Introduction
The first study on non-small cell lung cancer
(NSCLC) with the double expression of P40 and thyroid transcription
factor-1 (TTF-1) was reported by Pelosi et al (1) in 2015. Since then, only a few studies
on this topic have been published (2–5). In
these studies, immunohistochemistry (IHC) revealed the expression
of P40 and TTF-1 in the same tumor cells, while the ultrastructural
examination of the cells showed adenoid and squamous
differentiation characteristics (1,4). The
histological features were consistent with those of NSCLC. In all
cases, the high-grade tumor cells presented in solid nests with a
lamellar distribution, and exhibited distinct atypia, a high
nucleoplasmic ratio and mitotic figures; some were necrotic and
some exhibited squamous differentiation, adenoid differentiation
and peripheral palisade structures. The gene mutations accompanying
this disease vary; all six reported cases had a TP53 mutation or
genetic polymorphism, including epidermal growth factor receptor
gene (EGFR) (4), KRAS (1), PTEN (2) and neurofibromin 1 (NF1) (4) mutations, and fibroblast growth factor
receptor 1 (FGFR1) (1)
amplification with programmed death ligand 1 (PD-L1) (3) expression. Treatments administered
include surgery, radiotherapy, chemotherapy and targeted therapy,
the effects of which were inconsistent.
