Introduction
Population aging and exposure to carcinogenic
factors from industry and lifestyle choices, in addition to
improvements in detection have resulted in the reported cancer
incidence increasing in China (1).
Due to advances in cancer treatment, including molecular targeted
therapy, immunotherapy and heavy ion radiotherapy, the 5-year
survival rate of most types of cancer has improved substantially
over the past two decades, with an increase for all cancers
combined from 30.9% during 2003–2005 to 40.5% during 2012–2015
(2). In parallel with the
prolonged survival and growth of the first primary malignancy (FPM)
survivor population (3), the
occurrence of second primary malignancies (SPMs) has increased
markedly over recent decades in the USA (4) and other countries (5–8). Big
data analysis has revealed the clinical characteristics of SPMs in
the US population (9–11), which has aided in clinical
decisions and healthcare policies.
In China, case reports (12) and retrospective analyses (13,14)
have examined SPMs derived from first primary non-hematological
malignancies, but most of these have focused on one or more cases
of a single cancer type. Therefore, essential issues such as risk
factors for SPM, the FPM-SPM time interval for specific types of
cancer and the long-term efficacy of FPM treatment remain unclear
for the Chinese population. Therefore, the present retrospective
cohort study was conducted at a single center to assess the
clinical characteristics of Chinese patients with an SPM, focusing
on the site distributions of the FPM and SPM, the influence of
cancer treatment and pathological stage on the FPM-SPM interval,
the impact of SPM on the overall survival (OS) of patients with
lung or breast cancer, and the potential impacts of continued
alcohol consumption, biliary tract diseases and benign FPM on SPM
risk.
Materials and methods
Patient cohort
A retrospective patient cohort from the Oncology
Department of The Hospital of 81st Group Army PLA (Zhangjiakou,
China), a former national military center for the treatment of
cancer using integrated Chinese and Western medical methods, was
examined. A total of 1,423 patients with malignancy were enrolled
and 235 cases were excluded due to the lack of a pathological
diagnosis, according to clinical records dating from June 1, 2005
to December 30, 2020.
To ensure the quality of the data, inpatient cases
were selected and associated information was gathered from
inpatient records and from the medical staff via telephone. All
patients who did not meet the exclusion criteria were excluded. The
exclusion criteria were as follows: i) Patients with hematological
malignances, who were enrolled mainly in the Hematology Department;
ii) patients receiving outpatient chemo- or radiotherapy in this
hospital, with no access to long term follow-up; iii) patients
without histopathological confirmation of malignancy or whose
detailed follow-up was not available; and iv) patients at high risk
of the development of SPMs due to hereditary cancer syndromes.
The cohort was then established according to the
following inclusion criteria: i) Patients diagnosed with FPM and
SPM confirmed pathologically; ii) patients receiving at least one
course of inpatient radio- or chemotherapy; iii) patients who
developed SPM at an interval of ≥6 months, which is considered to
be the standard for distinguishing between synchronous malignancy
and SPM in the cohort of patients with breast or gastric cancer,
the two main types of cancer that were separately analyzed in the
present study (13,15); and iv) patients with regular follow
up (recorded every ~2 months during the course of therapy, and at
least every 3 months for 2 years, then every 6 months after the
first 2 years).
Data collection
The clinical data collected included the age of the
patient at diagnosis, the time interval between the FPM and SPM,
treatment of the FPM, sites of the FPM and SPM, complications of
diabetes, hypertension and biliary tract diseases, family history
of cancer, cigarette use, alcohol consumption and OS. The age of
the participants ranged from 20 to 78 years. As the average age of
FPM diagnosis was ~60 years, and drinking and smoking are not
common among teenagers, 40 years was regarded as the standard for
long-term consumption when investigating the association of
long-term tobacco use and excessive alcohol intake with the
occurrence of SPM. Data on family history of cancer and the
presence of thyroid and biliary tract diseases, namely
cholecystolithiasis, cholecystitis and cholecystectomy, were mainly
obtained from the records at first admission. Patients diagnosed
with hypertension or diabetes by an endocrinologist or cardiologist
before the first diagnosis of malignancy were regarded as patients
with hypertension while those who developed hypertension during
cancer treatment were not. Diabetes and hypertension were mostly
controlled during cancer treatment, with the exception of two
female patients with esophageal cancer, who developing uncontrolled
hypertension prior to lethal upper gastrointestinal bleeding, and
three patients with lung squamous cell carcinoma (LUSC) and one
patient with lung adenocarcinoma (LUAD) who were treated with
anti-angiogenic therapy and suffered from uncontrolled
hypertension, which eased with the withdrawal of the therapy. The
prescription of chemotherapy and intensity-modulated radiation
therapy was administered by colleagues in the oncology department
mainly according to guidelines from the National Comprehensive
Cancer Network (16).
Bioinformatics analysis
Gene profiles of SCLC cell populations were
investigated through the online database SynEcoSys (version V1.1.0;
Singleron Biotechnologies). The access numbers of the three
datasets involved in this paper were GSE129299, GSE150766 and
GSE161570. All datasets are visualized with UMAP plots in 2D. The
‘Explore gene expression’ tab plots the expression of a single gene
using UMAP. DEGs with log transformed fold change >1, expressed
in >10% of the cells and with P≤0.05 are considered significant
in this database.
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used to analyze the data. Basic characteristics were
summarized as counts and frequencies and comparisons of categorical
characteristics were made using Chi-square or Fisher's exact tests.
For continuous characteristics, analysis was performed using an
unpaired t-test or one-way ANOVA (with Turkey's multiple
comparisons test for the pairwise comparison of groups). The odds
ratio (OR) of each variable and the corresponding 95% confidence
interval (CI) were calculated using univariate analysis. OS was
defined as the period between the date of FPM diagnosis and the
last known date of follow-up or the date of death. Cumulative
survival was evaluated by Kaplan-Meier analysis and differences in
survival curves between groups of patients were assessed using the
log-rank (Mantel-Cox) test. The hazard ratio (HR) was calculated
using the Mantel-Haenszel test. A two-tailed P<0.05 was
considered to indicate a statistically significant result for all
tests.
Results
Baseline characteristics
A total of 1,188 patients were diagnosed with an FPM
between June 1, 2005 and December 30, 2020 and 102 (8.59%) of these
patients subsequently developed an SPM. The 1,086 patients who did
not develop an SPM were designated as the FPM group. In the
comparison of baseline characteristics between the SPM and FPM
groups (Table I), the patients
with an SPM presented at a significantly older age at diagnosis
(59.72±10.22 vs. 57.22±11.17 years, P<0.05), higher rate of
biliary tract disease (14.71 vs. 7.73, P<0.05) and thyroid
disease (7.84 vs. 1.01%, P<0.0001), lower rate of receiving
radiochemotherapy (14.71 vs. 34.90%, P<0.0001) and higher rate
of receiving post-operative chemotherapy (28.43 vs. 12.16%,
P<0.0001). In addition, no significant differences were detected
between the FPM and SPM groups in sex ratio, family history of
cancer and metabolic syndromes, including diabetes and
hypertension. Notably, the results on cigarette and/or alcohol
consumption revealed that the proportion of patients with ≥40
years' consumption of both cigarettes and alcohol in the SPM group
was much higher than that in the FPM group (25.00 vs. 8.95%,
P<0.05), while the long-term use of either cigarette or alcohol
alone was not found to be significantly different between the SPM
and FPM groups (Table II).
 | Table I.Clinical characteristics of patients
with and without SPM. |
Table I.
