Introduction
Primary central nervous system lymphoma (PCNSL) is a
highly aggressive non-Hodgkin lymphoma confined to the central
nervous system that accounts for merely 0.7–0.9% of all lymphomas,
and 0.3–1.5% of all intracranial lymphomas (1). Although PCNSL is a rare intracranial
tumor, its incidence has significantly increased in patients over
60 years of age in the past two decades (2). Immunodeficiency, HIV infection, and
the administration of immunosuppressive agents following organ
transplantation are risk factors for developing PCNSL; however,
there is also an increase in the incidence of this tumor in
immunocompetent individuals (1,2).
PCNSL classified as haematolymphoid tumors involving the central
nervous system (CNS), according to the World Health Organization
Classification of tumors of the CNS. The pathogenesis of PCNSL
suggests that the tumor cells correspond to mature, late
germinal-center exit B cells derived from
self-reactive/polyreactive precursor cells, which have escaped
elimination, possibly fostered by the early acquisition of MYD88
mutations (3). The majority of
PCNSLs are diffuse large B-cell lymphomas (DLBCL), regardless of
the patient's immunological status (4). Clinical presentations of PCNSL can
vary widely, only 50–70% of the patients present focal neurologic
deficits. Commonly, initial manifestations are nonspecific
cognitive or behavioral changes over a period of weeks to months,
signs of elevated intracranial pressure, including headache and
vomiting. Conversely, B symptoms, which are common clinical symptom
of systemic hematologic malignancies, such as fever, night sweats,
and weight loss, are rare in PCNSL (5). Lesions may be solitary or multiple,
and are commonly located in the cerebral white matter near the
corpus callosum, central grey matter, and basal
ganglia-thalamus-hypothalamus region, posterior fossa, and
periventricular region (1).
Conversely, cases of PCNSL confined to the third ventricle are
extremely rare, and can also radiologically mimic other third
ventricle lesions (1,6,7).
In many cases, conventional magnetic resonance
imaging (MRI) of PCNSL mimics that often high-grade glioma (HGG)
which could all appear as rim-like lesion with necrosis or could
manifest as homogenous enhancing masses (8,9).
Additionally, peritumoral edemas in PCNSL tend to mild compared
with in HGG, whereas, 23% of PCNSL lesions are lack peritumoral
edema, to establish definitive diagnosis using only radiological
features remains difficult, pathological and immunohistochemical
examination of the brain biopsy specimen is commonly required
(5,9,10).
Intratumoral hemorrhage (ITH) is accounts for
4.4–5.4% of gross intracerebral hemorrhage, and observed in
1.5–14.6% of intracranial neoplasms (11,12).
HGG, including glioblastoma, is most commonly associated with ITH,
following by metastatic brain tumors, meningiomas and low-grade
gliomas (12). Conversely, ITH in
PCNSL is extremely rare, the presence of ITH is even used to
exclude PCNSL from the differential diagnosis (13). According previous report, 6.25% of
the lateral ventricle tumors present with intraventricular
bleeding, however, only a few cases of the ventricle tumors
presenting ITH have been described (14–16).
Moreover, no cases of third ventricle PCNSL presenting with ITH
have not been reported previously.
Herein, we report a case of PCNSL confined to the
third ventricle with ITH mimicking HGG, and discuss the clinical
features and pitfalls of its radiological diagnosis.
