Introduction
Esophageal cancer (EC) is divided into two major
histological subtypes, squamous cell carcinoma (SCC) and
adenocarcinoma (AC), and is the eighth-most common type of tumor,
and the sixth leading cause of tumor-related death worldwide
(1). Esophageal squamous cell
carcinoma (ESCC) is the predominant subtype of EC in developing
eastern countries, including Turkey, Iran, Kazakhstan, and China.
The primary treatment approaches for ESCC include surgery,
radiotherapy, chemotherapy, targeted therapy, and multimodal
treatments (2–5). In China, 60–70% of patients with ESCC
are diagnosed with advanced-stage cancer, for whom surgery is no
longer possible (6).
Chemoradiotherapy with cisplatin- and 5-fluorouracil-based regimens
represent the standard mode of treatment for unresectable ESCC;
however, the overall survival (OS) times are <12 months
(7,8).
In the tumor microenvironment, stimulation and
inhibition of ligand-receptor interactions in macrophages,
dendritic cells, T-cells, and tumor cells regulate the activation
of T-cells as part of the immune defense against cancer (9). The ligand-receptor pairs that
negatively regulate T-cell activation, including cytotoxic
T-lymphocyte antigen 4 (CTLA4)-B7 and programmed death-1
(PD-1)-programmed death ligand 1 (PD-L1), are called ‘immune
checkpoints’ (10). Immune
checkpoint inhibitors (ICIs) have drastically improved the survival
rate of patients with several tumor types, including melanoma,
non-small cell lung cancer (NSCLC), renal cell cancer, ovarian
cancer, and gastrointestinal tract cancers (11–16).
Patients with high PD-L1 expression appear to benefit more from ICI
treatment for certain types of cancer (11,15).
The randomized phase III KEYNOTE-181 study on advanced EC
demonstrated that pembrolizumab monotherapy significantly improved
the objective response rate (ORR), disease control rate (DCR), and
OS in patients with PD-L1-positive (combined positive score ≥10) EC
as the second-line treatment (16). Clinical benefit has also been found
for pembrolizumab combined with chemotherapy as the first-line
treatment in patients with ESCC subtype owing to increased PD-L1
expression (17).
More than half of patients with cancer receiving ICI
treatment develop immune-related adverse events (irAEs), the
mechanisms of which depend on the type of ICIs used (18,19).
In addition to eliciting autoantibody formation by inducing a
cross-reaction between anti-tumor T-cells and healthy cell
antigens, CTLA-4 inhibitors can initiate the activation and
proliferation of T-cells, thereby impairing the survival of
regulatory T-cells (Tregs). PD-1 and PD-L1 inhibitors can reduce
the number and inhibit the function of Tregs by increasing cytokine
production (20). Multiple organ
injuries participate in the development of irAEs, including skin
reactions, hypothyroidism, pneumonitis, hepatitis, myositis,
adrenal insufficiency, and myocardial damage, because the T-cell
immune response is not tissue-specific (10,18).
Recently, irAEs have been shown to be positively
correlated with the efficacy of ICIs in patients with NSCLC and
hepatocellular carcinoma (21,22).
Therefore, here, the correlation between irAEs and ICI therapeutic
efficacy based on anti-PD-1 antibodies in patients with ESCC was
evaluated.
Materials and methods
Patients
Patients with ESCC treated with at least one cycle
of anti-PD-1 antibodies (monotherapy or combination therapy),
regardless of the treatment line, between October 2018 and May 2022
in the Fourth Hospital of Hebei Medical University were included in
this analysis. Patients who were alive and progression-free were
censored at the last follow-up date (September 30, 2022). Patients
who had previously received immunotherapy were excluded. The
following clinical data were collected: sex, age, Eastern
Cooperative Oncology Group performance status (ECOG PS) (23), treatment line number, stage of
disease (TNM), metastasis, history of radiotherapy or surgery, and
concurrent therapy.
