/HRP (cat. no.
K8002, Agilent Technologies, Inc.) was applied with incubation for
20 min at 25°C. The tissue sections were rinsed with EnVision FLEX
Wash Buffer (20X) (cat. no. K8007; Agilent Technologies, Inc.)
twice for 10 min each at 25°C. Every section was blown and 200 µl
Substrate Working Solution was applied with incubation for 5 min at
25°C. Substrate Working Solution was prepared by adding 20 drops of
EnVision FLEX DAB + Chromogen (cat. no. K8002; Agilent
Technologies, Inc.) to 20 ml EnVision FLEX Substrate Buffer (cat.
no. K8002; Agilent Technologies, Inc.). The specimen was stained
with Mayer's hematoxylin for 3 min at 25°C and then rinsed with
water. When the staining procedure was completed, the specimen was
dehydrated and permanent mounting was performed. The inspection
results indicated the following: CD21 (+) (Fig. 3A), CD23 (+) (Fig. 3B), CD35 (+) (Fig. 3C), CD34 (−) (Fig. 3D), Desmin (−) (Fig. 3E), S-100 (−) (Fig. 3F), ALK1 (−) (Fig. 3G), Vimentin (−), CD68 (−), CD16.3
(−), CD31 (−), CD4 (−), CD13 (−), CD10 (−), Lysozyme (−), CD1a (−),
Langerin (−), CD15 (−), Myeloperoxidase (−), CD117 (−), CK7 (−),
SMA (−), Myogenin (−), MyoD1 (−), MDM2 (−), CDK4 (−), CD30 (−),
SOX-10 (−), Melan-A (−), STAT6 (−), DOG1 (−), Ki-67 (+, 5–10%) and
EBV-encoded RNA (−). The patient was diagnosed with FDCS of the
spleen. The Affiliated Hospital of Chongqing Medical University of
China (Chongqing, China) obtained no different diagnosis when
re-examining the sections. The patient's abdominal computed
tomography indicated that more nodules and lumpy soft tissue
density shadows were present in the spleen area, intestinal space
and anterior abdominal wall incision area, and the measured
dimensions of the larger mass were 4.4×2.3 cm (Fig. 1D) 53 days after the splenectomy.
The patient preferred not to be treated. The patient's blood
parameters (ADVIA Centaur XP; Siemens Healthcare Diagnostics, Inc.)
indicated that the blood total prostate-specific antigen (PSA) was
5.58 ng/ml (normal range, 0–4 ng/ml), free PSA was 1.46 ng/ml
(normal range, 0–0.93 ng/ml) and neuron-specific enolase was 41.69
ng/ml (normal range, 0–16.3 ng/ml) 88 days after the splenectomy.
According to the advice of oncologists, the patient was treated
with cyclophosphamide, epirubicin, vindesin sulfate and prednisone
acetate. In addition, 200 mg of sintilimab injection was used for
targeted treatment (Table I). The
computed tomography scan of the patient's abdomen suggested that
the volume of the abdominal mass had increased, and the measured
dimensions were 10.6×10.2 cm (Fig.
1E) 114 days after the splenectomy. The patient was treated
again with cyclophosphamide, epirubicin, vindesin sulfate and
prednisone acetate, and 200 mg of sintilimab injection was used for
targeted treatment (Table I). The
computed tomography scan of the patient's abdomen indicated that
the size of abdominal mass had yet increased to 13.6×11.6 cm
(Fig. 1F) 135 days after the
splenectomy. Gene detection in the sarcoma tissue suggested that
the patient had no mutated tumor genes. The patient was treated
with 80 mg docetaxel and 1 g gemcitabine intravenously (Table I). The patient's body weight
decreased significantly, complicated by intraperitoneal infection
and bleeding. The patient began oral anlotinib hydrochloride
treatment (Table I) 151 days after
the splenectomy. At the same time, the patient was subjected to
several radiotherapy sessions for abdominal metastatic tumors, with
a total dose of 18 F/4,500 cGy. The patient's condition did not
improve, the body weight decreased significantly, a large amount of
intra-abdominal bleeding was present and basic vital signs were
abnormal. The patient died 9 months after the splenic mass was
found.