Clinical characteristics of patients
with and without SPM.
|
Characteristics | Total
(n=1,188) | Patients with SPM
(n=102) | Patients without
SPM (n=1,086) | P-value |
|---|
| Age, years, mean ±
SD (range) | 57.43±11.11
(31–84) | 59.72±10.22
(41–74) | 57.22±11.17
(31–84) | 0.0299 |
| Female, n (%) | 577 (48.57) | 50 (49.02) | 527 (48.53) | >0.9999 |
| DM, n (%) | 50 (4.21) | 4 (3.92) | 46 (4.24) | >0.9999 |
| HP, n (%) | 146 (12.29) | 14 (13.73) | 132 (12.15) | 0.6370 |
| DM and HP, n
(%) | 29 (2.44) | 2 (1.96) | 27 (2.49) | >0.9999 |
| Family history of
cancer, n (%) | 116 (9.76) | 11 (10.78) | 105 (9.67) | 0.7266 |
| Biliary tract
disease, n (%) | 99 (8.33) | 15 (14.71) | 84 (7.73) | 0.0228 |
| Thyroid disease, n
(%) | 19 (1.60) | 8 (7.84) | 11 (1.01) | <0.0001 |
| Cancer type, n
(%) |
|
|
|
|
|
NSCLC | 217 (18.27) | 11 (13.73) | 206 (18.97) | 0.0438 |
|
SCLC | 103 (8.67) | 0 | 103 (9.48) | NA |
| Other
types of lung cancer | 31 (2.61) | 3 (2.94) | 28 (2.58) | 0.7443 |
| Breast
cancer | 172 (12.09) | 16 (15.69) | 156 (11.81) | 0.6616 |
|
Esophageal cancer | 60 (5.05) | 5 (4.90) | 55 (5.06) | >0.9999 |
| Stomach
cancer | 39 (3.283) | 7 (6.863) | 32 (2.947) | 0.0717 |
|
Colorectal cancer | 88 (7.41) | 5 (4.90) | 83 (7.64) | 0.4279 |
|
Other | 478 (40.24) | 55 (53.92) | 423 (38.95) | 0.0042 |
| Treatment, n
(%) |
|
|
|
|
|
None | 26 (2.19) | 3 (2.94) | 23 (2.12) | 0.4835 |
| RT | 56 (4.71) | 4 (3.92) | 52 (4.79) | >0.9999 |
| CT | 144 (12.12) | 8 (7.84) | 136 (12.52) | 0.204 |
|
RCT | 394 (33.16) | 15 (14.71) | 379 (34.90) | <0.0001 |
| ST | 32 (2.69) | 4 (3.92) | 28 (2.58) | 0.3469 |
|
SRT | 46 (3.87) | 4 (3.92) | 42 (3.87) | >0.9999 |
|
SCT | 161 (13.55) | 29 (28.43) | 132 (12.16) | <0.0001 |
|
SRCT | 329 (27.69) | 35 (34.31) | 294 (27.07) | 0.1322 |
 | Table II.Influence of long-term cigarette
or/and alcohol consumption on the risk of developing an SPM for
male FPM patients. |
Table II.
Influence of long-term cigarette
or/and alcohol consumption on the risk of developing an SPM for
male FPM patients.
|
| A0 | A10 | A20 | A30 | A40 |
|---|
|
|
|
|
|
|
|
|---|
| Consumption | SPM | No SPM | SPM | No SPM | SPM | No SPM | SPM | No SPM | SPM | No SPM |
|---|
| C40 | 3 (5.77) | 74 (13.24) | 0 (0.00) | 5 (0.89) | 1 (1.92) | 11 (1.97) | 3 (5.77) | 15 (2.68) | 13
(25.00)a | 50 (8.95) |
| C30 | 6 (11.54) | 56 (10.02) | 0 (0.00) | 1 (0.18) | 3 (5.77) | 14 (2.50) | 1 (1.92) | 56 (10.02) | 0 (0.00) | 5 (0.89) |
| C20 | 2 (3.85) | 38 (6.80) | 0 (0.00) | 3 (0.54) | 0 (0.00) | 23 (4.11) | 0 (0.00) | 6 (1.07) | 1 (1.92) | 0 (0.00) |
| C10 | 0 (0.00) | 6 (1.07) | 1 (1.92) | 3 (0.54) | 0 (0.00) | 3 (0.54) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 00 (0.00) |
| C0 | 13 (25.00) | 157 (28.09) | 3 (5.77) | 3 (0.54) | 1 (1.92) | 6 (1.07) | 1 (1.92) | 6 (1.07) | 0 (0.00) | 8 (1.43) |
Clinical characteristics of the SPM
cohort
To visualize the characteristics of the SPM cohort,
the distribution of the primary and second cancer sites in the SPM
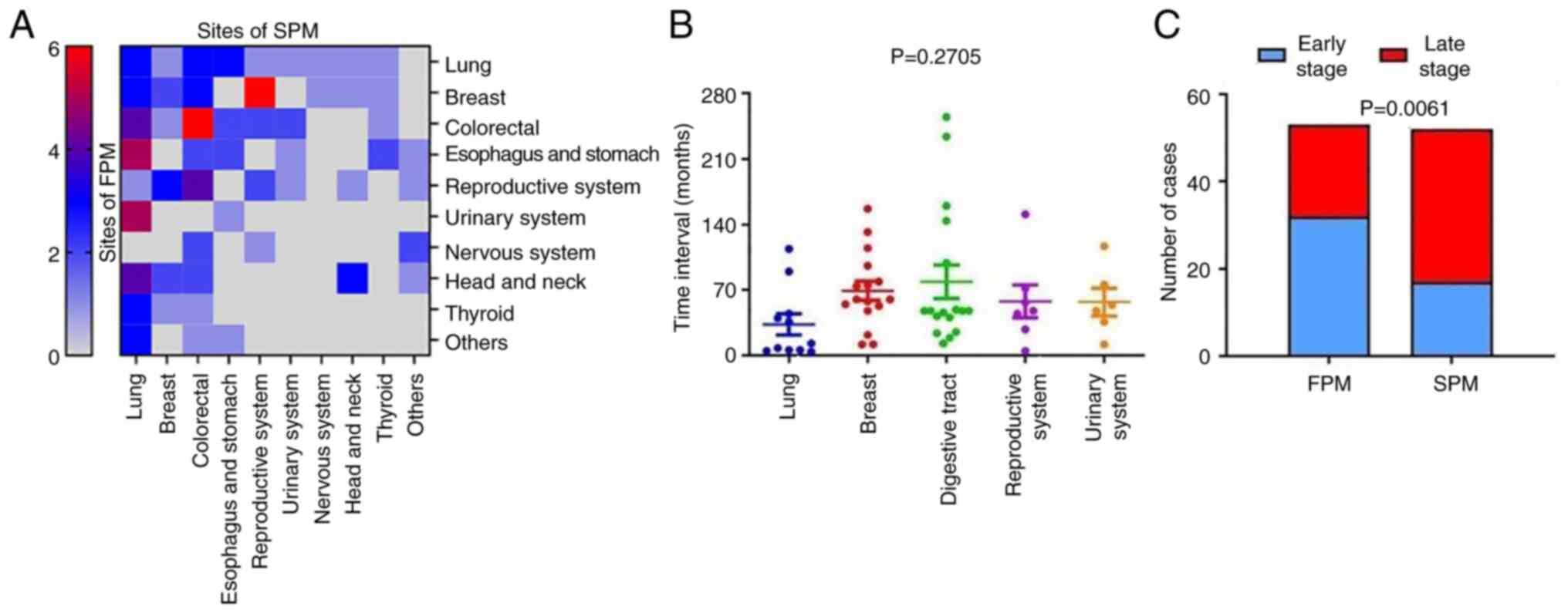
group was analyzed as a heat map (P=0.0014; Fig. 1A), and significant differences were
identified (Table I). Non-small
cell lung cancer (NSCLC; 13.73%) and breast cancer (15.69%) were
the two most common types of cancer for the first diagnosis in the
SPM group, followed by digestive tract malignancies (stomach
cancer, 6.86%; esophageal cancer, 4.90%; and colorectal cancer,
4.90%) for which details are shown in Table SI, Table SII, Table SIII. In addition, the occurrence
rate of SPM development in patients with primary NSCLC was 5.07%,
which was much lower than that of stomach cancer (17.95%),
esophageal cancer (8.33%) and breast cancer (9.30%). Moreover, the
results suggest potential associations between the distributions of
FPM and SPM. For example, patients with first primary colorectal
tumors were prone to SPM in the colon and rectum (6/18), while
patients with an FPM in the breast frequently developed
reproductive system tumors as the SPM (6/17). Due to the diverse OS
times of different types of cancers and the markedly different
constitution of the FPM and SPM cohorts, a comparison of the OS
between SPM and FPM groups was not performed. However, it was
observed that the TNM stage of the SPMs was more advanced than that
of the FPMs (P=0.0061; Fig. 1C).
This establishes an SPM as a life-threatening event, which was
subjected to further examination in specific types of cancer. These
results confirm that breast cancer is a common type of FPM with
high likelihood of SPM development, and SPMs tend to be more
advanced than FPMs.