Treatment and assessment
The patients were treated with standard anti-PD-1
antibodies (monotherapy or combined with chemotherapy, targeted
medicine, or radiotherapy) in a three-week cycle until disease
progression, unacceptable toxicity, clinical deterioration, or
patient rejection was observed. The anti-PD-1 antibody treatment
included toripalimab at a dose of 240 mg every 2 weeks as well as
sintilimab, camrelizumab, and pembrolizumab at a dose of 200 mg
every 3 weeks; the dose of combination chemotherapy drugs, target
drugs, and radiotherapy was adjusted by the clinicians according to
the guidelines of the Chinese Society of Clinical Oncology based on
the age, PS score, and degree of tolerance of the patients
(24). Computed tomography (CT),
magnetic resonance imaging (MRI), or endoscopy assessment were
repeated every 2 or 3 cycles to evaluate the objective tumor
response based on the New Response Evaluation Criteria in Solid
Tumours: revised RECIST guideline (version 1.1) (25). IrAEs were defined as inflammatory
side effects caused by an imbalance in immunological tolerance upon
ICI treatment. The National Cancer Institute Common Terminology
Criteria for Adverse Events ver. 4.03 was used for evaluating the
irAEs (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40).
We assigned patients to irAE and non-irAE groups based on the
occurrence of irAEs. Common Terminology Standard for Adverse Events
with a scale from grade 1 to grade 5 (1=mild, 2=moderate, 3=severe,
4=life-threatening, and 5=death associated with toxicity) was used
to grade irAEs.
Statistical analysis
A χ2 test was used to compare the
difference between the two groups of classified variables.
Continuous data presented as medians (ranges) were analyzed using a
Mann-Whitney U test. Progression-free survival (PFS) was defined as
the time from the start of immunotherapy to disease progression or
death from any cause. OS was defined as the time from the start of
immunotherapy to death or censoring at the latest follow-up in
surviving patients. Survival probability was estimated using the
Kaplan-Meier approach using a log-rank test. The Cox proportional
hazards regression model was used for univariate and multivariate
analyses. All statistical data were analyzed using SPSS (SPSS 21.0;
IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In total, 82 patients with ESCC were included in
this analysis, out of which four patients were lost to follow-up
and 46 (59.0%) of the remaining 78 patients died during the
follow-up period. The clinical characteristics of patients in the
irAE and non-irAE groups showed no significant differences
(Table I). The median OS and PFS
for all patients were 600 days [95% confidence interval (CI),
518–682 days] and 300 days (95% CI, 191–409 days), respectively.
Partial response (PR) was achieved in eight patients, whereas
stable disease (SD) was observed in 15 patients, which led to an
ORR of 10.3% (95% CI, 5.7-17.8%) and a DCR of 29.5% (95% CI,
21.5-39.1%) (Table II).
 | Table I.Characteristics of patients in the
irAEs and non-irAE groups. |
Table I.
Characteristics of patients in the
irAEs and non-irAE groups.
| Factor | Total, n (%) | non-irAE, n (%) | irAE, n (%) | P-value |
|---|
| Total patients | 78 | 39 | 39 |
|
| Sex |
|
|
|
|
|
Female | 28 (35.9) | 17 (43.6) | 22 (56.4) | 0.157a |
| Male | 50 (64.1) | 22 (56.4) | 28 (71.8) |
|
| Age, years |
|
|
|
|
|
<65 | 32 (41.0) | 16 (41.0) | 16 (41.0) | 1.000a |
| ≥65 | 46 (59.0) | 23 (59.0) | 23 (59.0) |
|
| ECOG PS |
|
|
|
|
| ≤1 | 66 (84.6) | 36 (92.3) | 30 (76.9) | 0.060a |
|
>1 | 12 (15.4) | 3 (7.7) | 9 (23.1) |
|
| Treatment line |
|
|
|
|
| ≤1 | 37 (47.4) | 16 (41.0) | 21 (53.8) | 0.257a |
| ≥2 | 41 (52.6) | 23 (59.0) | 16 (41.0) |
|
| TNM |
|
|
|
|
|
≤III | 28 (36.4) | 11 (28.2) | 17 (44.7) | 0.132a |
| IV | 49 (63.6) | 28 (71.8) | 21 (55.3) |
|
| Combined
chemotherapy or targeted therapy |
|
|
|
|
| No | 7 (9.0) | 2 (5.1) | 5 (12.8) | 0.428b |
|
Yes | 71 (91.0) | 37 (94.9) | 34 (87.2) |
|
| Combined
radiotherapy |
|
|
|
|
| No | 40 (51.3) | 16 (41.0) | 24 (61.5) | 0.070a |
|
Yes | 38 (48.7) | 23 (59.0) | 15 (38.5) |
|
| Postoperative
recurrence |
|
|
|
|
| No | 62 (79.5) | 33 (84.6) | 29 (74.4) | 0.262a |
|
Yes | 16 (20.5) | 6 (15.4) | 10 (25.6) |
|
| Metastasis |
|
|
|
|
| No | 17 (21.8) | 8 (20.5) | 9 (23.1) | 0.784a |
|
Yes | 61 (78.2) | 31 (79.5) | 30 (76.9) |
|
 | Table II.Response to immunotherapy. |
Table II.