Case report
Case presentation
A 75-year-old woman with no history of
immunosuppression presented with amnesia and gait disturbance that
gradually progressed in a short period. She was referred to our
hospital three weeks after the initial symptoms and following
identification of a brain tumor on MRI during screening at a nearby
clinic. On admission, her neurological symptoms progressed, and she
presented with mild disturbance of consciousness, headache, nausea,
and gait disturbance. Serological examination revealed mild
elevation of soluble interleukin 2 receptor levels (676 U/ml), and
the blood count status was normal. The human immunodeficiency virus
antibody test results were negative, and the patient had no history
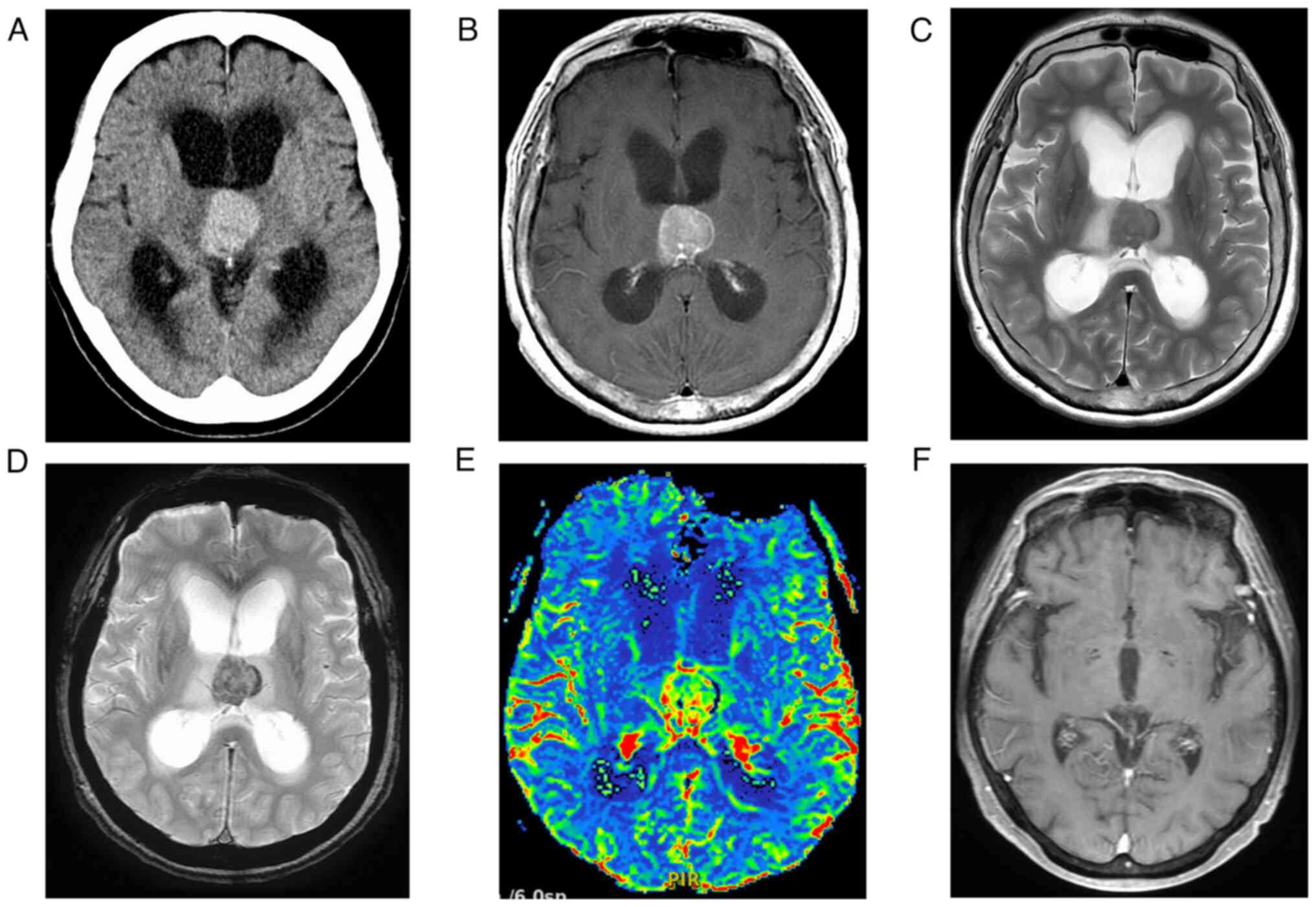
of immunosuppressive agents. Head CT revealed a tumor with high
attenuation confined to the third ventricle, without calcification
(Fig. 1A). MRI of the head
revealed a tumor confined to the third ventricle and with
obstructive hydrocephalus. Diffusion-weighted images and apparent
diffusion coefficient maps showed no obvious diffusion
restrictions. In contrast-enhanced T1 weighted imaging (CE-T1WI),
the tumor showed homogeneous enhancement (Fig. 1B). The marginal zone of the tumor
showed low intensity on T2 weighted image (T2WI) and T2*-weighted
images (T2*WI), suggesting ITH (Fig.
1C, D). DSC-MRI, a type of perfusion-weighted imaging, showed a
5 to 10 percent elevation of regional cerebral blood volume (rCBV)
in the tumor compared to the normal tissue (Fig. 1E). Based on the radiological
findings, we considered high-grade glioma as the preoperative
diagnosis.