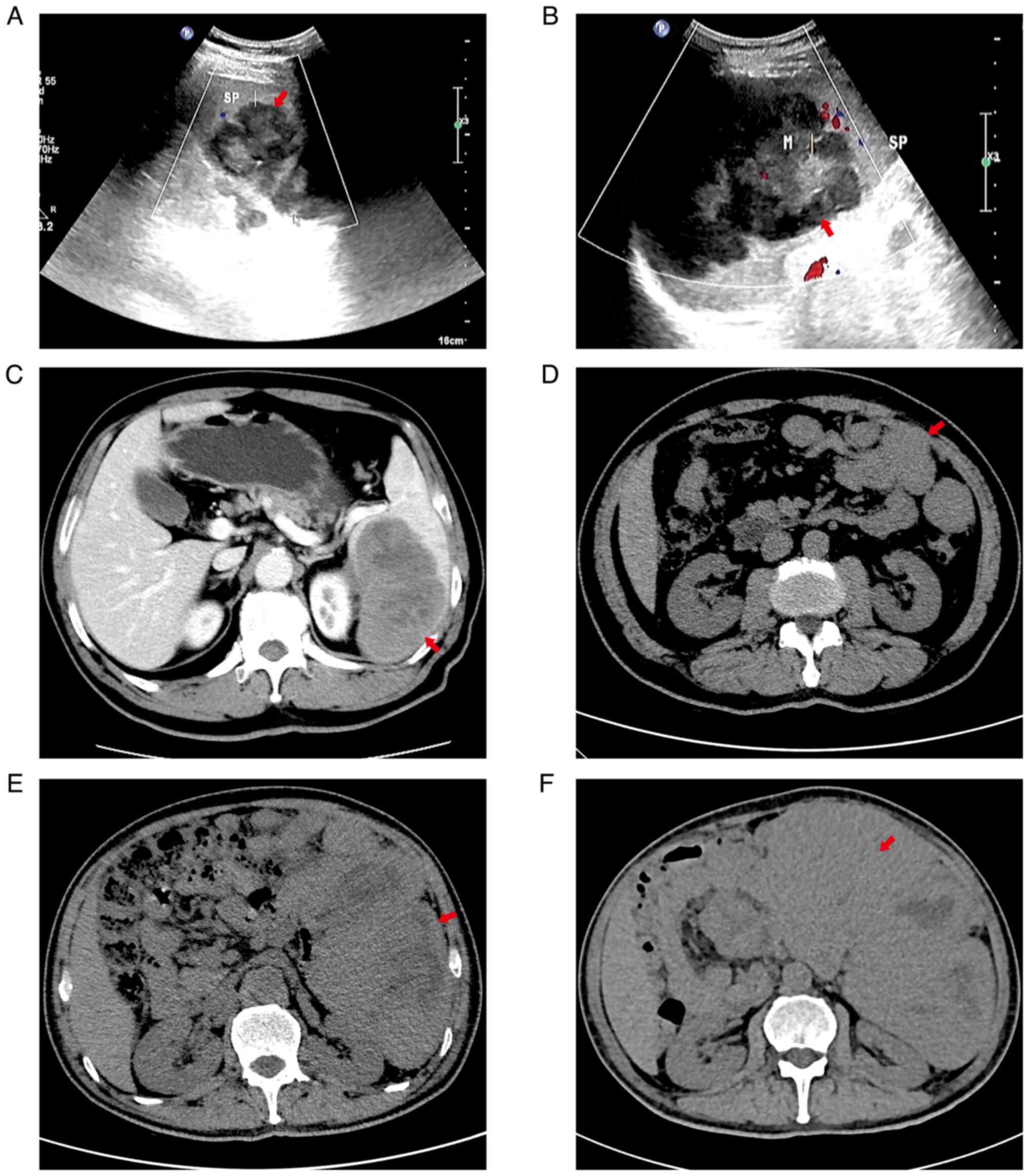 | Figure 1.(A) Spleen mass found on the day of
routine physical examination. Ultrasound examination revealed a
mass in the spleen. The mass measured 4.5×7.5 cm. The mass
displayed with hyperecho, clear boundary and irregular shape. CDFI
indicated blood flow signals. Red arrow denotes the mass. (B)
Ultrasound examination at 74 days after the spleen mass was found
indicated a mass in the spleen. The mass measured 10.4×8.5 cm. The
mass displayed with hyperecho, clear boundary and irregular shape.
