Introduction
Lung cancer is the second most common and most
lethal malignancy worldwide (1)
The development of targeted therapies against oncogenic driver
alterations of NSCLC, which affect several kinases, has
significantly improved outcomes of NSCLC, including for instance in
ALK+ mNSCLC (2).
Oncogenic rearrangements in the anaplastic lymphoma kinase gene
(ALK) account for 5% of non-small cell lung cancer (NSCLC)
(3–5).
ALK positive NSCLC represent 8% of all lung cancers
in never-smoker patients (6)
Treatment with an ALK inhibitor has significantly improved the
outcome of metastatic ALK positive NSCLC (ALK+ mNSCLC)
patients with median long term overall survival to 81 months
(7) Therefore, ALK inhibitors have
become a standard form of care in ALK+ mNSCLC (8). However, pregnant women are deprived
of using this drug, instructions of use stating that ALK inhibitors
are contraindicated during pregnancy (9).
Based on findings from animal studies and its
mechanism of action, alectinib can cause fetal harm when
administered to pregnant women. Administration of alectinib to
pregnant rats and rabbits during organogenesis period resulted in
embryo-fetal toxicity and abortion at maternally toxic doses with
exposures approximately 2.7 times of those observed in humans with
alectinib 600 mg twice daily. Fetal loss, low fetal weight and
malformations (vertebrae, thymic cord, ventricle, ureter and
subclavian artery) were reported (8). Effective contraception during
treatment and for several months after the last dose of ALK
inhibitors is thus recommended. Although a diagnosis of NSCLC
during pregnancy or the peripartum period is rare, ALK+
NSCLC accounts for 38% of NSCLC in women of childbearing age (18–45
years old) (10). Furthermore,
ALK+ NSCLC represent 12.5% of NSCLC diagnosed during
pregnancy or peripartum (10).
Younger age and prolonged survival of ALK+ mNSCLC bring
new challenges in this field, including questions associated with
pregnancy and family planning. We present the case of fetal
exposure to alectinib in an HIV infected patient diagnosed with
ALK+ mNSCLC.
Case report
An HIV infected, 24-year-old non-smoking patient was
diagnosed in April 2019 at the Clinique Saint-Pierre (Ottignies,
Belgium) with ALK+ mNSCLC cT2bN3M1c (axillary and
subdiaphragmatic nodes, no brain metastasis). Therapy with
alectinib 600 mg twice daily was initiated. The patient is treated
by Symtuza®, a combination of darunavir, cobicistat,
emtricitabine and tenofovir alafenamide. The HIV viral RNA blood
load was negative. The lung biopsy analysis identified an ALK
translocation, low expression of programmed death ligand 1 (PD-L1)
and no epidermal growth factor receptor (EGFR) mutation. Effective
contraception was recommended to prevent pregnancy during treatment
but the patient refused all forms of contraceptive method.
Her disease was stabilized after 6 months of
treatment without any adverse event, even in combination with the
retroviral treatment. Then, a pregnancy at 22 weeks of gestational
age was diagnosed. Alectinib treatment was continued despite a
formal contraindication. The patient was referred to a tertiary
center at 27 weeks and 5 days of pregnancy. A multidisciplinary
board consisting of medical oncologists, obstetricians and
pneumologists recommended stopping alectinib treatment until the
end of the pregnancy due to the teratogenic risks as well as the
risk of cognitive, psychological and psycho-motor development
disorders.
Fetal ultrasounds showed normal fetal growth and
morphology as well as placental aspect. Finally, the patient
decided to stop alectinib at 32 weeks of gestation.