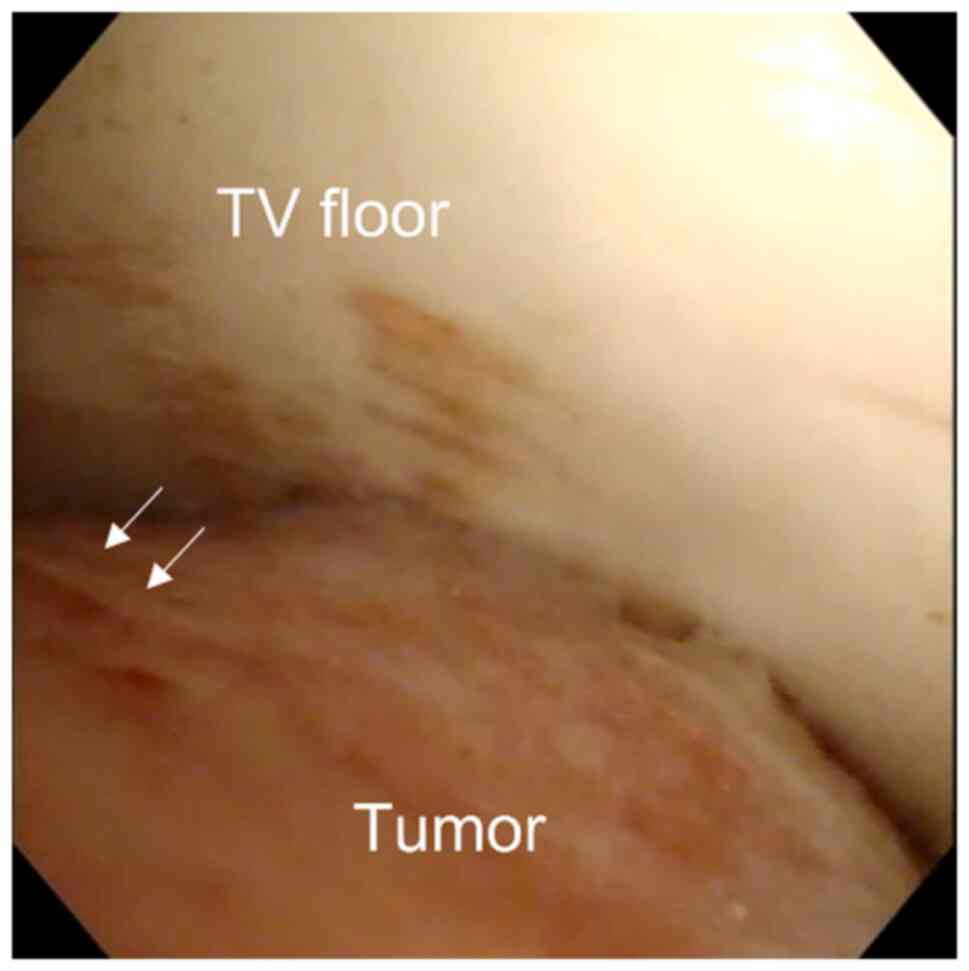
The patient's clinical symptoms of obstructive
hydrocephalus progressed rapidly. Subsequently, endoscopic third
ventriculostomy (ETV) and endoscopic tumor biopsy were performed
for pathological diagnosis (Fig.
2). The-third ventricle tumor was exposed using a
transventricular approach and a neuroendoscope. The tumor was
spherical, red, and hemorrhagic. ETV and tumor biopsies were
successfully performed without fatal tumor hemorrhage. Immediately
after surgery, a corticosteroid agent was administered, and her
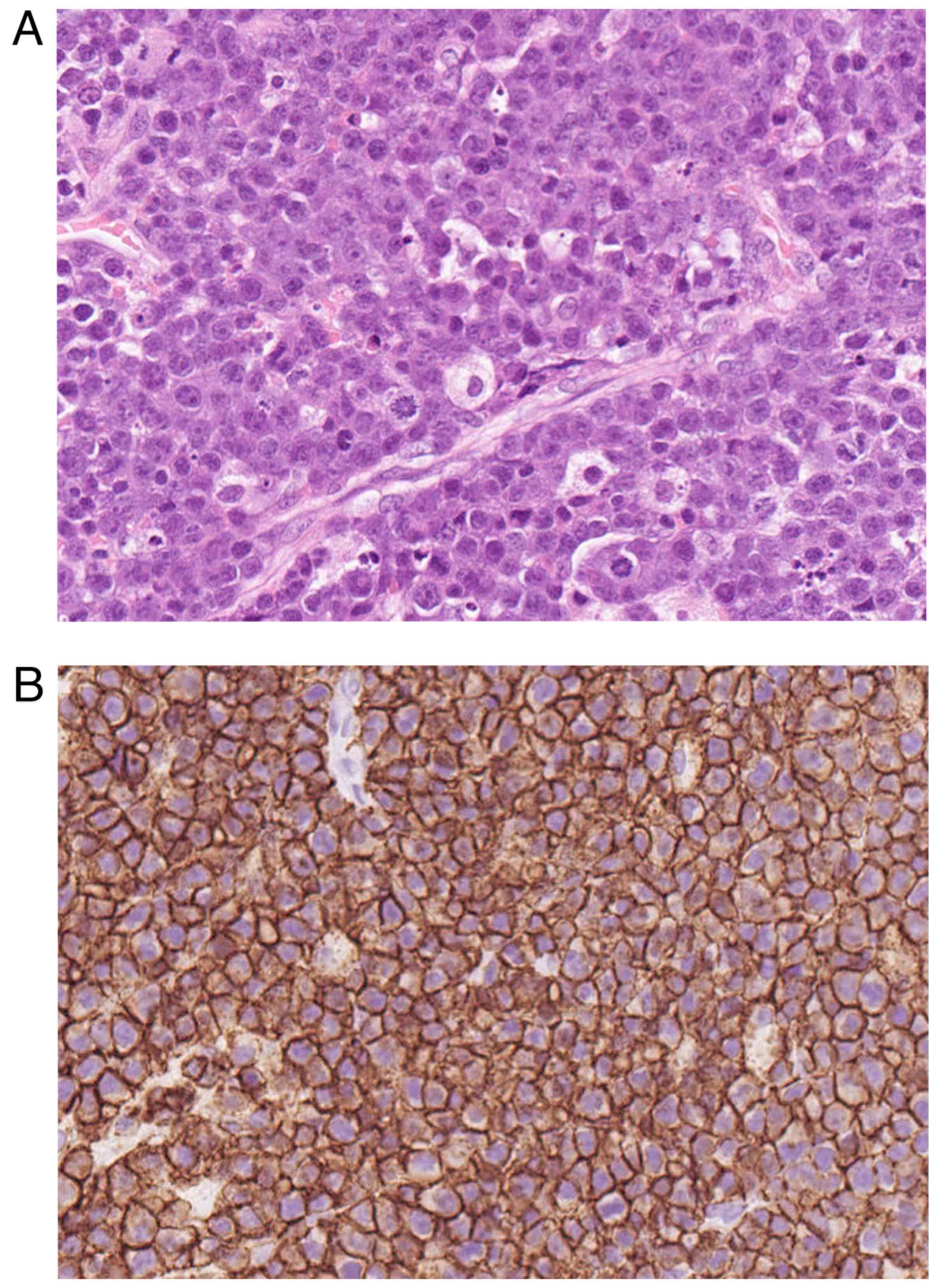
clinical symptoms improved. Histopathological examination of the
surgical specimens obtained intraoperatively prior to chemotherapy
revealed rounded cells with irregularly shaped hyperchromatic
nuclei, prominent nucleoli, and a small amount of eosinophilic
cytoplasm, often admixed with apoptotic cells and tingible body
macrophages (Fig. 3A).
Immunohistochemically, atypical cells were positive for CD20
(Fig. 3B). The diagnosis of DLBCL
was confirmed based on the pathological findings. The presence of
other lesions was excluded by whole-body contrast-enhanced CT and
ophthalmoscopy. Finally, we diagnosed the tumor as a PCNSL confined
to the third ventricle. Multiple drug chemotherapy using rituximab,
methotrexate, procarbazine, and vincristine (R-MPV) was
administered after surgery. After 5 Kur of chemotherapy, her
clinical status improved completely, and complete response was
confirmed 14 months after surgery (Fig. 1F).
Imaging data acquisition
CT was performed using a 320-row multidetector CT