Response to immunotherapy.
| Response | Total | irAE group | Non-irAE group | P-value |
|---|
| Progressive
disease, n | 55 | 22 | 33 | - |
| Stable disease,
n | 15 | 11 | 4 | - |
| Partial response,
n | 8 | 6 | 2 | - |
| Complete response,
n | 0 | 0 | 0 | - |
| Objective | 10.3% | 15.4% | 5.1% | 0.263b |
| response rate | (95% CI,
5.7-17.8) | (95% CI,
9.6-23.7) | (95% CI,
2.2-11.3) |
|
| Disease | 29.5% | 43.6% | 15.4% | 0.006a,c |
| control rate | (95% CI,
21.5-39.1) | (95% CI,
34.3-53.4) | (95% CI,
9.6-23.7) |
|
Comparison between irAE and non-irAE
groups
An ORR of 15.4% (95% CI, 9.6-23.7%) and a DCR of
43.6% (95% CI, 34.3-53.4%) was found for all 39 irAEs patients (6
PR, 11 SD), whereas the 39 patients in the non-irAE group (2 PR, 4
SD) achieved an ORR of 5.1% (95% CI, 2.2-11.3%) and a DCR of 15.4%
(95% CI, 9.6-23.7%). The DCR in the irAE group was higher than that
in the non-irAE group (P=0.006; Table
II).
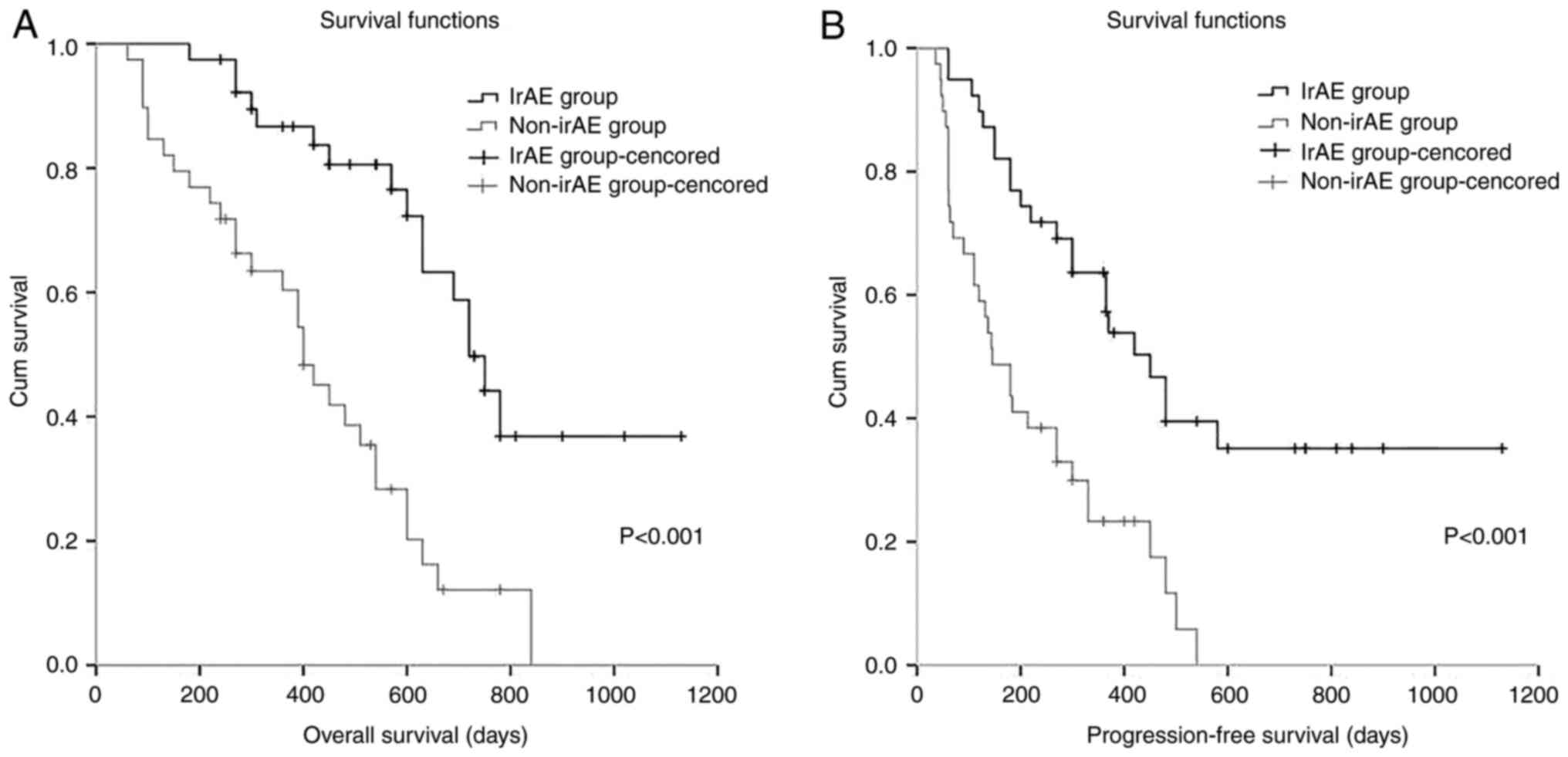
The median OS and PFS of the irAE and non-irAE
groups were calculated using the Kaplan-Meier method. The median OS
(P<0.001) and PFS (P<0.001) in the irAE group were higher
than those in the non-irAE group (Fig.
1). In the univariate analysis for OS and PFS with ECOG score,
treatment line number, TNM stage, therapy plan, postoperative
recurrence status, metastasis status, and irAE status as
covariates, only the irAE status displayed its association with OS
[hazard ratio (HR)=3.687, 95% CI, 1.974-6.888, P<0.001] and PFS
(HR=2.967, 95% CI, 1.691-5.204, P<0.001) at a significant level,
and irAEs were linked to relatively longer PFS and OS. TNM stage
also showed a trend for association with OS (HR=1.718, 95% CI,
0.918-3.214, P=0.090). There were no Stage I patients, although
there were eight Stage II patients; therefore Stage I, II, and III
patients were included in one group and Stage IV patients in a
separate group for comparison. Subsequent multivariate analysis
showed that the irAE status was an independent predictor of OS and
PFS (OS: HR=3.288, 95% CI, 1.636-6.606, P=0.001; PFS: HR=3.564, 95%
CI, 1.786-7.114, P<0.001) (Table
III). These data indicate that irAEs could extend the PFS and
OS of patients with ESCC.
 | Table III.Univariate and multivariate analyses
of OS and PFS with Cox regression models. |
Table III.
Univariate and multivariate analyses
of OS and PFS with Cox regression models.
