Introduction
Gastrointestinal tumors (GISTs) are the most common
mesenchymal tumors in the gastrointestinal (GI) tract. The tumors
arise from the interstitial cells of Cajal and are genetically
characterized by activating mutations in the c-kit or
platelet-derived growth factor receptor (PDGFR)
alpha gene (1,2). GISTs are considered ‘potentially
malignant’ tumors that pose a significant risk of metastasis,
although the extent of malignancy is dependent on tumor size,
mitotic index, and tumor site (3).
This concept implies that there is no such thing as an absolutely
benign GIST even though the tumor is devoid of morphological
features that would suggest a malignant nature from a classical
pathological perspective. Prior to the establishment of the
molecular biological and pathological concepts of GISTs in the
early 2000s, GISTs could not be clearly discriminated from other
mesenchymal tumors including leiomyoma, leiomyosarcoma, and
neurogenic tumors owing to the similarity in morphology (4).
We herein report a case of an 80-year-old man with
recurrent GIST. The primary tumor was diagnosed as a leiomyoma
arising from the jejunum 30 years ago, and hence patient follow-up
was discontinued. Although the patient did not know that the tumor
excised 30 years ago was malignant, immunohistochemical analysis of
archival pathological samples disclosed that the tumor was a
low-risk GIST. Literature review indicates that the disease-free
interval of this case is the second longest among the reported
cases of GIST recurrence. Our report offers evidence supporting the
concept of ‘potentially malignant’ GISTs and will help advance our
understanding of the management of GIST patients.
Case report
The patient was an 80-year-old man who suddenly
fainted and was transported to our hospital. Hemorrhage from the GI
tract was suspected because the patient presented with melena
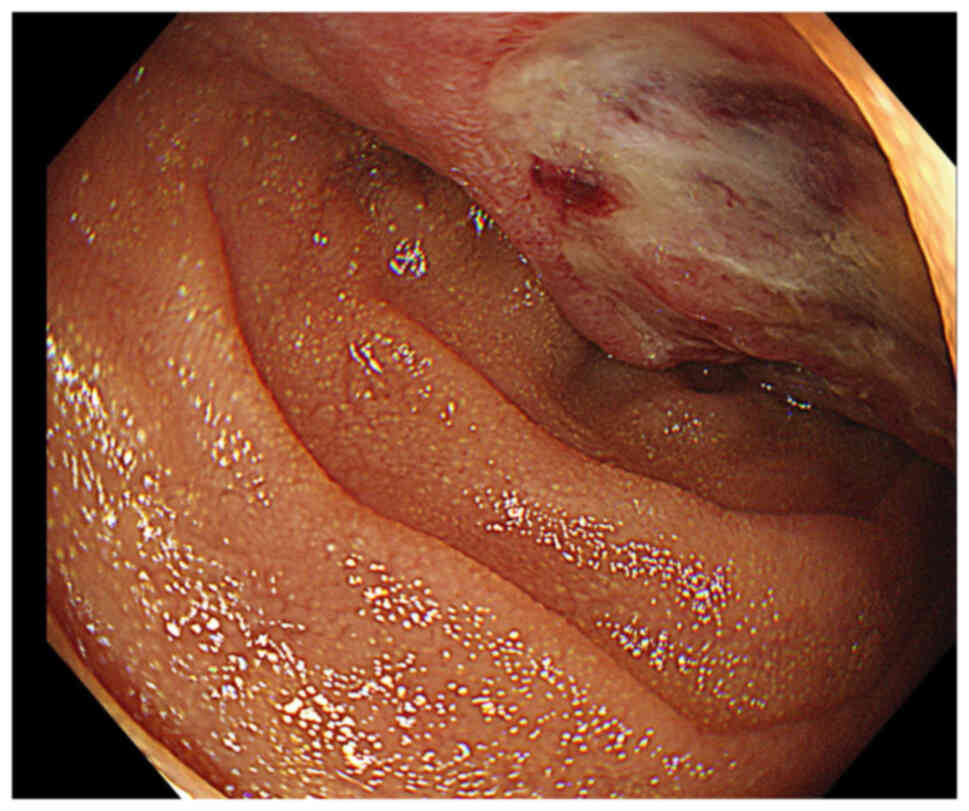
associated with a state of shock. Gastroduodenal endoscopy
performed immediately uncovered a large mass in the duodenum. The
tumor showed a plateau-like appearance and was widely covered with
normal mucosa. The tumor also formed shallow ulcers that were
identified as the source of bleeding (Fig. 1). Hemostasis was realized by
endoscopic cautery, and hemodynamics was stabilized after blood
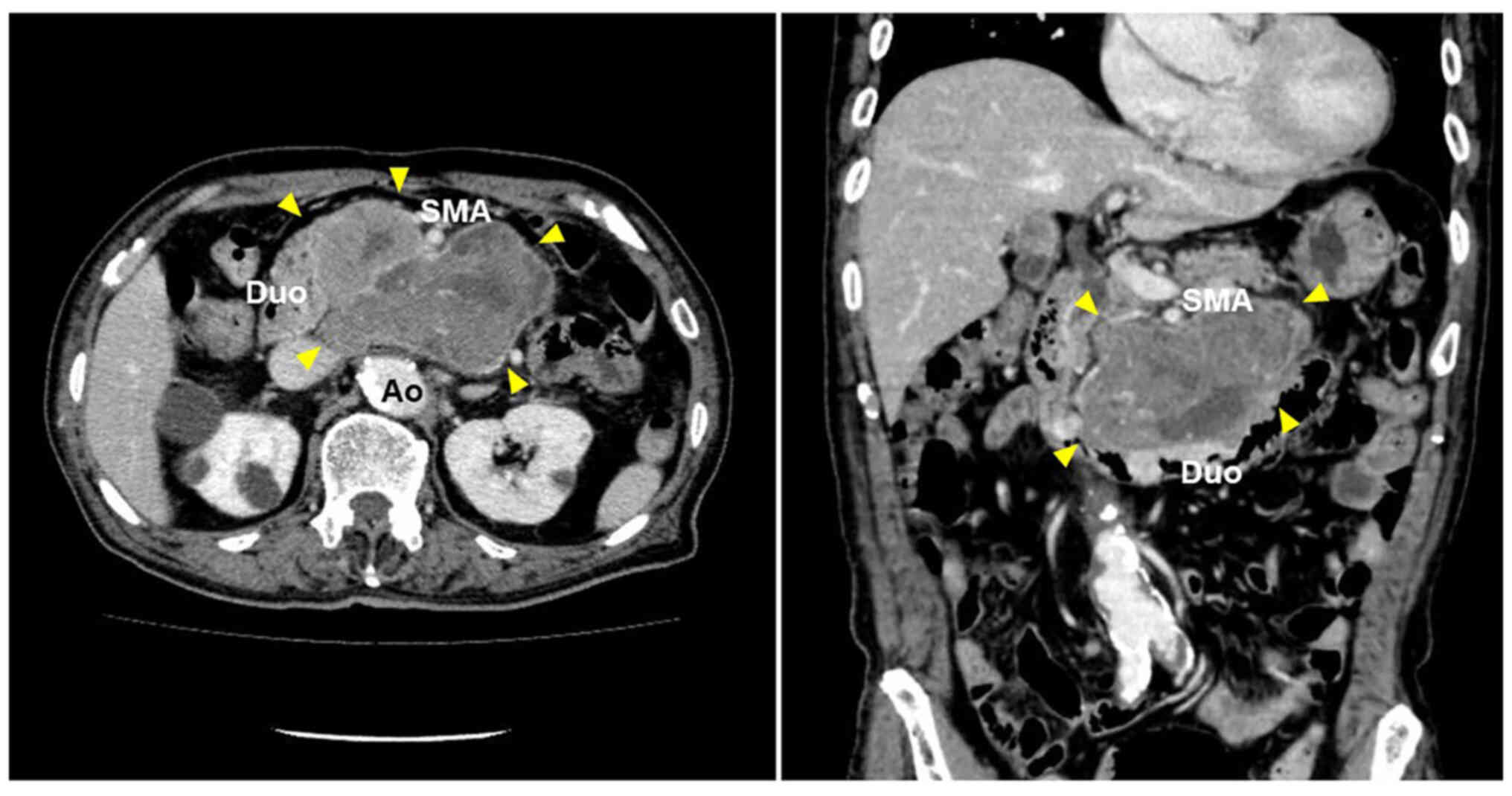
