Introduction
Renal cell carcinoma (RCC) is a common tumor,
accounting for 3–4% of malignant tumors and 90% of all renal
malignancies globally (1,2). More than 200,000 new RCC cases and
100,000 RCC-associated deaths occur each year, seriously affecting
human health (3). It is reported
that the occurrence of RCC is associated with genetic and
environmental factors, but the molecular mechanism of its
occurrence and development is still unclear (4). The primary treatment for RCC is
radical resection, but 30% of patients still have recurrence or
distant metastasis following the operation (5). Certain patients already have distant
metastasis at their first visit to a doctor and the recurrence and
metastasis rates in patients with advanced RCC are high (6). The mechanism of RCC metastasis is
still unclear. With the emergence of novel chemotherapeutic and
molecular targeted drugs, such as the advent of the tyrosine kinase
inhibitor sunitinib (7), progress
has been made in the drug treatment of RCC. However, due to the
heterogeneity of tumor cells and drug resistance of patients with
tumor, there are still problems in the treatment of RCC.
Hyaluronan-mediated mobility receptor (HMMR), also
known as CD168 on the cell surface (8), can combine with hyaluronic acid. HMMR
is not only expressed on the cell surface, but also distributed in
the cytoplasm and nucleus (9).
HMMR has the characteristics of an oncogene and can transform cells
(10). It is a
microtubule-associated protein, which can bind to microtubules,
microfilaments and calmodulin, affecting cytoskeleton assembly and
cell movement (11). It is also a
cell cycle regulator that regulates cell division in the
G2/M phase (12).
Moreover, HMMR is a centrosome and mitotic spindle-binding protein
that maintains the structural integrity of centrosome and the
number and structural integrity of spindle poles (13). All these biological characteristics
are associated with the occurrence, development and metastasis of
tumors. In clinical studies, HMMR has been shown to be expressed in
malignant glioma, as well as breast, bladder and endometrial cancer
(14–17). The HMMR gene is highly expressed in
mouse embryonic cardiomyocytes and regulates cell cycle as a
mitotic regulator (18). HMMR is
associated with, and may be a new marker for, cell proliferation
(15,19). Although HMMR is highly expressed in
a variety of tumor tissue, the mechanism of HMMR in the occurrence
and development of RCC is still unclear. The present study aimed to
investigate the mechanism of HMMR in RCC and provide a theoretical
basis for identifying novel molecular markers and gene therapy
targets of RCC.
Materials and methods
Subjects
A total of 30 patients (age range, 18–95 years;
males:females, 1:1) with RCC who received treatment at the
Department of Urology Surgery of Xintai People's Hospital (Xintai,
China) between January 2015 and October 2019 were included in the
study. Inclusion criteria were as follows: Histology or cytology
results confirming RCC; adult patients (≥18 years old), of either
sex, able to provide consent; suspected or confirmed RCC; written
informed consent provided by the patient. Exclusion criteria were a
patient age of ≤18 years old and an inability to provide informed
consent. Tumor and matched adjacent tissue (distance, ≥5 cm) were
collected and stored at −80°C. Incomplete clinical and pathological
data were obtained from the hospital biobank. All procedures
performed were approved by the Ethics Committee of Jinan Third
People's Hospital. Written informed consent was obtained from all
patients or their families.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to cleave 786-O, ACHN, 769-P and Caki-1
(all ATCC) cells and liver tissue, and total RNA was extracted by
chloroform and precipitated with isopropanol. The RNA concentration
was determined by Nanodrop 2000c ultraviolet spectrophotometer
(Thermo Fisher Scientific, Inc.). According to the instructions of
PrimeScript RT reagent with gDNA Eraser kit (Takara Biotechnology
Co., Ltd.), 1 µg RNA was reverse-transcribed into cDNA. An RT-qPCR
reaction system was set up according to the instructions of SYBR
Premix Ex Taq kit (Takara Biotechnology Co., Ltd.), and iQ5
(Bio-Rad Laboratories, Inc.) was used to assess the expression of
HMMR in RCC tissue and cells.
The forward primer for HMMR was
5′-CTGAGAGTGTCTTGGGAG-3′ and the reverse primer was
5′-CAGTGGGTGAGTGACTCTG-3′. GAPDH was used as an internal reference.
The forward primer for GAPDH was 5′-CCACTCCTCCACCTTTGACG-3′, and
the reverse primer was 5′-TGGTGGTCCAGGGGTCTTA-3′. The cycling
program was composed of an initial step to activate the enzyme at
95°C for 3 min, followed by 40 cycles of 95°C for 10 sec, 60°C for
20 sec and 72°C for 1 sec. The 2−ΔΔCq method (20) was used to calculate the expression
of the genes.
Cells
Human RCC ACHN and renal proximal convoluted tubule
epithelial HK-2 cells were cultured in MEM; human kidney clear cell
carcinoma 786-O, Caki-1 and 769-P cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.). Cell lines were
authenticated using STR profiling. All media were supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
100 U/ml penicillin/streptomycin, and all cells were incubated at
37°C and 5% CO2. When cell density reached 70–90%, cells
were digested with trypsin containing EDTA (Gibco), and the medium
was replaced every two days. The cells were seeded in 96-well
plates. After a density of 70–90% was reached, the cells were
transfected with small interfering (si)RNA-HMMR and its negative
control (siR-NC) or HMMR and its NC.
Cell Counting Kit (CCK)-8 assay
CCK-8 reagent (Dojindo Laboratories, Inc.) was added
to 786-O, ACHN, 769-P and Caki-1 cells, which were cultured for 1
h. Then, absorbance at 450 nm was measured at 0, 24, 48 and 72 h
using an enzyme-linked immunosorbent assay (ELISA) reader (Dynatech
Laboratories). Each sample was tested in 6 replicate wells.