system (Aquilion ONE Vision Edition; Canon Medical Systems). The
following imaging parameters were used: tube voltage, 120 kV; tube
current, 215 mA; gantry rotation speed, 1.0 s; detector
configuration, 80×0.5 mm; scan and display field of view (FOV),
240×240 mm; slice thickness, 5.0 mm; and image matrix, 512×512. All
MRI studies were performed using an MR system (SIGNA Premier 3.0T;
GE Healthcare) with a dedicated 48-channel phased-array coil (USA
Instruments). Dynamic susceptibility contrast-enhanced magnetic
resonance perfusion imaging (DSC-MRI) consisted of a GRE-EPI
sequence, which was acquired with the following parameters: field
of view 220 mm, slice thickness 5 mm, GAP 1 mm, repetition time
2000 ms, echo time 30 ms, flap angle 60; ASSET2; matrix, 160×160;
NEX 1; 60 phase, 2 min.
Histologic study
The tissues were fixed in 10% neutral formalin for
24 h and embedded in paraffin. Routine specimen processing involved
staining the slides with hematoxylin and eosin, followed by
immunohistochemical analyses with the monoclonal antibody CD20
(L26, 422441, Nichirei, Japan; 1:2) as a primary antibody and BOND
Polymer Refine Detection (DS9800, Leica Biosystems) including a
secondary antibody using a fully automated immunohistochemistry
staining system (BONDMAX; Leica Biosystems). Antigen activation was
performed using ER2 for 20 min. All specimens were scanned using a
vertical microscope system (NanoZoomer S360; Hamamatsu Photonics
K.K.) and images were acquired using NDP. View2 (freeware available
at http://www.hamamatsu.com/jp/ja/product/life-science-and-medical-systems/digital-slide-scanner/U12388-01.html).