CDFI indicated blood flow signals. Red arrow denotes the mass. (C)
Contrast-enhanced computed tomography at 74 days after the spleen
mass was found revealed enlargement of the spleen, a low-density
mass shadow was observed in the spleen parenchyma, and the mass
measured 10×7.5 cm; the boundary of the mass was clear and multiple
small patchy low-density areas were visible in the mass. Enhanced
scanning indicated that most areas of the mass had gradual moderate
enhancement and multiple patchy non-enhanced areas. Red arrow
denotes the mass. (D) Computed tomography at 53 days after the
splenectomy indicated that there were numerous masses of different
sizes in the spleen operation area, the gap between the intestines
and around the incision of the anterior abdominal wall, of which
the largest mass measured 4.4×3.2 cm. The boundary between the mass
and the small intestine was not clear. There was no peritoneal
effusion. Red arrow denotes the mass. (E) Computed tomography at
114 days after the splenectomy indicated that numerous masses were
located in the splenic surgical area, abdominal cavity, pelvic
cavity, bowel space and around the incision of the anterior
abdominal wall. The maximum measures of the mass were 10.6×10.2 cm
and the boundary was not clear. Certain masses fused and their
interior had low-density tissue necrosis areas. There was no clear
boundary between the masses and bilateral rectus abdominis,