The present article documents a case of lung
adenosquamous carcinoma (LASC), a subtype of NSCLC with P40 and
TTF-1 double expression and echinoderm microtubule-associated
protein-like 4-anaplastic lymphoma kinase (EML4-ALK) and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a
(PIK3CA) gene mutations. This case highlights that this tumor cell
can exhibit the characteristics of adenosquamous differentiation at
the cytological level, which differs from the current definition of
LASC recommended by the World Health Organization (WHO) (6).
Case report
A 38-year-old male patient with no history of
smoking was admitted to the First People's Hospital of Xiaoshan
District (Hangzhou, China) on June 24, 2019 with a cough and
expectoration that had persisted for 4 months and become aggravated
during the last month. On physical examination post-admission, the
patient had a respiratory rate of 18 breaths/min and the lymph
nodes on both sides of the clavicle were palpable; the larger nodes
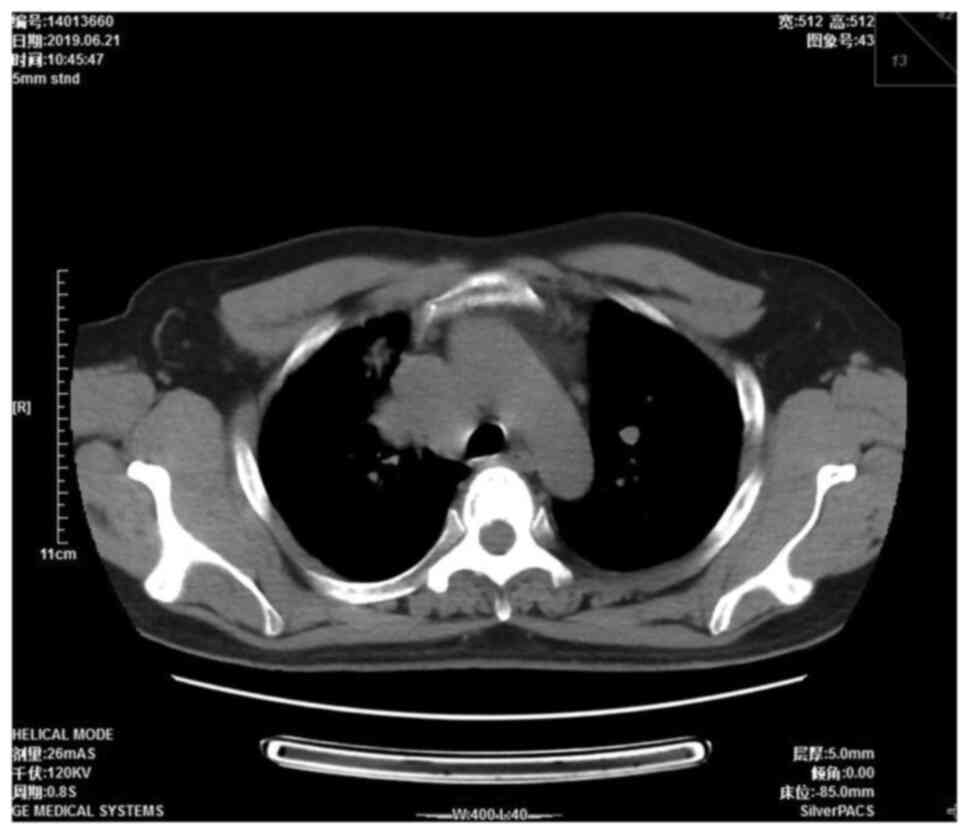
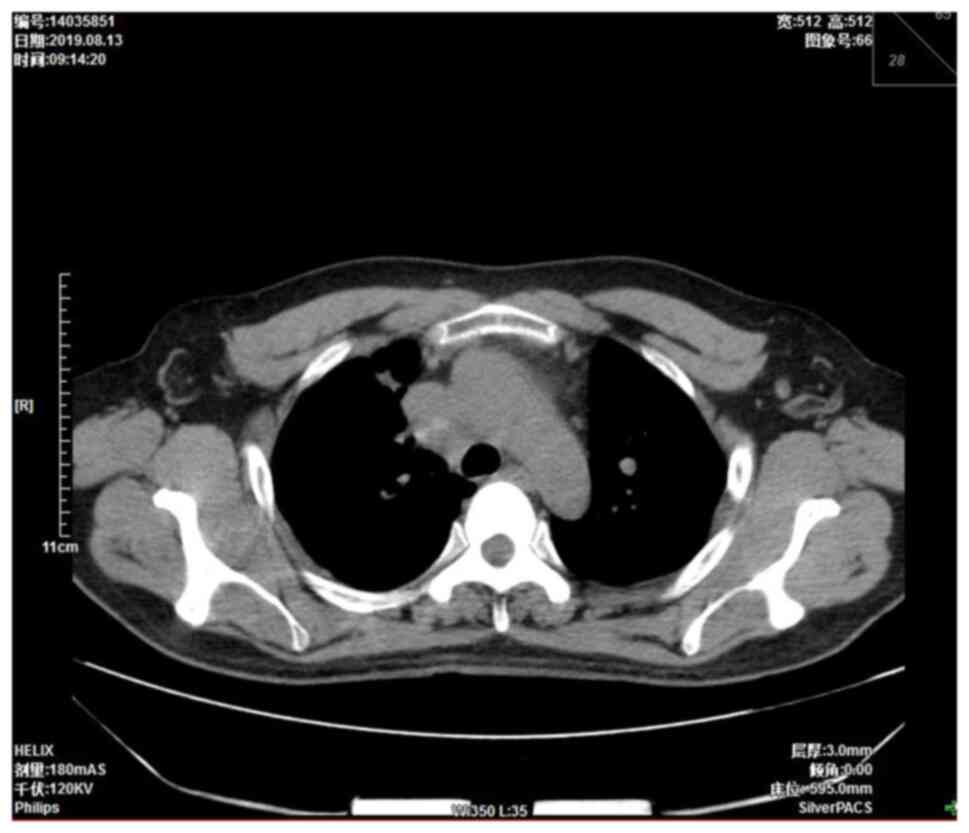
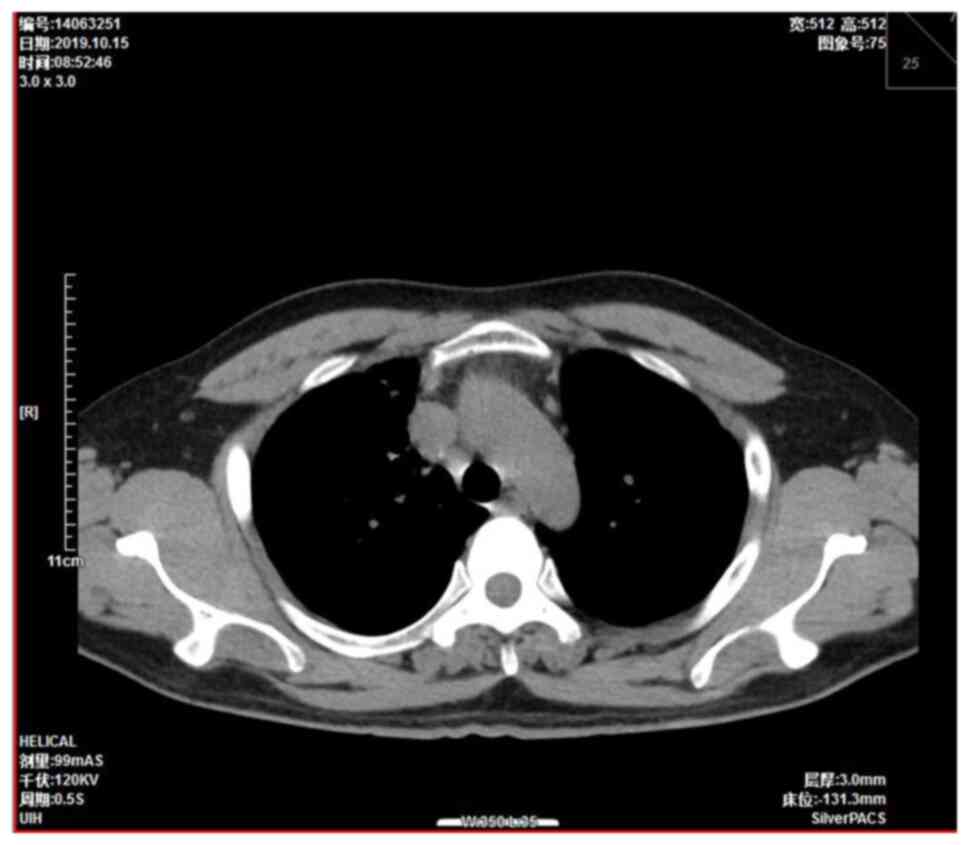
were approximately the size of a pea. A chest computed tomography
(CT) scan showed a right mediastinal mass 4.5×3.7 cm in size
(Fig. 1). Mediastinal lung cancer
with superior vena cava invasion was considered as a potential
diagnosis. The patient had multiple nodules and patulous shadows in
