Introduction
Advanced urothelial carcinoma (aUC), consisting of
locally progressive and metastatic disease, is generally considered
incurable (1). Since the 1980s,
cisplatin-based chemotherapy has been the standard of care for aUC.
A landmark regimen in systemic chemotherapy was the development of
a combination of methotrexate, vinblastine, doxorubicin, and
cisplatin (MVAC) (2). Patients on
MVAC demonstrated a good response against aUC, but its toxicity has
been known to be severe. A randomized control study on gemcitabine
and cisplatin (GC) vs. MVAC revealed the lower toxicity of GC
compared to standard MVAC, which resulted in GC becoming a new
standard regimen (3). Although the
majority of patients with metastatic UC initially respond to these
chemotherapy regimens, most such cancers eventually progress. To
establish systemic salvage therapy for patients who have progressed
after first-line chemotherapy is one of the unmet needs in the
field.
Pembrolizumab, a programmed death 1 inhibitor, was
approved as second-line therapy for aUC that had progressed after
chemotherapy. A randomized phase 3 KEYNOTE-045 trial showed a
superior overall survival (OS) benefit of pembrolizumab vs.
chemotherapy (paclitaxel, docetaxel or vinflunine) in patients with
aUC that progressed on platinum-based chemotherapy (4). However, its survival benefit was
relatively short (10.3 vs. 7.4 months). The objective response rate
[complete response (CR)+ partial response (PR), 21.1%] was also
unsatisfactory although it was significantly higher than that of
the chemotherapy group (11.4%). Unfortunately, only a minority of
patients benefits from pembrolizumab. Establishing biomarkers to
predict the efficacy of this drug is therefore an important
challenge (5).
Recently, the prognostic nutritional index (PNI) has
been studied as a potential biomarker that predicts patients'
response to immunotherapy for various cancers (6,7).
These works demonstrated that a low pretreatment PNI value was a
candidate prognostic biomarker of a poor objective response and
adverse prognosis in patients with advanced cancer treated with
immune checkpoint inhibitors (ICIs). In the field of aUC, a small
number of publications exist on PNI and pembrolizumab treatment
outcomes (8,9). However, their findings on the
predictive impact of a survival benefit were inconsistent.
Therefore, we planned a retrospective observational study of PNI
before and after the induction of pembrolizumab in our cohort. In
the present study, we elucidated that early posttreatment PNI after
the induction of pembrolizumab therapy predicts better clinical
outcomes in patients with aUC.
Materials and methods
Patients
Thirty-four consecutive patients who underwent
second-line or later pembrolizumab treatment for aUC from Jan 2018
to July 2022 at Shiga University of Medical Science Hospital were
included in this observational study. Clinical and pathological
data were collected from their medical records. Patients with
non-UC, or concurrent active cancer other than UC, were excluded.
This study was approved by the ethics committee of Shiga University
of Medical Science (approval number R2018-189), and it conforms to
the provisions of the Declaration of Helsinki. Information on the
present study was outlined on the website of our hospital in order
for patients to be able to opt-out as desired. The requirement for
written informed consent was waived because of the nature of the
study.
Treatment
Patients were intravenously administered 200 mg of
pembrolizumab every three weeks or 400 mg every six weeks. In
principle, treatment was continued until disease progression as
determined by imaging. However, some patients continued receiving
pembrolizumab after disease progression because no effective
salvage therapy existed at the time, such as enfortumab-vedotin.
When immune-related adverse events (irAE) occurred, pembrolizumab
treatment was terminated or interrupted.
Assessments
Hematological and biochemical laboratory tests were
performed every treatment cycle, and an imaging study (computed
tomography, magnetic resonance imaging, or
18F-fluorodeoxyglucose-positron emission tomography) was performed
every 2 to 3 months. The treatment response was determined
according to Response Evaluation Criteria in Solid Tumors, version
1.1 (10). Performance status was
determined in accordance with Eastern Cooperative Oncology Group
performance-status scores. The PNI was calculated by a formula
established by Onodera et al (11): PNI=[10 × serum albumin (g/dl)] +
[0.005 × lymphocyte count (/mm3)].
Pretreatment PNI values (pre-PNI) were determined by
hematological data within four weeks prior to the initiation of
pembrolizumab therapy; in most patients, data were obtained on the
starting day or the day before. Posttreatment PNI values (post-PNI)
were defined as the highest value within two months from the
initiation of pembrolizumab therapy. Prognostic nutritional index
values within one week after the start of treatment were excluded
because the observation period was considered too short. We
stratified our cohort into two categories (high and low PNI groups)
with cutoff values of pre- and post-PNI at 36 and 40,
respectively.
Preliminary analysis to determine the
optimal cutoff value
As a preliminary study to determine the optimal PNI
cutoff value, we applied several cutoff values (31 to 48) used in
previous studies described in the systematic review by Ni et
al (6). We selected the cutoff
value with the most significant difference in median overall
survival (OS) between the low and high groups and the smallest
P-value by log-rank test. Then, we considered that 36 (pre-PNI) and
40 (post-PNI) were the best cutoff values to discriminate OS in our
cohort (Table SI).