peritoneum and bowel, and the surgical area had strip-shaped
high-density images. Red arrow denotes the mass. (F) Computed
tomography at 135 days after the splenectomy indicated that
numerous masses were located in the splenic surgery area, abdominal
cavity, pelvic cavity, bowel space and around the incision of the
anterior abdominal wall. The maximum mass measured 13.6×11.6 cm and
the boundary was not clear. Certain masses fused and the interior
had a low-density tissue necrosis area. The boundary between the
mass and rectus abdominis, peritoneum and bowel was not clear and
the adjacent tissues were compressed and deformed by the mass. Red
arrow denotes the mass. CDFI, colour Doppler flow imaging. |
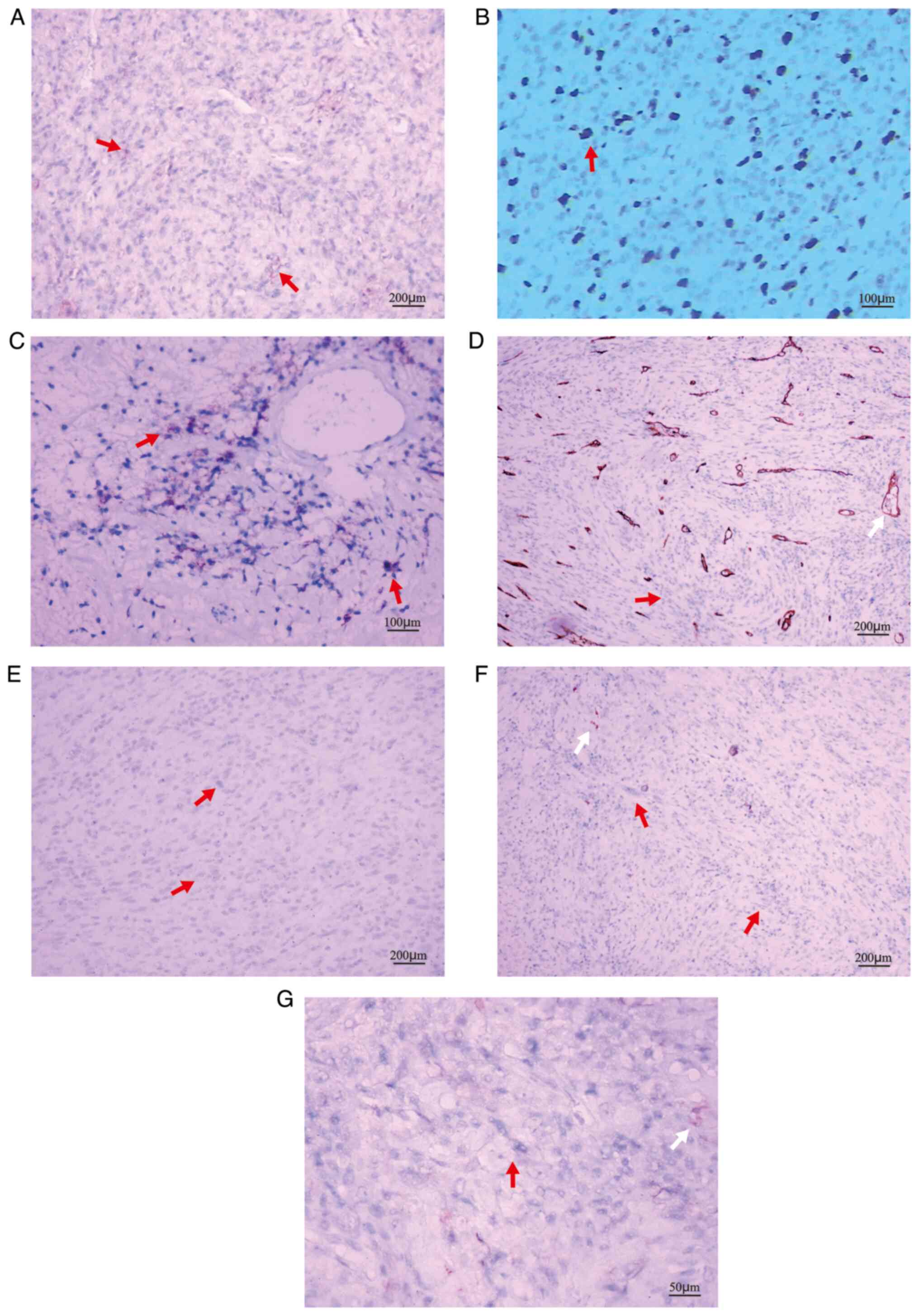 | Figure 3.Representative immunohistochemical
staining images. (A) CD 21 (+) (magnification, ×100; scale bar, 200
µm); (B) CD23 (+); (C) CD35 (+) (magnification, ×200; scale bar,
100 µm); red arrow denotes tumor cells with positive staining.
Positive expression of markers is indicated by pale brown staining.
(D) CD34 (−), the vascular endothelium was brown (white arrow); (E)
Desmin (−); (F) S100 (−) (magnification, ×100; scale bar, 200 µm);
(G) ALK1 (−) (magnification, ×400; scale bar, 50 µm). In D-G, red
arrow denotes tumor cells without special color and non-specific
staining (white arrow) was brown. |
 | Table I.Chemical drugs administered to the
patient (dose and number of days of treatment). |
Table I.