Cell transfection
siR-NC and siR-HMMR (siR-HMMR-1, siR-HMMR-2,
siR-HMMR-3), overexpression-NC and overexpression-HMMR were
obtained from Shanghai GenePharma Co., Ltd. and transfected into
786-O and ACHN cells by Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The sequences of HMMR siRNA were as follows: HMMR-1,
5′-CUGAUUUGCAGAACCAACUdTdT-3′; HMMR-2,
5′-GGAGAAUAUUGUUAUAUUAdTdT-3′ and HMMR-3,
5′-GGUGUAUAUAGAUAUAUUAdTdT-3′. The siR-NC was non-silencing siRNA
(siR-NC-1, 5′-UUAAUAUGCAGUGCCAUAUdTdT-3′; siR-NC-2,
5′-CCAGAAUACAGAUAUAUUAdTdT-3′ and siR-NC-3,
5′-GCAGUAUAUUAAUAUAACUdTdT-3′) with the same length as siRNA of
HMMR. The sequence of overexpression-NC sequence was
5′-GGATCTACACGAATGAGGAGC-3′ and the sequence of overexpression-HMMR
sequence was 5′-GTACGGTACAGGTCACTTGAT-3′. All 786-O and ACHN cells
were seeded in 6-well plates and cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for ≥24 h before transfection, and
rinsed with phosphate-buffered saline (PBS, pH 7.4) before
transient transfection. Transfections were performed with 10 nM
siR-NC or siR-HMMR mixed with Lipofectamine 2000 at 37°C for 24 h.
The cells were harvested for subsequent experimentation 24 h
later.
Transwell assay
Migration and invasion tests on 786-O and ACHN cells
were performed in 24-well Transwell chambers (Corning, Inc.) with a
polycarbonate membrane. In the Transwell migration assay,
1×105 786-O cells were seeded in serum-free DMEM in the
upper chamber and lower chamber contained DMEM with 10% FBS.
Following incubation at 37°C for ~10 h, the inserts were taken out
carefully. The cells were fixed on the lower side of the insert
membrane with 5% glutaraldehyde for 10 min, followed by staining
with 1% crystal violet in 2% ethanol for an addition 20 min (all at
25°C). The inserts were washed in PBS for several seconds to remove
excess dye, then observed under a light microscope (Nikon, 100×
magnification). Cells from five randomly selected fields were
counted. The procedure of Transwell invasion assay were the same as
aforementioned, except the upper chambers were coated with 20 µg
extracellular Matrix gel (Sigma-Aldrich; Merck KGaA). The Matrigel
temperature was 37°C and the precoating time was 10 h.
Flow cytometry
Cell cycle was analyzed by flow cytometry. 786-O
cells were collected, treated with trypsin, washed with PBS and
then fixed with cold ethanol at room temperature for 3 min. Then,
propidium iodide (Sigma-Aldrich; Merck KGaA) was used to stain the
cells at room temperature for 15 min, and cell proportions of each
phase were detected by flow cytometry (CytoFLEX; Beckman-Coulter,
Inc.). Lastly, flow cytometry data were analyzed by WinList 7.0
(Verity Software House).
Bioinformatics