Statistical analysis
Statistical analyses were carried out using EZR
software (12). We used Wilcoxon's
signed rank test to assess continuous variables. Fisher's exact
test was used to analyze the differences in categorical variables
of the groups. OS and progression-free survival (PFS) were
calculated using the Kaplan-Meier method and compared using the
log-rank test. Survival periods were calculated from the date of
initial administration of pembrolizumab to the events (death or
disease progression). A Cox proportional hazards model was used to
test the significance of predictive factors of OS and PFS.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients' demographics
Table I shows
patients' demographics. The median age of patients was 72.5 years,
and the male-to-female ratio was 62 vs. 38%. Twenty-two and twelve
patients were diagnosed with bladder and upper tract cancers as
primary lesions, respectively. With regard to the purpose of
pembrolizumab treatment, seven patients (21%) were treated for
early relapsing disease after the receipt of platinum-based
perioperative chemotherapy, and the remaining 27 patients (79%)
were administered pembrolizumab for the purpose of second-line or
later salvage therapy. Most patients (98%) received prior
cisplatin/carboplatin-containing chemotherapy. Liver metastasis was
present in nine cases (26%).
 | Table I.Patients' demographics |
Table I.
Patients' demographics
| Demographic | Values (n=34) |
|---|
| Median age, years
(range) | 72.5 (49–84) |
| Sex |
|
| Men | 21 (62%) |
|
Women | 13 (38%) |
| Performance status
(ECOG) |
|
| 0 | 18 (53%) |
| 1 | 11 (32%) |
| 2 | 5 (15%) |
| Primary site |
|
|
Bladder | 22 (65%) |
| Upper
tract | 12 (35%) |
| Histology |
|
| Pure
urothelial carcinoma | 32 (94%) |
|
Urothelial carcinoma with
sarcomatoid variant | 2 (6%) |
| Purpose of
pembrolizumab administration |
|
| For early
relapse after perioperative chemotherapy | 7 (21%) |
|
2nd-line | 25 (73%) |
|
3rd-line or
later | 2 (6%) |
| Prior systemic
therapy |
|
|
Gemcitabine/cisplatin or
gemcitabine/carboplatin | 33 (98%) |
|
Gemcitabine/paclitaxel | 4 (15%) |
|
Others | 2 (6%) |
| Metastatic
sites |
|
| Local
recurrence | 6 (18%) |
| Lymph
nodes | 23 (68%) |
|
Lung | 13 (38%) |
|
Liver | 9 (26%) |
|
Bone | 6 (18%) |
| Others
(peritoneal carcinomatosis, port-site recurrence) | 3 (9%) |
Treatment results
The median number of pembrolizumab administrations
was five (Table II). Thirty-two
patients (94%) were terminated from further treatment by reason of
disease progression (21), irAE
(7), fatigue (2) or having a long-term CR (2). The remaining two cases continued with
pembrolizumab treatment. The objective response rate was 29% (CR 3;
PR 7), and the disease control rate was 53% [CR 3; PR 7; stable
disease (SD) 8]. The irAEs that resulted in discontinuation of
treatment were interstitial pneumonia (2), liver dysfunction (2), severe diarrhea (1), encephalitis (1), and a worsening of rheumatoid
arthritis (1).
 | Table II.Treatment results. |
Table II.
Treatment results.
| Factor | Values (n=34) |
|---|
| Median cycles
(range) | 5 (1–33) |
| Objective response
of pembrolizumab |
|
| CR | 3 (9%) |
| PR | 7 (20%) |
| SD | 8 (24%) |
| PD | 16 (47%) |
| Reasons for
termination of pembrolizumab therapya |
|
|
Disease
progression | 21 (62%) |
|
irAE | 7 (21%) |
|
Long-term complete
response | 2 (6%) |
|
Treatment-related
fatigue 9+21+ | 2 (6%) |
Prognostic nutritional index
Median pre- and post-PNI values were 40.0
(21.6–52.7) and 41.4 (22.6–57.3), respectively (P=0.153, Wilcoxon's
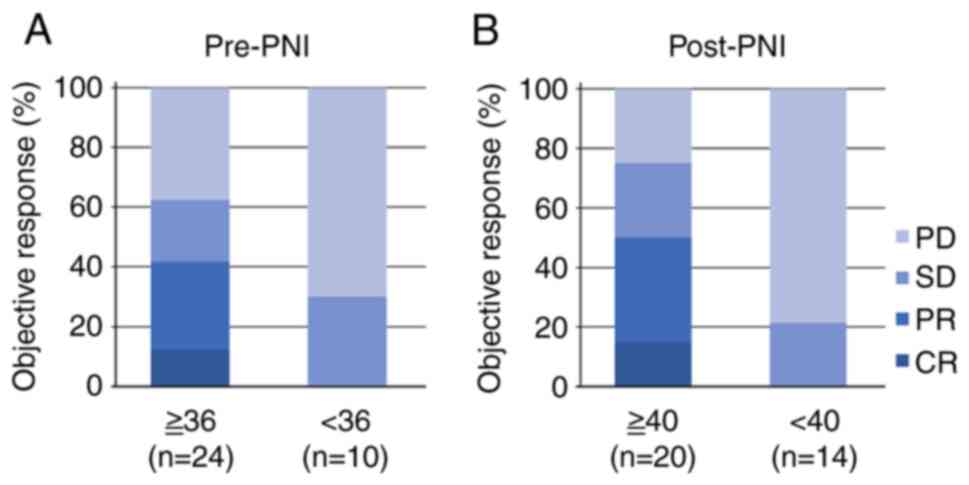
signed rank test). Fig. 1 shows
the objective response stratified by pre- and post-PNI values. In
pretreatment, the higher PNI group showed a better disease control
rate than the lower PNI group, but no significant difference was
observed (63 vs. 30%, P=0.134, Fisher's exact test). Whereas, in
posttreatment, the higher group demonstrated a significantly better
disease control rate than the lower group (75 vs. 21%, P=0.004,
Fisher's exact test).