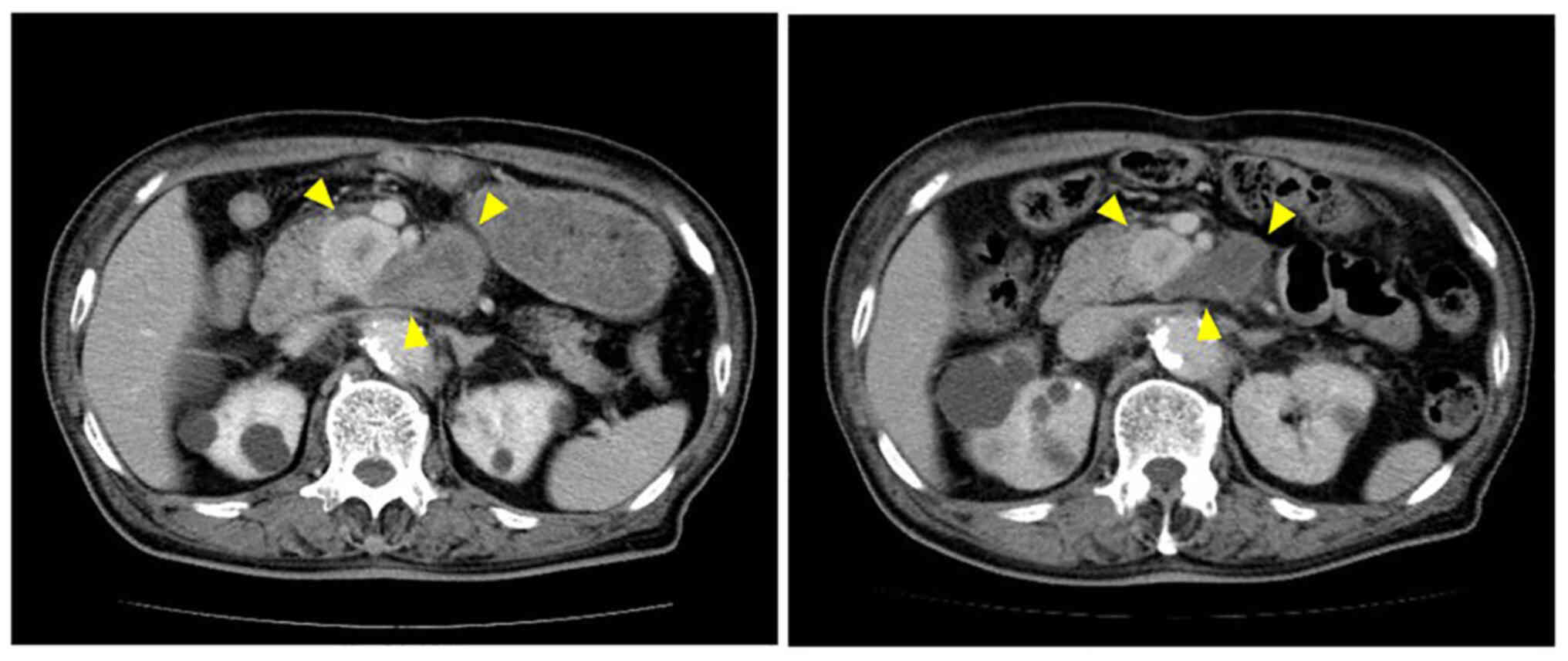
transfusion. Abdominal contrast-enhanced computed tomography (CT)
revealed that the tumor was 11.7×8.7 cm in size and located in the
retroperitoneum adjacent to the third portion of the duodenum. The
tumor exhibited as an irregularly shaped mass with heterogenous
enhancement and was encompassed by the abdominal aorta and the
superior mesenteric artery (Fig.
2). Although these radiological findings strongly suggested
that the mass was a metastatic tumor, the patient denied any
history of cancer diagnosis or treatment. Furthermore, endoscopy
and CT scans immediately conducted identified no possible primary
malignancy. Biopsied specimens obtained by endoscopy provided no
specific information owing to inappropriate sampling. Fine-needle
aspiration biopsy was abandoned because of the significant risk of
bleeding.
An in-depth interview revealed a history of surgery:
the patient had undergone resection of a ‘leiomyoma’ of the jejunum
30 years ago. The surgical records documented that the tumor was
located in the upper jejunum adjacent to the ligament of Treitz (4
cm distal) and excised en bloc with a short jejunal segment.