Literature search strategy
A literature search was conducted to review third
ventricle PCNSL cases. A MEDLINE search was performed using the
keywords ‘primary central nervous system lymphoma,’ ‘lymphoma,’
‘third ventricle,’ and ‘ventricle tumor.’ English language
publications in PubMed (https://pubmed.ncbi.nlm.nih.gov/) were analyzed. Cases
in which the lesion consisted of only a third ventricle were
included. Conversely, the cases in which the lesion did not clearly
consist of a third ventricle, such as multiple or disseminated
lesions, were excluded.
Discussion
Third-ventricle tumors are rare, accounting for 0.1
to 0.9% of all brain tumors (17–19).
These tumors are classified as involving either the anterior, the
posterior, or the entirety of the third ventricle. The division
between the anterior and posterior regions of the third ventricle
is an imaginary line connecting the foramen of Monro and the
aqueduct (20).
Differential considerations for third-ventricle
tumors are plenty. In cases where tumors show vivid enhancement in
adults, differential considerations include meningioma, ependymoma,
germinoma, lymphoma, subependymal giant-cell astrocytoma,
hypothalamic and optic astrocytoma, chordoma, and chordoid glioma
of the third ventricle (17,21,22).
HGG may also be present in the third ventricle, accounting for 1.4
to 2.2% (18,21). Although extremely rare, PCNSL may
also be confined to the third ventricle, but only four such cases
have been reported including present case (Table I) (1,6,7).
These cases include two men and two women with a median age of
54.25 y (range 32–72). One patient had a history of
immunocompromise and the other three did not. Symptoms associated
with hydrocephalus occur as the initial manifestations in most
cases. Tumors can occur in the anterior or posterior third
ventricles. In most cases, the tumors presented with low intensity
on T1WI; however, various intensities were observed on T2WI. Mild
diffusion restriction was described in two cases. All cases had a
good prognosis, which may be related to the fact that
third-ventricle PCNSL presents with hydrocephalus at an early
stage, which is easily detected. The possibility of improved
prognosis with early therapeutic intervention (before tumor
infiltration into the brain) has been reported, and clinicians
should consider PCNSL in the differential diagnosis of
third-ventricle tumors (2).
 | Table I.Summary of pure third ventricle
PCNSL. |
Table I.
Summary of pure third ventricle
PCNSL.
| Author, date | Age | Sex | Immunological
condition | Initial
manifestation | Surgery (duration
form initial manifestation) | Location in TV | Hydroce phalus | MRI features | Hemorrhage | Prognosis | (Refs.) |
|---|
| Sasani et
al, 2011 | 38 | M | Competent | Headache, gait
disturbance | Craniotomy (3
months) | Whole | Not severe | T1 low, T2
high | NM | Good (12
months) | (6) |
| Queiroz et
al, 2017 | 32 | M | Compromised | Headache | Biopsy (2
months) | Anterior | Not severe | - | NM | Good (NM) | (7) |
| Haddad et
al, 2019 | 72 | F | Competent | Confusion, urinary
incontinence | Biopsy (several
days) | Anterior | Not severe | T1 low, T2 low, DWI
high, SWI no blooming | - | Good (11
months) | (1) |
| Present case | 75 | F | Competent | Amnesia, gait
disturbance | Biopsy (3
weeks) | Whole | Severe | T1 low, T2 low, DWI
iso, T2* low, PWI high | + | Good (14
months) |
|
Although the preoperative differentiation of PCNSL
from other third-ventricle tumors, particularly with ITH, is
challenging, it is nevertheless crucial because the surgical
strategies for treating different tumors may be fundamentally
different. On conventional MRI, PCNSL usually presents with low
intensity on T1WI, low intensity on T2WI, and homogeneous
enhancement on CE-T1WI (23).
However, similar features are also observed for meningioma and
chordoid glioma, and differential diagnosis by conventional MRI
remains difficult in some cases (24). In these cases, DSC-MRI can help in
the differential diagnosis of these tumors. This technique involves
the calculation of rCBV maps and the subsequent correlation of
tumor vascularity with the histological grade of the malignancy
(25). On DSC-MRI, tumors can
present with different patterns of vascularization in chordoid
glioma (rCBV=1), intraventricular meningioma (rCBV 4.6±0.7), HGG
(rCBV 8.485±6.19), and PCNSL (1.38±0.64) (26–29).
