Introduction
For over 1,000 years, medicinal plants have
attracted considerable interest as valuable resources for the
development of novel therapies targeting various receptors and
signalling pathways (1). The World
Health Organization has recognised the importance of developing the
knowledge base for the active management of traditional and
complementary medicine (TCM) through national policies. Thus,
substantial funding has been provided for TCM research (2).
Traditional medicinal plants are an essential
component of indigenous medical systems globally. In Malaysia,
~1,300 plant species are suggested to have medicinal properties.
However, only ~100 have been investigated for their medicinal
potential. Thoroughly studying the benefits of these plants may
enable them to serve as practical alternative medicines (3). Alternative therapeutic approaches
have been applied using highly effective natural compounds that
possess pleiotropic properties, such as targeting signalling
mediators, transcription factors, growth factors and kinases that
modulate multiple signal transduction pathways (4). In this respect, phytochemical
extracts such as flavonoids, carotenoids and phenolic compounds
from medicinal plants have been extensively investigated for their
anticancer activities because they are often less toxic to healthy
cells. Some phytochemicals are less potent than synthetic drugs
(5–7). Therefore, phytochemicals are often
investigated in combination with other chemotherapeutic agents to
increase the efficiency of cancer treatment while reducing the
adverse effects on healthy cells (8). However, a cautious interpretation of
the benefits of phytochemicals is essential. Some phytochemicals
are toxins that may act as carcinogens or tumour promoters
(9). For example, studies have
shown that the leaves of betel [Piper betle (Piperaceae)]
cause oral cancer. However, the increased risk of oral cancer is
due to the consumption of betel quid, a concoction including other
carcinogenic components, while betel leaves themselves possess
anticancer properties (10).
The medicinal properties of betel leaves have long
been known (11,12). However, investigations into the
mechanism of action and the bioactive components with therapeutic
potential are ongoing (12,13).
P. betle is a perennial vine creeper commonly found in the
tropical rainforest. Its leaves have been used in native ceremonies
as spices and traditional medicine in India, Sri Lanka, Bangladesh,
Thailand, Malaysia and Indonesia (11,14–16).
Betel leaves are an excellent mouth freshener
(11). They are also considered to
be helpful treatments for various ailments, including boils and
abscesses, conjunctivitis, constipation, headache, itches, gum
swelling and rheumatism (17).
Furthermore, betel leaves have been shown to possess other
bioactivities, including antidiabetic, antiproliferative,
antitumour, anti-inflammatory and antimutagenic activities
(12,18–21).
Due to the potential of P. betle, research is proceeding on
its bioactive components, including hydroxychavicol, eugenol,
chavibetol and chavivol, which may be important for halting cancer
growth or killing cancer cells via their chemotherapeutic or
chemopreventive properties (10,22).
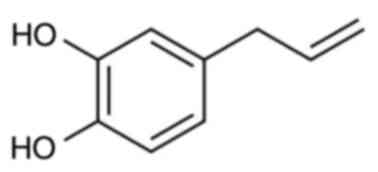
Among these, hydroxychavicol (HC), also known as 4-allyl-catechol
is a major catecholic component of betel leaves that has been shown
to have strong antimutagenic properties when compared with eugenol
(12).
HC has been reported to possess inhibitory
properties against prostate, colon, glioma and leukemic cancer
cells while leaving healthy cells unharmed (23). Notably, studies combining HC with
other drugs have shown this to be an advantageous approach, since
HC showed good results when integrated with other chemotherapeutic
agents as an adjuvant. For example, when combined with
γ-tocotrienol (GTT), HC exhibited multiple molecular effects on
glioma cancer cells, including the suppression of cell
proliferation by the endoplasmic reticulum (ER) untranslated
protein response pathway via activation of transcription factor 4
and DNA damage-inducible transcript 3 (DDIT3) protein (24). Due to the pleiotropic effects of HC
on various cancer cell lines and animal models (25–31),
the mechanisms of action of HC described in the literature were
examined in the present review to summarise the current study
scope. Thus, the present review may provide critical insights into
the cancer-preventive potential of HC, such as its antioxidant,
antiproliferative and anticancer effects. The study encompasses the
fundamental chemistry and biochemical activity of HC, laboratory
studies and the mechanism of action of HC in vitro and in
vivo.
Methods
The present scoping review followed five steps: i)
Definition of the research question; ii) identification of relevant
studies; iii) selection of articles to include in the review; iv)
charting the information; and v) summarising and reporting the
results (32). This scoping review
complies with the Preferred Reporting Items for Systematic Reviews
and Meta-Analysis (PRISMA) (33).
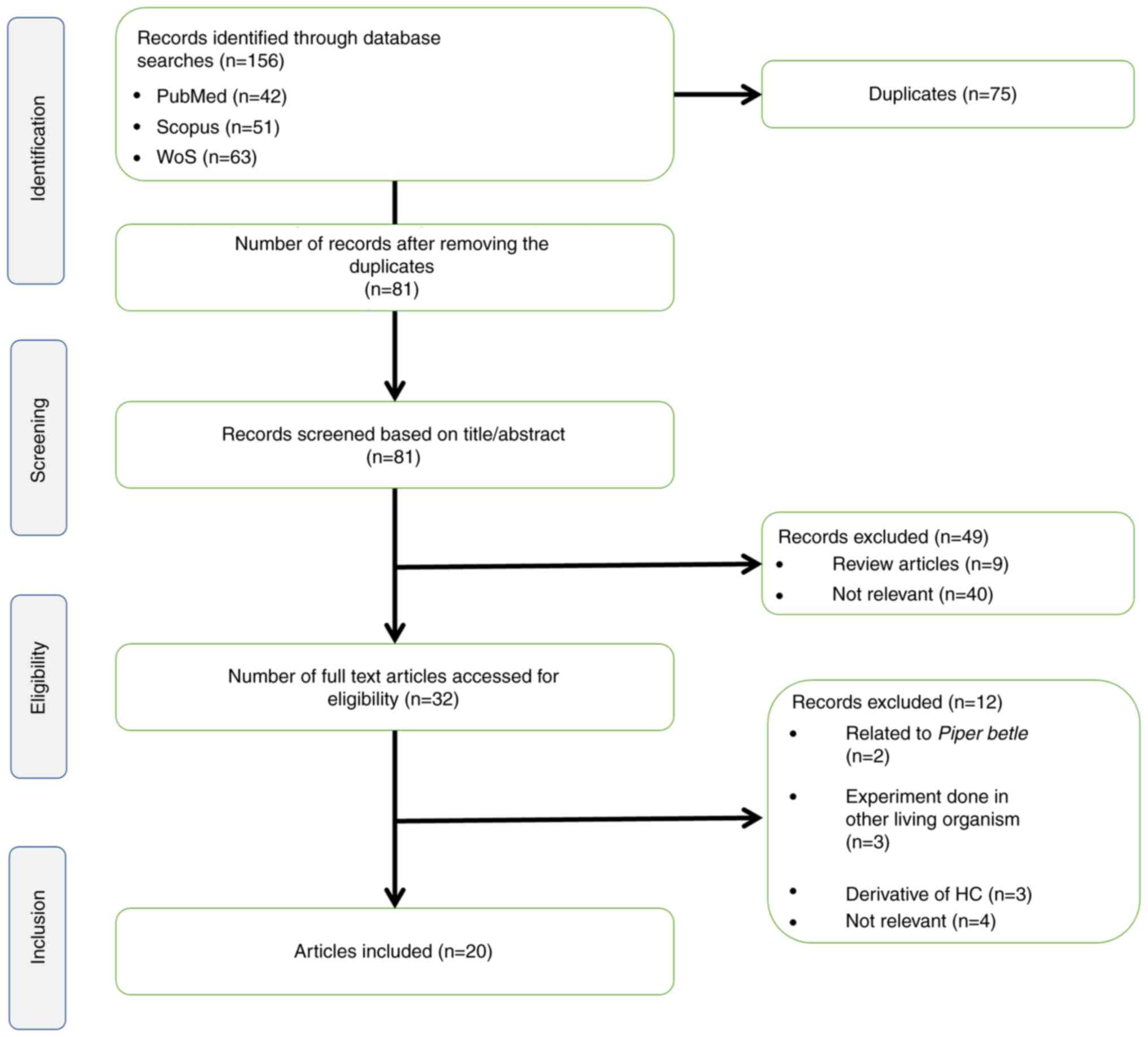
Articles were identified in August 2022 using PubMed, Scopus and
Web of Science using the search string (hydroxychavicol OR
4-allylpyrocatechol OR 4-allylcatechol) AND (cancer OR
carcinogenesis OR tumour OR carcinoma). From these databases, 156
articles were retrieved, encompassing publications between 1986 and
2022. After the removal of 75 duplicates, 81 unique articles were
identified and subjected to screening. Of these 81 articles, nine
were excluded due to being inappropriate article types, such as
chapters in books or review articles. Another 40 articles were
removed due to being on unrelated topics, such as those not on HC,
articles focused on P. betle only instead of HC, studies
focused on other properties of HC (for example as an antibacterial
or oral care agent) and studies on extraction methods. Following
evaluation of the full text of the remaining 32 articles, 12 were
rejected because they were not on cancer cells or cancerous animal
models or were studies associated with HC derivatives. Finally, the
present review included 20 articles for analysis, as shown in the
PRISMA flow chart in Fig. 1.