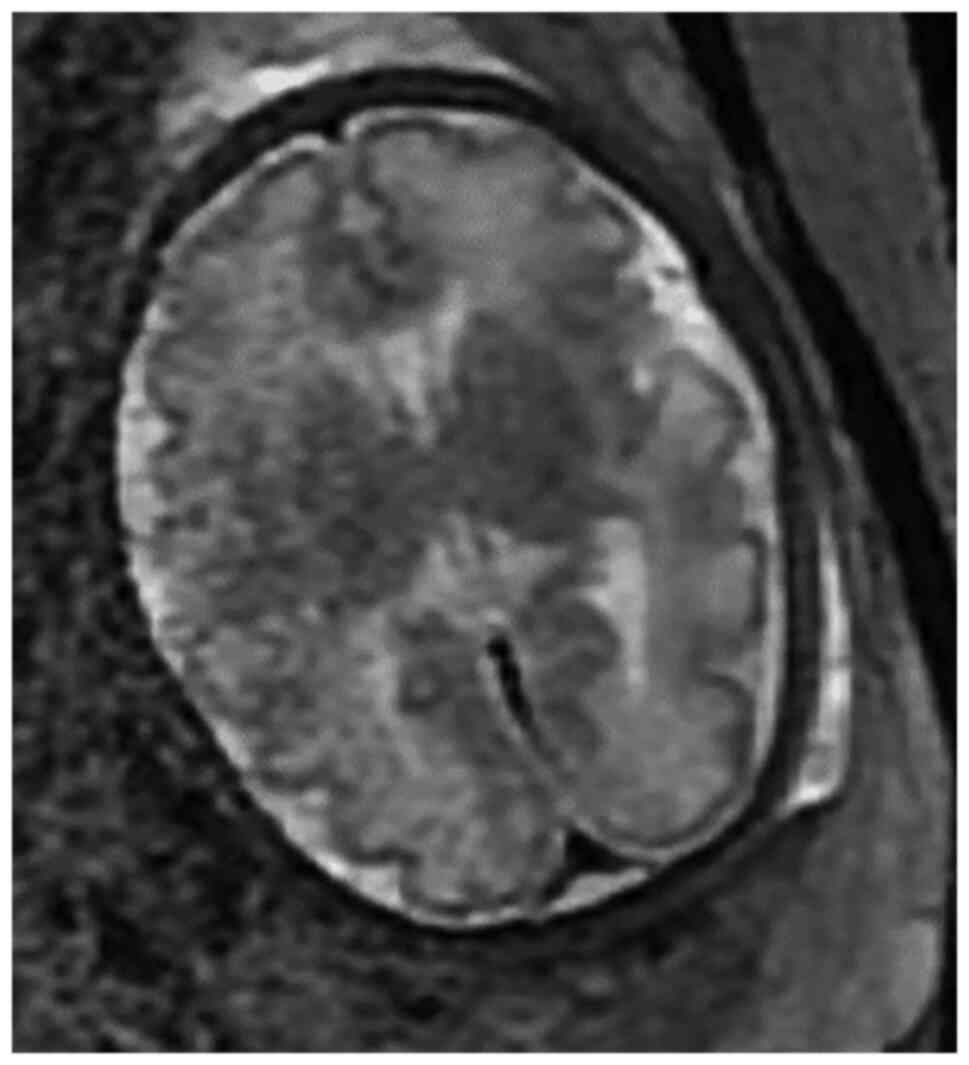
A fetal magnetic resonance imaging (MRI) at 32 weeks
did not show any developmental or cerebral abnormalities (Fig. 1).
Labor was induced at 36+6 weeks of gestation and a
eutrophic girl with an Apgar score of 7 at 5 min and 10 at 10 min
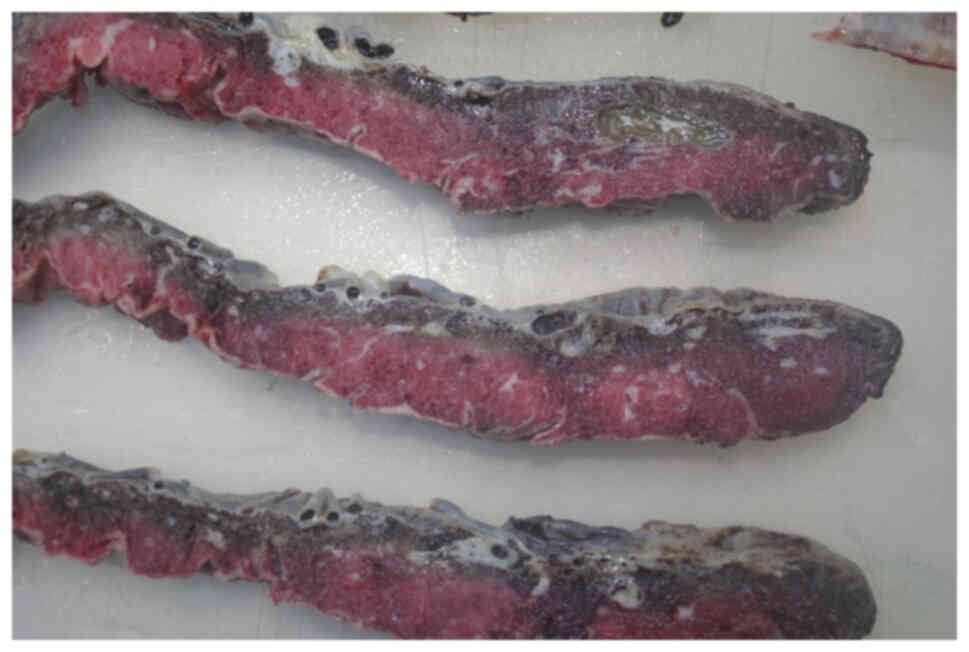
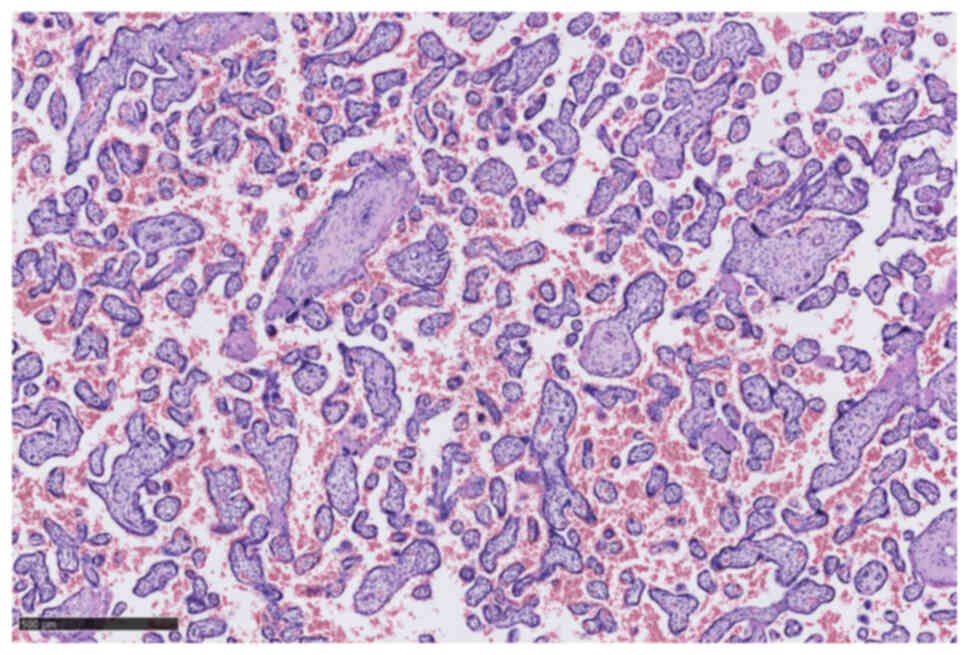