PCNSLs tend to have relatively low perfusion compared to HGG, and
elevation of rCBV, as evaluated by DSC-MRI, can distinguish HGG
from PCNSL (8). Using this
technique, the quantification of cerebral perfusion usually relies
on the assumption of an intact blood-brain barrier, which remains
relatively susceptible to the local magnetic field (26). Meanwhile, T2*-based DSC-MRI may
lead to inaccurate estimation of rCBV values, as in the present
case. In fact, the cases of ITH with PCNSL have been minimal, and
concomitant hemorrhage on CT was observed in 8% of patients with
PCNSL (9,30). In cases where it is difficult to
detect ITH using conventional MRI, it can be detected using more
sensitive hemorrhage-detecting sequences, such as
susceptibility-weighted imaging (SWI) and T2*-weighted imaging.
Previous reports have shown that SWI detects ITH more sensitively
when used in conjunction with CT to exclude calcification, and 21%
of lesions present with multiple intratumoral susceptibility
signals associated with gross ITH on SWI (4). ITH has also been observed as a silent
or micro-hemorrhage on T2*-weighted MRI (31). Therefore, the occurrence of silent
or microhemorrhage in PCNSL may have been underestimated. Thus,
clinicians should diagnose third ventricle tumors using MRI,
considering the possibility of silent or micro-ITH.
However, the mechanism of ITH in a third ventricle
PCNSL remains unclear. Various theories have been proposed
regarding the mechanism of ITH in brain tumors: vascular
obstruction due to endothelial obstruction, vessel compression
and/or distortion resulting in rapid tumor growth, vessel necrosis,
tumor invasion of vessel wall, and increased venous pressure
associated with increased intracranial pressure (ICP) (11). In parenchymal PCNSL, DLBCL tends to
develop ITH compared to other phenotypes (30), and fragile vessels traversing
necrotic areas and large vessels may also cause ITH owing to
thinning and rupture of the vessel wall (13). PCNSL with ITH reveals
overexpression of vascular endothelial growth factor (VEGF), and
progression of angiogenesis and breakdown of fragile vessels may
cause ITH (13,32). Meanwhile, in specific
intraventricular tumors, angiomatous lesions and abnormalities of
the draining vein, such as venostasis and venous thrombosis in the
subependymal layer arising from tumor extension may lead to ITH
(14,33,34).
In addition, a significant change in ICP may alter a cause of ITH
in intraventricular tumors (35).
Differences in ITH mechanisms between third ventricle PCNSL and
other ventricle lesions remain unclear. However, in recent years,
it has become known that CSF flow synchronized with the arterial
pulsation and morphological changes in the surrounding brain
parenchyma can cause CSF backflow from the third to the fourth
ventricle and alter local pressure gradients in the ventricular
system (36). The third ventricle,
which is contiguous to the foramen of Monro and the mesencephalic
aqueduct, undergoes more dynamic changes than the lateral
ventricles, and the third ventricle lesions might be continuous
exposed to their environment. In the present case, which contained
characteristics of both PCNSL and intraventricular tumor,
venostasis within the fragile vascular network might have caused
micro-ITH due to tumor extension surrounding the subependymal layer
and was further affected by continuously elevated ICP due to
obstructive hydrocephalus and dynamic change of CSF flows due to
these anatomical features, leading to micro-ITHs presenting as a
hypointense rim in the marginal region on T2*WI.