Overall and progression-free survival
rates stratified by PNI
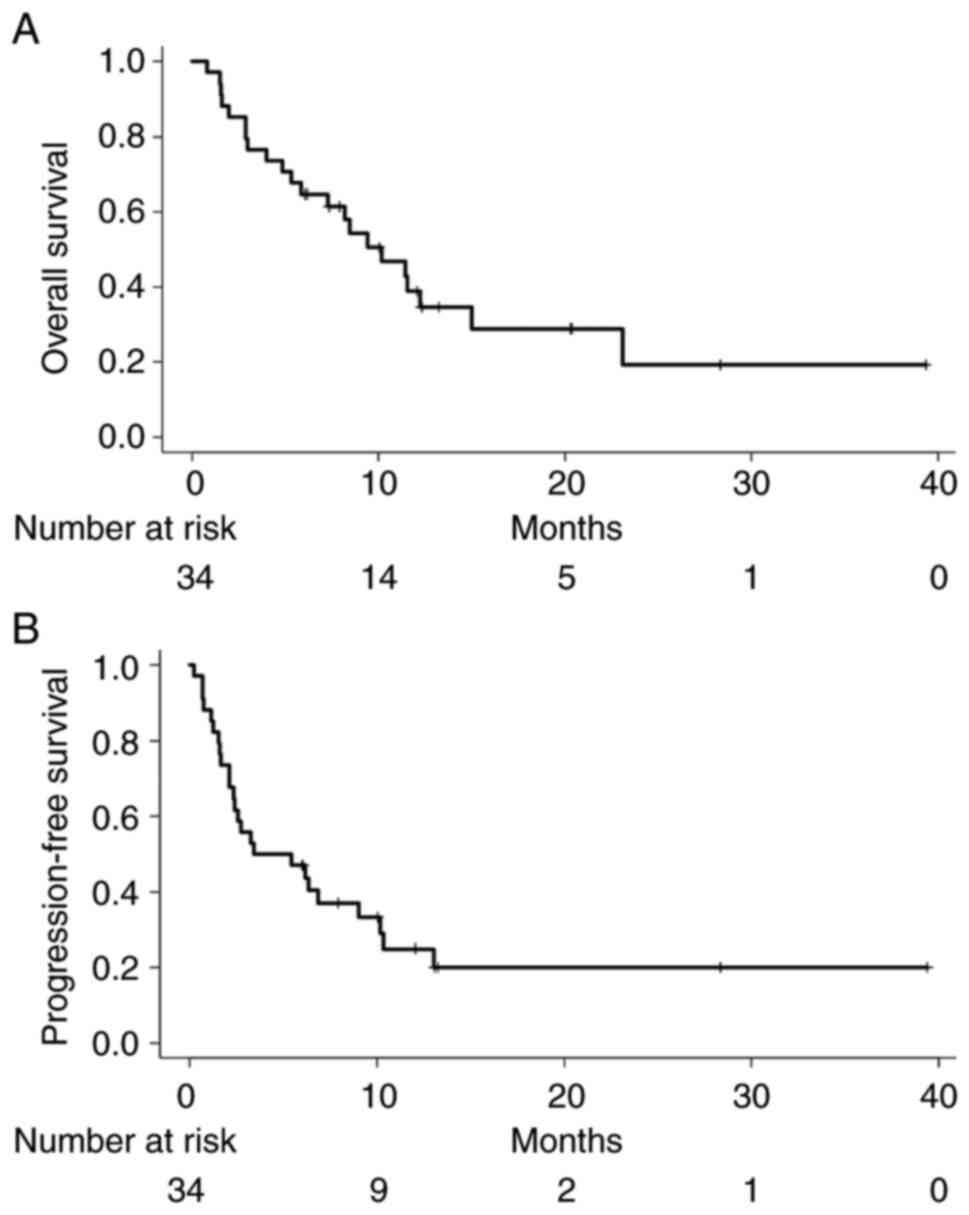
The median overall survival (OS) and
progression-free survival (PFS) of all patients were 10.2 and 3.5
months, respectively (Fig. 2). As
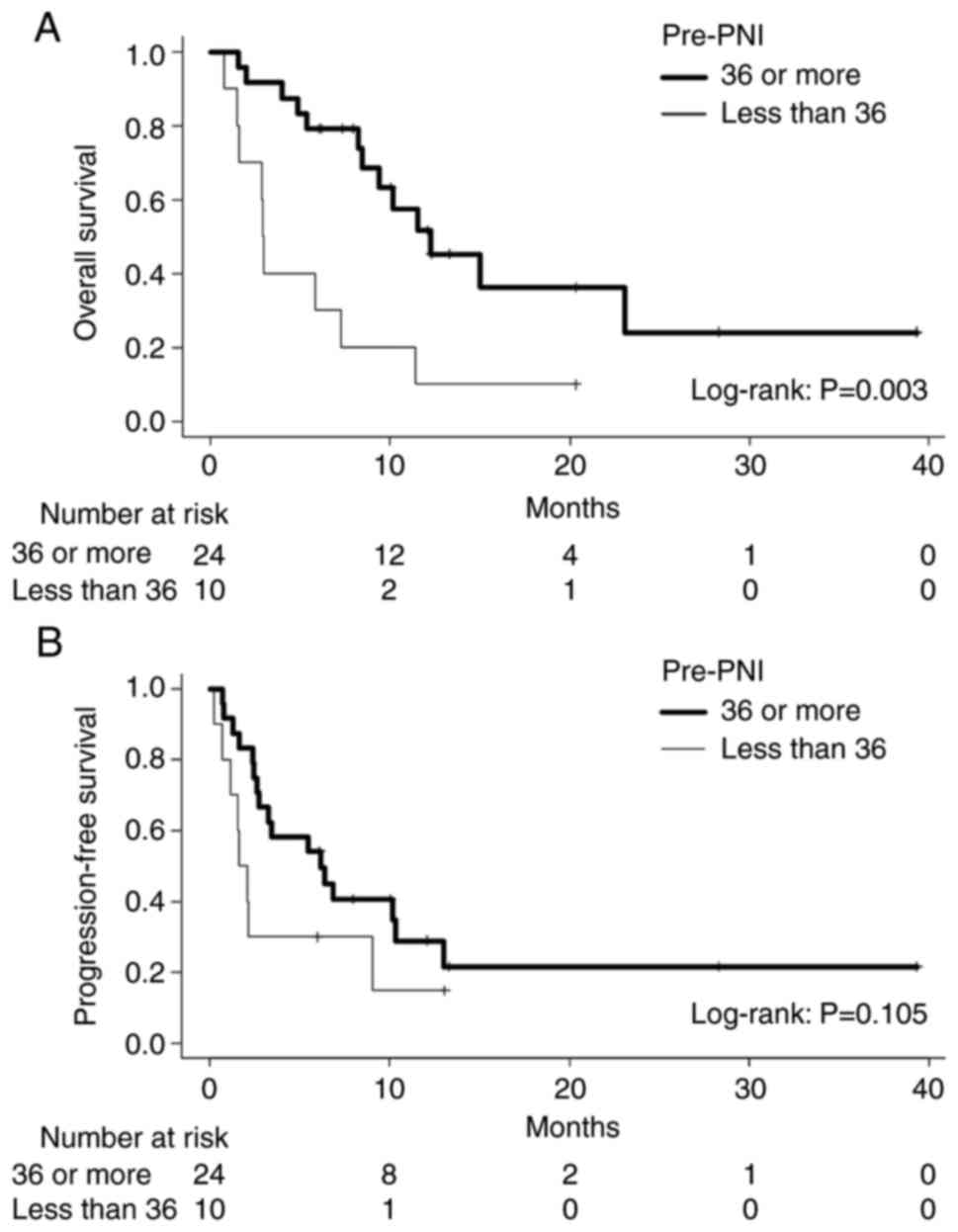
for pre-PNI, patients with a higher PNI showed a longer median OS
than the lower PNI group (12.2 vs. 3.0 months, P=0.003, log-rank;
Fig. 3). With regard to PFS, no
difference was observed between patients with higher and lower
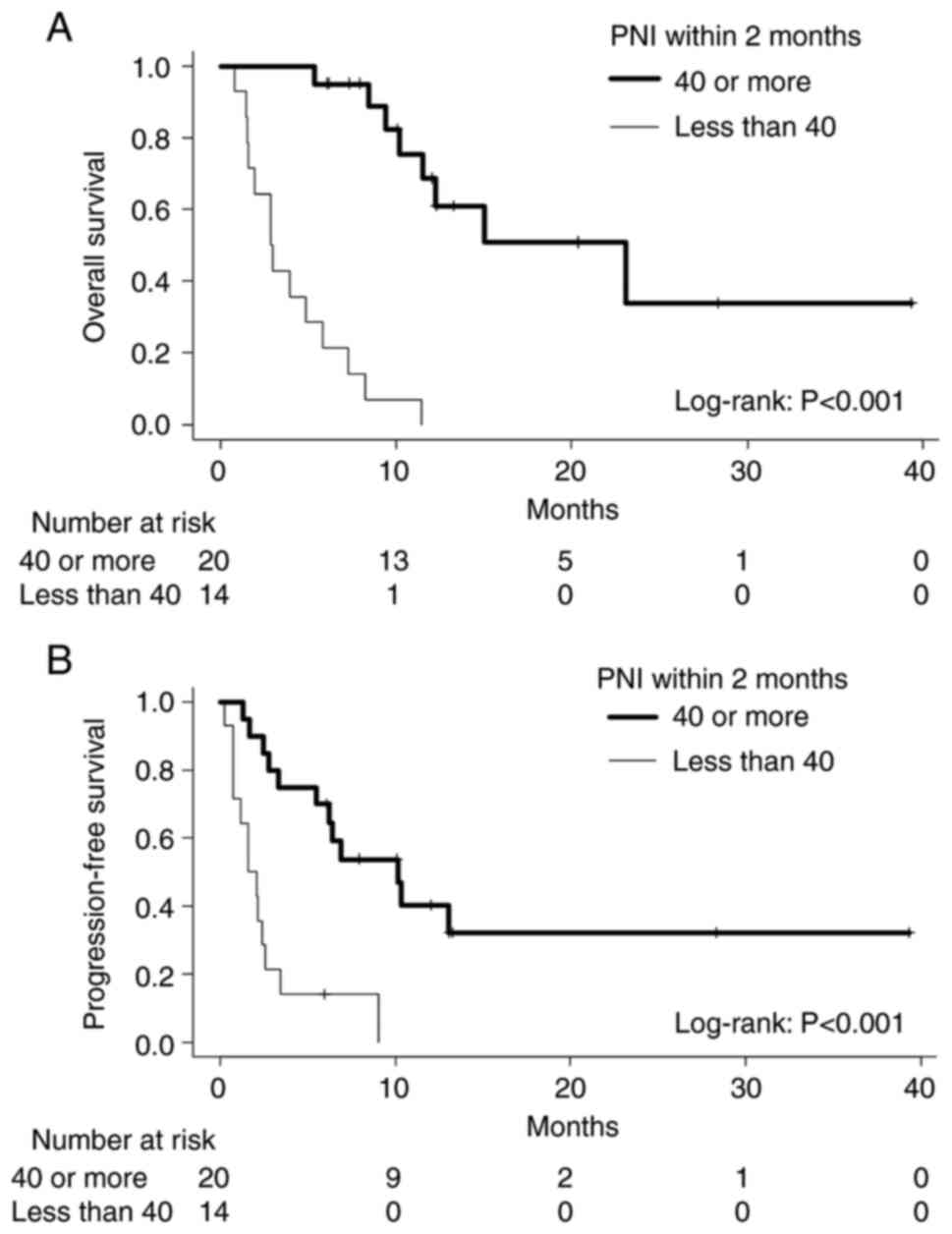
pre-PNI values (6.2 vs. 1.9 months, P=0.105). In terms of post-PNI,
higher PNI patients showed both better OS and PFS than the lower
post-PNI group; the median OS values for higher and lower PNI
patients were 23.1 and 2.9 months (P<0.001); the median PFS