The publications were organised and duplicate
articles were identified using EndNote 20 (Clarivate). Article
titles and abstracts were evaluated independently by two authors
(AAR and NAM) prior to retrieval of the full text for further
review based on the inclusion and exclusion criteria. Any
differences in opinion between the two authors regarding article
inclusion were discussed with the third author (SHSAK) to resolve
the conflict. Two authors (AAR and NAM) extracted data encompassing
information on authors, years, study design, experiment model type
and notable findings.
Composition of HC
HC is the most abundant component of betel leaves
(11), and can be extracted using
various chemical solvents such as ethanol, methanol and chloroform,
or by aqueous extraction. Methanol extraction has been shown to
yield a higher concentration of HC (25.035 mg/g) than ethanol,
ethyl acetate or n-hexane (34).
The ethanol extract of betel leaves reportedly contains 66.6% HC,
followed by 11.9% eugenol, indicating that HC is the primary
constituent of these leaves (35,36).
In another study comparing the extraction of HC with methanol,
boiling water, hexane (10% ethyl acetate), chloroform, solvent-free
microwave and hydrodistillation, it was found that boiling water
extraction provided the highest yield of HC (98%) while
hydrodistillation gave the lowest (2%) (37). In general, HC remains stable in the
dried extract when stored at 5°C in the dark, and its antioxidant
activity is stable after 180 days of storage under suitable
conditions (38). Furthermore, HC
is soluble in non-polar solvents due to the presence of two
hydroxyl (−OH) groups in its chemical structure (34) (Fig.
2). Table I summarises the
properties of HC.
 | Table I.Properties of hydroxychavicol. |
Table I.
Properties of hydroxychavicol.
| Property |
Hydroxychavicol |
|---|
| IUPAC Name |
4-Prop-2-enylbenzene-1,2-diol |
| Molecular
formula |
C9H10O2 |
| Molecular
weight | 150.17 g/mol |
| Boiling point | 316.77°C |
| Melting point | 166.14°C |
Structurally, HC has an aromatic core with
1,2-dihydroxyl substituents forming a catecholic structure with
redox properties, enabling it to behave as an electron acceptor or
hydrogen donor. These characteristics are due to radical structure
resonance and oxyanion stabilisation, respectively. In cancerous
cells, this redox feature may be crucial in reducing the levels of
reactive oxygen species (ROS) and preventing the cells from
becoming malignant (39). In
addition, HC has been suggested to induce DNA damage via the
chelation and reduction of iron or copper ions, or via the
ortho-hydroxy phenoxy radical produced by oxidation of the
catechol moiety of HC with 3O2 (40). Notably, HC can act as an
antioxidant for cells at different concentrations (39).
In vitro and in vivo
studies
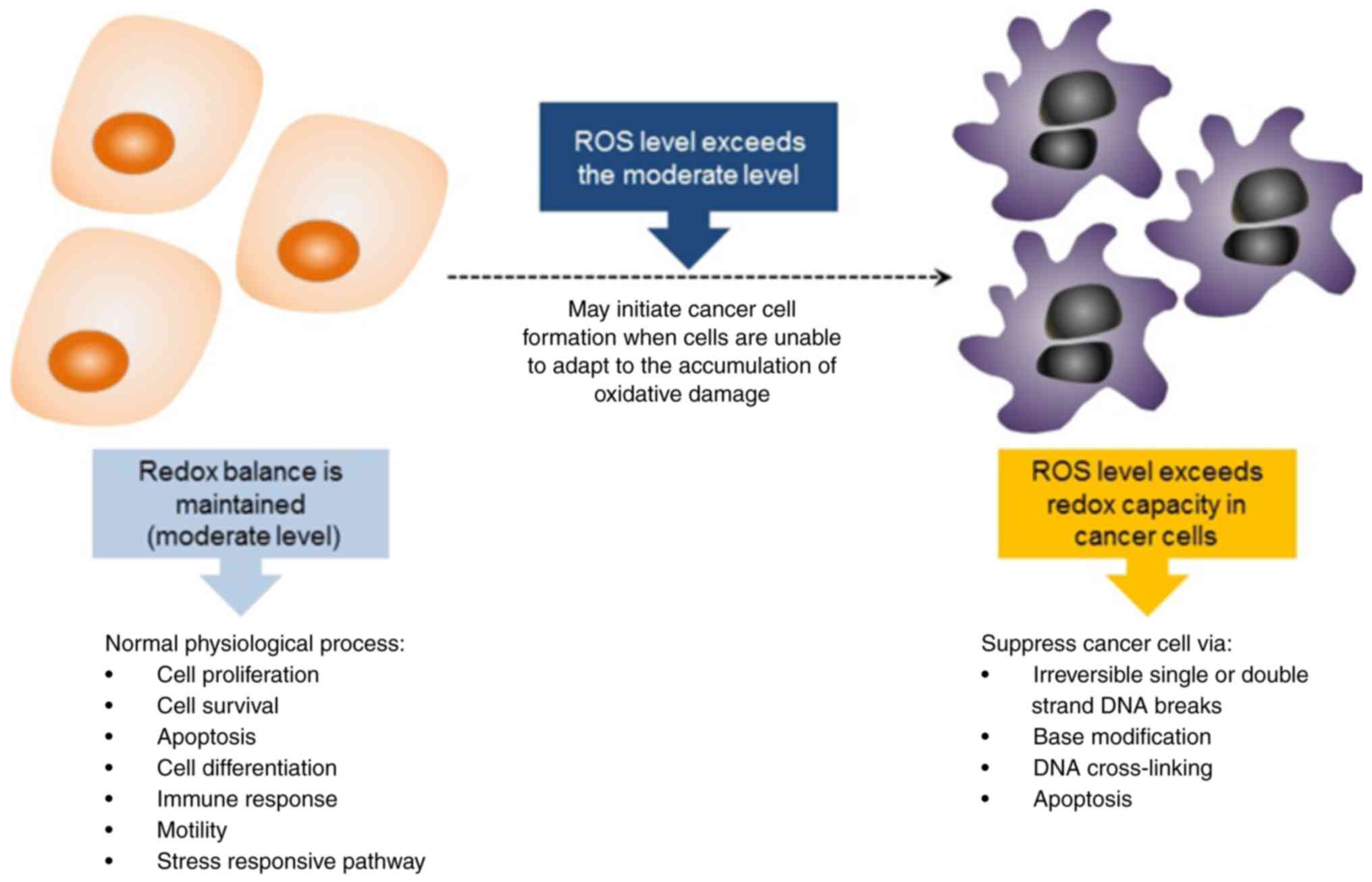
Cancer occurs partly due to imbalanced ROS levels
and an inefficient antioxidant defence system, which generate
unstable free radicals and create an oxidative stress environment
for cells (25,41). The detrimental effects of ROS on
DNA, combined with an aberrant DNA repair system and certain
signalling pathways, trigger mutations in cells and the development
of cancer. During normal metabolism, ROS are produced in living
organisms where they act as signalling molecules to activate cell
proliferation, survival, apoptosis, differentiation, immune
responses, motility and stress-responsive pathways (42,43).