both lungs, numerous enlarged lymph nodes in the mediastinum, right
hilum and bilateral supraclavicular region, but no pleural
effusion. Multiple ribs, thoracic vertebrae, scapula bone density
abnormalities and osteogenic metastases were considered. The
serological tumor markers were as follows: Carcinoembryonic antigen
9.20 µg/l (normal range, 0.00-5.00 µg/l), cytokeratin 19 fragment
5.15 ng/ml (normal range, <3.3 ng/ml), cancer antigen (CA) 125
30.80 kU/l (normal range, <35 kU/l), CA199 <2.00 kU/l (normal
range, <37 kU/l) and squamous cell carcinoma-associated antigen
1.30 µg/l (normal range <1.5 µg/l). Transbronchial endoscopic
ultrasound was employed to perform a biopsy of the level four
mediastinal lymph nodes. Cytopathologic investigation of the right
supraclavicular lymph nodes was performed using fine needle
aspiration. Gross pathological examination of the biopsy of the
right level four mediastinal lymph node revealed a large mass of
broken tissues that was gray, white and red in color (~1.0×0.8×0.3
cm3). The tissues were fixed with 4% neutral buffered
formalin (24 h at 25°C) and paraffinized to prepare 4-µm sections
for hematoxylin and eosin staining (according to a standard
protocol) and IHC staining. High-throughput next-generation
sequencing (NGS) was employed to detect the molecular
pathology.