The time interval between the FPM and SPM among
cancer types was investigated. A broad distribution of intervals
was observed for different types of cancer, among which the mean
time interval for digestive tract cancers was the longest (79.00
months, 95% CI: 40.71-117.3), while that for lung cancer was the
shortest (33.36 months, 95% CI: 8.121-58.61) without statistical
significance (P=0.2705; Fig. 1B).
Thus, patients with digestive tract cancer were not only the most
vulnerable to SPM, but also had the longest time interval for the
development of SPM in the present study population.
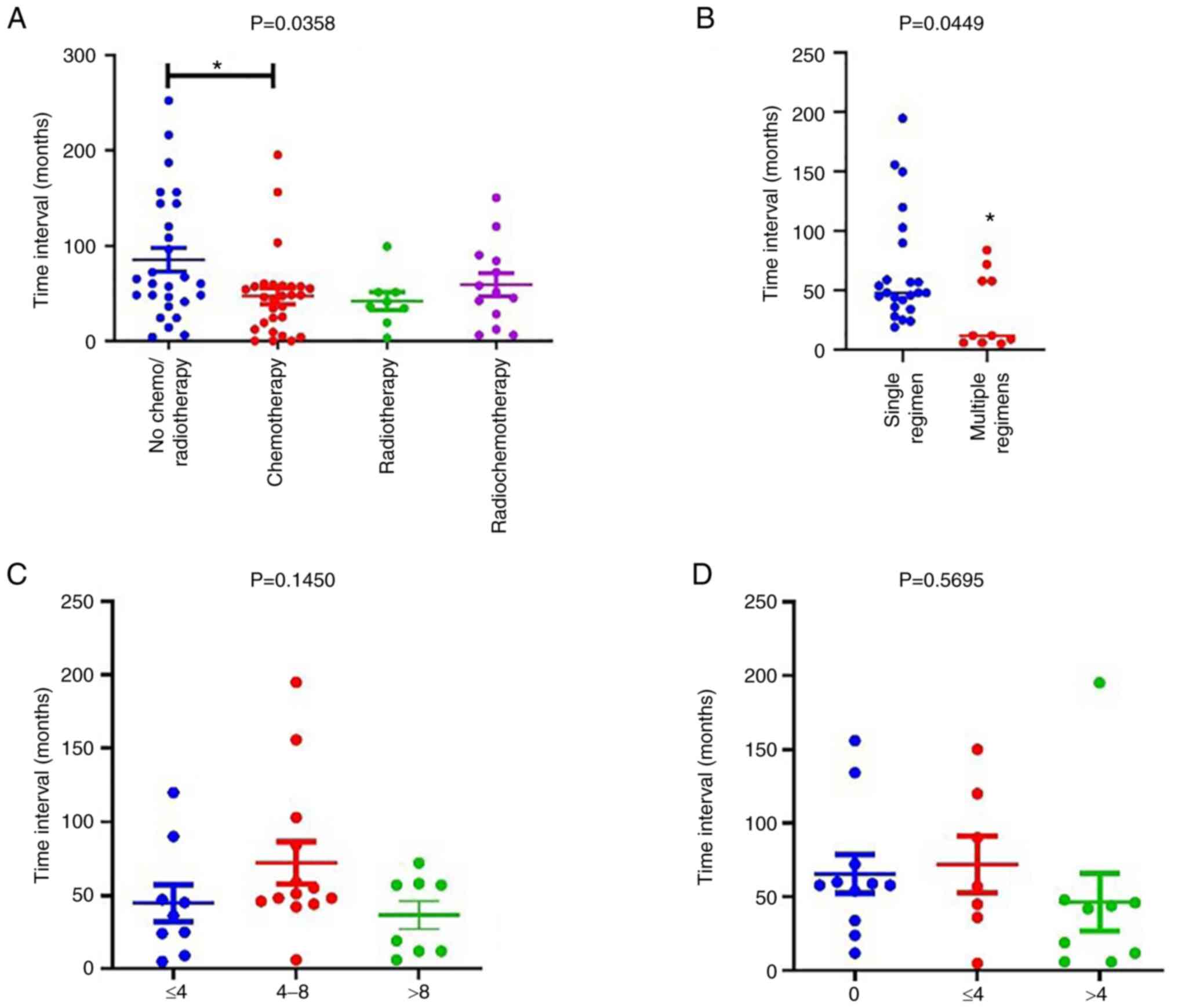
Although it has been reported that cancer treatment
increases the risk of SPM development (17,18),
no impact of radiotherapy, chemotherapy or surgical resection alone
on the risk of SPM was found in the present study. However,
adjuvant chemotherapy after surgery significantly increased the
risk of developing an SPM (OR, 2.871; 95% CI: 1.804-4.518, Table I). Furthermore, the results showed
that chemotherapy significantly reduced the FPM-SPM interval to
46.86±8.355 months compared with 85.15±12.66 months for patients
who received no chemo- or radiotherapy (P<0.05; Fig. 2A). However, no difference in the
FPM-SPM time interval was observed between patients receiving
radiotherapy or radiochemotherapy and those who received no chemo-
or radiotherapy. To explore the factors responsible for the
chemotherapy-associated reduction of the FPM-SPM time interval, the
impact of the numbers of chemotherapy regimens (Fig. 2B) and courses (Fig. 2C) on the time interval were then
investigated. The results revealed that an increase in the number
of regimens but not in the number of courses reduced the FPM-SPM
time interval. Given the ubiquity of platinum-based chemotherapy as
the first-line treatment for cancers, the effect of platinum-based
treatment was analyzed separately, but no significant difference
was detected among different numbers of courses (Fig. 2D). These results indicate that the
chemotherapy regimen, with the exception of platinum-based
chemotherapy, accelerated the occurrence of SPM, particularly in
postoperative patients.
Separate analysis of patients with
NSCLC or breast cancer
Lung and breast cancers were the most two common
FPMs in the present study; therefore, they were subjected to
further analysis. A total of 109 cases of LUSC, 96 cases of LUAD
and 103 cases of small cell lung cancer (SCLC), together with 28
cases of other types, including neuroendocrine, carcinoid and
sarcomatoid cancers, formed the cohort of lung cancer patients
(Table SIV). There were 11
patients among the 217 patients with NSCLC who developed a SPM
(Table SV), while none of the
patients with SCLC did so. In patients with NSCLC, when the
clinical characteristics were compared between patients with and
without an SPM (Table III), no
significant difference was identified in family history of
malignancy, the consumption of alcohol or cigarettes, treatment
strategy or biliary tract disease. In addition, the proportion of
cases with a pathological stage of ≥IIIA was significantly higher
for patients without an SPM than for those with an SPM (81.55 vs.