|
| OS | PFS |
|---|
|
|
|
|
|---|
|
| Univariate analysis
(n=54) | Multivariate
analysis (n=54) | Univariate analysis
(n=54) | Multivariate
analysis (n=54) |
|---|
|
|
|
|
|
|
|---|
| Covariate | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Group |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
irAE | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
|
Non-irAE | 3.687 | 1.974 | 6.888 | <0.001 | 3.288 | 1.636 | 6.606 | 0.001 | 2.967 | 1.691 | 5.204 | <0.001 | 3.564 | 1.786 | 7.114 |
<0.001a |
| ECOG PS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≤1 | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
| 1 | 0.689 | 0.291 | 1.629 | 0.396 | 0.565 | 0.206 | 1.549 | 0.267 | 0.939 | 0.460 | 1.920 | 0.864 | 0.976 | 0.394 | 2.421 | 0.959 |
| Treatment line |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≤1 | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
| ≥2 | 1.646 | 0.882 | 3.072 | 0.117 | 2.127 | 1.032 | 4.382 | 0.041 | 1.352 | 0.791 | 2.310 | 0.270 | 1.763 | 0.928 | 3.350 | 0.083 |
| TNM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≤III | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
| IV | 1.718 | 0.918 | 3.214 | 0.090 | 2.372 | 1.121 | 5.019 | 0.024 | 1.510 | 0.883 | 2.583 | 0.132 | 1.541 | 0.811 | 2.927 | 0.187 |
| Combine
chemotherapy or targeted therapy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
|
Yes | 1.557 | 0.554 | 4.376 | 0.401 | 1.286 | 0.418 | 3.954 | 0.661 | 1.931 | 0.694 | 5.373 | 0.207 | 1.263 | 0.429 | 3.719 | 0.671 |
| Combine
radiotherapy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
|
Yes | 1.303 | 0.727 | 2.337 | 0.374 | 1.101 | 0.566 | 2.142 | 0.777 | 0.893 | 0.526 | 1.518 | 0.676 | 0.667 | 0.360 | 1.236 | 0.198 |
| Postoperative
recurrence |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
|
Yes | 1.079 | 0.547 | 2.126 | 0.827 | 0.973 | 0.451 | 2.100 | 0.945 | 1.096 | 0.577 | 2.081 | 0.781 | 1.304 | 0.642 | 2.650 | 0.463 |
| Metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | Reference |
|
|
| Reference |
|
|
| Reference |
|
|
| Reference |
|
|
|
|
Yes | 1.348 | 0.649 | 2.800 | 0.423 | 0.958 | 0.431 | 2.129 | 0.916 | 1.813 | 0.885 | 3.712 | 0.104 | 1.334 | 0.620 | 2.870 | 0.461 |
Toxicity
The median time to irAE onset was 76 days (range:
5–570 days). Grade 3 irAEs were observed in eight patients and
grade 4 irAEs were observed in one patient (Table IV). Three patients discontinued
ICI treatment owing to the development of a grade 3 rash, grade 4
cutaneous capillary hyperplasia, and grade 3 myocardial damage.
Cutaneous capillary hyperplasia (n=15) was the most frequent
adverse event reported followed by hypothyroidism (n=12).
 | Table IV.Categorization of irAEs. |
Table IV.
Categorization of irAEs.
| irAE | No. (%) | Median days to
onset | Grade of irAEs, n,
1/2/3/4/5 |
|---|
| Hypothyroidism | 12 (30.8) | 155 | 3/9/0/0/0 |
| Cutaneous capillary
hyperplasia | 15 (38.5) | 60 | 3/10/1/1/0 |
| Rash | 8 (20.5) | 52 | 2/4/2/0/0 |
| Pneumonia | 1 (2.6) | 100 | 0/1/0/0/0 |
| Adrenal
insufficiency | 2 (5.1) | 70 | 0/0/2/0/0 |
|
Myositis/myocarditis | 3 (7.7) | 23 | 0/2/1/0/0 |
| AST/ALT/Bilirubin
increased | 2 (5.1) | 37 | 0/1/1/0/0 |
| Colitis | 1 (2.6) | 76 | 0/1/0/0/0 |
| Hypophysitis | 1 (2.6) | 156 | 0/1/0/0/0 |
| Type 1
Diabetes | 1 (2.6) | 50 | 0/0/1/0/0 |
Patients with irAEs were stratified into an irAE-A
group (patients with endocrine and cutaneous irAEs) and an irAE-B
group (patients with other irAEs) for survival analysis. The median
OS and PFS times in the irAE-A group were longer than those in the
non-irAE group (median OS: 720 vs. 400 days, P<0.001; median
PFS: 480 vs. 145 days, P<0.001), while no significant
differences were found in the median OS (P=0.080) and PFS (P=0.085)
between the irAE-B and the non-irAE groups (Table V). In addition, eight of the 39
patients with irAEs developed grade 3 irAEs, one developed grade 4
irAEs, and none had grade 5 irAEs. The 9 patients with grade 3 and
higher irAEs were assigned to group irAE-C, and the other 30
patients with grade 1 and 2 irAEs were assigned to group irAE-D.
There was no significant difference in the median OS and PFS
between these two groups (Fig.
S1). Of the 39 patients with irAEs, six developed ≥1 type of
irAE, and these were stratified into ‘single-site’ and
‘multiple-site’ groups; there was also no significant difference in
their median OS and PFS (Fig.