Cancer cells circumvent the ROS regulation mechanisms by avoiding
the usual thresholds for the induction of cell death by increasing
their antioxidant levels aberrantly, thus enabling them to maintain
high levels of ROS and optimise ROS-driven proliferation (44). However, the death of cancer cells
can be induced by disrupting their redox homeostasis via the strong
elevation of ROS levels or inhibition of the cells' antioxidant
processes (Fig. 3). These
disruptions can cause the extensive irreversible breakage of
single- or double-stranded DNA, base modifications and DNA
cross-links (45). HC potentially
suppresses cell growth and proliferation in several cancers,
including leukaemia, prostate, breast, pancreatic and brain
cancers, via pleiotropic mechanisms, including antioxidant
pathways. The effects of HC on various cancer models and the
affected pathways are addressed in the following sections.
Chronic myelogenous leukaemia cancer
(CML)
Approximately 90% of cases of CML in patients are
caused by the reciprocal translocation of chromosome 9 and
chromosome 22 t(9;22)(q34;q11) that produces a chimeric Bcr-Abl
gene (46). Patients with CML are
generally treated with the first-generation tyrosine kinase
inhibitor imatinib mesylate (IM). However, only 10% of the
recipients showed positive responses, while others were resistant
to IM treatment (47). Thus,
efforts have been made to identify potential alternative and/or
combined treatments for CML.
A study of imatinib-resistant CML cells (48) suggested that HC sensitised the
cells to apoptosis via the induction of
tumour-necrosis-factor-related-apoptosis-inducing ligand (TRAIL)
mediated by ROS homeostasis. In the study, imatinib-resistant K562
cells were treated with 4 µM of HC alone or combined with 200 ng/ml
TRAIL. Treatment with HC or TRAIL alone for 24 h induced only 5–10%
cell death, while combined HC and TRAIL treatment for 24 h induced
a significant cytotoxic effect with an increase in apoptosis of up
to 81% (48). ROS played a primary
role in the HC-mediated sensitisation of the cells to TRAIL,
probably acting via the anti-apoptotic proteins X-linked inhibitor
of apoptosis protein and FLICE-inhibitory protein. This was evident
by the dose-dependent increase in ROS levels when the
imatinib-resistant K562 cells were treated with HC and TRAIL
together compared with the single HC treatment. However, ROS
production was not affected by TRAIL (48).
Another study found that HC induced ROS in a
time-dependent manner, resulting in ROS build-up and a state of
high oxidative stress, which subsequently triggered phosphorylation
of the c-Jun N-terminal kinase (JNK) pathway. The activation of JNK
signalling by HC was suggested to induce the phosphorylation of
endothelial nitric oxide synthase (eNOS), resulting in the
generation of nitric oxide (NO) and, ultimately, cell death
(25). However, HC alone did not
increase extracellular signal-regulated kinase (ERK) levels or the
activation of p38 in K562 cells, suggesting that the anticancer
effect of HC is mediated by a mitochondrial ROS-dependent
eNOS-mediated route (25).
However, in another study, the activation of JNK with a combination
of HC and buthionine sulfoximine (BSO) for 24 h stimulated the ERK
pathway (49). Furthermore, the
intracellular glutathione (GSH) level of the K562 cells was not
affected by the HC treatment (25).
HC exhibited potent anti-CML effects in an in
vivo nude mouse model with xenografts formed from leukaemia
cell lines expressing wild-type and mutant Bcr-Abl. The results
showed that the oral administration of 100 mg/kg HC twice daily for
5–7 days reduced subcutaneous tumour growth in the
xenograft-bearing animals (25).
Furthermore, HC was able to kill CML cell lines harbouring mutant
T315I, one of the most common mutations causing imatinib resistance
in patients, via the activation of JNK, leading to increased NO
production via the phosphorylation of eNOS.
There is evidence that HC interacts synergistically
with other treatments; for example, a study showed that 100 µM BSO
combined with 10 µM HC potentiated CML cell death by disrupting the
redox equilibrium of cells via the reduction of intracellular GSH
and promotion of ROS generation. Furthermore, the combination of
BSO and HC acted via the GSH-ROS-JNK-ERK-iNOS pathway and the
partial activation of caspase-3 and poly (ADP-ribose) polymerase
(PARP) proteins (49). Reactive
nitrogen species produced by iNOS overexpression may also be
involved in the induction of apoptosis in CML (49). This combined treatment might
improve upon the efficacy of standard chemotherapeutics in halting
the progression of CML cells.
In another study, a combination of 5 µM HC with 5 µM
curcumin induced the accumulation of superoxide and
H2O2, activated JNK and p38 in the
mitogen-activated protein kinase (MAPK) cascade and induced the
phosphorylation of mammalian target of rapamycin (mTOR) and its
downstream mediators S6 kinase and eukaryotic translation
initiation factor 4E binding protein 1, causing apoptosis in K562
cells (50). Although it was
suggested that ER stress might induce apoptosis via activation of
the mTOR signalling pathway, the underlying mechanism remained
unclear. When used singly, these doses of HC and curcumin did not
induce apoptotic changes in the CML cells. Also, treatment of the
cells with apoptosis-mediating concentrations of HC alone inhibited
mTOR signalling, while the combined treatment with HC and curcumin
activated both mTOR and MAPK pathways; these effects were dependent
on the generation of ROS and led to the apoptosis of CML cells
(50).
Breast cancer
Breast cancer is the most frequently diagnosed
cancer worldwide, with an estimated number of cases of over two
million in females in 2020 (51).
Breast cancers are classified into several molecular subtypes based
on various characteristics, including their histology, metastatic
potential, mutations, disease progression and response to therapy
(52). Numerous chemotherapies
inhibit the development of breast cancer by preventing DNA damage
(53) or by blocking oestrogen
activity, with examples including tamoxifen (54) raloxifene (55), aromatase inhibitors (56), polymerase inhibitors (57), human epidermal growth factor
receptor 2 inhibitors and trastuzumab (58). Unfortunately, due to the severe
side effects or chemoresistance that they induce, some of these
treatments do not improve patients' morbidity or mortality
(59).
A recent study indicated that HC acted as an
antioxidant on Ehrlich ascites carcinoma (EAC) cells in an in
vivo experiment using Swiss albino mice. The EAC-inoculated
mice exhibited a high malondialdehyde level, indicating elevated
ROS generation (60). The study
showed that when administered orally for 21 days at doses of 200
and 400 mg/kg body weight, HC effectively reduced the volume of the
EAC tumours in mice, indicating its anticancer potential. In
addition, as the level of oxidative stress was significantly
reduced, the lifespan of the tumour-bearing mice was increased
(60).
Pancreatic cancer
Pancreatic cancer is the 14th most common cancer and
the 7th most frequent cause of cancer-associated death worldwide
(61). The standard treatment for
advanced pancreatic cancer is gemcitabine (62). However, adjuvant therapy using a
combination of oxaliplatin, irinotecan, leucovorin and
fluorouracil, known as FOLFIRINOX, is also used due to its efficacy
and ability to promote significantly longer survival than
gemcitabine (63). Unfortunately,
~80% of cases of pancreatic cancer progress into metastatic cancers
with the development of liver metastases within 2 years of the
treatment regime, inclusive of adjuvant therapy (64). Thus, the search for an alternative
treatment or adjuvant continues to be crucial.
The proliferation, migration and invasion of
pancreatic cancer cells can be inhibited by HC treatment, as is
evident by the inhibition of genes associated with
epithelial-mesenchymal transition, i.e., goosecoid homeobox and
snail family transcriptional repressor 2, and increased DNA damage
as revealed by the comet assay (27). The HC concentration used for the
migration assay and gene expression analysis was 25 µM for 24 or 48
h. However, higher HC doses were used for protein analysis, i.e.,
50 and 100 µM for 48 h. The induction of apoptosis was confirmed by
the activation of caspase-3, −8, and −9, the cell cycle proteins
cyclin B1 and cell division control 2, cell division cycle 25C and
apoptotic-associated proteins, including PARP, BH3 interacting
domain death agonist, B-cell lymphoma 2 (Bcl2), Bcl2 associated X
and survivin. The induction of apoptosis was suggested to be
JNK-dependent. Furthermore, proteins associated with DNA damage,
including Ser-139-phosphorylated H2A histone family member X and
p53 binding protein 1, and genes DDIT3 and DNA polymerase β were
upregulated in MIA PaCa-2 and PANC-1 pancreatic ductal
adenocarcinoma (PDAC) cells treated with HC. Notably, HC exhibited
higher half-maximal inhibitory concentration (IC50)
values, indicating lower toxicity, in a panel of non-cancerous
cells comprising L929 and NIH-3T3 mouse fibroblasts and human 293
cells compared with PDAC cells (27).