Although the tumor showed exophytic growth, serosal status was not
mentioned in the pathological report. That event was published as a
case report because of a unique manifestation of GI bleeding
(5). This history raised the
possibility that the tumor excised 30 years ago might be a GIST and
the current tumor was a locoregional recurrence of the jejunal
tumor. We obtained archival pathological samples of that tumor and
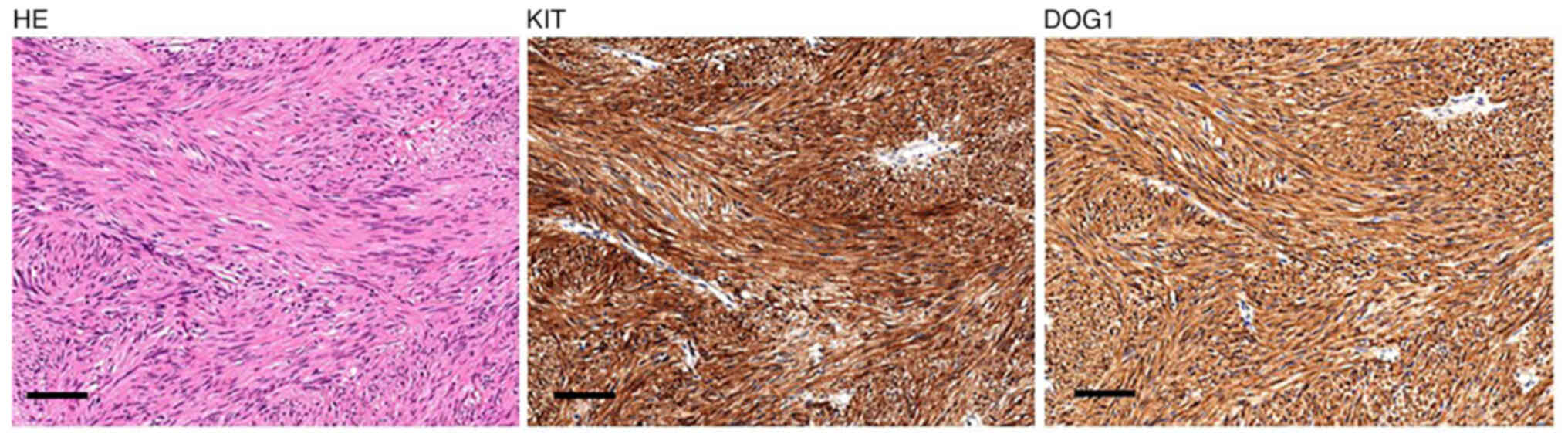
reanalyzed them with current standard pathology including
immunohistochemistry for KIT and DOG1. KIT is a standard cellular
marker for GISTs, and DOG1 is a currently identified marker for
GISTs, which is more highly diagnostic than KIT. Specific
antibodies were purchased from MBL, Tokyo (no. 566, dilution,
1:400) for KIT and Nichirei, Tokyo (no. 718041, dilution, 1:1) for
DOG1. The tumor showed diffuse, strong immunoreactivity for both
KIT and DOG1 (Fig. 3). Ki-67
labeling index was lower than 5%. On the basis of these findings,
the previously excised jejunal tumor was diagnosed as a low-risk
GIST [tumor size, 3.5 cm; mitotic index, 4/50 high-power fields
(HPF)]. Using the formalin-fixed and paraffin-embedded (FFPE)
tissues, we performed the polymerase chain reaction for
c-kit gene analysis but were unable to obtain sufficient
amplicons for sequencing owing to the low-DNA quality.
The patient consented to undergoing molecularly