For the prediction of HMMR gene expression, The
Cancer Genome Atlas (TCGA) online database (https://portal.gdc.cancer.gov/) was utilized to
compare differential expression of the HMMR gene in normal renal
and RCC tissue. For survival analysis, TCGA (cancergenome.nih.gov/) database was used to study the
association between gene expression of HMMR and survival time and
recurrence rate of RCC. For Gene Ontology (GO) terms and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses, the Metascape (metascape.org) database was used to find the GO
classification items and associated pathways of enriched HMMR and
associated proteins, and to search for their possible functions.
The STRING database was applied to construct the protein-protein
interaction network. For proteomics data analysis, The Human
Protein Atlas database (proteinatlas.org) was used to obtain data on the
expression of HMMR protein in normal renal and cancer tissue.
Statistical analysis
Data were analyzed using GraphPad Prism 7 software
(GraphPad Software, Inc.). All data are expressed as the mean ± SEM
from three independent experiments. Statistical significance was
analyzed using a paired two-tailed Student's t-tests for two groups
and one-way ANOVA followed by Tukey's post hoc test for multiple
groups. χ2 test was performed to test differences in prognosis
between groups. Survival curves were analyzed by Kaplan-Meier and
log-rank analysis. Cox model was used to analyze the association
between characteristic data (including age, sex, grade and stage)
and patient survival. HMMR expression was ranked from low to high.
The lowest and highest 25% were defined as low- and high-expression
group, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HMMR is increased in RCC
tissue and renal cancer cell lines
Table I shows the
clinical and pathological data of patients with RCC included in the
study (Table I). To examine the
expression of HMMR in tissue and cells, RT-qPCR was performed. The
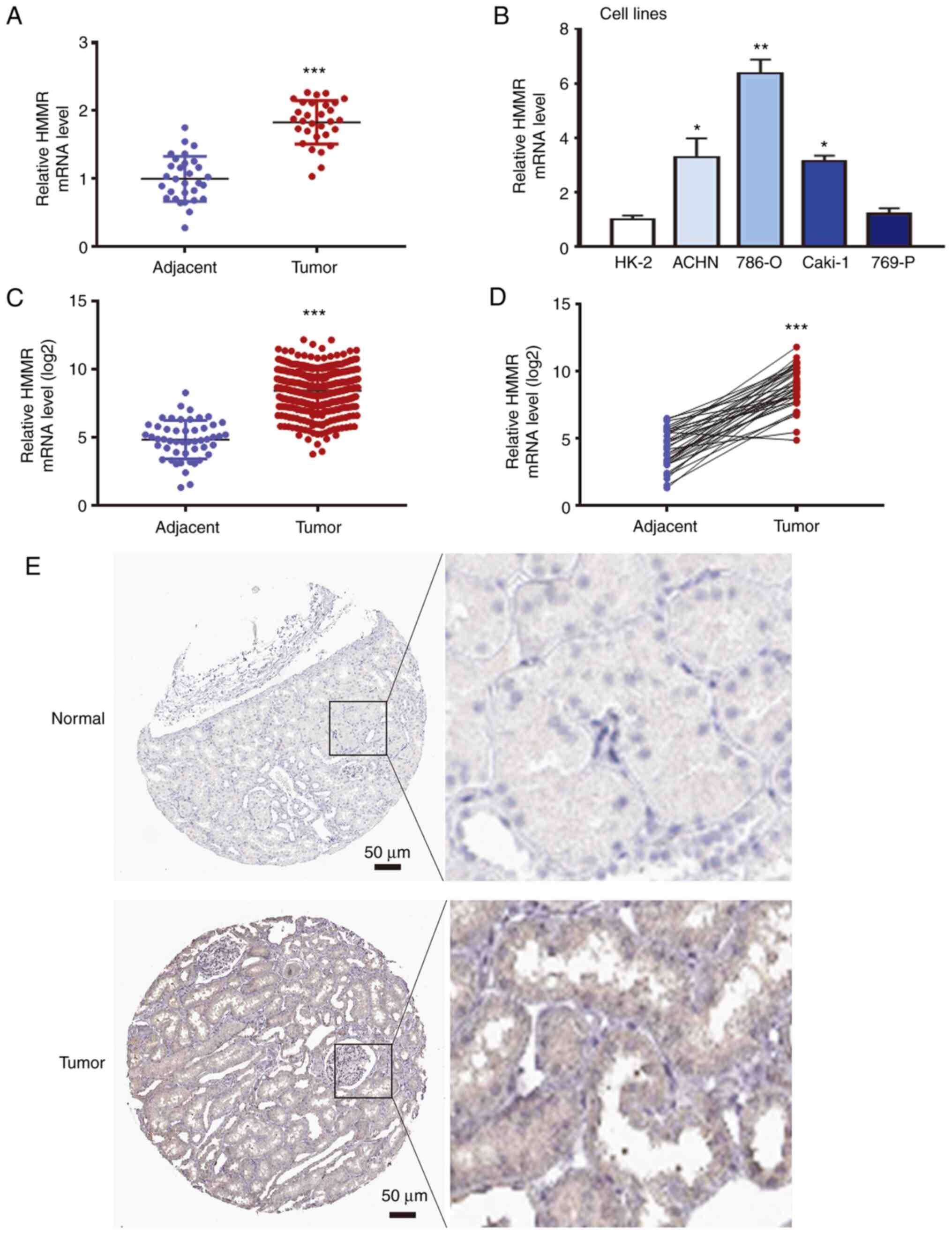
data showed that HMMR mRNA level in RCC tissue was significantly
higher than that in adjacent tissue (Fig. 1A). Moreover, HMMR mRNA levels in
renal cancer ACHN, 786-O and Caki-1 cells were significantly higher
than in HK-2 cells (Fig. 1B). A
total of 530 patients with RCC were screened through TCGA database
and the expression of HMMR was detected in RCC tissue. The data
showed that the expression of HMMR mRNA in RCC was significantly
increased compared with normal tissue (Fig. 1C). In addition, the expression of
HMMR mRNA in RCC was significantly higher than that in
corresponding adjacent tissue (Fig.
1D). The Human Protein Atlas (proteinatlas.org/) showed that