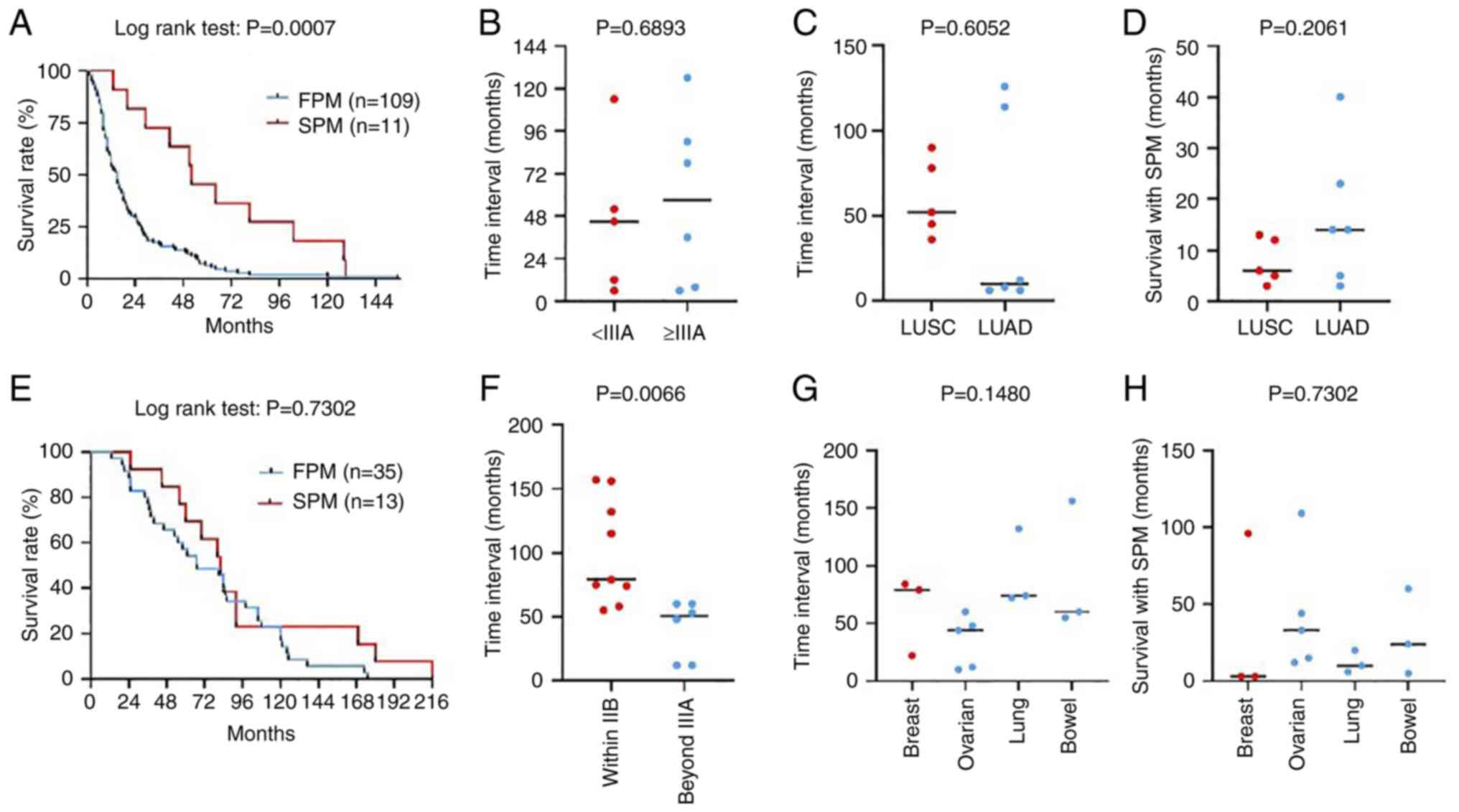
54.55%, P<0.05). Furthermore, the results of Kaplan-Meier
analysis revealed a significant reduction in OS for patients with
NSCLC without an SPM compared with those with an SPM, with a
markedly reduced median survival time (15 vs. 52 months,
P<0.001; Fig. 3A). However, the
proportion of SPM-associated deaths was only 45.45% (5/11) for the
SPM group and 2.30% for the entire NSCLC cohort. The impact of
pathological stage (Fig. 3B) and
type (Fig. 3C) on the time
interval of SPM development in patients with an NSCLC FPM was
further analyzed and no significant difference was detected. In
addition, no significant difference in the time of survival with
SPM was detected between patients with LUSC and LUAD FPMs (Fig. 3D). Therefore, the improved OS of
the SPM group may largely be associated with the early stage at
which the primary NSCLC was treated with the SPM itself showing
limited impact on the OS of the NSCLC cohort.
 | Table III.Clinical and pathological
characteristics of patients with NSCLC. |
Table III.
Clinical and pathological
characteristics of patients with NSCLC.
|
Characteristics | Total (n=217) | Patients with SPM
(n=11) | Patients without
SPM (n=206) | P-value |
|---|
| Age, years, mean ±
SD (range) | 60.91±0.45
(31–77) | 59.71±2.84
(41–71) | 60.95±0.45
(31–77) | 0.6406 |
| Sex, n (%) |
|
|
| 0.2451 |
|
Female | 45 (20.74) | 4 (36.36) | 41 (19.90) |
|
|
Male | 172 (79.26) | 7 (63.64) | 165 (80.10) |
|
| Cigarette and
alcohol consumption, n (%) |
|
|
|
|
|
None | 23 (10.60) | 4 (36.36) | 19 (9.22) |
|
|
C30 | 26 (11.98) | 2 (18.18) | 24 (11.65) | 0.4002 |
|
C30A30 | 41 (18.89) | 5 (45.45) | 36 (17.48) | 0.6972 |
| Treatment, n
(%) |
|
|
|
|
| RT | 8 (3.69) | 1 (9.09) | 7 (3.40) | 0.5517 |
| CT | 32 (14.75) | 1 (9.09) | 31 (15.05) | 0.6431 |
|
RCT | 48 (22.12) | 4 (36.36) | 44 (21.36) |
|
|
SCT | 16 (7.37) | 1 (9.09) | 15 (7.28) | >0.9999 |
|
SRT | 33 (15.21) | 1 (9.09) | 32 (15.53) | 0.5892 |
|
SRCT | 28 (12.90) | 2 (18.18) | 26 (12.62) |
|
| Family history of
malignancy, n (%) | 36 (16.59) | 4 (36.36) | 32 (15.53) | 0.0889 |
| DM, n (%) | 12 (5.53) | 1 (9.09) | 11 (5.34) | 0.4733 |
| Biliary tract
disease, n (%) | 15 (6.91) | 2 (18.18) | 13 (6.31) | 0.1707 |
| TNM stage, n
(%) |
|
|
| 0.0442 |
|
≤IIB | 43 (29.82) | 5 (45.45) | 38 (18.45) |
|
|
≥IIIA | 174 (80.18) | 6 (54.55) | 168 (81.55) |
|
| Median survival in
months, n (%) | 15.5, 120
(55.30) | 52, 11 (100) | 15, 109
(52.91) | 0.0007 |
| Succumbed to SPM, n
(%) | 5 (2.30) | 5 (45.45) | NA | NA |
In contrast with NSCLC, a family history of
malignancy was screened out as a risk factor of the development of
an SPM in patients with breast cancer (OR, 6.167; 95% CI:
1.819-22.68). The proportion of patients with an SPM that received
post-operative chemotherapy was significantly higher than of
patients without an SPM and was accompanied by a reduction in the
proportion of patients that received radiochemotherapy after
surgery (Table IV). Moreover,
although no difference in OS was detected between the FPM and SPM
groups (Fig. 3E), the mortality
rate for patients with breast cancer who did not develop an SPM was
only 22.44% and 13/16 (81.25%) patients with an SPM succumbed to
the second malignancy. The pathological stage exhibited no
significant effect on the occurrence of an SPM in patients with
breast cancer (Table IV), whereas
a higher stage (≥IIIA) in the SPM group was found to be associated
with a significant reduction in the time interval for SPM
development (Fig. 3F). Differences
among the sites at which an SPM occurred showed no significant
impact on the time interval for SPM development (Fig. 3G) or the time of survival with an
SPM (Fig. 3H) in patients with
breast cancer. These results support the idea that patients with
breast cancer who have a family history of malignancy or have
received postoperative chemotherapy are more vulnerable to the
development of an SPM and a shortened time interval for SPM
development is expected for patients with late-stage breast
cancer.
 | Table IV.Clinical and pathological
characteristics of patients with breast cancer. |
Table IV.
Clinical and pathological
characteristics of patients with breast cancer.
|
Characteristics | Total (n=172) | Patients with SPM
(n=16) | Patients without
SPM (n=156) | P-value |
|---|
| Age, years, mean ±
SD (range) | 50.90±0.70
(24–72) | 52.00±2.86
(24–67) | 50.78±0.71
(28–72) | 0.6131 |
| Treatment, n
(%) |
|
|
| 0.0371 |
|
SCT | 48 (27.91) | 8 (50.00) | 40 (25.64) |
|
|
SRCT | 119 (69.19) | 7 (43.75) | 112 (71.79) |
|
| Family history of
malignancy, n (%) | 12 (6.98) | 4 (25.00) | 8 (5.13) | 0.0159 |
| Biliary tract
disease, n (%) | 14 (8.14) | 3 (18.75) | 11 (7.05) | 0.1271 |
| DM, n (%) | 9 (5.23) | 1 (6.25) | 8 (5.13) | 0.5938 |
| HP, n (%) | 16 (9.30) | 3 (18.75) | 13 (8.33) | 0.1737 |
| TNM stage, n
(%) |
|
|
| 0.5508 |
|
≤IIB | 61 (35.47) | 9 (56.25) | 57 (36.54) |
|
|
≥IIIA | 65 (37.79) | 7 (43.75) | 61 (39.10) |
|
| Median survival in
months, n (%) | 80.50, 48
(27.91) | 82, 13 (81.25) | 67, 35 (22.44) | 0.3526 |
| Succumbed to SPM, n
(%) | 13 (7.56) | 13 (81.25) | NA | NA |
Thyroid adenoma maybe a sign of SCLC
development
In the present study, patients with an SPM presented
a higher rate of biliary tract or thyroid disease than those
without an SPM, as shown in Table
I. Excluding one case with hypothyroidism, the present study
included 7 patients with thyroid adenoma, all of whom received
excision surgery and also developed an SPM (Fig. 4A). Of these SPMs, SCLC was the most
common type (42.86%) and the FPM-SPM interval was relatively short
at 40.67±2.91 months. However, only one of the 5 patients with
thyroid carcinoma who subsequently had an SPM developed SCLC. This
indicates that benign tumors may also increase the risk of
subsequent malignancy at a different site.