Chemical drugs administered to the
patient (dose and number of days of treatment).
|
| Time of starting drug
treatment after splenectomy, days |
|---|
|
|
|
|---|
| Drug | 94 | 114 | 136 | 137 | 151 | 157 | 178 |
|---|
| Cyclophosphamide | 1 g/d; 1 d | 1.2 g/d; 1 d |
|
|
|
|
|
| Epirubicin | 80 mg/d; 1 d | 80 mg/d; 1 d |
|
|
|
|
|
| Vindesin sulfate | 4 mg/d; 1 d | 4 mg/d; 1 d |
|
|
|
|
|
| Prednisone
tablets | 100 mg/d; 5 d | 100 mg/d; 5 d |
|
|
|
|
|
| Sintilimab
injection |
| 200 mg/d; 1 d | 200 mg/d; 1 d |
|
| 200 mg/d; 1 d | 200 mg/d; 1 d |
| Docetaxel |
|
|
| 80 mg/d; 1 d |
|
|
|
| Gemcitabine |
|
|
| 1 g/d; 1 d |
|
|
|
| Anlotinib |
|
|
|
| 12 mg/d; 21 d |
|
|
| hydrochloride |
|
|
|
|
|
|
|
Discussion
FDCS is the proliferation of spindle or oval cells.
These cells have histomorphological and immunophenotypic
characteristics similar to follicular dendritic cells. The age span
of patients with FDCS is wide, the prevalence rate in adults is
higher than that in children and there is no significant difference
between males and females in terms of incidence rate. Epstein Barr
virus (EBV) infection may be one of the predisposing factors for
FDCS (9). EBV may carry latent
membrane protein 1, which is frequently detected in the spleen and
liver of patients with FDCS. It has the function of promoting viral
oncogene transformation (10,11).
A small number of cases may be accompanied by Castelman disease,
which may also occur several years prior to FDCS (12). The etiology of FDCS has remained to
be fully elucidated. In most cases, FDCS occurs in lymph nodes, and
one-third to two-thirds of patients present with lymphadenopathy,
the most common of which is cervical lymphadenopathy. FDCS may also
occur in organs outside lymph nodes, such as the mouth, tonsils,
mediastinum, gastropancreas, liver, spleen, intestine and soft
tissue. Lymph nodes, lungs and liver are common metastatic sites.
Most tumors grow slowly. Patients have no obvious symptoms and
signs in the early stage of the disease and the tumor is detected
at the late stage. Abdominal tumors are frequently uncovered due to
abdominal discomfort. When abdominal tumors are found, the tumor
volume is relatively large (13).
The clinical manifestations with FDCS vary greatly among patients.
The disease may involve lymph nodes and extranodal parts of the
body (14,15).
FDCS is composed of spindle to oval cells, arranged
in bundles, bamboo mat stripes, whirlpools, diffuse flakes or fuzzy
nodules. A single tumor cell has clear boundaries and increased
cytoplasm and is eosinophilic. The nucleus is oval or long
spindle-shaped, the chromatin is vacuolated or fine granular and
the nucleolus of the nucleus is small and clear. It is common for
giant cells with double nuclei or multinucleated tumors to be
present. Certain cases have obvious cell atypia, with a higher
proportion of nuclear division. Atypical mitotic images and
coagulative necrosis are common. FDCS express one or more
follicular dendritic cell markers, such as CD21, CD23, CD35 and
KiM4P. Clusterin is almost always strongly positive. Fascin and
aodoplanin are uniformly positive. In addition, the diagnosis of
FDCS outside lymph nodes is more difficult (16–18).
The pathological images of the patient of the present study
indicated a clear boundary between the tumor tissue and the
surrounding normal tissue. The spindle-shaped tumor cells were
arranged in bundles with a large number of lymphocytes and plasma
cells in the background. On IHC, CD21 (+), CD23 (+) and CD35 (+)
are characteristic markers of FDCS (18). Positive expression of the markers
is indicated by a pale brown stain. Vimentin (−), CD68 (−), CD163
(−), CD31 (−), CD4 (−), CD13 (−), CD10 (−), Lysozyme (−), CD34 (−),
CD1a (−), Langerin (−), CD15 (−), myeloperoxidase (−), CD117 (−),
CK7 (−), SMA (−), Desmin (−), Myogenin (−), MyoD1 (−), S-100 (−),
MDM2 (−), CDK4 (−), CD30 (−), ALK1 (−), SOX-10 (−), Melan-A (−),
STAT6 (−) and DOG1 (−) are used to identify other tumors and no
misdiagnosis occurred. There are limited research data on genetic
changes of FDCS. Its origin cells are follicular dendritic cells in
lymphoid follicles. FDCS has high histological similarity with
sarcoma, non-Hodgkin's lymphoma, melanoma, undifferentiated cancer
and other dendritic cell and histiocytic diseases, which may lead
to misdiagnosis of FDCS as other tumors (19).