HMMR protein expression levels in patients with RCC were higher
than in normal renal tissue (Fig.
1E). These results suggested that the expression of HMMR is
increased in RCC tissue and renal cancer cell lines.
 | Table I.Clinical and pathological data of
patients with renal cell carcinoma. |
Table I.
Clinical and pathological data of
patients with renal cell carcinoma.
| Case | Sex | Age, years | Tumor stage |
|---|
| 1 | Male | 85 | III |
| 2 | Male | 52 | III |
| 3 | Female | 87 | I |
| 4 | Female | 56 | II |
| 5 | Male | 49 | IV |
| 6 | Male | 72 | III |
| 7 | Male | 31 | III |
| 8 | Female | 45 | II |
| 9 | Male | 64 | II |
| 10 | Female | 39 | III |
| 11 | Male | 55 | I |
| 12 | Female | 48 | II |
| 13 | Male | 66 | III |
| 14 | Male | 60 | IV |
| 15 | Female | 33 | II |
| 16 | Female | 73 | III |
| 17 | Female | 53 | I |
| 18 | Female | 41 | IV |
| 19 | Female | 54 | II |
| 20 | Male | 37 | III |
| 21 | Male | 51 | III |
| 22 | Male | 95 | III |
| 23 | Female | 51 | IV |
| 24 | Male | 29 | II |
| 25 | Male | 55 | II |
| 26 | Male | 89 | III |
| 27 | Male | 85 | I |
| 28 | Female | 82 | IV |
| 29 | Female | 77 | II |
| 30 | Female | 34 | III |
Expression of HMMR is an independent
prognostic factor for 5-year and disease-free survival in patients
with RCC
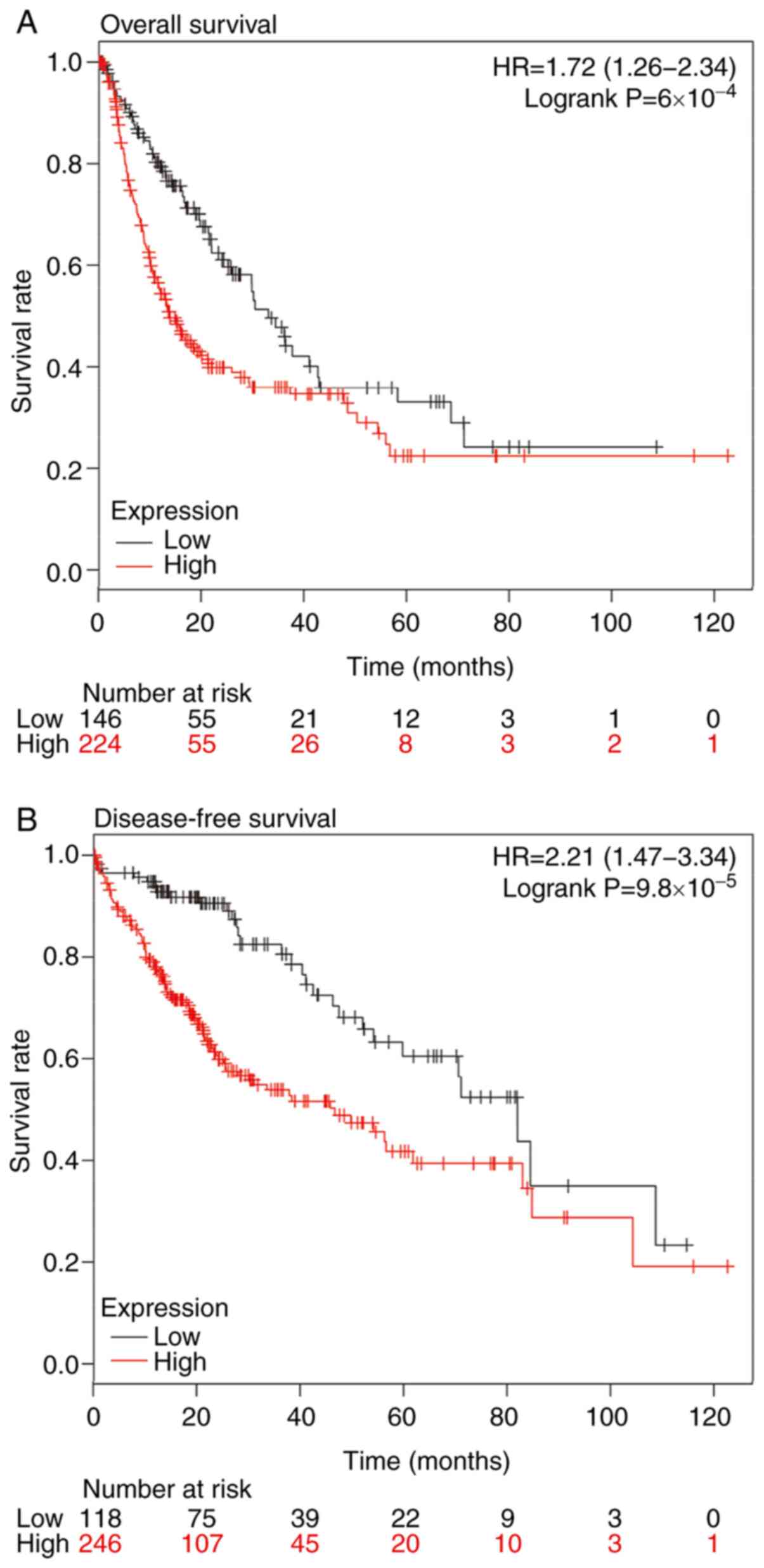
To investigate the association between HMMR and the
prognosis of patients with RCC, survival analysis of 530 patients
with RCC was performed using the expression of HMMR and 5-year
overall and disease-free survival rate. The data showed that the
5-year overall and disease-free survival rate of patients with RCC
with high expression of HMMR were both decreased (Fig. 2A and B). To analyze whether HMMR
was an independent factor affecting the prognosis of patients with
RCC, all samples were divided into low- and high-expression groups
according to the mean HMMR expression in RCC tissue. All the
samples were divided into two age groups (using 60 years as the
cut-off point) and χ2 test was performed. Univariate
analysis showed that there was no significant difference in 5-year
survival rate between different genders and ages, and the prognosis
of patients with RCC with high expression of HMMR was worse than
that of patients with low expression of HMMR. Multivariate
regression analysis showed that high expression of HMMR was an
independent prognostic factor for RCC (Table II). These results suggested that
expression of HMMR is an independent prognostic factor for 5-year
overall and disease-free survival in patients with RCC.
 | Table II.Cox regression model analysis of
overall survival in renal cell carcinoma. |
Table II.
Cox regression model analysis of