Prostate cancer (PCa)
Aside from active surveillance, which is a viable
method of management for localised PCa, the two primary curative
forms of therapy are radical prostatectomy and radiotherapy
(65). A review of prostate cancer
therapies has shown that majority of patients receiving
androgen-ablative or -deprivation therapy initially responded to
treatment but became resistant over time and the cancer progressed
(66).
In one study, the antiproliferative action of HC
against a panel of prostate cancer cells comprising PC-3, C4-2,
DU145 and 22Rv1 was shown to be dose- and time-dependent, with
IC50 values ranging from 30 to 320 µM (67). The HC treatment caused the PCa
cells to accumulate in the G1 phase, signalling the onset of
apoptosis (67), as evidenced by
the upregulated levels of cleaved caspase-3 and cleaved PARP
observed in PC-3 cells treated with HC for 18 h. HC was suggested
to trigger cell death by generating high levels of ROS, which was
further supported by a reduction in the mitochondrial transmembrane
potential of PC-3 cells (67).
Autophagic signalling was also postulated as an alternative pathway
for the mechanism of action of HC in PCa cells since acidic
vesicular organelles and the elevated expression of the autophagic
markers light chain 3-IIb and beclin-1 were observed. Furthermore,
the oral administration of 150 mg/kg reduced the tumour volume of
PCa xenografts in mice by ~72% (67). In addition, the HC dosage was well
tolerated with no signs of toxicity, and the IC50 value
of HC in RWPE normal prostate epithelial cells was 398 µM, which
was much higher than that in the PCa cell lines (67).
Colorectal cancer (CRC)
As of 2020, CRC ranked third and second among the
most common cancers in men and women, respectively (51,68).
Chemotherapeutic drugs for CRC include 5-fluorouracil and
oxaliplatin. However, over time multidrug resistance may develop in
some patients (69). Therefore,
the identification of alternative compounds with anticancer
potential that can overcome the multidrug resistance pathway is
crucial.
Similar to findings in other types of cancer
(67), HC induced cell cycle
arrest at the G0/G1 and G2/M phases in TP53-resistant HT-29 colon
cancer cells, causing cells to accumulate in the G0/G1 phase
(70). The apoptotic cell death
observed in HT-29 cells following treatment with 30 µg/ml HC was
higher than that of HT-29 cells treated with 50 µmol/l
5-fluorouracil for 12, 18, 24 and 30 h (70). The HT-29 cells treated with HC
exhibited increased activation of JNK and p38 MAPK following 12 h
of treatment while the 5-fluorouracil-treated cells did not
(70).
Glioblastoma
Glioblastoma multiforme (GBM) is a primary malignant
brain neoplasia that occurs in intracranial tissue or glial cells,
which contribute to the supply of functional nutrients and oxygen
to neurons (71). GBM patients
typically have a poor prognosis, partly due to the infiltrative
nature of glioma cells (72).
Despite clinical and technological advances in the treatment of
brain tumours over the last three decades, the survival of patients
with GBM has not notably improved, and resistance to chemotherapy
with agents such as temozolomide, is commonplace (73).
In an in vitro study, HC was shown to
synergise with GTT, an isomer of vitamin E, to increase the death
of glioma cells. This combined treatment activated caspase-3 by
7.1-79.0% and induced cell cycle arrest at the G2M and S-phases
(74). In another study, the
effect of HC and GTT in reducing the migration, invasion and colony
formation of glioma cells was evidenced by a reduction in the
downregulation of several genes in these pathways, namely
cyclooxygenase (COX)-2, VEGFA, Jun and Wnt family member 5A
(24). The ability of 1321N1,
SW1783 and LN18 glioma cells to migrate was reduced by 16.1, 36.3
and 16.7%, respectively, when HC was combined with GTT (24,75).
The ER-unfolded protein response (ER-UPR) pathway was postulated to
contribute to the underlying mechanism for the HC and GTT
combination. In addition, the expression of forkhead box protein
M1, a crucial gene in pro-oncogenic signalling, was decreased in
glioma by these combined treatments (24).
Liver cancer
Liver cancer was ranked as the sixth most common
cancer in Malaysia and worldwide and the third most common cause of
cancer-associated death in Malaysia in 2020 (51). The risk factors for liver cancer
include hepatitis B virus, hepatitis C virus, fatty liver disease,
alcohol-associated cirrhosis, smoking, obesity, diabetes, iron
overload and various dietary exposures (76). Most patients with liver cancer have
a poor prognosis, with only 5–15% of patients at an early stage
without cirrhosis being eligible for surgical excision (77). Patients with late-stage liver
cancer are commonly treated with trans-arterial chemoembolisation
(TACE) and the kinase inhibitor sorafenib as chemotherapy. As it
the case with most chemotherapeutics, the long-term usage of TACE
or sorafenib tends to cause side effects, toxicity and drug
inefficacy (77).
A study revealed that the application of HC to HepG2
liver cancer cells pre-treated with BSO had cytotoxic effects,
suggesting that the mechanism of action of HC was dependent on
endogenous GSH. Although a low concentration of HC (12.5 µM) failed
to reduce the viability of HepG2 cells when incubated for 24 h, 100
µM HC induced apoptosis over the same treatment period. These
findings were confirmed by the observation of condensed chromatin
by fluorescence microscopy and nuclear fragmentation using gel
electrophoresis (78). It was
concluded that these findings indicate that HC induces oxidative
DNA damage and apoptosis in GSH-depleted HepG2 cells.
Oral cancer
Oral cancer is one of the most common head-and-neck
cancers, which includes cancers of the lip, tongue, gum, palate,
mouth, floor of the mouth, gingiva and other parts of the oral
cavity (79). In 2020, India had
the highest number of oral cancer cases and Malaysia was ranked
sixth among Southeast Asian countries for the prevalence of this
type of cancer (51). Oral cancer
may occur due to infection with human papillomavirus (HPV), poor
hygiene, poor dental care and the consumption of unhealthy food
(79). The most common treatment
for oral cancer is surgical resection alone or combined with
radiotherapy or adjuvant therapy. Despite the advancement of
surgical techniques, the number of cases of oral cancer in certain
Asian regions continues to rise, partly due to habits including
alcohol consumption, smoking, tobacco chewing and areca nut
chewing, as well as the limited availability of tertiary healthcare
for most of the population (80–85).
A study indicated that at concentrations of <0.1
mM, HC exhibited anti-oxidative properties, while at higher
concentrations, it inhibited oral KB carcinoma cell growth and cell
cycle progression (31).
Furthermore, at concentrations of 10 and 50 µM HC acted as a
scavenger of H2O2, reducing
H2O2-induced chemiluminescence by 53 and 75%,
respectively (31). In addition,
0.02 µM HC effectively scavenged superoxide created by
xanthine/xanthine oxidase (31).
HC was a more potent scavenger of superoxide than of
H2O2. The treatment of oral KB cells with
≥0.1 mM HC for 24 h induced cell cycle arrest at the late S and
G2/M phases and reduced the GSH levels of the cells. Interestingly,
at the low concentration of 0.01 mM, HC inhibited the production of
ROS in oral KB cells. However, the intracellular accumulation of
ROS occurred at a higher concentration of HC (0.1 mM) (31). In another study, 50 and 100 µM HC
effectively inhibited the expression of matrix metallopeptidase
(MMP-9) induced by areca nut extract in squamous cell carcinoma of
the tongue epithelial cells. Although these concentrations of HC
inhibited the expression of MMP-9, which plays a vital role in
cancer invasion and metastasis, it could not halt the proliferation
of the cells (86).
In a study of the effect of HC on KB epithelial
cells (87), exposure to HC at
concentrations of 0.1 and 0.3 mM for 6 h resulted in S-phase
arrest. However, when treated with 0.1 mM HC for 24 h, some KB
cells progressed to the G2/M and G0/G1 phases, and when exposed to
0.3 mM HC for 24 h, KB cells were either arrested in the S phase or
became apoptotic (87). HC also
induced mitochondrial depolarisation in the cells as demonstrated
by impaired rhodamine uptake. At a concentration of 0.3 mM, HC
caused GSH depletion and the generation of intracellular ROS in KB
cells, while at concentrations of 0.1 and 0.2 mM, HC raised the GSH
levels of the KB cells (87).