values for higher and lower PNI patients were 10.2 and 1.9 months
(P<0.001; Fig. 4). These
results suggest that post-PNI has a better prognostic potential
than the pre-PNI.
Table III shows
univariate and multivariate analyses by a Cox hazard model
regarding OS. In univariate analysis, the Eastern Cooperative
Oncology Group performance status and post-PNI were revealed as
significant predictors of OS. In multivariate analysis, a higher
post-PNI value represented an independent prognostic factor for
longer OS. Similarly, a higher post-PNI value indicated a
predictive factor for better PFS (Table IV).
 | Table III.Cox hazard model with regard to
OS. |
Table III.
Cox hazard model with regard to
OS.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤72 | 1 |
|
|
|
|
>72 | 0.66
(0.28–1.55) | 0.340 |
|
|
| Sex |
|
|
|
|
|
Men | 1 |
|
|
|
|
Women | 0.83
(0.34–2.02) | 0.688 |
|
|
| ECOG performance
status |
|
|
|
|
| 0,
1 | 1 |
| 1 |
|
| 2 | 3.69
(1.31–10.44) | 0.014 | 2.03
(0.59–6.97) | 0.262 |
| Primary lesion |
|
|
|
|
|
Bladder | 1 |
|
|
|
| Upper
tract | 0.87
(0.35–2.13) | 0.757 |
|
|
| Purpose of
pembrolizumab administration |
|
|
|
|
| For
early relapsing after perioperative chemotherapy | 1 |
|
|
|
|
2nd-line or later | 1.11
(0.37–3.33) | 0.859 |
|
|
| Liver
metastasis |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 1.62
(0.61–4.30) | 0.334 |
|
|
| Post-PNI (within 2
months) |
|
|
|
|
| Less
than 40 | 1 |
| 1 |
|
| 40 or
more | 0.06
(0.02–0.20) | <0.001 | 0.04
(0.01–0.14) | <0.001 |
 | Table IV.Cox hazard model with regard to
PFS. |
Table IV.