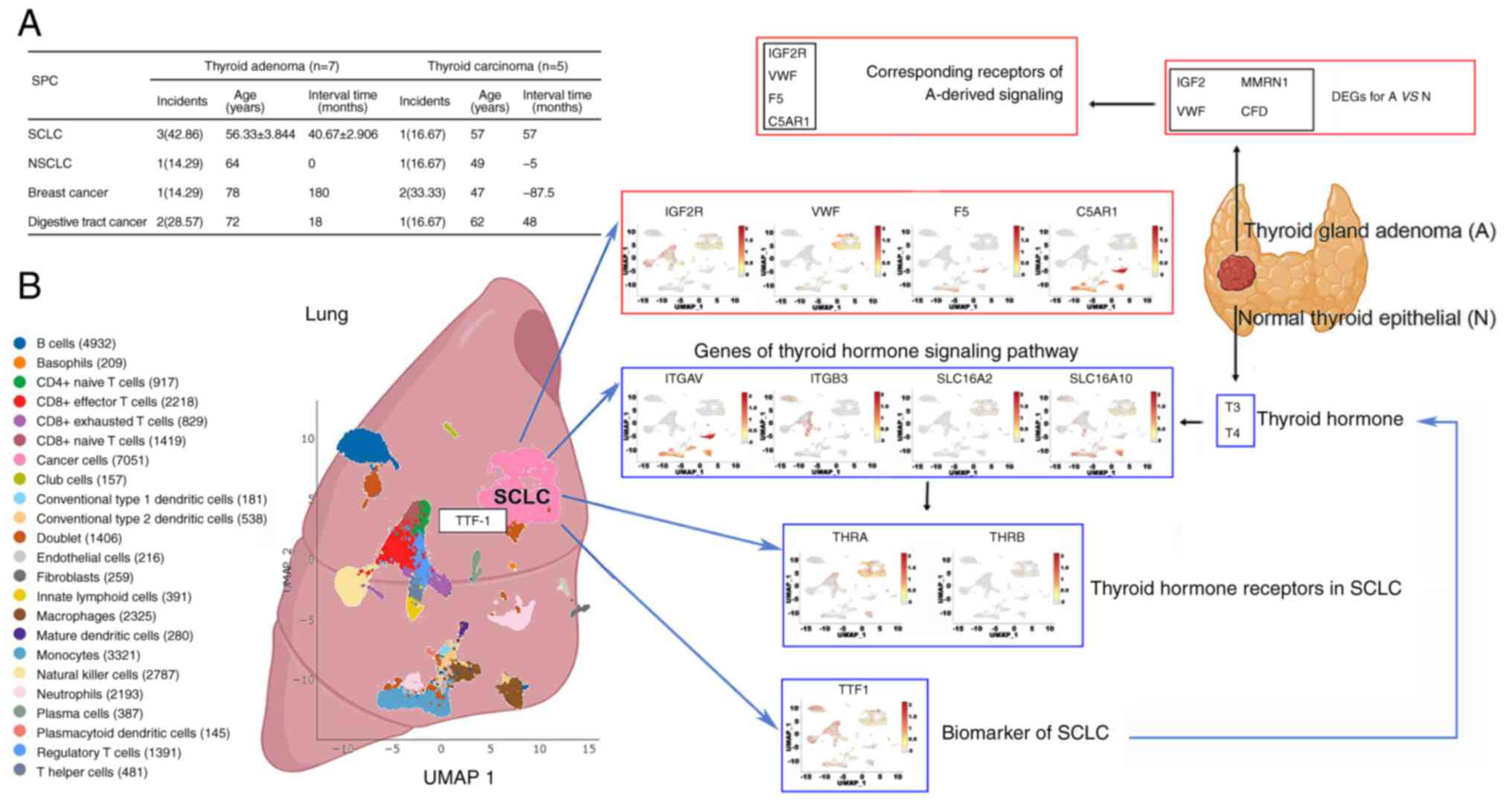 | Figure 4.Potential association between thyroid
gland adenoma and SCLC. (A) Summary of the clinical characteristics
of the SPMs for thyroid gland adenoma and thyroid carcinoma. (B)
Analysis of the expression profiles of the receptors corresponding
to the DEG-mediated signaling from thyroid gland adenoma. Vital
genes involved in the thyroid hormone signaling pathway and TTF-1
in SCLC cells, based on a reported single cell RNA-sequencing
dataset analyzed using SynEcoSys (Singeron). SCLC, small cell lung
cancer; NSCLC, non-SCLC; SPM, second primary malignancy; DEG,
differentially expressed gene; TTF-1, thyroid transcription factor
1; IGF2, insulin-like growth factor 2; IGF2R, IGF2 receptor; VWF,
von Willebrand factor; MMRN1, multimerin 1; F5, coagulation factor
V; CFD, complement factor D; C5AR1, complement C5a receptor 1;
ITGAV, integrin subunit α V; ITGB3, integrin subunit β 3;
SLC16A2/10, solute carrier family 16 member 2/10; THRA, thyroid
hormone receptor α; THRB, thyroid hormone receptor β; T3,
triiodothyronine; T4, thyroxine. |
To clarify this potential association, the thyroid
adenoma signaling-associated gene profiles of SCLC cell populations
from three different studies were investigated using bioinformatics
analysis. The differentially expressed genes (DEGs) of thyroid
adenoma are reported to be insulin-like growth factor 2 (IGF2), von
Willebrand factor (VWF), complement factor D (CFD) and multimerin 1
(MMRN1), which are involved in the regulation of IGF and the
complement and collagen systems (19). Therefore, an investigation of the
corresponding receptors for DEG-mediated signaling [the IGF2
receptor (IGF2R) for IGF2, complement C5a receptor 1 (C5AR1) for
CFD and coagulation factor V (F5) for MMRN1] and the VMF expression
profile was performed using a dataset from an orthotopic SCLC mouse
model available on SynEcoSys, with the commonly used SCLC biomarker
thyroid transcription factor 1 as the reference (20). The results showed that the
expression of IGF2R and VWF was relatively high in cancer cells,
while the expression of F5 and C5AR1 was high in the surrounding
immune cells. In addition, the expression profiles of integrin
subunit α V (ITGAV), integrin subunit β 3, solute carrier family 16
member 2/10 (SLC16A2/10) and thyroid hormone receptor α/β (THRA and
THRB) were explored, as these molecules function as transporters or
receptors of thyroid hormones and regulate the thyroid hormone
signaling pathway. The cancer cells were positive for SLC16A2,
SLC16A10 and THRA while the other genes were enriched in
paracancerous cells (Fig. 4B).
Further analysis of single cell RNA sequencing
(scRNA-Seq) datasets from another two independent studies was
conducted. The first dataset revealed that IGF2R, ITGAV or THRA
positive cancer cells gradually increase over time at a similar
rate to that at which SCLC cells evolve from being neurogenic
differentiation factor 1 positive to being Yes1 associated
transcriptional regulator positive (Fig. 5A and B) (21). The second dataset revealed that
despite their abundant expression in orthotopic SCLC cells,
relatively high expression levels of IGF2R, ITGAV and THRA were
also detected in liver metastases, whereas THRB was absent
(Fig. 5C and D) (22). These results suggest that thyroid
adenoma signals via IGF, the complement system, collagen system and
thyroid hormones, and SCLC cells are well-prepared to receive these
signals. Moreover, this potential connection may affect the
evolution, metastasis and immunotherapy of SCLC.