targeted therapy with imatinib mesylate after careful explanation
of the high possibility of metastatic GIST. Imatinib therapy was
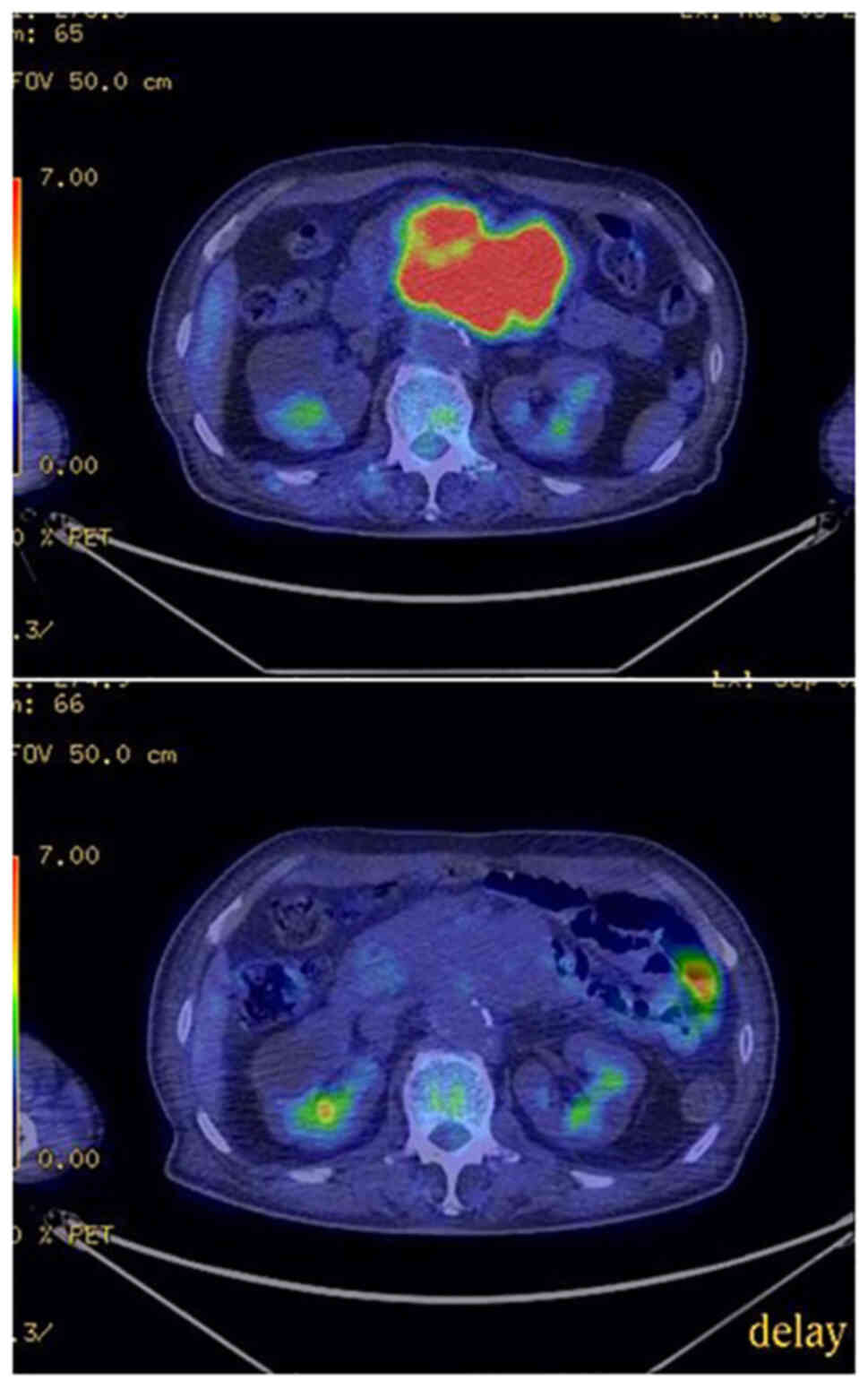
started with the dosage of 400 mg daily once a day. Positron
emission tomography (PET)/CT conducted on the 28th treatment day
revealed that imatinib therapy completely shut down
18F-fluorodeoxyglucose (FDG) uptake in the tumor
(Fig. 4), confirming that the
tumor was imatinib-sensitive.
Despite a short interruption due to an adverse event
(grade-3 eruption), the patient continued imatinib therapy for 24
months and has shown partial response so far (Fig. 5).
Literature review
To characterize late-recurrence of GISTs, we
searched the literature using PubMed database, designating
‘gastrointestinal stromal tumor/GIST’ and ‘late
recurrence/metastasis’ as key words. The search gave twenty reports
in English; careful examination revealed that one case series
(6) and four case reports
(7–10) met our research purpose. In
addition, we reviewed the references of those reports and found
further six case reports (11–16).
Finally, we found a total of 12 cases of GIST recurrence that was
diagnosed more than 10 years after the primary resection. A summary
of their clinicopathological characteristics is presented in
Table I.
 | Table I.Summary of cases of late recurrence of
GIST diagnosed more than 10 years after primary tumor
resection. |
Table I.