Cox hazard model with regard to
PFS.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤72 | 1 |
|
|
|
|
>72 | 0.76
(0.34–1.67) | 0.492 |
|
|
| Sex |
|
|
|
|
|
Men | 1 |
|
|
|
|
Women | 1.17
(0.51–2.65) | 0.711 |
|
|
| ECOG performance
status |
|
|
|
|
| 0,
1 | 1 |
| 1 |
|
| 2 | 1.94
(0.66–5.75) | 0.231 | 2.36
(0.67–8.32) | 0.183 |
| Primary lesion |
|
|
|
|
|
Bladder | 1 |
|
|
|
| Upper
tract | 1.01
(0.48–2.54) | 0.821 |
|
|
| Purpose of
pembrolizumab administration |
|
|
|
|
| For
early relapsing after perioperative chemotherapy | 1 |
|
|
|
|
2nd-line or later | 0.99
(0.37–2.68) | 0.991 |
|
|
| Liver
metastasis |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 1.38
(0.57–3.34) | 0.475 |
|
|
| Post-PNI (within 2
months) |
|
|
|
|
| Less
than 40 | 1 |
| 1 |
|
| 40 or
more | 0.18
(0.07–0.44) | <0.001 | 0.12
(0.04–0.35) | <0.001 |
Discussion
The era of cancer immunotherapy for the treatment of
aUC commenced with the introduction of pembrolizumab (4). In the first report of the KEYNOTE-045
trial, median OS and PFS were 10.3 and 2.1 months for a
pembrolizumab treatment group compared with 7.4 and 3.3 months for
a chemotherapy group, respectively. The trial revealed longer OS
for the pembrolizumab group compared to the chemotherapy group
(hazard ratio = 0.73). After >2 years of follow-up, the
long-term results were consistent with those of previously reported
analyses, which showed that median OS and PFS were 10.1 and 2.1
months, respectively (13). Our
cohort yielded similar results to those of the KEYNOTE-045 trial;
the median OS and PFS were 10.2 and 3.5 months, respectively.
Although our sample size was very small, the clinical outcome of
our cohort is considered standard quality of care.
Not all patients benefit from cancer immunotherapy.
Therefore, many researchers have made efforts to identify
biomarkers to predict the therapeutic benefit of pembrolizumab.
Histological markers, such as programmed death-ligand 1 (PD-L1),
are generally used to predict the response during immunotherapy for
some types of cancer (14).
Bellmunt et al have shown that multiple biomarkers that
characterize the tumor microenvironment, such as PD-L1, tumor
mutational burden, and the T-cell-inflamed gene expression profile
(TcellinfGEP), may be clinically useful in better
selecting patients with UC in KEYNOTE-045 and 052 cohorts for
treatment with pembrolizumab (15). However, the role of PD-L1
expression as second-line immunotherapy for aUC is uncertain. In a
subgroup analysis of KEYNOTE-045, a survival benefit was observed
in patients who had a tumor PD-L1 combined positive score of less
than 1% as well as 1% or more (4).
PD-L1 thresholds vary due to tumor types and the use of different
assays. PD-L1 expression was also measured in a variable fashion
either on tumor cells, tumor-infiltrating immune cells, or both. To
date, PD-L1 expression as a predictive biomarker appears to have
limitations (14).
Along with the search for histological biomarkers,
hematological biomarkers have also been explored (5). Pretreatment baseline hematological
parameters, such as the neutrophil-to-lymphocyte ratio (NLR),
platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte
ratio (LMR), have been studied (16–19).
Such prior works described how low NLR and PLR were associated with
a favorable outcome of pembrolizumab therapy. With regard to the
correlation between pretreatment PNI and the outcome of
pembrolizumab treatment in aUC, several studies have been reported
(8,9). Ishiyama et al described how a
low PNI group showed significant shorter OS and PFS; they concluded
that PNI is a useful predictor of prognosis in patients with aUC
treated with pembrolizumab (9). Ni
et al performed a meta-analysis of several types of cancers
(gastric, lung, esophageal, urothelial, among others) and reported
that low PNI might be an effective biomarker of poor outcome in
patients with advanced cancer administered ICIs (6). Chemotherapy suppresses the numbers of
neutrophils, platelets, and monocytes severely, and the use of
granulocyte colony-stimulating factor and platelet transfusion may
influence NLR/PLR/LMR before the start of salvage pembrolizumab. In
comparison, serum albumin levels and lymphocyte counts change
moderately. Therefore, we considered that PNI to be suitable as a
prognostic biomarker after chemotherapy.