Due to the rarity of the disease, small number of
reported cases and limited research on prognosis, there is
currently no unified standard and no guideline for the treatment of
FDCS. In most cases, patients with FDCS receive surgery and
adjuvant radiotherapy or chemotherapy. Radical resection is an
important treatment for local masses (20). However, adjuvant radiotherapy had
no significant effect on survival outcomes. The development of
medical molecular genetics may contribute to research on tumor
targeted therapies. There is no conclusion as to whether patients
with FDCS should receive radiotherapy or chemotherapy after
surgery. The choice rather depends on the experience of clinicians
and the comprehensive condition of the patients. Current treatment
options include surgery, radiotherapy and chemotherapy alone or in
combination. Although surgical resection is the preferred
treatment, for cases that cannot be resected or patients with tumor
recurrence and metastasis after resection, chemotherapy or
radiotherapy is still required (21). In addition, FDCS is regarded as
lymphoma or sarcoma according to its tumor cell origin, and thus,
cyclophosphamide, epirubicin, oncovin, prednisone (CHOP)
chemotherapy is used in certain patients; however, the therapeutic
effect of CHOP chemotherapy in patients with FDCS is lower than
that in patients with non-Hodgkin's lymphoma. The reason for the
decreased treatment effect may be that the drugs used in the CHOP
regimen do not directly break down follicular dendritic cells
(22). Follicular dendritic cells
have an important role in the formation of systemic follicular B
cells during chronic inflammation (23). However, the case of the present
study is different. The spleen and surrounding lymph nodes were
removed. Pathological images indicated no infiltration of sarcoma
cells in lymph nodes and distant organs. The patient relapsed again
after a short period. After chemotherapy, tumor targeted drug
therapy and radiotherapy, the condition was not improved.
FDCS is rare in the clinic and the effect of
radiotherapy and chemotherapy is not significant. FDCS is inert to
moderately malignant. Its biological behavior and prognosis are
related to patient age, tumor size, lymphoplasmacyte infiltration,
tumor cell nuclear division count, necrosis and lymph node
metastasis (2). Patients with
large tumors outside lymph nodes or intraperitoneal tumors have a
poor prognosis (24). In general,
the prognosis of FDCS is favorable (25–27).
Wang et al (12) reported
that a patient with FDCS of the spleen was in good condition after
3 years of surgical treatment. Their study indicated that the
prognosis of FDCS is generally favorable. However, the present case
is different from common FDCS. Although Ki-67 was 5–10%, the tumor
of the patient soon spread to the whole abdominal cavity. The size
of the sarcoma of >6 cm and intra-abdominal involvement in the
patient may be associated with poor prognosis. The mass protruded
from the splenic membrane and attached to the left renal anterior
fascia. It is possible for the cancer to spread to the abdominal
cavity via this way. The poor prognosis of this patient may be
related to the mass of splenic capsule protrusion. Even for cases
with no tumor-cell infiltration in perisplenic lymph nodes, it may
be necessary to remove the tissues or organs around the spleen,
such as the left kidney fascia and the left kidney, while removing
the spleen. More data and further research are urgently needed. As
the disease is rare and the treating clinician was inexperienced,
the patient did not undergo any fine-needle biopsy prior to
surgery. One limitation of the present case report is that the
photos of the mass attached to the spleen were not retained, so
that they could not be provided to be viewed in the present study.