overall survival in renal cell carcinoma.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (<60
vs. ≥60) | 1.031 | 0.981-1.051 | 0.588 | 1.030 | 0.988-1.049 | 0.421 |
| Sex (male vs.
female) | 0.681 | 0.587-1.249 | 0.441 | 0.769 | 0.575-1.343 | 0.876 |
| Grade (G1 + G2 vs.
G3 + G4) | 1.215 | 0.784-1.588 | 0.467 | 1.263 | 0.639-1.611 | 0.791 |
| Stage (I–II vs.
III–IV) | 1.664 | 1.426-2.369 |
2.67×10−5 | 1.317 | 0.572-3.527 | 0.622 |
| T1 + T2 vs. T3 +
T4 | 1.573 | 1.297-2.588 |
3.58×10−5 | 1.239 | 0.474-3.249 | 0.775 |
| M1 vs. M0 | 4.634 | 2.246-6.473 | 0.037a | 1.436 | 0.394-5.312 | 0.157 |
| N0 + N1 vs. N2 +
N3 | 3.371 | 0.599-6.482 | 0.511 | 1.966 | 0.571-8.175 | 0.387 |
| HMMR (low vs.
high) | 1.235 | 1.187-1.244 |
6.63×10−5b | 1.216 | 1.041-1.273 |
2.46×10−5b |
Analysis of HMMR gene enrichment and
protein interaction
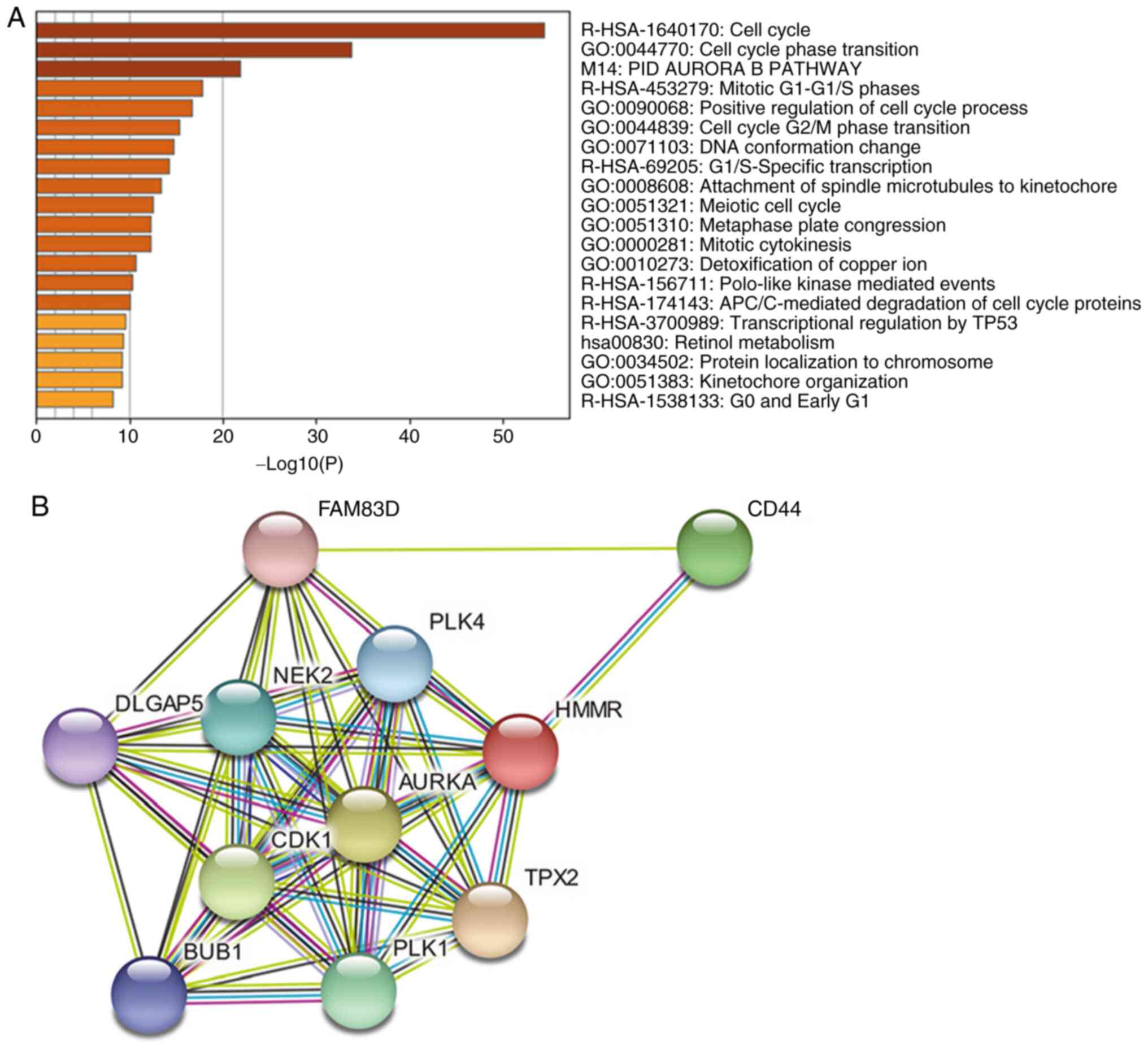
GO enrichment analysis was performed on the
differentially genes between the high- and low-HMMR expression
groups. The data showed that differentially expressed genes were
primarily enriched in biological behaviors such as ‘cell cycle’,
‘cell cycle G2/M phase transition’ and ‘DNA conformation
change’ (Fig. 3A; Table III). Kyoto Encyclopedia of Genes
and Genomes pathway analysis of HMMR-associated differential genes
showed that the high HMMR expression group involved ‘PID AURORA B
PATHWAY’, ‘mitotic G1-G1/S phases’,
‘transcriptional regulation by TP53’ and ‘retinol metabolism’
(P<0.05 vs. low HMMR expression group; Fig. 3A; Table III). Prediction of
HMMR-interacting proteins using STRING database showed that 10
proteins, including NIMA-related kinase 2, DLG-associated protein
5, CDK1, BUB1 mitotic checkpoint serine/threonine kinase, polo-like
kinase (PLK)1, TPX2 microtubule nucleation factor, aurora kinase A,
PLK4, family with sequence similarity 83 member D and CD44,
interact with HMMR (Fig. 3B).
These genes were shown to be upregulated in RCC.
 | Table III.Top 20 function pathways enriched in
patients with high expression of HMMR. |
Table III.
Top 20 function pathways enriched in
patients with high expression of HMMR.
| GO | Category | Description | Count | % | Log10(P) | Log10(q) |
|---|
| R-HSA-1640170 | Reactome Gene
Sets | Cell cycle | 87 | 22.54 | −54.46 | −50.14 |
| GO:0044770 | GO Biological
Processes | Cell cycle phase
transition | 66 | 17.10 | −33.82 | −30.58 |
| M14 | Canonical
Pathways | PID AURORA B
PATHWAY | 18 | 4.66 | −21.85 | −18.98 |
| R-HSA-453279 | Reactome Gene
Sets | Mitotic
G1-G1/S phases | 25 | 6.48 | −17.82 | −15.07 |
| GO:0090068 | GO Biological
Processes | Positive regulation
of cell cycle process | 32 | 8.29 | −16.71 | −14.02 |
| GO:0044839 | GO Biological
Processes | Cell cycle
G2/M phase transition | 29 | 7.51 | −15.36 | −12.78 |