S2). No significant differences were found in the median OS and
PFS between patients whose irAE onset was within 90 days and those
with an onset >90 days (Table
SI).
 | Table V.Kaplan-Meier survival curve of OS and
PFS. |
Table V.
Kaplan-Meier survival curve of OS and
PFS.
| Comparison | Median OS,
days | P-value | Median PFS,
days | P-value |
|---|
| irAE group vs.
non-irAE group |
|
|
|
|
| irAE
group | 720 (95% CI:
639–801) |
<0.001a | 450 (95% CI:
340–560) |
<0.001a |
|
Non-irAE group | 400 (95% CI:
320–480) |
| 145 (95% CI:
86–204) |
|
| irAE-A group vs.
non-irAE group |
|
|
|
|
| irAE-A
group | 720 (95% CI:
637–803) |
<0.001a | 480 (95% CI:
351–609) |
<0.001a |
|
Non-irAE group | 400 (95% CI:
320–480) |
| 145 (95% CI:
86–204) |
|
| irAE-B group vs.
non-irAE group |
|
|
|
|
| irAE-B
group | - | 0.080 | 300 (95% CI:
161–439) | 0.085 |
|
Non-irAE group | 400 (95% CI:
320–480) |
| 145 (95% CI:
86–204) |
|
Discussion
In the present study, it was confirmed that irAEs
are concordantly correlated with a higher DCR, longer PFS, and
longer OS in patients with ESCC undergoing immunotherapy, which was
comparable with the treatment efficiency for irAEs in other types
of cancer including melanoma, head and neck squamous cell
carcinoma, NSCLC, renal cell carcinoma, and urothelial carcinoma
(26–28). The sample size was small in the
present study; however, cases where irAEs are associated with
better treatment efficacy of ICIs still exist even after adjustment
for other prognostic factors by multiple analyses. The KEYNOTE-590
clinical trial enrolled patients with unresectable locally advanced
(TNM stage III) or metastatic EC who were treated with a first-line
treatment. The results showed that pembrolizumab combined with
chemotherapy was significantly superior to chemotherapy alone
regarding OS, PFS, ORR, and DOR (29). The patients with TNM stage III in
the present study included a subset of patients with first-line
unresectable locally advanced disease as well as a subset of older
patients or patients with stage II ESCC who could not tolerate
surgery.
IrAEs are induced by a non-specifically activated
immune system involving almost any organ system. Cutaneous,
gastrointestinal, pulmonary, endocrine, and musculoskeletal irAEs
are common, whereas renal, hematological, neurological,
cardiovascular, and ophthalmological irAEs occur less frequently
(30,31). Dermatological, endocrine, and
gastrointestinal irAEs are associated with a favorable prognosis,
whereas other irAEs are not (32).
Here, it was similarly found that endocrine and dermatological
irAEs were associated with a favorable prognosis in patients with
ESCC. Previous reports on NSCLC indicated that ‘single-site’ irAEs
are associated with relatively better clinical results (ORR, PFS,
and OS) when compared with ‘multi-site’ irAEs (19); however, there was no significant
difference in the data of patients with ESCC in the present study.
The incidence of grade 3 and 4 irAEs in the present study was lower
than that reported in other studies, which may be attributed to our
comprehensive baseline examination, close monitoring, and early
treatment of irAEs (18,33). Since irAEs predicted better ECSS
treatment efficiency, the prevention of fatal irAEs should be
prioritized over all irAEs, thereby making ICI therapy efficient
and uninterrupted.
The mutual effect of PD-1 with its ligands PD-L1 and
PD-L2, which generates negative costimulatory signals, can weaken
T-cell activation via tyrosine phosphatase 2, thereby facilitating
the immune escape of tumor cells. ICIs can block the binding of
PD-1 and PD-L1 to enhance T-cell activation and kill tumors in the
tumor microenvironment (9). IrAEs
develop owing to the destruction of autoimmune tolerance, which is
at least partly mediated by antigen-specific T-cell responses.
Activated T-cells can initiate a series of inflammatory reactions
in multiple organs to induce irAEs when they recognize and kill
tumor cells. Despite the unclear pathogenesis of immune toxicity,
the inflammatory toxicity induced by activated CD8 T-cells overlaps
with the immunotherapeutic effects induced by activated CD8 T-cells
(30,34). This may explain the enhanced
treatment efficiency for irAEs in patients with ESCC to a certain
extent.