Recently, several reports have focused on the early
hematological response after the induction of pembrolizumab therapy
(19–22). These studies demonstrated that
changes between pre- and posttreatment NLR, or the absolute value
of NLR after treatment, were significantly associated with patient
outcomes. We observed trends in PNI values for our patients, before
and during pembrolizumab treatment. In cases with a good prognosis,
PNI values increased soon after the start of treatment, even when
the pre-PNI value was low. Thus, we speculated that the PNI value
after pembrolizumab initiation was a good prognostic indicator. In
previous reports regarding posttreatment NLR, observation points
varied according to investigator: Three weeks, six weeks, and two
cycles (approximately 4 weeks) after the start of pembrolizumab
(18–21). Although the timing of a rise in
post-PNI values in our patients with good outcomes showed a wide
distribution, most were observed within two months (8–58 days,
median 25.5 days). Therefore, we designated the observation point
as within two months after the initiation of pembrolizumab. To our
knowledge, the present study is the first report regarding post-PNI
in patients with aUC treated with pembrolizumab.
The exact reason why a low PNI value was associated
with a poor outcome in cancer immunotherapy has not been fully
elucidated (6). Ryman and Meibohm
stated that the increased catabolism associated with malnutrition
might accelerate the clearance of monoclonal antibodies, which are
eliminated primarily by catabolic degradation (23). Turner et al also reported
that patients showing high clearance of pembrolizumab were
associated with cachexia and increased protein turnover secondary
to chronic inflammation; they showed a shorter OS (24). In our patients, the rapid increase
in absolute lymphocyte counts mainly contributed to the increase in
post-PNI values. Elevation of absolute lymphocyte counts after the
induction of ICIs has been reported in several cancers, including
melanoma, non-small cell lung cancer, and renal cell carcinoma
(25–29). These studies demonstrated that
higher absolute lymphocyte counts after the start of ICI (3–6
weeks) led to longer survival. We speculated that the induction of
an immune response with pembrolizumab leads to an early increase in
lymphocyte count. Moreover, an improvement in the patient's
nutritional condition results in an increase in albumin levels,
which may cause an increase in the post-PNI level.
Several limitations existed in the present study.
First, this was a single-institutional retrospective study with a
small patient number, and thus may have been prone to selection
bias. Further study with a larger and more diverse cohort is
required to validate our results. In fact, we are currently
planning a multi-institutional validation study using a larger
number of patients. Second, the timing of blood tests was not
strictly defined, since it varied from physician to physician. The
optimal sampling time to determine the best post-PNI value should
be validated in future studies. Third, the PNI does not reflect the
inflammatory state of the host, although cancer-related
inflammation is considered to indicate a worsened prognosis. A
comparison in future of PNI with inflammation-based markers, such
as NLR, PLR, LMR and c-reactive protein, should be performed in
terms of their ability to discriminate prognosis.
In conclusion, higher post-PNI values within two
months predict both longer OS and PFS. Our findings may help
identify good responders with aUC to salvage pembrolizumab therapy
in an early phase of treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI [grant no. 21K09342
(grant provided to SKa)].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKa and TY designed the study, analyzed the data and
drafted the manuscript. SKa and TY confirm the authenticity of all
the raw data. KK, AW, MaN, SKu, TK, FJ and SN acquired clinical
data. KJ and MiN analyzed the data and reviewed the manuscript. AK
interpreted data and supervised the study. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Shiga University of Medical Science (approval no.
R2018-189). This study was undertaken according to the provisions
of The Declaration of Helsinki. The requirement for written
informed consent was waived because of the nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel VG, Oh WK and Galsky MD: Treatment
of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J
Clin. 70:404–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Ahmed T, Weiselberg LR, Geller N, Hollander PS, Herr HW and
Sogani PC: Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CN, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent LA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanagisawa T, Mori K, Katayama S,
Mostafaei H, Quhal F, Laukhtina E, Rajwa P, Motlagh RS, Aydh A,
König F, et al: Pretreatment clinical and hematologic prognostic
factors of metastatic urothelial carcinoma treated with
pembrolizumab: A systematic review and meta-analysis. Int J Clin
Oncol. 27:59–71. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni L, Huang J, Ding J, Kou J, Shao T, Li
J, Gao L, Zheng W and Wu Z: Prognostic nutritional index predicts
response and prognosis in cancer patients treated with immune
checkpoint inhibitors: A systematic review and meta-analysis. Front
Nutr. 9:8230872022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johannet P, Sawyers A, Qian Y, Kozloff S,
Gulati N, Donnelly D, Zhong J and Osman I: Baseline prognostic
nutritional index and changes in pretreatment body mass index
associate with immunotherapy response in patients with advanced
cancer. J Immunother Cancer. 8:e0016742020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimizu T, Miyake M, Hori S, Ichikawa K,
Omori C, Iemura Y, Owari C, Itami Y, Nakai W and Anai S: Clinical
impact of sarcopenia and inflammatory/nutritional markers in
patients with unresectable metastatic urothelial carcinoma treated
with pembrolizumab. Diagnostics (Basel). 10:E3102020. View Article : Google Scholar
|
|
9
|
Ishiyama Y, Kondo T, Nemoto Y, Kobari Y,
Ishihara H, Tachibana H, Yoshida K, Hashimoto Y, Takagi T and
iizuka J: Predictive impact of prognostic nutritional index on
pembrolizumab for metastatic urothelial carcinoma resistant to
platinum-based chemotherapy. Anticancer Res. 41:1607–1614. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenhauer EA, Rherasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
12
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of >2 years of follow-up. Ann Oncol.