was born. The placenta histopathology did not show any mutations or
metastatic invasion by cancerous cells (Figs. 2 and 3). Neonatal HIV PCR-test was negative.
The child is being monitored on a regular basis for any behavioral
or cognitive delays.
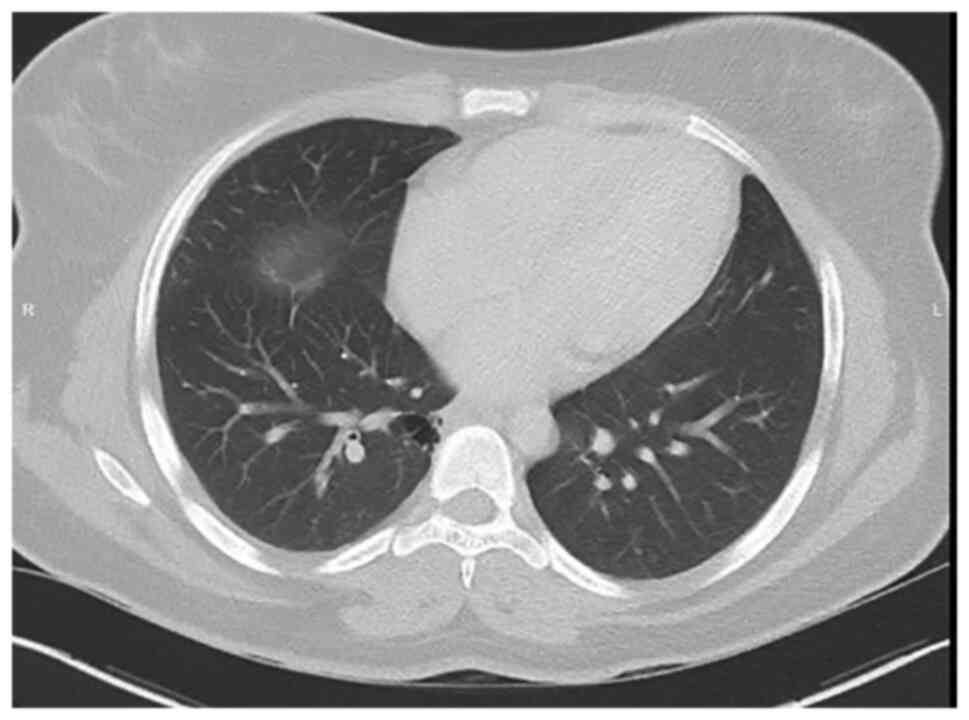
Alectinib treatment was reintroduced on the day
after the delivery. A CT scan (Fig.