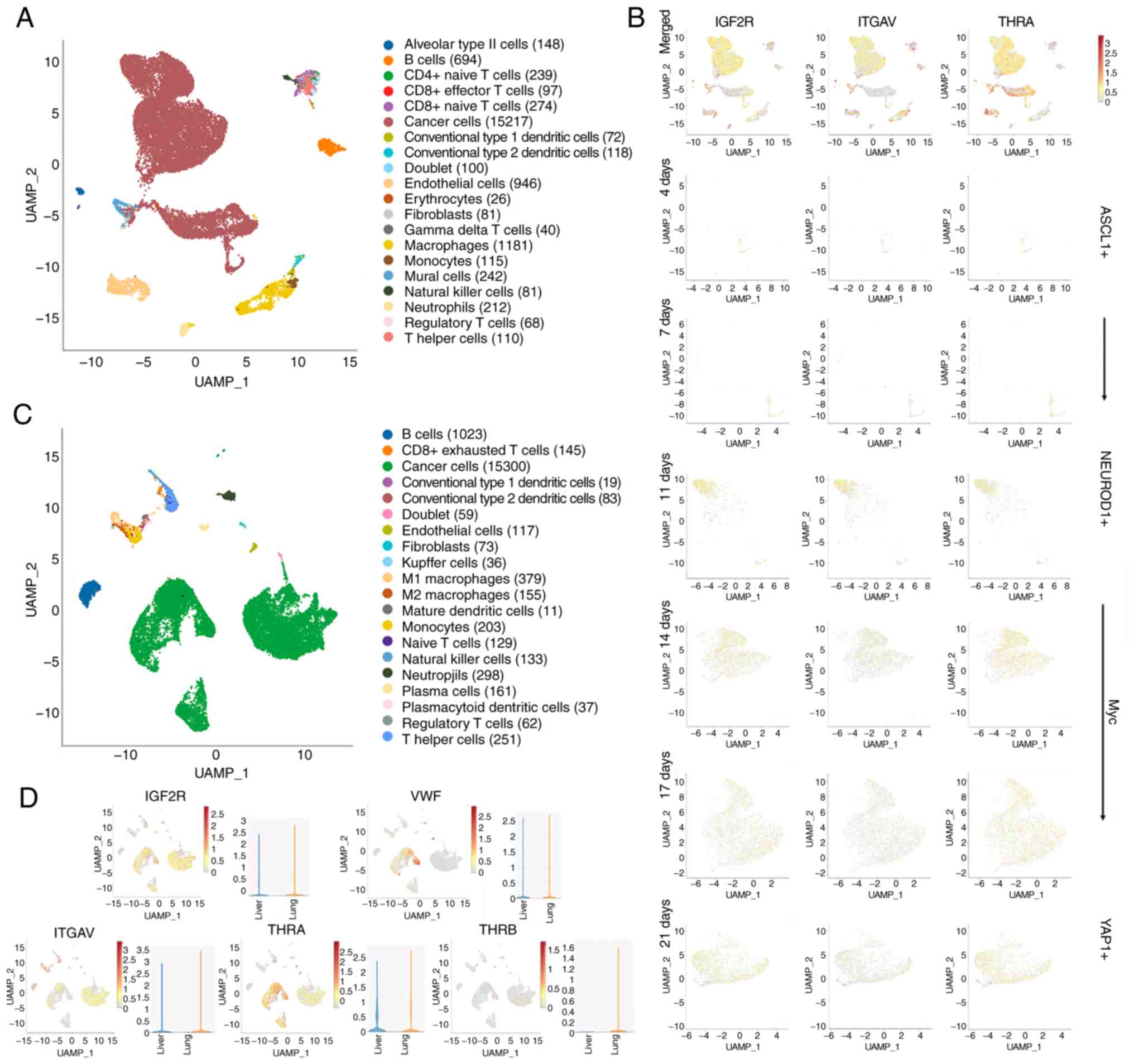 | Figure 5.Signaling associated with thyroid
gland adenoma affects the evolution and metastasis of SCLC. (A) The
UAMP projection of main clusters in human SCLC samples. (B)
Investigation of the changes of IGF2R, ITGAV and THRA expression
over time in the Myc-driven evolution of SCLC, through SynEcoSys
based on scRNA-Seq of human SCLC samples. (C) The UAMP projection
of main clusters in orthotopic and metastatic SCLC tissues from
mice. (D) Analysis of expression profiles of genes responsive for
signaling from thyroid gland adenoma in orthotopic and metastatic
SCLC tissues, through SynEcoSys based on scRNA-Seq of mouse SCLC
samples. SCLC, small cell lung cancer; IGF2R, insulin-like growth
factor 2 receptor; ITGAV, integrin subunit α V; THRA, thyroid
hormone receptor α; scRNA-Seq, small conditional RNA-sequencing;
VWF, von Willebrand factor; THRB, thyroid hormone receptor β;
ASCL1, achaete-scute homolog 1; NEUROD1, neurogenic differentiation
factor 1; YAP1, Yes1 associated transcriptional regulator. |
Discussion
To the best of our knowledge, this is the first
description of SPM characteristics in Chinese patients with diverse
non-hematological malignancies (23). The study population comprised only
patients with solid tumors as those with hematological malignances
were treated by other departments. Furthermore, due to the
relatively small number of FPM cases, it was not possible to
describe SPMs following less common types of primary tumor.
However, the distribution of FPMs was sufficient to examine cancers
of the lung, breast, colon and rectum, esophagus and stomach,
reproductive system, urinary system, nervous system, head and neck
and thyroid. The results of this study provide valuable information
on the potential sites and time to recurrence of SPMs for specific
FPMs in Chinese patients.
The relative incidences of common types of cancer in
China, including lung, colorectal and female breast cancer, are
comparable to those in the USA (24). Therefore, it is rational to compare
differences in SPM characteristics from the present study to US
data. In a previous study it was reported that nearly 1 in 12
patients (8.1%) diagnosed with a common cancer in the US develop a
second malignancy, with the majority of the patients with an SPM
being >65 years old (60%) and significantly older than those
without an SPM, with a well differentiated or moderately
differentiated first cancer (55%) (4). In the present study, the SPM rate at
8.59% was comparable with and within the range of previous reports
(5.5–16%) (25,26). In addition, the median age at first
diagnosis of the SPM cohort was significantly older than that of
patients without an SPM (59.72±10.22 vs. 57.22±11.17 years,
P<0.05). Consistent with previous reports (4,9,21), a
relatively large proportion of the SPMs were lung cancer, and none
of the SCLC cases developed SPM; however, the proportion of cases
of SCLC rose markedly to 15.69% (16/102) as the second diagnosis of
the SPM cohort.
In contrast to previous reports (9,27,28),
prostate cancer was rare as the FPM in the present study. Also, the
present study demonstrated that patients with gastrointestinal
malignances were most vulnerable to SPM and had a relatively long
FPM-SPM interval. In addition, a longer OS of patients with an SPM
was observed for the NSCLC cohort. However, patients with a FPM who
have a longer survival time are more likely to develop an SPM.
Notably, the TNM stage of 6/11 NSCLC patients with an SPM was
≥IIIA, while 168/206 of those without an SPM were in a relatively
late stage in terms of the diagnosis at first admission.
Furthermore, 16 patients with breast cancer developed an SPM and 13
of them succumbed to the SPM (81.25%). By contrast, the mortality
rate for patients with breast cancers without an SPM was only
35/156 (22.44%). Thus, having an early-stage cancer allows for
longer survival and the development of an SPM, which results in a
relatively longer OS for patients with an SPM than those without an
SPM. However, the SPM itself increases the risk of death. In light
of these distinct characteristics of Chinese SPM cases, larger
scale studies including patients from different regions, with
different FPM types, FPM treatments and comorbidity/complication
profiles are required.
Treatment- (26,29),
syndrome- and exposure-related risk factors, particularly tobacco
and excessive alcohol intake, are regarded as the three major
etiological factors for SPMs (25). Ionizing radiation is carcinogenic
and it has been reported that ~8% of SPMs may be attributed to
previous radiotherapy, although the proportion of cases varies
according to the age at diagnosis, FPM site and exposure dose
(30). However, the present study
observed little effect of radiotherapy on SPM risk. This
discrepancy from previous findings may stem from the shorter and
later follow-up period compared with that in the previous study
(31), which was ~30 years
starting from 1978. Modern radiotherapeutic technology has
developed to mitigate excessive damage to non-target tissues and
thus reduce the delayed carcinogenic effects of radiation.