Summary of cases of late recurrence of
GIST diagnosed more than 10 years after primary tumor
resection.
| Author, year | DFI, years | Age at metastasis,
years | Sex | Primary tumor | Size of
metastasis | Diagnosis of primary
tumor | Site of primary
tumor, cm | Mitotic count, /50
HPF | Risk of primary
tumor, modified Fletcher classification | Genotype | (Refs.) |
|---|
| Nannini et al,
2012 | 11 | 76 | Female | Small bowel | Liver | n.m. | 7 | <1/30 HPF | n.m. | KIT exon
11 | (6) |
|
| 11 | 70 | Male | Rectum | Liver | n.m. | 4 | 16 | High | KIT exon
11 |
|
| Ballarini et
al, 1998 | 11 | 62 | Male | Stomach | Liver | Benign
leiomyoblastoma | 4 | 1 | Low | n.m. | (7) |
| Furukawa et
al, 2012 | 11 | 71 | Male | Stomach | Port-site | Leiomyosarcoma | 4 | 1 | Low | PDGFRA | (8) |
| Whang et al,
2017 | 11 | 45 | Female | Small bowel | Liver | GIST | 11 | n.m. | High | KIT exon
11 | (11) |
| Masuoka et al,
2003 | 12 | 58 | Male | Rectum | Liver | Low-grade
leiomyosarcoma | 4 | 2/10 HPF | High | n.m. | (12) |
| Nowain et al,
2005 | 17 | 56 | Female | Small bowel | Liver | Leiomyosarcoma | 10 | n.m. | High | n.m. | (13) |
| Matsuoka et
al, 2007 | 17 | 55 | Female |
Retroperitoneum | Liver | Leiomyosarcoma | 14 | n.m. | High | n.m. | (14) |
| Cahill et
al, 2009 | 20 | 59 | Female | Small bowel | Liver | Benign
leiomyosarcomaa | 6 | 5 | High | n.m. | (15) |
| Grossi et
al, 2019 | 23 | 79 | Male | Stomach | Liver | Benign
leiomyoblastoma | n.m | >10 | High | PDGFRA | (16) |
| Ginori et
al, 2015 | 29 | 71 | Male | Duodenum | Liver | Schwannoma | 2.5 | 1 | Low | KIT exon
11 | (9) |
| Ishizaki et
al, 2020 | 32 | 72 | Female | Small bowel | Liver | Leiomyosarcoma | n.m | <1 | Low | n.m. | (10) |
| Current case | 30 | 80 | Male | Jejunum | Locoregional | Leiomyoma | 3.5 | 4 | Low | n.a. |
|
Discussion
We present herein a case of a patient who suffered
from recurrence of jejunal GIST 30 years after the primary tumor
resection. We started imatinib therapy despite the lack of
histological evidence by endoscopic biopsy because we were able to
comprehensively make the diagnosis of locoregional recurrence of
GIST on the basis of atypical imaging presentation on endoscopy and
CT in addition to the patient's medical history of surgery for GIST
of the upper jejunum. The diagnosis of GIST was finally confirmed
by 18F-FDG-PET, which showed that imatinib therapy
definitely shut down 18F-FDG uptake in the tumor. On the
other hand, we could not obtain direct evidence that the tumor in
the present case was a recurrence of the previously excised GIST
and not a second primary one. Matching genotypes between the
surgically excised tumor and the current one would offer strong
evidence of ‘recurrence’. Unfortunately, c-kit gene analysis
was unsuccessful owing to low DNA quality. The diagnosis-treatment
process in the present case was extraordinary and did not meet
current clinical standards. However, the patient was at risk of
re-bleeding and there was an urgent need to start treatment
swiftly. This diagnosis-treatment process together with
18F-FDG-PET may be warranted in suspected cases of GIST,
particularly in an oncologic emergency.
GISTs are considered ‘potentially malignant’ tumors
(17). Although it sounds
ambiguous, this concept indicates that all GISTs have a significant
risk of metastasis and none can be definitely labeled as benign.
The primary tumor in the present case had been histologically
diagnosed as leiomyoma, a benign myogenic tumor, on the basis of
spindle-cell morphology. Utilizing current diagnostic standards
including immunohistochemistry for KIT and DOG1, we re-evaluated
archival FFPE samples and found that the tumor was a GIST. In 1990,
the year the diagnosis was made, molecular understanding of GIST
had been not established yet (1).
Moreover, CD117 (KIT), a determinative immunohistochemical marker
for GIST, was unavailable (18).
The primary tumor in the present case was categorized as low
malignant potential even by re-evaluation: mitotic index was 4/50
HPF and Ki-67 labeling index was lower than 5%. Our literature
review also revealed that in four of 12 cases, the primary tumors
were originally diagnosed as benign ones (Table I). These findings suggest that a
clear-cut distinction between benign and malignant is impossible in
the pathology of GISTs and support the current understanding that
all GISTs should be dealt with as having a significant risk of
recurrence.