4) and brain imaging showed stable disease. To this day, the
patient is tolerating the treatment well.
Discussion
We present a case of ALK+ mNSCLC lung
cancer in combination with unplanned pregnancy. The alectinib
treatment was started a few months before the pregnancy, resulting
in a good response and stability of the disease. It was
discontinued at 32 weeks. In absence of safe administration during
pregnancy, alectinib is not recommended during pregnancy. Lung
cancers are mostly treated by surgery, chemotherapy, radiotherapy
and systemic therapy (11,12).
Surgery is not uncommon during pregnancy and needs
close collaboration between surgeons, anesthesiologists,
gynecologists and pediatricians. Surgeries under local or general
anesthesia are performed during pregnancy, but non-emergent
procedures should be postponed until after the first trimester to
avoid miscarriages (13,14). Chemotherapy can be administered
after the first trimester of pregnancy (15). The short and long-term child
development, the cognitive behavior and the cardiac function seem
to be reassuring (16,17). Radiotherapy for curative or
palliative treatment is also feasible after the first trimester of
pregnancy while balancing the fetal and maternal risks (18). Immunotherapy affects the pregnant
woman's immune system, so that materno-fetal adverse outcomes may
occur. Therefore, it is not recommended during pregnancy and should
be discussed case by case (19).
Treatment by alectinib is nevertheless not recommended during
pregnancy because of rarity of cases.
Over the last years, cancer incidence among pregnant
women is increasing due to a high rate of smokers and an increased
maternal age (20–24). Although mild side-effects with this
targeted therapy are observed, pregnancy is discouraged considering
the fetal toxicity observed in preliminary animal studies.
Regarding the paucity of these cancers during pregnancy, these
treatments are currently not recommended (22).
Although a diagnosis of NSCLC during pregnancy or
the peripartum period is rare, ALK+ NSCLC represents 38% of NSCLC
diagnosis performed in women of childbearing age (10). Because pregnant women are excluded
from almost all clinical trials, available data on the teratogenic
risks of tyrosine kinase inhibitors including ALK inhibitor are
limited to case reports and animal studies.
The European Society for Medical Oncology (ESMO)
recommends close monitoring until the second trimester. If urgent
treatment is required, pregnancy termination should be discussed.
In the last trimester, preterm delivery or initiation of
carboplatin plus weekly dose of paclitaxel is therefore recommended
in the context of lung cancer (25). The young age of patients in the
context of prolonged survival of ALK+ mNSCLC raises new
questions associated with maternity and family planning of these
patients.
The ALK inhibitor may impact not only the fetal
development but also the cognitive (memory impairment, disturbance
in attention, and amnesia) and psychological development (anxiety,
depression, and affect lability) of the child (26). Only one other case of pregnancy
during an alectinib treatment has been described in the literature,
with a continuation of the treatment until delivery. The fetal and
child development until 20 months were normal and uneventful
(27). The balance between fetal
and maternal benefits and risks illustrates the dilemma faced in
this specific and rare situation. Breastfeeding was discouraged
according to drug maker recommendations. Other cases reports with
ALK+ lung tumors associated with pregnancy are
summarized in Table I.
 | Table I.Clinicopathologic features of
ALK+ MNSCLC lung cancers reported in literature. |
Table I.