| GO:0071103 | GO Biological
Processes | DNA conformation
change | 31 | 8.03 | −14.67 | −12.13 |
| R-HSA-69205 | Reactome Gene
Sets |
G1/S-specific
transcription | 12 | 3.11 | −14.25 | −11.75 |
| GO:0008608 | GO Biological
Processes | Attachment of
spindle microtubules to kinetochore | 12 | 3.11 | −13.4 | −10.96 |
| GO:0051321 | GO Biological
Processes | Meiotic cell
cycle | 25 | 6.48 | −12.49 | −10.1 |
| GO:0051310 | GO Biological
Processes | Metaphase plate
congression | 14 | 3.63 | −12.3 | −9.93 |
| GO:0000281 | GO Biological
Processes | Mitotic
cytokinesis | 15 | 3.89 | −12.27 | −9.9 |
| GO:0010273 | GO Biological
Processes | Detoxification of
copper ion | 8 | 2.07 | −10.64 | −8.36 |
| R-HSA-156711 | Reactome Gene
Sets | Polo-like kinase
mediated events | 8 | 2.07 | −10.34 | −8.07 |
| R-HSA-174143 | Reactome Gene
Sets | APC/C-mediated
degradation of cell cycle proteins | 14 | 3.63 | −10.05 | −7.81 |
| R-HSA-3700989 | Reactome Gene
Sets | Transcriptional
regulation by TP53 | 26 | 6.74 | −9.59 | −7.38 |
| hsa00830 | KEGG Pathway | Retinol
metabolism | 12 | 3.11 | −9.36 | −7.15 |
| GO:0034502 | GO Biological
Processes | Protein
localization to chromosome | 13 | 3.37 | −9.22 | −7.03 |
| GO:0051383 | GO Biological
Processes | Kinetochore
organization | 8 | 2.07 | −9.17 | −6.98 |
| R-HSA-1538133 | Reactome Gene
Sets | G0 and
Early G1 | 8 | 2.07 | −8.17 | −6.05 |
HMMR promotes proliferation of RCC
cells
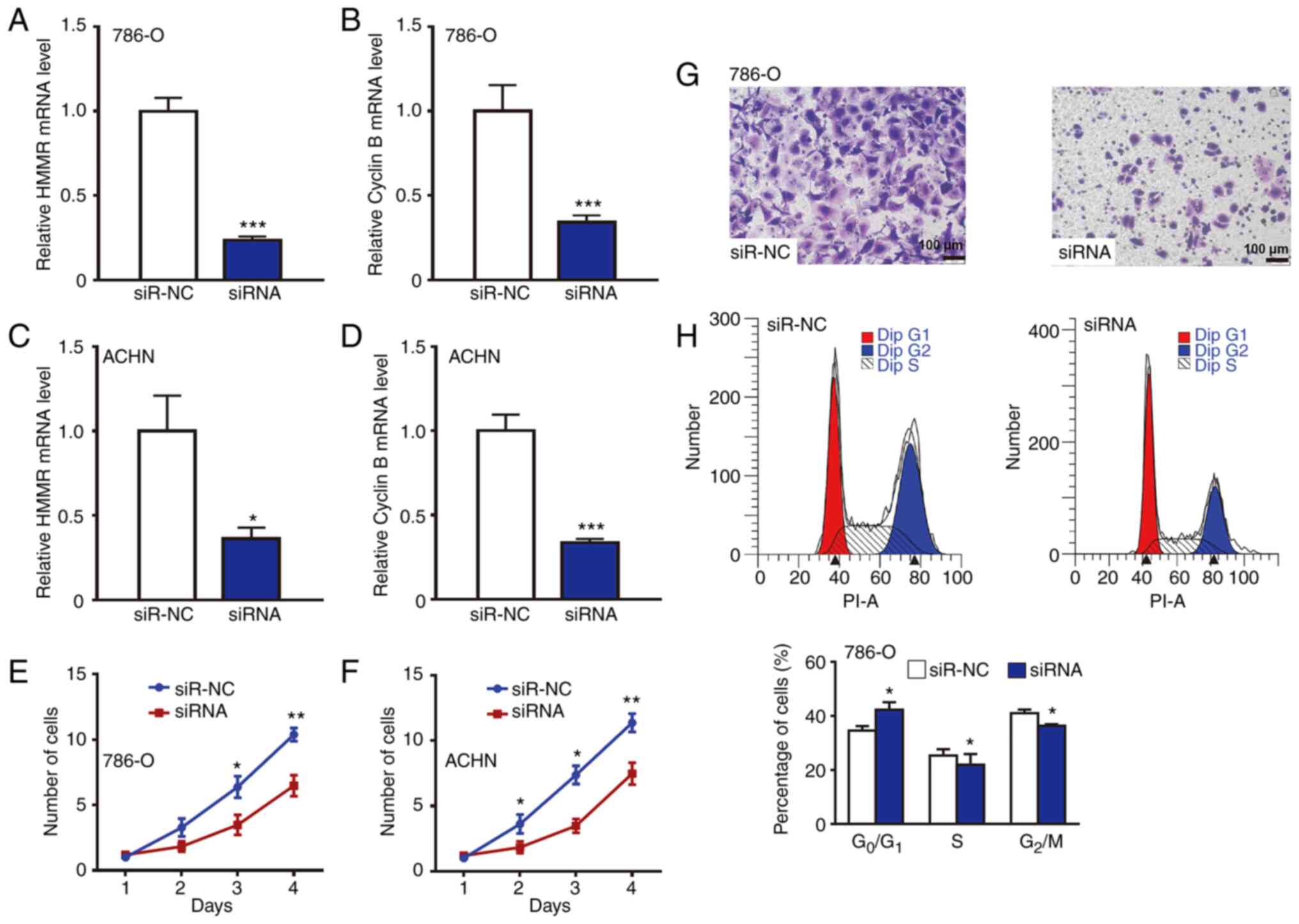
To examine the effect of HMMR on RCC cell
proliferation, 786-O and ACHN cells were first transfected by siRNA
of HMMR and Cyclin B1 to inhibit their expression. RT-qPCR showed
that HMMR and Cyclin B1 mRNA levels in transfected 786-O or ACHN
cells were significantly lower than those in the siR-NC group
(Fig. 4A-D). CCK-8 assay showed
that the proliferation of 786-O and ACHN cells with HMMR knockdown
was decreased compared with that in siR-NC group (Fig. 4E and F). Transwell assay showed
that 786-O cells with HMMR knockdown exhibited decreased migration
and invasion ability (Fig. 4G).
Flow cytometry showed that 786-O cells with HMMR knockdown
exhibited an increased percentage of cells in
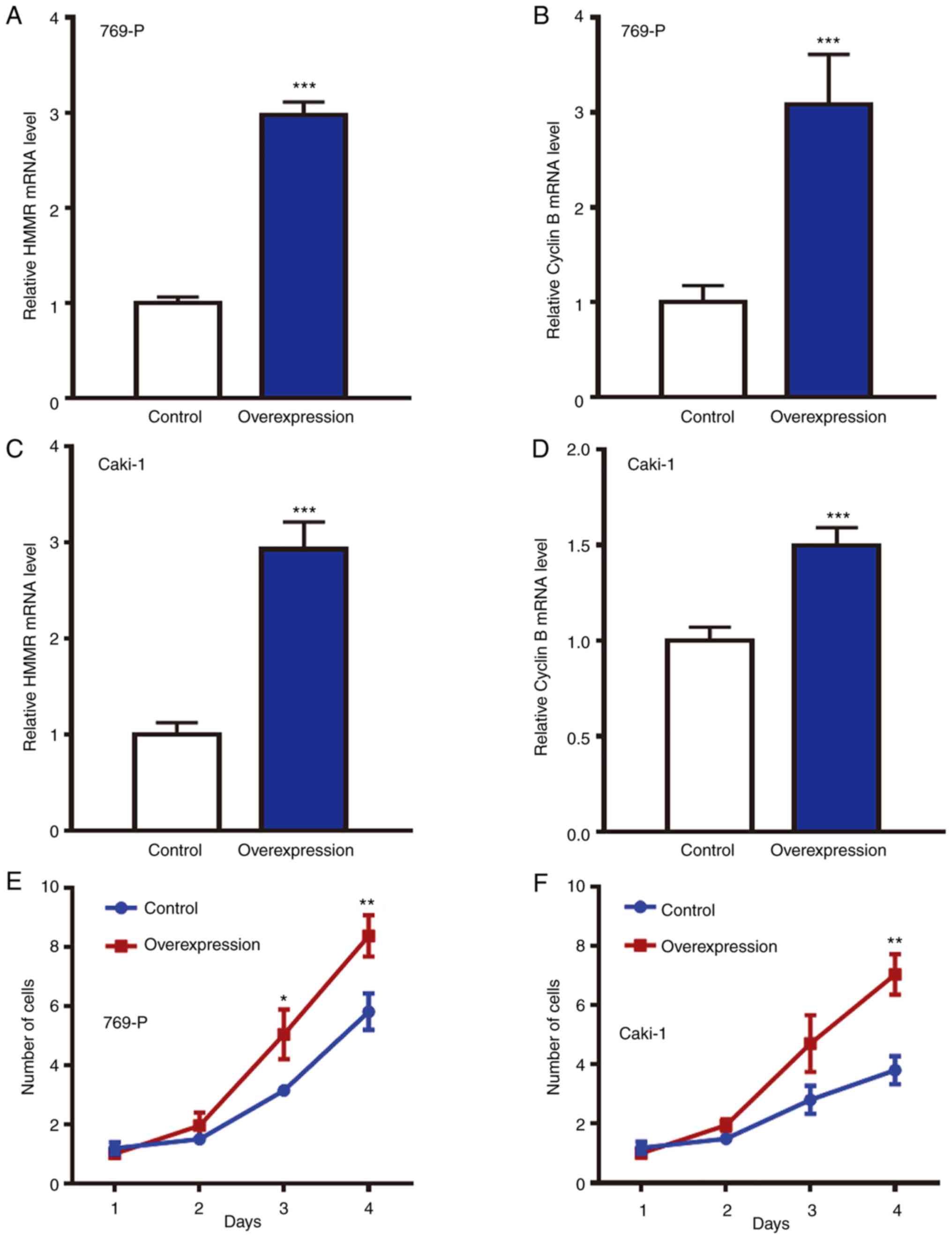
G0/G1 phase (Fig. 4H). Moreover, HMMR was overexpressed
in 769-P and Caki-1 cells via lentiviral infection. RT-qPCR showed
that expression of HMMR and Cyclin B1 mRNA in the overexpression
group was significantly higher than that in control group (Fig. 5A-D). CCK-8 assay showed that
proliferation of 769-P and Caki-1 cells with overexpression of HMMR
was enhanced compared with that in control group (Fig. 5E and F). These results indicated
that HMMR promoted the proliferation of RCC cells.