Chemotherapy can also increase the risks of hematologic and solid
malignancies, particularly chemotherapy using platinum-based drugs
and alkylating agents (32,33).
In the present study, postoperative chemotherapy not only increased
the SPM risk but also shortened the FPM-SPM interval. Moreover, the
results revealed that the number of chemotherapy regimens but not
the number of courses affected the FPM-SPM interval. Furthermore,
platinum-based chemotherapy, ubiquitously used as the first line
treatment against cancers, showed a limited impact on the FPM-SPM
interval. Given the number of new anticancer agents, drug
combinations and radiation techniques, larger-scale studies with
long-term follow-up are required to further assess the
treatment-associated risk factors for SPM.
Through analysis of the SEER database, Adjei Boakye
et al (34) found that 1/12
of patients who survived a smoking-associated cancer developed an
SPM, a large proportion of which were lung cancers However, in the
current study, the influence of long-term smoking (≥40 years) on
SPM development was limited for the entire cohort and among
patients with lung cancer. Alcohol intake has been linked to an
increased risk of SPM among patients with upper aerodigestive tract
cancer (35) and female patients
with keratinocyte carcinoma (36).
However, relatively few females in China regularly smoke tobacco or
consume alcohol, so the effects of these behaviors on the
occurrence of SPM after breast cancer were not analyzed in the
present study. However, the long-term consumption of alcohol and
cigarettes was observed to significantly increase the risk of SPM
(OR, 3.140, 95% CI: 1.346-7.298, P=0.0089).
Diabetes, hypertension and family history of
malignancy were also analyzed in the present study and, in contrast
to previous studies (37,38), no differences were observed in
these factors according to whether the patients had an SPM or not.
However, the proportion of patients with a biliary tract or thyroid
disease was significantly higher for patients with an SPM than
those without. In addition, data from the separate analysis of
patients with breast cancer supported the proposition that a family
history of malignancy is a risk factor of SPM. Together, these
findings indicate that long-term alcohol and cigarette consumption
as well as dysfunction of the biliary tract or thyroid increased
the risk of developing SPM. Furthermore, postoperative chemotherapy
appeared to accelerate the development of SPMs in the whole patient
population. However, larger multiple-center studies are necessary
to assess the effects of treatment, syndromes and environmental
risk factors for SPM.
Multiple malignancies in different site are not
uncommon; however, it is unclear whether benign tumors predict SPMs
at other sites. The patient population in the present study
included 7 cases of thyroid adenoma with an SPM at a different
site, most frequently cancer of the lung, including 3 patients with
SCLC and a single NSCLC case. Thus, thyroid adenoma may predict
future lung cancer, although there is as yet no direct evidence for
such an association. It has been reported that higher free
thyroxine 4 levels are associated with greater lung cancer risk
(HR, 2.33; 95% CI, 1.39-3.92) (39). Furthermore, elevated thyrotropin
levels suggestive of hyperthyroid function were also shown to be
associated with increased risks of lung and prostate cancer in
another prospective population study (40). Together with the bioinformatics
analysis based on scRNA-Seq datasets, we hypothesize that thyroid
adenoma could be a potential risk factor for lung cancer. In the
present study, the FPM-SPM interval between a thyroid adenoma FPM
and SCLC SPM was 3–3.5 years. Therefore, a longer follow-up for
patients with thyroid adenoma is recommended to ensure the early
detection of SCLC. Further study is required on this issue to guide
the follow-up of patients with thyroid adenoma and certain other
benign tumors.
The present study is a single-center study and due
to the limited number of cases, some malignancies were categorized
according to the system affected, i.e., the reproductive system,
which covered different pathological types. Therefore, it was only
possible to analyze the relationships between the site
distributions of FPM and SPM, and not to determine the risk factors
and influence of SPMs on OS in specific systems. Further analysis
was only performed for patients with BC and NSCLC, the two most
common types of FPM. The results revealed the influence of
chemotherapy on the interval of diagnosis; however, it was not
possible to investigate each regimen. Only the role of
platinum-based chemotherapy was observed, since it is ubiquitously
used in chemotherapy, and the exact regimen was not definite
(gemcitabine, taxol or some other agents in company with platinum).
In addition, some results in the present study may be affected by
the small sample size. The study did not evaluate whether a
reduction in OS occurred for patients with an SPM in general, and
no influence of radiotherapy on the occurrence of an SPM was
detected, which may conflict with previous studies (17,18).
A larger sample size including patients from multiple centers is
necessary to understand whether these differences are due to the
patients being from different regions or the limited number of
cases.
The present study is a retrospective study and thus
selection bias is inevitable. Based on the exclusion criteria, only
solid malignancies were included and information on SPMs associated
with hematological FPMs was not gathered. In addition, the included
cases are mostly low- and middle-income patients who mainly
accepted primary examination and chemo- and/or radiotherapy.
Therefore, the roles of genetic testing, immunotherapy and other
targeted therapies on SPM were not investigated. Advanced diagnosis
and treatment methods should improve the prognosis of malignancies
and their role in SPMs merits further attention.
In conclusion, the present single-center
retrospective study showed that the malignancy of SPMs was higher
than that of FPMs and post-surgical chemotherapy shortened the time
taken for SPM development. Furthermore, the sites at which SPMs
developed were demonstrated to be associated with the site of the
FPM. Moreover, certain potential independent risk factors for SPM
were screened out, namely biliary tract disease, thyroid disease
and long-term cigarette and alcohol consumption through general
analysis, and a family history of malignancy for patients with
breast cancer. In addition, clinical and experimental evidence for
the potential connection between thyroid adenoma and SCLC was
obtained. These findings may provide valuable guidance for the
close monitoring of cancer survivors. However, a comprehensive
investigation based on a larger population is necessary to develop
long-term screening programs for SPM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant nos. 31770909 and 82203973) and the
Military Logistics Research Program, Science Foundation for
Distinguished Young Scholars of Shaanxi Province (grant no.
2018JC-013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
JZ and JY were responsible for the concept and
design of the study. FL, YG, JYZ, JS, LG and SW collected the data.
ST and JC analyzed the data. JC and CY performed the literature
review. FG and ML interpreted the data and wrote the manuscript. YW
revised the statistical analysis and CY helped with the
bioinformatics analysis. All authors read and approved the final
version of the manuscript. JZ and FG confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
This study was approved by The Hospital of 81st
Group Army PLA, Human Research Ethics Committee. Written informed
consent was obtained from all individual participants involved in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y,
Zhao R, Duan Y, Zeng Z, Li X, et al: Analysis of status and
countermeasures of cancer incidence and mortality in china. Sci
China Life Sci. 62:640–647. 2019. View Article : Google Scholar
|
|
2
|
Zeng H, Chen W, Zheng R, Zhang S, Ji JS,
Zou X, Xia C, Sun K, Yang Z, Li H, et al: Changing cancer survival
in China during 2003-15: A pooled analysis of 17 population-based
cancer registries. Lancet Glob Health. 6:e555–e567. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donin N, Filson C, Drakaki A, Tan HJ,
Castillo A, Kwan L, Litwin M and Chamie K: Risk of second primary
malignancies among cancer survivors in the United States, 1992
through 2008. Cancer. 122:3075–3086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rasmussen LA, Jensen H, Virgilsen LF,
Falborg AZ, Møller H and Vedsted P: Healthcare utilisation in
general practice and hospitals in the year preceding a diagnosis of
cancer recurrence or second primary cancer: A population-based
register study. BMC Health Serv Res. 19:9412019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barclay ME, Lyratzopoulos G, Walter FM,
Jefferies S, Peake MD and Rintoul RC: Incidence of second and
higher order smoking-related primary cancers following lung cancer:
A population-based cohort study. Thorax. 74:466–472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jo JH, Cho IR, Jung JH, Lee HS, Chung MJ,
Bang S, Park SW, Chung JB, Song SY and Park JY: Clinical
characteristics of second primary pancreatic cancer. PLoS One.