Clinicopathologic features of
ALK+ MNSCLC lung cancers reported in literature.
| First author,
year | Lung tumor type and
diagnosis | Treatment during
pregnancy | Antineoplasic
treatment during pregnancy | Timing of
delivery | Fetal outcome | Maternal outcome | Follow-up in
years | (Refs.) |
|---|
| Present study | ALK+
NSCLC, diagnosis before pregnancy | Alectinib and HIV
treatment | Start of pregnancy
until 32 weeks | 36+6 weeks | Normal, 19
months | Alive | 1.6 | - |
| Scarfone, 2021 | ALK+ lung
adenocarcinoma diagnosis before pregnancy | Alectinib | Whole pregnancy | 35+5 weeks | Normal, 32
months | Alive, mother in
partial remission | 2.6 | (27) |
| Komura, 2018 | Lung adenocarinoma
ALK+ diagnosis after delivery | No | No treatment | 37 weeks | Normal, 12
months | Alive | 1 | (22) |
| Neves, 2014 | ALK+ lung
adenocarcinoma diagnosis at 27 weeks | Corticosteroid
injection for fetal lung maturation | No treatment | 29 weeks | Normal at 19
months | Progression-free
survival of 9 months | Died 19 months after
diagnosis | (23) |
| Sariman, 2013 | ALK+ lung
adenocarcinoma diagnosis after delivery | Antibiotics for
pneumonia | No treatment | 28 weeks | NA | Stable at 6
months | NA | (24) |
Our case also shows the importance of including the
patient in the treatment decision. Despite being advised of the
risks, the patient decided to become pregnant regardless of the
potential consequences for her and the child and to continue the
treatment until 32 weeks of pregnancy. This case illustrates a
normal pregnancy under ALK-inhibitor without fetal and placental
abnormalities and no relapse of the mother at one year after the
birth. Given the rarity of case reports and information on fetal
adverse effects, further analysis is needed to confirm these
observations and this positive feto-maternal development.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMG and FDS conceived the idea for this paper. MMG,
FDS, FD, LC, PB, FAN, PC and VB collected the data. FDS, MMG, LC,
PB, FAN and PC prepared, created and wrote the initial draft. MGM
drafted the figure. MMG, FDS, FD and FA analyzed the data. MMG, FDS
and FD confirm the authenticity of all the raw data. MMG, FDS,
FDLC, PB, FAN, PC, VB, FA and MMG wrote and reviewed the draft. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
the Cliniques Universitaires Saint-Luc, Brussels (Belgian number
2014/28Mar/142). Patient data were registered after written
informed consent.
Patient consent for participation
The patient consented to the data and images being
taken for the purpose of research and also consented to their
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Recondo G, Facchinetti F, Olaussen KA,
Besse B and Friboulet L: Making the first move in EGFR-driven or
ALK-driven NSCLC: First-generation or next-generation TKI? Nat Rev
Clin Oncol. 15:694–708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pacheco JM, Gao D, Smith D, Purcell T,
Hancock M, Bunn P, Robin T, Liu A, Karam S, Gaspar L, et al:
Natural History and Factors Associated with Overall Survival in
Stage IV ALK-Rearranged Non-small cell lung cancer. J Thorac Oncol.
14:691–700. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shokoohi A, Al-Hashami Z, Moore S, Pender
A, Wong SK, Wang Y, Leung B, Wu J and Ho C: Effect of targeted
therapy and immunotherapy on advanced nonsmall-cell lung cancer
outcomes in the real world. Cancer Med. 11:86–93. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cameron LB, Hitchen N, Chandran E, Morris
T, Manser R, Solomon BJ and Jordan V: Targeted therapy for advanced
anaplastic lymphoma kinase (ALK)-rearranged non-small cell
lung cancer. Cochrane Database Syst Rev. 1:CD0134532022.PubMed/NCBI
|
|
6
|
Bittner N, Ostoros G and Geczi L: New
treatment options for lung adenocarcinoma-in view of molecular
background. Pathol Oncol Res. 20:11–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishio M, Nakagawa K, Mitsudomi T,
Yamamoto N, Tanaka T, Kuriki H, Zeaiter A and Tamura T: Analysis of
central nervous system efficacy in the J-ALEX study of alectinib
versus crizotinib in ALK-positive non-small-cell lung cancer. Lung
Cancer. 121:37–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larkins E, Blumenthal GM, Chen H, He K,
Agarwal R, Gieser G, Stephens O, Zahalka E, Ringgold K, Helms W, et
al: FDA approval: Alectinib for the treatment of metastatic,
ALK-positive non-small cell lung cancer following crizotinib. Clin
Cancer Res. 22:5171–5176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paik J and Dhillon S: Alectinib: A review