Skin cancer
Skin cancer is a frequently occurring cancer for
which sun exposure is the leading cause (88–90).
Malignant melanoma (MM) and non-melanoma skin cancer (NMSC) are the
two main types of skin cancer (88). Over the last 50 years, the
incidence rates of both MM and NMSC have increased, with MM showing
a 0.6% increase among adults (91).
In a study investigating the effect of HC on DNA
biosynthesis in a female Swiss mice model of skin cancer, exposing
the mice to 1 mg/day of HC demonstrated the ability to restore
normal DNA biosynthesis in skin exposed to the carcinogen
dimethylbenz[a]anthracene (92).
In summary, HC has shown anticancer effects in
various cancer cell lines, including CML, glioma, breast,
pancreatic, prostate, oral and colorectal cancer cells. In general,
HC affects the JNK pathway, induces ROS, inhibits the cell cycle
and lowers the viability of the cancer cells. However, data on the
bioavailability of HC in cancer cells and animal models remains
scarce. Bioavailability is the extent and rate at which an active
compound enters the systemic circulation to reach the site of
action (93). It remains unknown
if HC goes directly to target sites or affects the organs, and the
effects of HC in the body may be reduced as it migrates to the
target site. Another aspect to consider is the inflammatory process
that HC may induce in cancer cells. Unfortunately, little
information is available on this in the literature, although the
anti-inflammatory effects of HC have been studied in healthy cells.
Table II summarises the
anticancer activity of HC in various cancers based on in
vitro and in vivo studies.
 | Table II.Anticancer activities of HC in
various cancers based on in vitro and in vivo
studies. |
Table II.
Anticancer activities of HC in
various cancers based on in vitro and in vivo
studies.
| First author/s,
year | Cancer type | Experimental
models | Treatment | Major findings | (Refs.) |
|---|
| Paul et al,
2019 | CML | K562-imatinib
resistant | HC (2, 4 and 6
µg/ml) + TRAIL (200 ng/ml) | ↑ROS, sensitised to
TRAIL-induced apoptosis, ↑cytotoxic effect and induced potent
anti-CML activity, induced caspase-8 cleavage, ↓XIAP and FLIP
protein expression (4 and 6 µmol/l HC) via proteasomal
degradation | (48) |
| Chakraborty et
al, 2012 | CML | Mouse xenografts
with mutated or wild-type Bcr-Abl | HC (100 mg/kg) | ↑Apoptosis,
lifespan of tumour-bearing mice and ↓tumor growth | (25) |
| Chakraborty et
al, 2012 | CML | K562, KU812 and
KCL22; K562 | HC (0.5-20 µg/ml);
HC (5 µg/ml) | ↓Cell viability in
a dose-dependent manner, ↑ROS, ↑NO, ↑apoptosis, no depletion of
GSH; induced cleavage of caspase-9 and caspase-3, degraded PARP,
induced JNK and eNOS phosphorylation (2.5 µg/ml) | (25) |
| Chakraborty et
al, 2012 | CML | Primary CML cells
and leukaemic cells expressing mutated Bcr-Abl | HC (5 µg/ml) | ↓Cell
viability | (25) |
| Chowdhury et
al, 2013 | CML | K562 | 10 µM + 100 µM
BSO | Disrupted the ROS
balance and induced the production of RNS, cleaved PARP and
caspase-3, triggered apoptosis-inducing factor-translocation,
induced phosphorylation of JNK and ERK1/2, ↑iNOS | (49) |
| Chaudhari et
al, 2014 | CML | K562 | HC (0, 5 and 15 µM)
+ curcumin (1, 2.5 and 5 µM) | ↓Cell viability,
activated mTOR, MAPK and mitochondrial signalling pathway, induced
cleavage of caspase-3, caspase-9 and PARP, ↓Bad, Bax and BID
protein expression, ↑phosphorylation of JNK1, p38, mTOR and S6K-1,
and ↑4E-BP1 protein expression | (50) |
| Hemamalini et
al, 2020 | Breast cancer | Ehrlich ascites
carcinoma mouse model | HC (200 and 400
mg/kg) | ↓Tumour volume,
↑oxidative stress markers GSH, superoxide dismutase and catalase,
↓MDA levels, indicated to inhibit chemokine receptor type 4 via
in silico receptor docking | (60) |
| Majumdar and
Subramanian, 2019 | Pancreatic
cancer | MIA PaCa-2 and
PANC-1 | HC (25–100 µM) | ↓Cell viability,
↓colony formation, ↑cell cycle arrest at G2/M, ↑mitotic
catastrophe, induced EMT by ↓migration and invasion,
↓EMT-associated genes (Snail1, MMP9 and ICAM-1), ↓G2/M checkpoint
protein cyclin B1 and CDC2 phosphorylation, ↑DNA response protein
γH2AX and ↑phosphorylation of ATM and Chk1 | (27) |
| Gundala et
al, 2014 | Prostate
cancer | PC-3, C4-2, DU145
and 22Rv1; PC-3 | IC50
values 100, 35, 316 and 33 µM, respectively; 100 µM HC | ↓Cell viability,
↑apoptosis, ↓clonogenicity, ↑ROS, ↑induced release of cytochrome
c from mitochondria, inhibited cell cycle progression, ↑DNA
damage, ↑cleavage of caspase-3 and PARP, ↑γH2AX, and ↑activation of
autophagy protein markers LC3-IIB and beclin-1 | (67) |
| Gundala et
al, 2014 | Prostate
cancer | PC-3-luc tumor
xenografts in C47BL6/J mice | HC (150 mg/kg) | ↓Tumour weight,
↑cleavage of caspase-3 and PARP | (67) |
| Rajedadram et
al, 2021 | Colorectal
cancer | HT-29 | HC (30 µg/ml) | ↓Cell viability,
↑apoptosis, induced cell cycle arrest at G0/G1 and G2/M phases,
induced activation of JNK and p38 MAPK | (70) |
| Rahman et
al, 2019; Rahman et al, 2014 | Glioblastoma | 1321N1 (grade II);
SW1783 (grade III); LN18 (grade IV) | 12 µg/ml HC + 40
µg/ml GTT; 2 µg/ml HC + 40 µg/ml GTT; 29 µg/ml HC + 20 µg/ml
GTT | ↑Apoptosis,
arrested cell cycle, ↓migration, invasion and colony formation, ↑ER
stress pathway (XBP1, ATF4 and DDIT3), ↓FOXM1, E2F1 and PARP1,
↓cell cycle-associated genes CDC20 and CAV1, altered the splicing
expression of RRAS2, SRSF3 and ADAMTS2 | (24,74) |
| Rahman et
al, 2022 | Glioblastoma | 1321N1 (grade II);
SW1783 (grade III); LN18 (grade IV) | 50 µg/ml EGCG + 20
µg/ml HC; 100 µg/ml EGCG + 25 µg/ml HC; 50 µg/ml EGCG + 10 µg/ml
HC | ↑Apoptosis,
arrested cell cycle, ↓migration, invasion and colony formation,
↑apoptosis by activating caspase-3, regulation of ER-UPR activated
inflammatory response pathway, ↓regulation of SEMA3A and SEMA3F,
inhibited PLXNA1 and PLXNB2 | (75) |
| Chen et al,
2000 | Liver cancer | HepG2 | 100-200 µM HC,
pretreated with 100 µM BSO | GSH dependent,
↑apoptosis, ↑DNA damage by 8-OH-dG formation, ↓cell viability;
effect of HC was suppressed by catalase pretreatment | (78) |
| Chang, 2002 | Oral cancer | Oral KB carcinoma
cells | HC (10 µM-0.3
mM) | ↓Cell viability,
↑apoptosis, ↑cell cycle arrest at late S and G2/M phases, ↑ROS,
↓reduced form of GSH, ↓adhesion to collagen type I and fibronectin
at 100 and 250 µM HC | (31) |
| Chang et al,
2019 | Oral cancer | SAS tongue cancer
epithelial cells | HC (50 and 100
µM) | Inhibited
ANE-induced MMP-9 production | (86) |
| Jeng et al,
2004 | Oral cancer | KB epithelial
cells | HC (0.1, 0.2 and
0.3 mM) | ↑Cell cycle arrest
at late S and G2/M phases, ↑ROS and ↓reduced form of GSH | (87) |
| Moushumi and Sumati
V, 1994 | Skin cancer | Female Swiss
mice | HC (1 mg/day) | Restored normal DNA
biosynthesis in skin exposed to DMBA | (92) |
Anti-inflammatory activity of HC
Chronic inflammation is associated with cancer.