In conclusion, there are numerous differential
diagnoses for a third-ventricle tumor, and PCNSL only rarely
presents as a pure third-ventricle tumor. Evaluation of rCBV values
on DSC-MRI may be useful in aiding preoperative differentiation
diagnosis; however, the presence of ITH can lead to an inaccurate
estimation of rCBV values. Silent or micro-hemorrhage on PCNSL may
be underestimated, and clinicians should carefully evaluate tumor
vascularity. Moreover, it is difficult to differentiate
third-ventricle PCNSL with ITH from other third ventricle
tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and KS drafted the manuscript and wrote the final
paper. SN, YN and JY made substantial contribution to conception
and design. SN, YN and JY drafted parts of the manuscript, revised
the content critically and provided constructive feedback. KS and
YN performed the surgery. YM and KS analyzed all images. KS and JY
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Oral and written informed consent was obtained from
the patient for the publication of the case details and any
associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PCNSL
|
primary central nervous system
lymphoma
|
|
ITH
|
intratumoral hemorrhage
|
|
HGG
|
high-grade glioma
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
CE-T1WI
|
contrast-enhanced T1 weighted
imaging
|
|
T2WI
|
T2-weighted imaging
|
|
T2*WI
|
T2*-weighted imaging
|
|
DSC-MRI
|
dynamic susceptibility
contrast-enhanced magnetic resonance perfusion imaging
|
|
rCBV
|
regional cerebral blood volume
|
|
ETV
|
endoscopic third ventriculostomy
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
R-MPV
|
rituximab, methotrexate, procarbazine
and vincristine
|
|
T1WI
|
T1-weighted imaging
|
|
SWI
|
susceptibility-weighted imaging
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Haddad R, Alkubaisi A, Al Bozom I, Haider
A and Belkhair S: Solitary primary central nervous system lymphoma
mimicking third ventricular colloid cyst-case report and review of
literature. World Neurosurg. 123:286–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okano R, Suzuki K, Nakano Y and Yamamoto
J: Primary central nervous system lymphoma presenting with
Parkinsonism as an initial manifestation: A case report and
literature review. Mol Clin Oncol. 14:952021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
WHO Classification of Tumours Editorial
Board, . Central Nervous System Tumours. WHO Classification of
Tumors. 5th Edition. International Agency for Research on Cancer;
Lyon: 2022
|
|
4
|
Sakata A, Okada T, Yamamoto A, Kanagaki M,
Fushimi Y, Dodo T, Arakawa Y, Takahashi JC, Miyamoto S and Togashi
K: Primary central nervous system lymphoma: Is absence of
intratumoral hemorrhage a characteristic finding on MRI? Radiol
Oncol. 49:128–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaff LR and Grommes C: Primary central
nervous system lymphoma. Blood. 140:971–979. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasani M, Bayhan M, Sasani H, Kaner T,
Oktenoglu T, Cakiroglu G and Ozer AF: Primary central nervous
system lymphoma presenting as a pure third ventricular lesion: A
case report. J Med Case Rep. 5:2132011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Queiroz RM, Abud LG, Abud TG, Miyake CH
and Dos Santos AC: Burkitt-like lymphoma of the brain mimicking an
intraventricular colloid cyst. Radiol Bras. 50:413–414. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu W, Wang Q, Shao A, Xu B and Zhang J:
The performance of MR perfusion-weighted imaging for the
differentiation of high-grade glioma from primary central nervous
system lymphoma: A systematic review and meta-analysis. PLoS One.
12:e01734302017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haldorsen IS, Kråkenes J, Krossnes BK,
Mella O and Espeland A: CT and MR imaging features of primary
central nervous system lymphoma in Norway, 1989–2003. AJNR Am J
Neuroradiol. 30:744–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bataille B, Delwail V, Menet E,
Vandermarcq P, Ingrand P, Wager M, Guy G and Lapierre F: Primary
intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg.
92:261–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondziolka D, Bernstein M, Resch L, Tator
CH, Fleming JF, Vanderlinden RG and Schutz H: Significance of
hemorrhage into brain tumors: Clinicopathological study. J
Neurosurg. 67:852–857. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Licata B and Turazzi S: Bleeding cerebral
neoplasms with symptomatic hematoma. J Neurosurg Sci. 47:201–10;
discussion 210. 2003.PubMed/NCBI
|
|
13
|
Matsumoto Y, Kashimura H, Aso K, Saura H,
Osakabe M and Kurose A: Primary central nervous system lymphoma
presenting as growing intracerebral hemorrhage. World Neurosurg.
116:155–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schartz D, D'Agostino E, Makler V, Hickey
WF and Bauer DF: Third ventricle World Health Organization Grade II
meningioma presenting with intraventricular hemorrhage and
obstructive hydrocephalus: A case report and literature review.
Surg Neurol Int. 10:732019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tosaka M, Sato K, Amanuma M, Higuchi T,
Arai M, Aishima K, Shimizu T, Horiguchi K, Sugawara K and Yoshimoto
Y: Superficial siderosis of the central nervous system caused by
hemorrhagic intraventricular craniopharyngioma: Case report and
literature review. Neurol Med Chir (Tokyo). 55:89–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dogan I, Ucer M and Başkaya MK: Gross
total resection of chordoid glioma of the third ventricle via
anterior interhemispheric transcallosal transforaminal approach at
two stages. J Neurol Surg B Skull Base. 79 (Suppl 3):S281–S282.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brain tumor registry of Japan (2005–2008).