The present case raises one clinical question: how
long we should follow patients with low-risk GIST after potentially
curable surgery (R0 resection)? The latest clinical practice
guidelines of Europe (19)
recommend a long follow-up of 10 to 13 years for high-risk GIST
patients. Meanwhile, they propose a five-year follow-up for
low-risk GIST patients after acknowledging lack of evidence on the
clinical usefulness of that management.
Early retrospective studies on GIST recurrence
(20,21) have revealed that the disease-free
interval between primary tumor resection and diagnosis of
recurrence is approximately two years in median and not largely
different from that of common GI malignancies overall. On the
contrary, two retrospective studies focusing on the late recurrence
of GISTs have disclosed that considerable numbers of metastases
occurred even after five years. In one study conducted by Italian
researchers (6), reviews of 42
patients who underwent treatment for GIST recurrence in their
institution indicated that the incidence of patients with late
recurrence of five years or later was 14%. One Japanese study of
115 patients who developed recurrence after surgery (22) revealed that the incidence of late
recurrence was 12.2%. These findings suggest that five-year
follow-up is insufficient to determine the oncological outcomes of
GIST patients, and longer follow-up is required. On the other hand,
it was reported that the recurrence-free survival of low-risk GIST
patients is 95% or higher (23),
and the risk of late recurrence is extremely low in overall cases
of low-risk GISTs. In addition, a study of the cost-effectiveness
of follow-up of GIST patients showed that low-risk GIST patients
needed 98 CT examinations and that it cost 15,484 euros to find one
recurrence, which is approximately 7.5 times higher than that of
high-risk GIST patients (24).
Follow-up of selected patients would be the best solution. However,
there is no known clinicopathological feature that can enable the
effective selection of patients requiring long follow-up of more
than five years. Although the above-mentioned Japanese study
(22) revealed that small and
low-risk GISTs were frequently found in cases of late GIST
recurrence, those features are substantially useless for patient
selection.
In conclusion, we have presented a case of late
recurrence of jejunal GIST. The patient's history of surgical
resection of ‘leiomyoma’ 30 years ago was a valuable hint that led
to the diagnosis of recurrent GIST. Literature review of the late
recurrence of GISTs indicated that a considerable number of tumors
were previously diagnosed as benign mesenchymal tumors or low-risk
GISTs. The present case, although anecdotal, offers supporting
evidence that all GISTs have a significant risk of metastasis and
therefore require longer follow-up than other malignancies.
Clinical and surgical oncologists should keep in mind that disease
relapse occurring 10 years or later after curative surgery for
GISTs is possible.
Acknowledgements
The authors would like to thank Dr Kenta Sasaki
(Department of Medical Oncology, Niigata University Graduate School
of Medical and Dental Sciences, Niigata, Japan) for assistance in
the literature review.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TK conceptualized the study and drafted the
manuscript. TN and AW contributed to acquisition of data and
prepared the images used in the manuscript. YI provided valuable
information leading to a precise diagnosis and contributed to
interpretation of data. SH conducted gene analysis of the archival
samples. YA conducted immunohistochemical analysis and was
responsible for pathological diagnosis. TK, TN, and YI confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case report and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
FDG
|
fluorodeoxyglucose
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
GI
|
gastrointestinal
|
|
GIST
|
gastrointestinal stromal tumor
|
|
HPF
|
high-power field
|
|
PET
|
positron emission tomography
|
References
|
1
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miettinen M, Sarlomo-Rikala M and Lasota
J: Gastrointestinal stromal tumors: Recent advances in
understanding of their biology. Hum Pathol. 30:1213–1220. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwafuchi Y, Arai F, Tsuruya T, Honda K,
Ito T, Hasegawa A, Kamimura A, Takii Y, Kanahara H, Narisawa R, et
al: A case of jejunal leiomyoma (in Japanese with English
abstract). Endoscopic Forum for Digestive Disease. 7:223–227.
1991.
|
|
6
|
Nannini M, Biasco G, Pallotti MC, Di
Battista M, Santini D, Paterini P, Maleddu A, Mandrioli A, Lolli C,
Saponara M, et al: Late recurrences of gastrointestinal stromal
tumours (GISTs) after 5 years of follow-up. Med Oncol. 29:144–150.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ballarini C, Intra M, Ceretti AP,
Prestipino F, Bianchi FM, Sparacio F, Berti E, Perrone S and Silva
F: Gastrointestinal stromal tumors: A ‘benign’ tumor with hepatic
metastasis after 11 years. Tumori. 84:78–81. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furukawa M, Izumi S, Asano H, Tokumo M,
Mano S and Shiota K: Late umbilical port-site recurrence of a
gastrointestinal stromal tumor with an acquired PDGFRα mutation
after laparoscopic resection: Report of a case. Surg Laparosc
Endosc Percutan Tech. 22:e109–e111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ginori A, Scaramuzzino F, Marsili S and
Tripodi S: Late hepatic metastasis from a duodenal gastrointestinal
stromal tumor (29 years after surgery): Report of a case and review
of the literature. Int J Surg Pathol. 23:317–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishizaki M, Uno F, Yoshida R, Miyauchi S
and Honda O: Very delayed liver metastasis from small bowel
gastrointestinal stromal tumor (32 years after resection of the
small bowel GIST): Report of a case. Int J Surg Case Rep.