The present study has several limitations. First,
the study was conducted in only one center with a small sample
size. Second, this study had a short follow-up period, and several
patients did not reach the point of death; therefore, these
patients will continue to be followed up. Third, we could not
exclude the effects of combination therapy, such as radiotherapy,
chemotherapy, and target therapy. However, this is the first study
to indicate a relationship between irAEs and efficacy in treating
patients with ESCC, to the best of our knowledge. Thus, the
findings of this pilot study provide a foundation for future
studies with larger sample sizes.
In conclusion, the present study demonstrated that
the occurrence of irAEs is concordantly correlated with a
relatively higher DCR, longer PFS, and longer OS in patients with
ESCC undergoing ICI treatment, and that irAEs may serve as
biomarkers for predicting improved treatment efficacy for ICIs in
patients with ECSS.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
China of Hebei (grant no. H2019206428).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FY and JL designed the study. YLL, SX, and CZL were
responsible for collecting the clinical data of the patients. RJC
and CSW analyzed the data and wrote the manuscript. RJC, JL and FY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Fourth Hospital of Hebei
Medical University (Shijiazhuang, China) reviewed and approved the
research protocol (approval no. 2021136).
Patient consent for publication
All patients provided informed consent prior to
inclusion.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uhlenhopp DJ, Then EO, Sunkara T and
Gaduputi V: Epidemiology of esophageal cancer: Update in global
trends, etiology and risk factors. Clin J Gastroenterol.
13:1010–1021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domper Arnal MJ, Ferrández Arenas Á and
Lanas Arbeloa Á: Esophageal cancer: Risk factors, screening and
endoscopic treatment in western and eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Q, Yu J and Meng X: A good start of
immunotherapy in esophageal cancer. Cancer Med. 8:4519–4526. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borggreve AS, Kingma BF, Domrachev SA,
Koshkin MA, Ruurda JP, van Hillegersberg R, Takeda FR and Goense L:
Surgical treatment of esophageal cancer in the era of multimodality
management. Ann N Y Acad Sci. 1434:192–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe M, Otake R, Kozuki R, Toihata T,
Takahashi K, Okamura A and Imamura Y: Recent progress in
multidisciplinary treatment for patients with esophageal cancer.
Surg Today. 50:12–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Fang W, Yu Z, Mao Y, Chen L, He J,
Rong T, Chen C, Chen H, Chen K, et al: Chinese expert consensus on
mediastinal lymph node dissection in esophagectomy for esophageal
cancer (2017 edition). J Thorac Dis. 10:2481–2489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirano H and Boku N: The current status of
multimodality treatment for unresectable locally advanced
esophageal squamous cell carcinoma. Asia Pac J Clin Oncol.
14:291–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park R, Williamson S, Kasi A and Saeed A:
Immune therapeutics in the treatment of advanced gastric and
esophageal cancer. Anticancer Res. 38:5569–5580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan S and Gerber DE: Autoimmunity,
checkpoint inhibitor therapy and immune-related adverse events: A
review. Semin Cancer Biol. 64:93–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Wang P and Pang Q: Immune
checkpoint inhibitors for esophageal squamous cell carcinoma: A
narrative review. Ann Transl Med. 8:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Long-term outcomes with nivolumab plus ipilimumab
or nivolumab alone versus ipilimumab in patients with advanced
melanoma. J Clin Oncol. 40:127–137. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rathmell WK, Rumble RB, Van Veldhuizen PJ,
Al-Ahmadie H, Emamekhoo H, Hauke RJ, Louie AV, Milowsky MI, Molina
AM, Rose TL, et al: Management of metastatic clear cell renal cell
carcinoma: ASCO guideline. J Clin Oncol. 40:2957–2995. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng J, Li H, Yu X, Yu S, Chen K, Pan G,
Xie M, Li N, Zhou Z and Fan Y: Efficacy of PD-1/PD-L1 inhibitors in
patients with non-small cell lung cancer and brain metastases: A
real-world retrospective study in China. Thorac Cancer.