Discussion
To the best of our knowledge, the present study was
the first to observe high expression of HMMR in RCC tissue and then
verify this result in RCC cell lines. Analysis of data from TCGA
database showed that HMMR expression was associated with sex, tumor
grade and prognosis of patients with RCC. HMMR is highly expressed
in mouse embryonic cardiomyocytes, and high expression of HMMR is
associated with proliferation of hepatocytes (21,22).
However, when cardiomyocytes are exposed to oxygen, expression of
HMMR is decreased significantly (21,22).
HMMR is overexpressed in numerous types of tumor, including breast,
bladder and endometrial cancer (14–17),
and knockdown of HMMR inhibits the growth of tumor cells (23,24).
However, the specific molecular regulatory mechanism is still
unclear.
As a protooncogene, HMMR promotes cell cycle and
proliferation through G2/M phase (25). HMMR may be a novel marker for cell
proliferation. To confirm the association between HMMR and RCC cell
proliferation, RCC cell lines with high or low HMMR expression were
selected as research models. Following HMMR knockdown, the
proliferation of 786-O and ACHN cells decreased; following HMMR
overexpression, the proliferation of 769-P and Caki-1 cells was
enhanced. Cyclin B1 is a key marker for G2/M phase, and
high expression of Cyclin B1 accelerates cell cycle and promotes
cell proliferation. Following overexpression of HMMR in 769-P and
Caki-1 cells, Cyclin B1 level was increased. In 786-O and ACHN cell
lines, by knocking out HMMR, Cyclin B1 level was lowered. These
results suggested that HMMR affected cell cycle and proliferation
of RCC by regulating the expression of Cyclin B1. However, the
molecular mechanism of action of HMMR is unclear. P53 inhibits HMMR
expression via hyaluronic acid-mediated signaling and metabolic
pathways (26,27). In prostate cancer, estrogen
receptor regulates HMMR expression (28,29).
A study of estrogen-dependent tumor cell lines demonstrated that
HMMR is a downstream molecule in the signaling pathway by which
estrogen promotes tumor formation (28). Therefore, HMMR expression in RCC
cells may be associated with hormone regulation (23). HMMR exerts a variety of functions
in cells, including the phosphorylation of PTK2/FAK1 (29), but its specific molecular mechanism
and associated signaling pathways are unclear. Further work is
needed to determine the molecular mechanism of HMMR.
The present study had several limitations. First,
only Caki-1 cell line was available in the laboratory. Future
experiments should investigate the role of HMMR in Caki-2 cells.
The lack of rescue experiments is another limitation of the present
study and will be performed in future. Third, human liver samples
were obtained primarily from the hospital biobank. Therefore, only
data for sex, age and classification was available.
In conclusion, the present study demonstrated that
HMMR was significantly upregulated in RCC tissue and an independent
prognostic factor for RCC. HMMR may be involved in the occurrence
of RCC by regulating Cyclin B1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL, DW and HZ contributed to the design of the
study. LL performed the experiments. LL and DW analyzed the data.
LL and HZ interpreted results and prepared the manuscript. All
authors have read and approved the final version of the manuscript.
LL, DW and HZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of Jinan Third People's Hospital (approval no. 20200201081).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Terrone C, Guercio S, De Luca S, Poggio M,
Castelli E, Scoffone CM, Tarabuzzi R, Scarpa RM, Fontana D and
Rossetti SR: Number of nodes examined and staging accuracy in renal
cell carcinoma (RCC). BJU Int. 91:37–40. 2003. View Article : Google Scholar
|
|
2
|
Motzer RJ, Russo P, Nanus DM and Berg WJ:
Renal-cell carcinoma. Curr Probl Cancer. 21:185–232. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oosterwijk E, Stillebroer AB and Mulders
PFA: Carbonic Anhydrase IX: Its Role as a Biomarker, Diagnostic,
and Therapeutic Target in Renal Cell Carcinoma. Renal Cell
Carcinoma. Figlin R, Rathmell W and Rini B: Springer; Boston, MA:
2012, View Article : Google Scholar
|
|
4
|
Rini BI, Rathmell WK and Godley P: Renal
cell carcinoma. Curr Opin Oncol. 20:300–306. 2008. View Article : Google Scholar
|
|
5
|
Trump DL: 1. Sorafenib in advanced
clear-cell renal-cell carcinoma. Escudier B, Eisen T, Stadler WM,
Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E,
Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S,
Schwartz B, Shan M, Simantov R and Bukowski RM; TARGET Study Group,
: Department of Medicine, Institut Gustave Roussy; Villejuif,
France: Urol Oncol Semin Orig Investig. 25. pp. 443–445. 2007
|
|
6
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Snauwaert S, Vanhee S, Goetgeluk G,
Verstichel G, Van Caeneghem Y, Velghe I, Philippé J, Berneman ZN,
Plum J, Taghon T, et al: RHAMM/HMMR (CD168) is not an ideal target
antigen for immunotherapy of acute myeloid leukemia. Haematologica.