Leukocytes and other phagocytic cells cause DNA damage in
proliferating cells via the production of ROS and reactive nitrogen
species to fight infection (94).
The inflammatory process is mediated by two enzymes, namely COX and
lipoxygenase (95). The COX and
lipoxygenase cascades can lead to the development of multiple types
of cancer (96,97).
In one study, HC decreased the generation of
superoxide ions and the release of elastase by human neutrophils
induced by the leukocyte chemotactic factor
formyl-methionyl-leucyl-phenylalanine and the mycotoxin
cytochalasin B (98). In another
study, HC was shown to be a potent COX1/COX2 inhibitor, ROS
scavenger and inhibitor of platelet calcium signalling, thromboxane
B2 synthesis and aggregation (30). However, the anti-inflammatory
effects of HC in the study were on non-cancer model organisms
(30). In addition, 10, 50 and 100
µM HC was shown to elevate COX-2 expression in normal human oral
keratinocytes in a concentration-dependent manner, increasing COX-2
mRNA expression by 2.81, 3.68 and 9.25 folds, respectively, when
treated with HC for 18 h, and increasing COX-2 protein expression
by 0.96, 2.81 and 4.09 folds, respectively compared with the
untreated control (29).
Only a few studies have shown an anti-inflammatory
effect of HC on malignant cells, and no specific molecular
mechanism has been elucidated. For instance, when HC combined with
epigallocatechin gallate was used to treat 1321N1 and LN18 glioma
cells, the UPR pathway was induced, followed by activation of an
inflammatory response. However, the molecular mechanism underlying
the anti-inflammatory effect of HC was not elucidated (75). Future research may therefore focus
on the anti-inflammatory properties of HC in cancer cells.
Conclusions and prospects
The efficacy of HC varies among cancers owing to
different effects on pleiotropic pathways. Nevertheless, common
pathways targeted by HC have been identified, namely the JNK, MAPK
and eNOS signalling pathways. HC may exert its anticancer effect by
altering proteins in apoptotic pathways, including caspase-3 and
PARP. Overall, it appears that the anticancer potential of HC is
dependent on the accumulation of ROS in cancer cells, which
eventually lead to apoptotic cell death. However, despite numerous
efforts to elucidate the physicochemical and biological
characteristics of HC, various issues on bioavailability, potency
and tissue and dose selectivity require clarification. Also, the
adsorption of HC and the appropriate dosage remain uncertain due to
limited studies using animal models. Therefore, future research
should emphasise in vivo experimentation to ensure the
safety and bioavailability of HC in humans. Furthermore, combining
HC with other chemotherapeutics might be viable to determine
whether a synergistic positive interaction occurs that facilitates
the killing of cancer cells or sensitises them to chemotherapy.
Acknowledgements
Not applicable.
Funding
This study was funded by Ministry of Education Malaysia (grant
no. RACER/1/2019/SKK08/UITM//3) and an UiTM Supervisory Incentive
Grant (GIP) [grant no. 600-RMC/GIP 5/3 (038/2021)].
Availability of data and materials
Not applicable.
Authors' contributions
NAM and AAR drafted the manuscript. SHAK reviewed
and revised the manuscript content. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent of
participants
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan SY, Litscher G, Gao SH, Zhou SF, Yu
ZL, Chen HQ, Zhang SF, Tang MK, Sun JN and Ko KM: Historical
perspective of traditional indigenous medical practices: The
current renaissance and conservation of herbal resources. Evid
Based Complement Alternat Med. 2014:5253402014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization (WHO), . WHO
Global Report on Traditional and Complementary Medicine 2019. WHO;
Geneva: 2019
|
|
3
|
Ismail SF, Azmi IMAG, Daud M, Jalil JA and
Safuan S: Regulatory control of herbal and traditional medicines in
malaysia: Issues and concerns. Int J Business Society. 21:192–204.
2020.
|
|
4
|
Shehzad A, Islam SU, AL-Suhaimi EA and Lee
YS: Pleiotropic effects of bioactive phytochemicals (Polyphenols
and Terpenes). Functional Foods, Nutraceuticals and Natural
Products. DEStech Publications Inc.; 2018
|
|
5
|
Nishino H, Tokuda H, Satomi Y, Masuda M,
Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Takemura M, Ii T, et
al: Cancer chemoprevention by phytochemicals and their related
compounds. Asian Pac J Cancer Prev. 1:49–55. 2000.PubMed/NCBI
|
|
6
|
Amin ARMR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nisar B, Sultan A and Rubab SL: Comparison
of medicinally important natural products versus synthetic drugs-a
short commentary. Nat Prod Chem Res. 6:22018. View Article : Google Scholar
|
|
8
|
Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q,
Zou L, Sui X, Xie T, Zhang J and Hu Y: Opportunities and challenges
for co-delivery nanomedicines based on combination of
phytochemicals with chemotherapeutic drugs in cancer treatment. Adv
Drug Deliv Rev. 188:1144452022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bode AM and Dong Z: Toxic phytochemicals
and their potential risks for human cancer. Cancer Prev Res
(Phila). 8:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudva AK, Rao S, Rao P, Periera R,
Bhandari G, Mathew JM, Ashwini K, Pais M, Swamy MK and Baliga M:
Piper betle Linn, in cancer: Past, present, and future. Anticancer
plants: Properties and Application. Vol 1. Springer; Singapore: pp.
327–347. 2018
|
|
11
|
Guha P: Betel leaf: The neglected green
gold of india. J Hum Ecol. 19:87–93. 2006. View Article : Google Scholar
|
|
12
|
Amonkar AJ, Nagabhushan M, D'Souza AV and
Bhide SV: Hydroxychavicol: A new phenolic antimutagen from betel
leaf. Food Chem Toxicol. 24:1321–1324. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amonkar AJ, Padma PR and Bhide SV:
Protective effect of hydroxychavicol, a phenolic component of betel
leaf, against the tobacco-specific carcinogens. Mutat Res.
210:249–253. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaveerach A, Mokkamul P, Sudmoon R and
Tanee T: Ethnobotany of the Genus Piper (Piperaceae) in
Thailand. Ethnobotany Research Applications. 4:2232006. View Article : Google Scholar
|
|
15
|
Shah GA, Shah TI, Telang S, Science G,
Nehru J and Hospital C: Anti-proliferative efficacy of piper betle
leaf extracts against B16F10 melanoma in an in vivo. World J Pharm
Pharm Sci. 5:835–843. 2016.
|
|
16
|
Haslan H, Suhaimi FH, Thent ZC and Das S:
The underlying mechanism of action for various medicinal properties
of Piper betle (betel). Clin Ter. 166:208–214. 2015.PubMed/NCBI
|
|
17
|
Agarwal T, Singh R, Shukla AD, Waris I and
Gujrati A: Comparative analysis of antibacterial activity of four
Piper betel varieties. Adv Appl Sci Res. 3:698–705. 2012.
|
|
18
|
Chakraborty D and Shah B: Antimicrobial,
anti-oxidative and anti-hemolytic activity of Piper betel leaf
extracts. Int J Pharm Pharm Sci. 3:192–199. 2011.
|
|
19
|
Kumar N, Misra P, Dube A, Bhattacharya S,
Dikshit M and Ranade S: Piper betle Linn. A maligned pan-asiatic
plant with an array of pharmacological activities and prospects for
drug discovery. Curr Sci. 99:922–932. 2010.
|
|
20
|
Punuri JB, Sharma P, Sibyala S, Tamuli R
and Bora U: Piper betle-mediated green synthesis of biocompatible
gold nanoparticles. Int Nano Lett. 2:182012. View Article : Google Scholar
|
|
21
|
Sharma S, Khan IA, Ali I, Ali F, Kumar M,
Kumar A, Johri RK, Abdullah ST, Bani S, Pandey A, et al: Evaluation
of the antimicrobial, antioxidant, and anti-inflammatory activities
of hydroxychavicol for its potential use as an oral care agent.
Antimicrob Agents Chemother. 53:216–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madhumita M, Guha P and Nag A: Bio-actives
of betel leaf (Piper betle L.): A comprehensive review on
extraction, isolation, characterization, and biological activity.