12:e01797842017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina-Montes E, Pollán M, Payer T, Molina
E, Dávila-Arias C and Sánchez MJ: Risk of second primary cancer
among women with breast cancer: A population-based study in Granada
(Spain). Gynecol Oncol. 130:340–345. 2013. View Article : Google Scholar
|
|
9
|
Zheng X, Li X, Wang M, Shen J, Sisti G, He
Z, Huang J, Li YM and Wu A: Second primary malignancies among
cancer patients. Ann Transl Med. 8:6382020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keegan THM, Bleyer A, Rosenberg AS, Li Q
and Goldfarb M: Second Primary malignant neoplasms and survival in
adolescent and young adult cancer survivors. JAMA Oncol.
3:1554–1557. 2017. View Article : Google Scholar
|
|
11
|
Simard JL, Kircher SM, Didwania A and Goel
MS: Screening for recurrence and secondary cancers. Med Clin North
Am. 101:1167–1180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Cui P, Yang Z, Zhang P, Guo R and
Shao G: Right lower lobectomy eight years after left pneumonectomy
for a second primary lung cancer. J Cardiothorac Surg. 8:462013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Dong C and Chen L: The
clinicopathological features of second primary cancer in patients
with prior breast cancer. Medicine (Baltimore). 96:e66752017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Zhong WZ, Niu FY, Zhao N, Yang JJ,
Yan HH and Wu YL: Multiple primary malignancies involving lung
cancer. BMC Cancer. 15:6962015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bian X, Wang K, Wang Q, Yang L, Xia J, Wu
W and Li L: The impact of a prior malignancy on outcomes in gastric
cancer patients. Cancer Med. 10:1457–1470. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
NCCN, . https://www.nccn.org/guidelines/category_1
|
|
17
|
Toma-Dasu I, Wojcik A and Kjellsson
Lindblom E: Risk of second cancer following radiotherapy. Phys Med.
42:211–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang F, Zhang S, Xue H and Chen Q: Risk
of second primary cancers in cancer patients treated with
cisplatin: A systematic review and meta-analysis of randomized
studies. BMC Cancer. 17:8712017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Shen Y, Ye B, Hu H, Fan C, Wang T,
Zheng Y, Lv J, Ma Y and Xiang M: Gene expression differences
between thyroid carcinoma, thyroid adenoma and normal thyroid
tissue. Oncol Rep. 40:3359–3369. 2018.PubMed/NCBI
|
|
20
|
Zhang H, Christensen CL, Dries R, Oser MG,
Deng J, Diskin B, Li F, Pan Y, Zhang X, Yin Y, et al: CDK7
Inhibition potentiates genome instability triggering anti-tumor
immunity in small cell lung cancer. Cancer Cell. 37:37–54.e9. 2020.
View Article : Google Scholar
|
|
21
|
Ireland AS, Micinski AM, Kastner DW, Guo
B, Wait SJ, Spainhower KB, Conley CC, Chen OS, Guthrie MR, Soltero
D, et al: MYC drives temporal evolution of small cell lung cancer
subtypes by reprogramming neuroendocrine fate. Cancer Cell.
38:60–78. e122020. View Article : Google Scholar
|
|
22
|
Na F, Pan X, Chen J, Chen X, Wang M, Chi
P, You L, Zhang L, Zhong A, Zhao L, et al: KMT2C deficiency
promotes small cell lung cancer metastasis through DNMT3A-mediated
epigenetic reprogramming. Nat Cancer. 3:753–767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong P, Deng L, Xin X, Luo D, Liu Z, Sun H
and Meng F: Risks of second primary malignancies among Chinese
cancer survivors at a single center during 2002–2016. Transl Cancer
Res. 7:257–267. 2018. View Article : Google Scholar
|
|
24
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
Global Cancer Statistics? Cancer Commun (Lond). 39:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin
P and Travis LB: Second malignant neoplasms: Assessment and
strategies for risk reduction. J Clin Oncol. 30:3734–3745. 2012.
View Article : Google Scholar
|
|
26
|
Li Z, Wang K, Shi Y, Zhang X and Wen J:
Incidence of second primary malignancy after breast cancer and
related risk factors-Is breast-conserving surgery safe? A nested
case-control study. Int J Cancer. 146:352–362. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Zhang P, Zhang Y, Zheng L, Xu W,
Hou D and Kang Z: Clinical characteristics and overall survival
nomogram of second primary malignancies after prostate cancer, a
SEER population-based study. Sci Rep. 11:12932021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zang Y, Qi F, Cheng Y, Xia T, Xiao R, Li X
and Yang N: Survival outcomes in prostate cancer patients with a
prior cancer. Transl Androl Urol. 10:741–753. 2021. View Article : Google Scholar
|
|
29
|
Bright CJ, Reulen RC, Winter DL, Stark DP,
McCabe MG, Edgar AB, Frobisher C and Hawkins MM: Risk of subsequent
primary neoplasms in survivors of adolescent and young adult cancer
(Teenage and Young Adult Cancer Survivor Study): A
population-based, cohort study. Lancet Oncol. 20:531–545. 2019.
View Article : Google Scholar
|
|
30
|
Mahmood S, Vu K, Tai P, Joseph K, Koul R,
Dubey A and Yu E: Radiation-induced second malignancies. Anticancer
Res. 35:2431–2434. 2015.PubMed/NCBI
|
|
31
|
Berrington de Gonzalez A, Curtis RE, Kry
SF, Gilbert E, Lamart S, Berg CD, Stovall M and Ron E: Proportion
of second cancers attributable to radiotherapy treatment in adults:
A cohort study in the US SEER Cancer Registries. Lancet Oncol.
12:353–360. 2011. View Article : Google Scholar
|
|
32
|
Turcotte LM, Liu Q, Yasui Y, Henderson TO,
Gibson TM, Leisenring W, Arnold MA, Howell RM, Green DM, Armstrong
GT, et al: Chemotherapy and risk of subsequent malignant neoplasms
in the Childhood Cancer Survivor Study Cohort. J Clin Oncol.
37:3310–3319. 2019. View Article : Google Scholar
|
|
33
|
Morton LM, Swerdlow AJ, Schaapveld M,
Ramadan S, Hodgson DC, Radford J and van Leeuwen FE: Current
knowledge and future research directions in treatment-related
second primary malignancies. EJC Suppl. 12:5–17. 2014. View Article : Google Scholar
|
|
34
|
Adjei Boakye E, Buchanan P, Hinyard L,
Osazuwa-Peters N, Simpson MC, Schootman M and Piccirillo JF: Trends
in the risk and burden of second primary malignancy among survivors
of smoking-related cancers in the United States. Int J Cancer.
145:143–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Druesne-Pecollo N, Keita Y, Touvier M,
Chan DS, Norat T, Hercberg S and Latino-Martel P: Alcohol drinking
and second primary cancer risk in patients with upper aerodigestive
tract cancers: A systematic review and meta-analysis of
observational studies. Cancer Epidemiol Biomarkers Prev.
23:324–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SM, Li T, Wu S, Li WQ, Qureshi AA,
Stampfer M and Cho E: Risk of second primary cancer associated with
pre-diagnostic smoking, alcohol, and obesity in women with
keratinocyte carcinoma. Cancer Epidemiol. 47:106–113. 2017.
View Article : Google Scholar
|
|
37
|
Halamkova J, Kazda T, Pehalova L, Gonec R,
Kozakova S, Bohovicova L, Slaby O, Demlova R, Svoboda M and Kiss I:
The impact of diabetes mellitus on the second primary malignancies
in colorectal cancer patients. Front Oncol. 10:5733942021.
View Article : Google Scholar
|
|
38
|
Park SM, Lim MK, Jung KW, Shin SA, Yoo KY,
Yun YH and Huh BY: Prediagnosis smoking, obesity, insulin
resistance, and second primary cancer risk in male cancer
survivors: National Health Insurance Corporation Study. J Clin
Oncol. 25:4835–4843. 2007. View Article : Google Scholar
|
|
39
|
Khan SR, Chaker L, Ruiter R, Aerts JG,
Hofman A, Dehghan A, Franco OH and Stricker BH: Thyroid function
and cancer risk: The Rotterdam Study. J Clin Endocrinol Metab.
101:5030–5036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hellevik AI, Asvold BO, Bjøro T,
Romundstad PR, Nilsen TI and Vatten LJ: Thyroid function and cancer
risk: A prospective population study. Cancer Epidemiol Biomarkers
Prev. 18:570–574. 2009. View Article : Google Scholar : PubMed/NCBI
|