97:1539–1547. 2012. View Article : Google Scholar
|
|
9
|
Sohr S and Engeland K: RHAMM is
differentially expressed in the cell cycle and downregulated by the
tumor suppressor p53. Cell Cycle. 7:3448–3460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tilghman J, Wu H, Sang Y, Shi X,
Guerrero-Cazares H, Quinones-Hinojosa A, Eberhart CG, Laterra J and
Ying M: HMMR maintains the stemness and tumorigenicity of
glioblastoma stem-like cells. Cancer Res. 74:3168–3179. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esguerra KV, Tolg C, Akentieva N, Price M,
Cho CF, Lewis JD, McCarthy JB, Turley EA and Luyt LG:
Identification, design and synthesis of tubulin-derived peptides as
novel hyaluronan mimetic ligands for the receptor for
hyaluronan-mediated motility (RHAMM/HMMR). Integr Biol (Camb).
7:1547–1560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang C, Li C, Zhang P, Wu W and Jiang X:
Redox responsive hyaluronic acid nanogels for treating RHAMM
(CD168) over-expressive cancer, both primary and metastatic tumors.
Theranostics. 7:1719–1734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad S, Kolli S, Li DQ, de Paiva CS,
Pryzborski S, Dimmick I, Armstrong L, Figueiredo FC and Lako M: A
putative role for RHAMM/HMMR as a negative marker of stem
cell-containing population of human limbal epithelial cells. Stem
Cells. 26:1609–1619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamilton SR, Fard SF, Paiwand FF, Tolg C,
Veiseh M, Wang C, McCarthy JB, Bissell MJ, Koropatnick J and Turley
EA: The hyaluronan receptors CD44 AND Rhamm (CD168) form complexes
with ERK1,2, that sustain high basal motility in breast cancer
cells. J Biol Chem. 282:16667–16680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jöhrens K, Anagnostopoulos I, Dommerich S,
Raguse JD, Szczepek AJ, Klauschen F and Stölzel K: Expression
patterns of CD168 correlate with the stage and grade of squamous
cell carcinoma of head and neck. Mol Clin Oncol. 6:597–602. 2017.
View Article : Google Scholar
|
|
16
|
Lin SL, Chang D, Chiang A and Ying SY:
Androgen receptor regulates CD168 expression and signaling in
prostate cancer. Carcinogenesis. 29:282–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Connell M, Mei L, Gsd R and
Maxwell CA: The nonmotor adaptor HMMR dampens Eg5-mediated forces
to preserve the kinetics and integrity of chromosome segregation.
Mol Biol Cell. 29:786–796. 2018. View Article : Google Scholar
|
|
18
|
Greiner J, Ringhoffer M, Li L, Barth T,
Wölfel T, Döhner H and Schmitt M: The receptor for hyaluronic acid
mediated motility (RHAMM/CD168) is a leukemia associated antigen
eliciting both humoral and cellular immune responses in patients
with acute myeloid leukemia (AML). Cancer Cell Int. 4 (Suppl
1):S552004. View Article : Google Scholar
|
|
19
|
Ishigami S, Ueno S, Nishizono Y, Matsumoto
M, Kurahara H, Arigami T, Uchikado Y, Setoyama T, Arima H, Yoshiaki
K, et al: Prognostic impact of CD168 expression in gastric cancer.
BMC Cancer. 11:1062011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Missinato MA, Tobita K, Romano N, Carroll
JA and Tsang M: Extracellular component hyaluronic acid and its
receptor Hmmr are required for epicardial EMT during heart
regeneration. Cardiovasc Res. 107:487–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heikinheimo K, Kurppa KJ, Laiho A,
Peltonen S, Berdal A, Bouattour A, Ruhin B, Catón J, Thesleff I,
Leivo I and Morgan PR: Early dental epithelial transcription
factors distinguish ameloblastoma from keratocystic odontogenic
tumor. J Dent Res. 94:101–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bidadi B, Liu D, Kalari KR, Rubner M, Hein
A, Beckmann MW, Rack B, Janni W, Fasching PA, Weinshilboum RM and
Wang L: Pathway-based analysis of genome-wide association data
identified SNPs in HMMR as biomarker for chemotherapy-induced
neutropenia in breast cancer patients. Front Pharmacol. 9:1582018.
View Article : Google Scholar
|
|
24
|
Stevens LE, Zhao M, Liu Z and Nguyen D:
Abstract 2269: A novel molecular subset of metastatic lung
adenocarcinoma is defined by the function of the proteoglycan
receptor HMMR. Cancer Res. 75 (Suppl 15):S22692015. View Article : Google Scholar
|
|
25
|
Tang XH, Osei-Sarfo K, Urvalek AM, Zhang
T, Scognamiglio T and Gudas LJ: Combination of bexarotene and the
retinoid CD1530 reduces murine oral-cavity carcinogenesis induced
by the carcinogen 4-nitroquinoline 1-oxide. Proc Natl Acad Sci USA.
111:8907–8912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinaire N, Johnson P, Chari N, Spurgers K,
Meyn R and McDonnell T: Abstract #5312: Novel transcriptional
targets of p53 may inhibit cell migration. Cancer Res. 69
(Suppl):S53122009. View Article : Google Scholar
|
|
27
|
Keane M, Craig T, Alföldi J, Berlin AM,
Johnson J, Seluanov A, Gorbunova V, Di Palma F, Lindblad-Toh K,
Church GM and de Magalhães JP: The naked mole rat genome resource:
Facilitating analyses of cancer and longevity-related adaptations.
Bioinformatics. 30:3558–3560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu TLH, Connell M, Zhou LX, He ZC, Won J,
Chen H, Rahavi SMR, Mohan P, Nemirovsky O, Fotovati A, et al: Cell
Cycle-Dependent Tumor Engraftment and Migration Are Enabled by
Aurora-A. Mol Cancer Res. 16:16–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy JM, Park H and Lim STS: FAK and
Pyk2 in disease. Front Biol. 11:1–9. 2016. View Article : Google Scholar
|