76:156–160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whang IY, Seo KJ, Kim HY, Kim CW and Won
HS: A huge necrotic liver mass in a 45-year-old woman: Delayed
hepatic metastasis of a gastrointestinal stromal tumor. Korean J
Intern Med. 32:378–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masuoka H, Kawagishi N, Inoue T, Ohkohchi
N, Fujimori K, Koyamada N, Sekiguchi S, Tsukamoto S and Satomi S:
Giant hepatic metastasis from gastrointestinal stromal tumor of the
rectum 12 years after surgery. Hepatogastroenterology.
50:1454–1456. 2003.PubMed/NCBI
|
|
13
|
Nowain A, Bhakta H, Pais S, Kanel G and
Verma S: Isolated hepatic metastasis from a gastrointestinal
stromal tumor (GIST) 17 years after initial resection: Need for
long-term surveillance. J Clin Gastroenterol. 39:9252005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuoka L, Stapfer M, Mateo R, Jabbour N,
Naing W, Selby R and Gagandeep S: Left extended hepatectomy for a
metastatic gastrointestinal stromal tumor after a disease-free
interval of 17 years: Report of a case. Surg Today. 37:70–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cahill RA, Mutter D, Bailey C, Varela D,
Neuville A and Marescaux J: Primary resection of late, isolated
secondary GIST. J Clin Gastroenterol. 43:288–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossi U, Ardito F, Petracca Ciavarella L,
Goglia M and Giuliante F: Ultra-late recurrence of gastrointestinal
stromal tumour: Case report and literature review. ANZ J Surg.
89:E224–E225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demetri GD, Benjamin R, Blanke CD, Choi H,
Corless C, DeMatteo RP, Eisenberg BL, Fletcher CD, Maki RG, Rubin
BP, et al: NCCN Task Force report: Optimal management of patients
with gastrointestinal stromal tumor (GIST)-expansion and update of
NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2 (Suppl
1):S-1-26; quiz. 27–30. 2004.PubMed/NCBI
|
|
18
|
Sarlomo-Rikala M, Kovatich AJ,
Barusevicius A and Miettinen M: CD117: A sensitive marker for
gastrointestinal stromal tumors that is more specific than CD34.
Mod Pathol. 11:728–734. 1998.PubMed/NCBI
|
|
19
|
Casali PG, Blay JY, Abecassis N, Bajpai J,
Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JV,
et al: Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 33:20–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Pruitt A, Nicol TL, Gorgulu S and
Choti MA: Complete hepatic resection of metastases from
leiomyosarcoma prolongs survival. J Gastrointest Surg. 2:151–155.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeMatteo RP, Shah A, Fong Y, Jarnagin WR,
Blumgart LH and Brennan MF: Results of hepatic resection for
sarcoma metastatic to liver. Ann Surg. 234:540–547; discussion
547-8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wada N, Takahashi T, Kurokawa Y, Nakajima
K, Masuzawa T, Nakatsuka R, Kawada J, Nishida T, Kimura Y, Tanaka
K, et al: Appropriate follow-up strategies for gastrointestinal
stromal tumor patients based on the analysis of recurrent interval
and patterns. Digestion. 95:115–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagimoto Y, Takahashi T, Muguruma K,
Toyokawa T, Kusanagi H, Omori T, Masuzawa T, Tanaka K, Hirota S and
Nishida T: Re-appraisal of risk classifications for primary
gastrointestinal stromal tumors (GISTs) after complete resection:
Indications for adjuvant therapy. Gastric Cancer. 18:426–433. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Ambrosio L, Palesandro E, Boccone P,
Tolomeo F, Miano S, Galizia D, Manca A, Chiara G, Bertotto I, Russo
F, et al: Impact of a risk-based follow-up in patients affected by
gastrointestinal stromal tumour. Eur J Cancer. 78:122–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|