Phytother Res. 34:2609–2627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das S, Parida R, Sandeep IS, Nayak S and
Mohanty S: Biotechnological intervention in betelvine (Piper betle
L.): A review on recent advances and future prospects. Asian Pac J
Trop Med. 9:938–946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rahman AA, Mokhtar NM, Harun R, Jamal R
and Ngah WZ: Transcriptome analysis reveals the molecular
mechanisms of combined gamma-tocotrienol and hydroxychavicol in
preventing the proliferation of 1321N1, SW1783, and LN18 glioma
cancer cells. J Physiol Biochem. 75:499–517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakraborty JB, Mahato SK, Joshi K, Shinde
V, Rakshit S, Biswas N, Mukherjee IC, Mandal L, Ganguly D,
Chowdhury AA, et al: Hydroxychavicol, a Piper betle leaf component,
induces apoptosis of CML cells through mitochondrial reactive
oxygen species-dependent JNK and endothelial nitric oxide synthase
activation and overrides imatinib resistance. Cancer Sci.
103:88–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prabhu MS, Platel K, Saraswathi G and
Srinivasan K: Effect of orally administered betel leaf (Piper betle
Linn.) on digestive enzymes of pancreas and intestinal mucosa and
on bile production in rats. Indian J Exp Biol. 33:752–756.
1995.PubMed/NCBI
|
|
27
|
Majumdar AG and Subramanian M:
Hydroxychavicol from Piper betle induces apoptosis, cell cycle
arrest, and inhibits epithelial-mesenchymal transition in
pancreatic cancer cells. Biochem Pharmacol. 166:274–291. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee-Chen SF, Chen CL, Ho LY, Hsu PC, Chang
JT, Sun CM, Chi CW and Liu TY: Role of oxidative DNA damage in
hydroxychavicol-induced genotoxicity. Mutagenesis. 11:519–523.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang DW, Lin SC, Chang KW, Chi CW, Chang
CS and Liu TY: Elevated expression of cyclooxygenase (COX)-2 in
oral squamous cell carcinoma-evidence for COX-2 induction by areca
quid ingredients in oral keratinocytes. J Oral Pathol Med.
32:522–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang MC, Uang BJ, Tsai CY, Wu HL, Lin BR,
Lee CS, Chen YJ, Chang CH, Tsai YL, Kao CJ and Jeng JH:
Hydroxychavicol, a novel betel leaf component, inhibits platelet
aggregation by suppression of cyclooxygenase, thromboxane
production and calcium mobilization. Br J Pharmacol. 152:73–82.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang MC, Uang BJ, Wu HL, Lee JJ, Hahn LJ
and Jeng JH: Inducing the cell cycle arrest and apoptosis of oral
KB carcinoma cells by hydroxychavicol: Roles of glutathione and
reactive oxygen species. Br J Pharmacol. 135:619–130. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arksey H and O'Malley L: Scoping studies:
Towards a methodological framework. International J Social Research
Methodology: Theory and Practice. 8:19–32. 2005. View Article : Google Scholar
|
|
33
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. J Clin Epidemiol.
62:e1–e34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thi Truc Nguyen L, Thi Nguyen T, Ngoc
Nguyen H and Thi Phuong Bui Q: Simultaneous determination of active
compounds in Piper betle Linn. leaf extract and effect of
extracting solvents on bioactivity. Eng Reps. 2:e122462020.
|
|
35
|
Ali A, Lim XY, Chong CH, Mah SH and Chua
BL: Optimization of ultrasound-assisted extraction of natural
antioxidants from Piper betle using response surface methodology.
LWT. 89:681–688. 2018. View Article : Google Scholar
|
|
36
|
Pin KY, Chuah AL, Rashih AA, Mazura MP,
Fadzureena J, Vimala S and Rasadah MA: Antioxidant and
anti-inflammatory activities of extracts of betel leaves (Piper
betle) from solvents with different polarities. J Tropical Forest
Sci. 22:448–455. 2010.
|
|
37
|
Zamakshshari N, Ahmed IA and Nasharuddin
MNA: Effect of extraction procedure on the yield and biological
activities of hydroxychavicol from Piper betle L. leaves. J Appl
Res Med Aromat Plants. 24:1003202021.
|
|
38
|
Ali A, Chong CH, Mah SH, Abdullah LC,
Choong TSY and Chua BL: Impact of storage conditions on the
stability of predominant phenolic constituents and antioxidant
activity of dried piper betle extracts. Molecules. 23:4842018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gundala SR and Aneja R: Piper betel leaf:
A reservoir of potential xenohormetic nutraceuticals with
cancer-fighting properties. Cancer Prev Res (Phila). 7:477–486.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mira L, Fernandez MT, Santos M, Rocha R,
Florêncio MH and Jennings KR: Interactions of flavonoids with iron
and copper ions: A mechanism for their antioxidant activity. Free
Radic Res. 36:1199–1208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Halliwell B: Biochemistry of oxidative
stress. Biochem Soc Trans. 35:1147–1150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Song M, Zhang B and Zhang Y:
Reactive oxygen species regulate T cell immune response in the
tumor microenvironment. Oxid Med Cell Longev. 2016:15809672016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Juan CA, de la Lastra JM, Plou FJ and
Pérez-Lebeña E: The chemistry of reactive oxygen species (ROS)
revisited: Outlining their role in biological macromolecules (DNA,
Lipids and Proteins) and induced pathologies. Int J Mol Sci.
22:46422021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim SJ, Kim HS and Seo YR: Understanding
of ROS-inducing strategy in anticancer therapy. Oxid Med Cell
Longev. 2019:53816922019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ureshino H, Shindo T and Kimura S: Role of
cancer immunology in chronic myelogenous leukemia. Leuk Res.
88:1062732020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hochhaus A, Larson RA, Guilhot F, Radich
JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F,
Fujihara S, et al: Long-term outcomes of imatinib treatment for
chronic myeloid leukemia. N Engl J Med. 376:917–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paul T, Banerjee A, Reddy SVB, Mahato SK
and Biswas N: Hydroxychavicol sensitizes imatinib-resistant chronic
myelogenous leukemia cells to TRAIL-induced apoptosis by
ROS-mediated IAP downregulation. Anticancer Drugs. 30:167–178.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chowdhury AA, Chaudhuri J, Biswas N, Manna
A, Chatterjee S, Mahato SK, Chaudhuri U, Jaisankar P and
Bandyopadhyay S: Synergistic apoptosis of CML cells by buthionine
sulfoximine and hydroxychavicol correlates with activation of AIF
and GSH-ROS-JNK-ERK-iNOS pathway. PLoS One. 8:e736722013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chaudhuri J, Chowdhury AA, Biswas N, Manna
A, Chatterjee S, Mukherjee T, Chaudhuri U, Jaisankar P and
Bandyopadhyay S: Superoxide activates mTOR-eIF4E-Bax route to
induce enhanced apoptosis in leukemic cells. Apoptosis. 19:135–148.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
World Health Organization (WHO), .
GLOBOCAN 2020. WHO; Geneva: 2020
|
|
52
|
Perou CM, Sørile T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cazzaniga M and Bonanni B: Breast cancer
chemoprevention: Old and new approaches. J Biomed Biotechnol.
2012:985620S2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Neven P, Jongen L, Lintermans A, Van Asten
K, Blomme C, Lambrechts D, Poppe A, Wildiers H, Dieudonné AS,
Brouckaert O, et al: Tamoxifen metabolism and efficacy in breast
cancer: A prospective multicenter trial. Clin Cancer Res.
24:2312–2318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Waters EA, McNeel TS, Stevens WM and
Freedman AN: Use of tamoxifen and raloxifene for breast cancer
chemoprevention in 2010. Breast Cancer Res Treat. 134:875–880.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chumsri S, Howes T, Bao T, Sabnis G and
Brodie A: Aromatase, aromatase inhibitors, and breast cancer. J
Steroid Mol Biol. 125:13–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tong CWS, Wu M, Cho WCS and To KKW: Recent
advances in the treatment of breast cancer. Front Oncol. 8:2272018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kreutzfeldt J, Rozeboom B, Dey N and De P:
The trastuzumab era: Current and upcoming targeted HER2+ breast
cancer therapies. Am J Cancer Res. 10:1045–1067. 2020.PubMed/NCBI
|
|
59
|
Sinha D, Biswas J, Sung B, Aggarwal BB and
Bishayee A: Chemopreventive and chemotherapeutic potential of
curcumin in breast cancer. Curr Drug Targets. 13:1799–1819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hemamalini V, Velayutham DPM, Lakshmanan
L, Muthusamy K, Sivaramakrishnan S and Premkumar K: Inhibitory
potential of Hydroxychavicol on Ehrlich ascites carcinoma model and
in silico interaction on cancer targets. Nat Prod Res.