12:3019–3031. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Awada A, Ahmad S, McKenzie ND and Holloway
RW: Immunotherapy in the treatment of platinum-resistant ovarian
cancer: Current perspectives. Onco Targets Ther. 15:853–866. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SY, Yang CY, Liao BC, Ho CC, Liao WY,
Chen KY, Tsai TH, Hsu CL, Hsu WH, Su KY, et al: Tumor PD-L1
expression and clinical outcomes in advanced-stage non-small cell
lung cancer patients treated with nivolumab or pembrolizumab:
Real-world data in Taiwan. J Cancer. 9:1813–1820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chennamadhavuni A, Abushahin L, Jin N,
Presley CJ and Manne A: Risk factors and biomarkers for
immune-related adverse events: A practical guide to identifying
high-risk patients and rechallenging immune checkpoint inhibitors.
Front Immunol. 13:7796912022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cortellini A, Chiari R, Ricciuti B, Metro
G, Perrone F, Tiseo M, Bersanelli M, Bordi P, Santini D, Giusti R,
et al: Correlations between the immune-related adverse events
spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients.
Clin Lung Cancer. 20:237–247.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suárez-Almazor ME: Immune-related adverse events of
checkpoint inhibitors. Nat Rev Dis Primers. 6:382020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu S, Lai R, Zhao Q, Zhao P, Zhao R and
Guo Z: Correlation between immune-related adverse events and
prognosis in hepatocellular carcinoma patients treated with immune
checkpoint inhibitors. Front Immunol. 12:7940992021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toi Y, Sugawara S, Kawashima Y, Aiba T,
Kawana S, Saito R, Tsurumi K, Suzuki K, Shimizu H, Sugisaka J, et
al: Association of immune-related adverse events with clinical
benefit in patients with advanced non-small-cell lung cancer
treated with nivolumab. Oncologist. 23:1358–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mischel AM and Rosielle DA: Eastern
cooperative oncology group performance status #434. J Palliat Med.
25:508–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Health Commission Of The People's
Republic Of China, . Chinese guidelines for diagnosis and treatment
of esophageal carcinoma 2018 (English version). Chin J Cancer Res.
31:223–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suo A, Chan Y, Beaulieu C, Kong S, Cheung
WY, Monzon JG, Smylie M, Walker J, Morris D and Cheng T:
Anti-PD1-induced immune-related adverse events and survival
outcomes in advanced melanoma. Oncologist. 25:438–446. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaddepally R, Doddamani R, Sodavarapu S,
Madam NR, Katkar R, Kutadi AP, Mathew N, Garje R and Chandra AB:
Review of immune-related adverse events (irAEs) in non-small-cell
lung cancer (NSCLC)-their incidence, management, multiorgan irAEs,
and rechallenge. Biomedicines. 10:7902022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ricciuti B, Genova C, De Giglio A,
Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi
F and Chiari R: Impact of immune-related adverse events on survival
in patients with advanced non-small cell lung cancer treated with
nivolumab: long-term outcomes from a multi-institutional analysis.
J Cancer Res Clin Oncol. 145:479–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato K, Shah MA, Enzinger P, Bennouna J,
Shen L, Adenis A, Sun JM, Cho BC, Özgüroğlu M, Kojima T, et al:
KEYNOTE-590: Phase III study of first-line chemotherapy with or
without pembrolizumab for advanced esophageal cancer. Future Oncol.
15:1057–1066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the society for
immunotherapy of cancer (SITC) toxicity management working group. J
Immunother Cancer. 5:952017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okiyama N and Tanaka R: Immune-related
adverse events in various organs caused by immune checkpoint
inhibitors. Allergol Int. 71:169–178. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou X, Yao Z, Yang H, Liang N, Zhang X
and Zhang F: Are immune-related adverse events associated with the
efficacy of immune checkpoint inhibitors in patients with cancer? A
systematic review and meta-analysis. BMC Med. 18:872020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sangro B, Chan SL, Meyer T, Reig M,
El-Khoueiry A and Galle PR: Diagnosis and management of toxicities
of immune checkpoint inhibitors in hepatocellular carcinoma. J
Hepatol. 72:320–341. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weinmann SC and Pisetsky DS: Mechanisms of
immune-related adverse events during the treatment of cancer with
immune checkpoint inhibitors. Rheumatology (Oxford). 58 (Suppl
7):vii59–vii67. 2019. View Article : Google Scholar : PubMed/NCBI
|