In the late stage of the disease, the patient refused to accept
next-generation gene sequencing. Thus, no further genetic
information for treatment that may have been provided by genetic
testing was available. The lack of these assays is another
limitation of the present case report.
In conclusion, the prognosis of FDCS is not
necessarily favorable. Even if no tumor cells are present in the
perisplenic lymph nodes, tumor cells may metastasize to distant
sites via different ways. The patient's regret was that no surgical
treatment was performed as soon as possible after the spleen mass
was found in the physical examination. Whether the delayed
operation time is the cause of tumor recurrence requires further
study. The accumulation of case reports provides evidence support
for clarifying the pathophysiology of splenic FDCS and formulating
the best treatment strategy.
Acknowledgements
The author would like to acknowledge Mr. He
Xingzhuang and Ms. Mou Hui, Pathologists at the Department of
Pathology, The First People's Hospital of Guangyuan (Guangyuan,
China) for performing parts of the immunohistochemistry
experiments.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ZYX has completed the work of designing the study
and writing the manuscript, treating the patient, accumulating and
analyzing data and images and revising the manuscript. The author
has read and approved the final manuscript. ZYX confirmed the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
As the patient died before the study was written,
the patient's wife provided written informed consent for the
publication of the case data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferede A, O'Connor R, Stafford A and Swan
N: Follicular dendritic cell sarcoma of the duodenum: An extremely
rare entity. BMJ Case Rep. 2018:bcr20172215052018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saygin C, Uzunaslan D, Ozguroglu M,
Senocak M and Tuzuner N: Dendritic cell sarcoma: A pooled analysis
including 462 cases with presentation of our case series. Crit Rev
Oncol Hematol. 88:253–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perkins SM and Shinohara ET:
Interdigitating and follicular dendritic cell sarcomas: A SEER
analysis. Am J Clin Oncol. 36:395–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soriano AO, Thompson MA, Admirand JH,
Fayad LE, Rodriguez AM, Romaguera JE, Hagemeister FB and Pro B:
Follicular dendritic cell sarcoma: A report of 14 cases and a
review of the literature. Am J Hematol. 82:725–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalia S, Shao H, Sagatys E, Cualing H and
Sokol L: Dendritic cell and histiocytic neoplasms: Biology,
diagnosis, and treatment. Cancer Control. 21:290–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalia S, Jaglal M, Chervenick P, Cualing H
and Sokol L: Clinicopathologic characteristics and outcomes of
histiocytic and dendritic cell neoplasms: The moffitt cancer center
experience over the last twenty five years. Cancers. 6:2275–2295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shinagare AB, Ramaiya NH, Jagannathan JP,
Hornick JL and Swanson RS: Primary follicular dendritic cell
sarcoma of liver treated with cyclophosphamide, doxorubicin,
vincristine, and prednisone regimen and surgery. J Clin Oncol.