34:1591–1596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Conroy T, Hammel P, Hebbar M, Abdelghani
MB, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, et
al: FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic
cancer. N Eng J Med. 379:2395–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Neuzillet C, Gaujoux S, Williet N, Bachet
JB, Bauguion L, Durand LC, Conroy T, Dahan L, Gilabert M, Huguet F,
et al: Pancreatic cancer: French clinical practice guidelines for
diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER,
SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis. 50:1257–1271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hamdy FC, Donovan JL, Lane JA, Mason M,
Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, et
al: 10-year outcomes after monitoring, surgery, or radiotherapy for
localized prostate cancer. N Eng J Med. 375:1415–1424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Armstrong CM and Gao AC: Adaptive pathways
and emerging strategies overcoming treatment resistance in
castration resistant prostate cancer. Asian J Urol. 3:185–194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gundala SR, Yang C, Mukkavilli R, Paranjpe
R, Brahmbhatt M, Pannu V, Cheng A, Reid MD and Aneja R:
Hydroxychavicol, a betel leaf component, inhibits prostate cancer
through ROS-driven DNA damage and apoptosis. Toxicol Appl
Pharmacol. 280:86–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJH and Watanabe
T: Colorectal cancer. Nat Rev Dis Primers. 1:50652015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yalcin-Ozkat G: Molecular modeling
strategies of cancer multidrug resistance. Drug Resist Updat.
59:1007892021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rajedadram A, Pin KY, Ling SK, Yan SW and
Looi ML: Hydroxychavicol, a polyphenol from Piper betle leaf
extract, induces cell cycle arrest and apoptosis in TP53-resistant
HT-29 colon cancer cells. J Zhejiang Univ Sci B. 22:112–122. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bahadur S, Sahu AK, Baghel P and Saha S:
Current promising treatment strategy for glioblastoma multiform: A
review. Oncol Rev. 13:4172019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Koshy M, Villano JL, Dolecek TA, Howard A,
Mahmood U, Chmura SJ, Weichselbaum RR and McCarthy BJ: Improved
survival time trends for glioblastoma using the SEER 17
population-based registries. J Neurooncol. 107:207–212. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rahman AA, Jamal AR, Harun R, Mokhtar NM
and Ngah WZ: Gamma-tocotrienol and hydroxy-chavicol synergistically
inhibits growth and induces apoptosis of human glioma cells. BMC
Complement Altern Med. 14:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rahman AA, Ngah WZ, Jamal R, Makpol S,
Harun R and Mokhtar N: Inhibitory mechanism of combined
hydroxychavicol with epigallocatechin-3-gallate against glioma
cancer cell lines: A transcriptomic analysis. Front Pharmacol.
13:8441992022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Center MM and Jemal A: International
trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers
Prev. 20:2362–2368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen CL, Chi CW and Liu TY: Enhanced
hydroxychavicol-induced cytotoxic effects in glutathione-depleted
HepG2 cells. Cancer Lett. 155:29–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Harsha C, Banik K, Ang HL, Girisa S,
Vikkurthi R, Parama D, Rana V, Shabnam B, Khatoon E, Kumar AP and
Kunnumakkara AB: Targeting AKT/mTOR in oral cancer: Mechanisms and
advances in clinical trials. Int J Mol Sci. 21:32852020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sawhney M, Rohatgi N, Kaur J, Shishodia S,
Sethi G, Gupta SD, Deo SVS, Shukla NK, Aggarwal BB and Ralhan R:
Expression of NF- kappaB parallels COX-2 expression in oral
precancer and cancer: Association with smokeless tobacco. Int J
Cancer. 120:2545–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Baek SH, Ko JH, Lee H, Jung J, Kong M, Lee
JW, Lee J, Chinnathambi A, Zayed ME, Alharbi SA, et al:
Phytomedicine Resveratrol inhibits STAT3 signaling pathway through
the induction of SOCS-1: Role in apoptosis induction and
radiosensitization in head and neck tumor cells. Phytomedicine.
23:566–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sinha N, Panda PK, Naik PP, Das DN,
Mukhopadhyay S, Maiti TK, Shanmugam MK, Chinnathambi A, Zayed ME,
Alharbi SA, et al: Abrus agglutinin promotes irreparable DNA damage
by triggering ROS generation followed by ATM-p73 mediated apoptosis
in oral squamous cell carcinoma. Mol Carcinog. 56:2400–2413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Monisha J, Roy NK, Padmavathi G, Banik K,
Bordoloi D, Khwairakpam AD, Arfuso F, Chinnathambi A, Alahmadi TA,
Alharbi SA, et al: NGAL is downregulated in oral squamous cell
carcinoma and leads to increased survival, proliferation, migration
and chemoresistance. Cancers (Basel). 10:2282018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hashim D, Genden E, Posner M, Hashibe M
and Boffetta P: Head and neck cancer prevention : From primary
prevention to impact of clinicians on reducing burden. Ann Oncol.
30:744–756. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Behera AK, Kumar M, Shanmugam MK,
Bhattacharya A, Rao VJ, Bhat A, Vasudevan M, Gopinath KS,
Mohiyuddin A, Chatterjee A, et al: Functional interplay between YY1
and CARM1 promotes oral carcinogenesis. Oncotarget. 10:3709–3724.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang MC, Pan YH, Wu HL, Lu YJ, Liao WC,
Yeh CY, Lee JJ and Jeng JH: Stimulation of MMP-9 of oral epithelial
cells by areca nut extract is related to TGF-ß/Smad2-dependent
and-independent pathways and prevented by betel leaf extract,
hydroxychavicol and melatonin. Aging. 11:11624–11639. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jeng JH, Wang YJ, Chang WH, Wu HL, Li CH,
Uang BJ, Kang JJ, Lee JJ, Hahn LJ, Lin BR and Chang MC: Reactive
oxygen species are crucial for hydroxychavicol toxicity toward KB
epithelial cells. Cell Mol Life Sci. 61:83–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Garbe C and Leiter U: Melanoma
epidemiology and trends. Clin Dermatol. 27:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lomas A, Leonardi-Bee J and Bath-Hextall
F: A systematic review of worldwide incidence of nonmelanoma skin
cancer. Br J Dermatol. 166:1069–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Green A, Whiteman D, Frost C and
Battistutta D: Sun exposure, skin cancers and related skin
conditions. J Epidemiol. 9:S7–S13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
American Cancer Society, . Cancer Facts
& Figures 2016. American Cancer Society; Atlanta, GA: 2016
|
|
92
|
Moushumi L and Sumati B: Studies on
possible protective effect of plant derived phenols and the vitamin
precursors-β-carotene and α-tocopherol on 7, 12 Dimethylbenz (a)
anthracene induced tumour initiation events. Phytotherapy Res.
8:237–240. 1994. View Article : Google Scholar
|
|
93
|
Vinusri S, Gnanam R, Caroline R,
Santhanakrishnan VP and Kandavelmani A: Anticancer potential of
hydroxychavicol derived from piper betle L: An in silico and
cytotoxicity study. Nutr Cancer. 74:3701–3713. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Maeda H and Akaike T: Nitric oxide and
oxygen radicals in infection, inflammation, and cancer.
Biochemistry (Mosc). 63:854–865. 1998.PubMed/NCBI
|
|
95
|
Kennedy BM and Harris RE: Cyclooxygenase
and lipoxygenase gene expression in the inflammogenesis of breast
cancer. Inflammopharmacology. 26:909–923. 2018. View Article : Google Scholar
|
|
96
|
Romano M and Clària J: Cyclooxygenase-2
and 5-lipoxygenase converging functions on cell proliferation and
tumor angiogenesis: implications for cancer therapy. FASEB J.
17:1986–1995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schneider C and Pozzi A: Cyclooxygenases
and lipoxygenases in cancer. Cancer Metastasis Rev. 30:277–294.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lin CF, Hwang TL, Chien CC, Tu HY and Lay
HL: A new hydroxychavicol dimer from the roots of Piper betle.
Molecules. 18:2563–2570. 2013. View Article : Google Scholar : PubMed/NCBI
|