in advanced, ALK-Positive NSCLC. Drugs. 78:1247–1257. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dagogo-Jack I, Gainor JF, Porter RL,
Schultz KR, Solomon BJ, Stevens S, Azzoli CG, Sequist LV, Lennes IT
and Shaw AT: Clinicopathologic features of NSCLC diagnosed during
pregnancy or the peripartum period in the era of molecular
genotyping. J Thorac Oncol. 11:1522–1528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boussios S, Han SN, Fruscio R, Halaska MJ,
Ottevanger PB, Peccatori FA, Koubková L, Pavlidis N and Amant F:
Lung cancer in pregnancy: Report of nine cases from an
international collaborative study. Lung Cancer. 82:499–505. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pavlidis N: Lung cancer during pregnancy:
An emerging issue. Lung Cancer. 59:279–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Calsteren K, Heyns L, De Smet F, Van
Eycken L, Gziri MM, Van Gemert W, Halaska M, Vergote I, Ottevanger
N and Amant F: Cancer during pregnancy: An analysis of 215 patients
emphasizing the obstetrical and the neonatal outcomes. J Clin
Oncol. 28:683–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moran BJ, Yano H, Al Zahir N and
Farquharson M: Conflicting priorities in surgical intervention for
cancer in pregnancy. Lancet Oncol. 8:536–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cardonick E and Iacobucci A: Use of
chemotherapy during human pregnancy. Lancet Oncol. 5:283–291. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amant F, Van Calsteren K, Halaska MJ,
Gziri MM, Hui W, Lagae L, Willemsen MA, Kapusta L, Van Calster B,
Wouters H, et al: Long-term cognitive and cardiac outcomes after
prenatal exposure to chemotherapy in children aged 18 months or
older: An observational study. Lancet Oncol. 13:256–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Haan J, Verheecke M, Van Calsteren K,
Van Calster B, Shmakov RG, Mhallem Gziri M, Halaska MJ, Fruscio R,
Lok CAR, Boere IA, et al: Oncological management and obstetric and
neonatal outcomes for women diagnosed with cancer during pregnancy:
A 20-year international cohort study of 1170 patients. Lancet
Oncol. 19:337–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stovall M, Blackwell CR, Cundiff J, Novack
DH, Palta JR, Wagner LK, Webster EW and Shalek RJ: Fetal dose from
radiotherapy with photon beams: Report of AAPM Radiation Therapy
Committee Task Group No. 36. Med Phys. 22:63–82. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borgers JSW, Heimovaara JH, Cardonick E,
Dierickx D, Lambertini M, Haanen JBAG and Amant F: Immunotherapy
for cancer treatment during pregnancy. Lancet Oncol. 22:e550–e561.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amant F, Han SN, Gziri MM, Vandenbroucke
T, Verheecke M and Van Calsteren K: Management of cancer in
pregnancy. Best Pract Res Clin Obstet Gynaecol. 29:741–753. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acosta Rojas A, Collazo-Lorduy A, Remon J,
Hernando Requejo O, Jiménez-Munarriz B, Rubio Rodríguez MC and De
Castro J: Lung Adenocarcinoma during Pregnancy: 11-Year Follow-Up.
Case Rep Oncol. 13:892–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komura M, Yagishita S, Nakamura K, Arano
N, Takeshige T, Muraki K, Nagashima O, Izumi H, Tomita S, Sasaki S
and Takahashi K: A case of a pregnant woman diagnosed as having
ALK-rearranged lung adenocarcinoma. In Vivo. 32:1205–1209. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neves I, Mota PC and Hespanhol VP: Lung
cancer during pregnancy: An unusual case. Rev Port Pneumol.
20:46–49. 2014.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sariman N, Levent E, Yener NA, Orki A and
Saygi A: Lung cancer and pregnancy. Lung Cancer. 79:321–323. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peccatori FA, Azim HA Jr, Orecchia R,
Hoekstra HJ, Pavlidis N, Kesic V and Pentheroudakis G; ESMO
Guidelines Working Group, : Cancer, pregnancy and fertility: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 (Suppl 6):vi160–vi170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Webb TR, Slavish J, George RE, Look AT,
Xue L, Jiang Q, Cui X, Rentrop WB and Morris SW: Anaplastic
lymphoma kinase: Role in cancer pathogenesis and small-molecule
inhibitor development for therapy. Expert Rev Anticancer Ther.
9:331–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scarfone G, Fumagalli M, Imbimbo M, Ceruti
T, Cribiù FM, Di Loreto E, D'Incalci M, Facchin F, Fontana C,
Garassino MC, et al: First case report of pregnancy on alectinib in
a woman with metastatic ALK-Rearranged lung cancer: A case report.
J Thorac Oncol. 16:873–877. 2021. View Article : Google Scholar : PubMed/NCBI
|