29:e849–e851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi BS, Baek JH, Shin YM, Kim JH, Kim HW,
Lee SJ and Cha HJ: Follicular dendritic cell sarcoma: A case report
and review of the literature. Cancer Res Treat. 42:121–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun J, Wang C, Wang D, Wu J, Wang L, Zhao
L and Teng L: Follicular dendritic cell sarcoma (FDCS) of urinary
bladder with coexisting urothelial carcinoma-a case report. BMC
Urol. 19:832019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haranhalli N, Ammar AE, Weidenheim KM,
Rosenblum MK and Altschul DJ: Hemorrhagic intracranial follicular
dendritic cell sarcoma: A case report. Surg Neurol Int. 8:2482017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mondal SK, Bera H, Bhattacharya B and
Dewan K: Follicular dendritic cell sarcoma of the tonsil. Natl J
Maxillofac Surg. 3:62–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Xu D, Qiao Z, Shen L, Dai H and Ji
Y: Follicular dendritic cell sarcoma of the spleen: A case report
and review of the literature. Oncol Lett. 12:2062–2064. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarkar R, Sharma S, Roy N, Shankar A and
Basu S: Follicular dendritic cell sarcoma of Ileoceacal region in a
young woman: A rare case report with review of literature. Oman Med
J. 28:e0552013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohtake H and Yamakawa M: Interdigitating
dendritic cell sarcoma and follicular dendritic cell sarcoma:
Histopathological findings for differential diagnosis. J Clin Exp
Hematop. 53:179–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pai VD, Desai S, Desouza A and Saklani AP:
Extranodal follicular dendritic cell sarcoma: A frequently
misdiagnosed entity. J Postgrad Med. 61:55–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Liu G, Liu J, Fang T, Zeng Y, Zhang
H, Yang S, Wang Y, Zhang J, Wei J, et al: Paraneoplastic pemphigus
associated with follicular dendritic cell sarcoma: Report of a case
and review of literature. Int J Clin Exp Pathol. 8:11983–11994.
2015.PubMed/NCBI
|
|
17
|
Zhang BX, Chen ZH, Liu Y, Zeng YJ and Li
YC: Inflammatory pseudotumor-like follicular dendritic cell
sarcoma: A brief report of two cases. World J Gastrointest Oncol.
11:1231–1239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan T, Yang Q, Zhang H, Li J and Zhang X:
A 46-year-old Chinese woman presenting with retroperitoneal
follicular dendritic cell sarcoma: A case report. J Med Case Rep.
8:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiaofei C, Juan W, Qingxin X, Fangfang G,
Zhandong Z, Shuke Z, Yanyan L, Jianbo Z and Ling M:
Clinicopathological analysis of colon follicular dentritic cell
sarcomawith metastasis. J Basic Clin Oncol. 30:209–212. 2017.
|
|
20
|
De Pas T, Spitaleri G, Pruneri G,
Curigliano G, Noberasco C, Luini A, Andreoni B, Testori A and de
Braud F: Dendritic cell sarcoma: An analytic overview of the
literature and presentation of original five cases. Crit Rev Oncol
Hematol. 65:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu HL, Chen B, Jiang CW, Yang ZL and Wang
KR: Follicular dendritic cell sarcoma in the right chest wall: A
case report. Medicine (Baltimore). 99:e219352020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conry RM: Response of follicular dendritic
cell sarcoma to gemcitabine and docetaxel: Report of two cases and
literature review. Clin Sarcoma Res. 4:62014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vermi W, Giurisato E, Lonardi S, Balzarini
P, Rossi E, Medicina D, Bosisio D, Sozzani S, Pellegrini W,
Doglioni C, et al: Ligand-dependent activation of EGFR in
follicular dendritic cells sarcoma is sustained by local production
of cognate ligands. Clin Cancer Res. 19:5027–5038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jain P, Milgrom SA, Patel KP, Nastoupil L,
Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J, et al:
Characteristics, management, and outcomes of patients with
follicular dendritic cell sarcoma. Br J Haematol. 178:403–412.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clement P, Saint-Blancard P, Minvielle F,
Le Page P and Kossowski M: Follicular dendritic cell sarcoma of the
tonsil: A case report. Am J Otolaryngol. 27:207–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakashima T, Kuratomi Y, Shiratsuchi H,
Yamamoto H, Yasumatsu R, Yamamoto T and Komiyama S: Follicular
dendritic cell sarcoma of the neck; a case report and literature
review. Auris Nasus Larynx. 29:401–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Domínguez-Malagón H, Cano-Valdez AM,
Mosqueda-Taylor A and Hes O: Follicular dendritic cell sarcoma of
the pharyngeal region: Histologic, cytologic, immunohistochemical,
and ultrastructural study of three cases. Ann Diagn Pathol.
8:325–332. 2004. View Article : Google Scholar : PubMed/NCBI
|