Neurol Med Chir (Tokyo). 57 (Suppl 1):S9–S102. 2017. View Article : Google Scholar
|
|
18
|
Ostrom QT, Patil N, Cioffi G, Waite K,
Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2013–2017. Neuro Oncol. 22 (12 Suppl
2):iv1–iv96. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chibbaro S, Di Rocco F, Makiese O, Reiss
A, Poczos P, Mirone G, Servadei F, George B, Crafa P, Polivka M and
Romano A: Neuroendoscopic management of posterior third ventricle
and pineal region tumors: Technique, limitation, and possible
complication avoidance. Neurosurg Rev. 35:331–338; discussion
338–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmed SI, Javed G, Laghari AA, Bareeqa SB,
Aziz K, Khan M, Samar SS, Humera RA, Khan AR, Farooqui MO and
Shahbaz A: Third ventricular tumors: A comprehensive literature
review. Cureus. 10:e34172018.PubMed/NCBI
|
|
21
|
Yılmaz B, Ekşi MŞ, Demir MK, Akakın A,
Toktaş ZO, Yapıcıer Ö and Kılıç T: Isolated third ventricle
glioblastoma. Springerplus. 5:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suetens K, Swinnen J, Stessens L, Van
Cauter S and Gelin G: Chordoid glioma as a differential diagnosis
of anterior third ventricle tumours: A rare case report and
five-year follow-up. Case Rep Radiol. 2019:35848372019.PubMed/NCBI
|
|
23
|
Slone HW, Blake JJ, Shah R, Guttikonda S
and Bourekas EC: CT and MRI findings of intracranial lymphoma. AJR
Am J Roentgenol. 184:1679–1685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pomper MG, Passe TJ, Burger PC,
Scheithauer BW and Brat DJ: Chordoid glioma: A neoplasm unique to
the hypothalamus and anterior third ventricle. AJNR Am J
Neuroradiol. 22:464–469. 2001.PubMed/NCBI
|
|
25
|
Holveck A, Grand S, Boini S, Kirchin M, Le
Bas JF, Dietemann JL, Bracard S and Kremer S: Dynamic
susceptibility contrast-enhanced MRI evaluation of cerebral
intraventricular tumors: Preliminary results. J Neuroradiol.
37:269–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saini J, Gupta RK, Kumar M, Singh A, Saha
I, Santosh V, Beniwal M, Kandavel T and Cauteren MV: Comparative
evaluation of cerebral gliomas using rCBV measurements during
sequential acquisition of T1-perfusion and T2-perfusion MRI. PLoS
One. 14:e02154002019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holveck A, Grand S, Boini S, Kirchin M, Le
Bas, JF, Dietemann JL, Bracard S and Kremer S: Dynamic
susceptibility contrast-enhanced MRI evaluation of cerebral
intraventricular tumors: Preliminary results. J Neuroradiol.
37:269–275. 2010.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grand S, Pasquier B, Gay E, Kremer S, Remy
C and Le Bas JF: Chordoid glioma of the third ventricle: CT and
MRI, including perfusion data. Neuroradiology. 44:842–846. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaganti J, Taylor M, Woodford H and Steel
T: Differentiation of primary central nervous system lymphoma and
high-grade glioma with dynamic susceptibility contrast-derived
metrics: Pilot study. World Neurosurg. 151:e979–e987. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bureta C, Higa N, Makino R, Takajo T,
Yonezawa H, Uchida H and Yoshimoto K: Diffuse Large B-Cell lymphoma
of the central nervous system manifesting with intratumoral
hemorrhage: A case report and literature review. World Neurosurg.
143:490–494. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang XZ, Ni J and Cui LY: Multifocal
hemosiderin depositions on T2*-weighted magnetic resonance imaging
in a patient with pathology-proven systemic diffuse large B-cell
lymphoma. BMC Neurol. 14:1842014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rubenstein J, Fischbein N, Aldape K,
Burton E and Shuman M: Hemorrhage and VEGF expression in a case of
primary CNS lymphoma. J Neurooncol. 58:53–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuchinoue Y, Uchino K, Terazono S, Harada
N, Kondo K and Sugo N: A case of lateral ventricular subependymoma
with intratumoral hemorrhage via neuroendoscopic surgery.
NMC Case Rep J. 9:231–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sumi K, Suma T, Yoshida R, Kajimoto R,
Kobayashi M, Katsuhara T, Hirayama K, Tang X, Otani N and Yoshino
A: Massive intracranial hemorrhage caused by intraventricular
meningioma: Case report. BMC Neurol. 21:252021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taguchi A, Kinoshita Y, Amatya VJ,
Takayasu T, Takano M, Yonezawa U, Tominaga A, Takeshima Y, Sugiyama
K and Yamasaki F: Intratumoral hemorrhage after endoscopic third
ventriculostomy for obstructive hydrocephalus caused by brain
tumors. World Neurosurg. 158:e256–e264. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masoumi N, Framanzad F, Zamanian B,
Seddighi AS, Moosavi MH, Najarian S and Bastani D: 2D computational
fluid dynamic modeling of human ventricle system based on
fluid-solid interaction and pulsatile flow. Basic Clin Neurosci.
4:64–75. 2013.PubMed/NCBI
|