30:970–976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US Food
and Drug Administration (FDA) approvals of immune checkpoint
inhibitors. J Immunother Cancer. 7:2782019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bellmunt J, de Wit R, Fradet Y, Climent
MA, Petrylak DP, Lee JL, Fong L, Necchi A, Sternberg CN, O'Donnell
PH, et al: Putative biomarkers of clinical benefit with
pembrolizumab in advanced urothelial cancer: Results from the
KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res.
28:2050–2060. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogihara K, Kikuchi E, Shigeta K, Okabe T,
Hattori S, Yamashita R, Yoshimine S, Shirotake S, Nakazawa R,
Matsumoto K, et al: The pretreatment neutrophil-to-lymphocyte ratio
is a novel biomarker for predicting clinical responses to
pembrolizumab in platinum-resistant metastatic urothelial carcinoma
patients. Urol Oncol. 38:602.e1–602.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi T, Ito K, Kojima T, Maruyama S,
Mukai S, Tsutsumi M, Miki J, Okuno T, Yoshio Y, Matsumoto H, et al:
Pre-pembrolizumab neutrophil-to-lymphocyte ratio (NLR) predicts the
efficacy of second-line pembrolizumab treatment in urothelial
cancer regardless of the pre-chemo NLR. Cancer Immunol Immunother.
71:461–471. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurashina R, Ando K, Inoue M, Izumi K,
Maruyama R, Mitani K, Takenobu H, Haruta M, Iizuka T, Kamijo T, et
al: Platelet-to-lymphocyte ratio predicts the efficacy of
pembrolizumab in patients with urothelial carcinoma. Anticancer
Res. 42:1131–1136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto Y, Yatsuda J, Shimokawa M, Fuji
N, Aoki A, Sakano S, Yamamoto M, Suga A, Tei Y, Yoshihiro S, et al:
Prognostic value of pre-treatment risk stratification and
post-treatment neutrophil/lymphocyte ratio change for pembrolizumab
in patients with advanced urothelial carcinoma. Int J Clin Oncol.
26:169–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomioka-Inagawa R, Nakane K, Enomoto T,
Tomioka M, Taniguchi T, Ishida T, Ozawa K, Takagi K, Ito H,
Takeuchi S, et al: The impact of Neutrophil-to-Lymphocyte ratio
after two courses of pembrolizumab for oncological outcomes in
patients with metastatic urothelial carcinoma. Biomedicines.
10:16092022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujiwara M, Fujiwara R, Urasaki T, Oguchi
T, Komai Y, Numao N, Yamamoto S, Yonese J and Yuasa T: Early serum
and hematological responses to pembrolizumab therapy as predictors
of survival in metastatic urothelial cancer. Anticancer Res.
42:2045–2051. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchimoto T, Nakamura K, Komura K,
Fukuokaya W, Yano Y, Nishimura K, Kinoshita S, Nishio K, Fukushima
T, Nakamori K, et al: Prognostic value of the fluctuation in the
neutrophil-lymphocyte ratio at 6 weeks of pembrolizumab treatment
is specific to the clinical response in metastatic urothelial
carcinoma. Urol Oncol. 40:344.e11–344.e17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryman JT and Meibohm B: Pharmacokinetics
of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol.
6:576–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turner DC, Kondic AG, Anderson KM,
Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J,
Ebbinghaus SW, et al: Pembrolizumab Exposure-response assessments
challenged by association of cancer cachexia and catabolic
clearance. Clin Cancer Res. 24:5841–5849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delyon J, Mateus C, Lefeuvre D, Lanoy E,
Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E and Robert C:
Experience in daily practice with ipilimumab for the treatment of
patients with metastatic melanoma: An early increase in lymphocyte
and eosinophil counts is associated with improved survival. Ann
Oncol. 24:1697–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khunger M, Patil PD, Khunger A, Li M, Hu
B, Rakshit S, Basu A, Pennell N, Stevenson JP, Elson P, et al:
Post-treatment changes in hematological parameters predict response
to nivolumab monotherapy in non-small cell lung cancer patients.
PLoS One. 13:e01977432018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karantanos T, Karanika S, Seth B and
Gignac G: The absolute lymphocyte count can predict the overall
survival of patients with non-small cell lung cancer on nivolumab:
A clinical study. Clin Transl Oncol. 21:206–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueda K, Suekane S, Kurose H, Ogasawara N,
Hiroshige T, Chikui K, Uemura K, Nakiri M, Nishihara K, Matsuo M,
et al: Absolute lymphocyte count is an independent predictor of
survival in patients with metastatic renal cell carcinoma treated
with nivolumab. Jpn J Clin Oncol. 52:179–186. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Park YS, Lee HW, Park TY, Lee JK
and Heo EY: Peripheral lymphocyte count as a surrogate marker of
immune checkpoint inhibitor therapy outcomes in patients with
non-small-cell lung cancer. Sci Rep. 12:6262022. View Article : Google Scholar : PubMed/NCBI
|