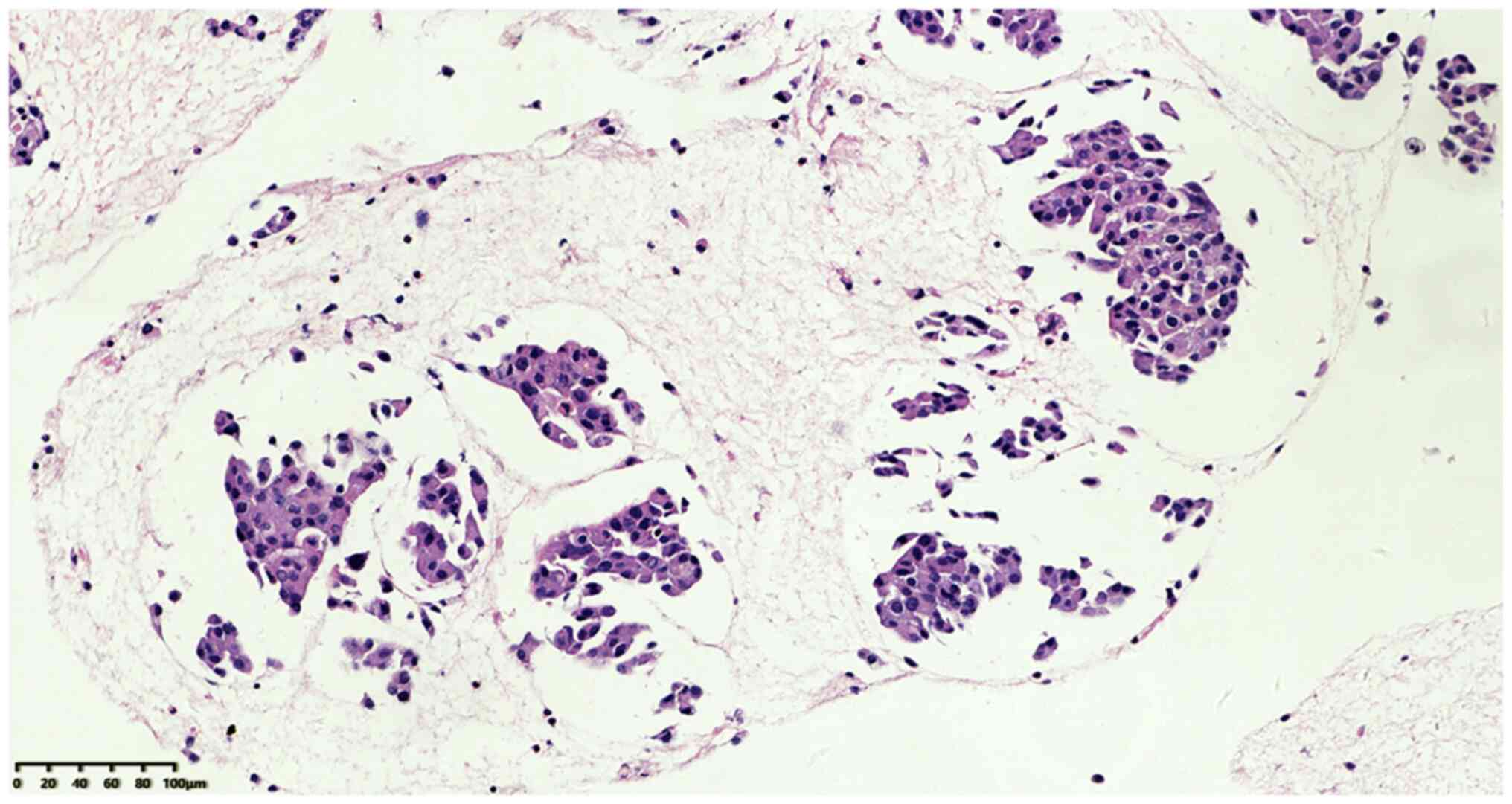
Microscopic examination of the tissues at low power
revealed that the broken tumor tissue was distributed in clusters
and nests (Fig. 2), and a small
number of lymphocytes were present in a loose cellulose exudate
between tumor nests without evident fibrous stroma. At high
magnification, solid flake tumor cells were observed with obvious
cell atypia, different cell sizes and clear boundaries in some
parts. In addition, the cells had a high nucleus-plasma ratio,
varying nuclear sizes, coarse chromatin and indistinct nucleoli.
Mitotic figures were visible, along with abundant eosinophilic
cytoplasm and some nuclear deviation. However, no salt and
pepper-like particles, keratinization or necrosis were observed
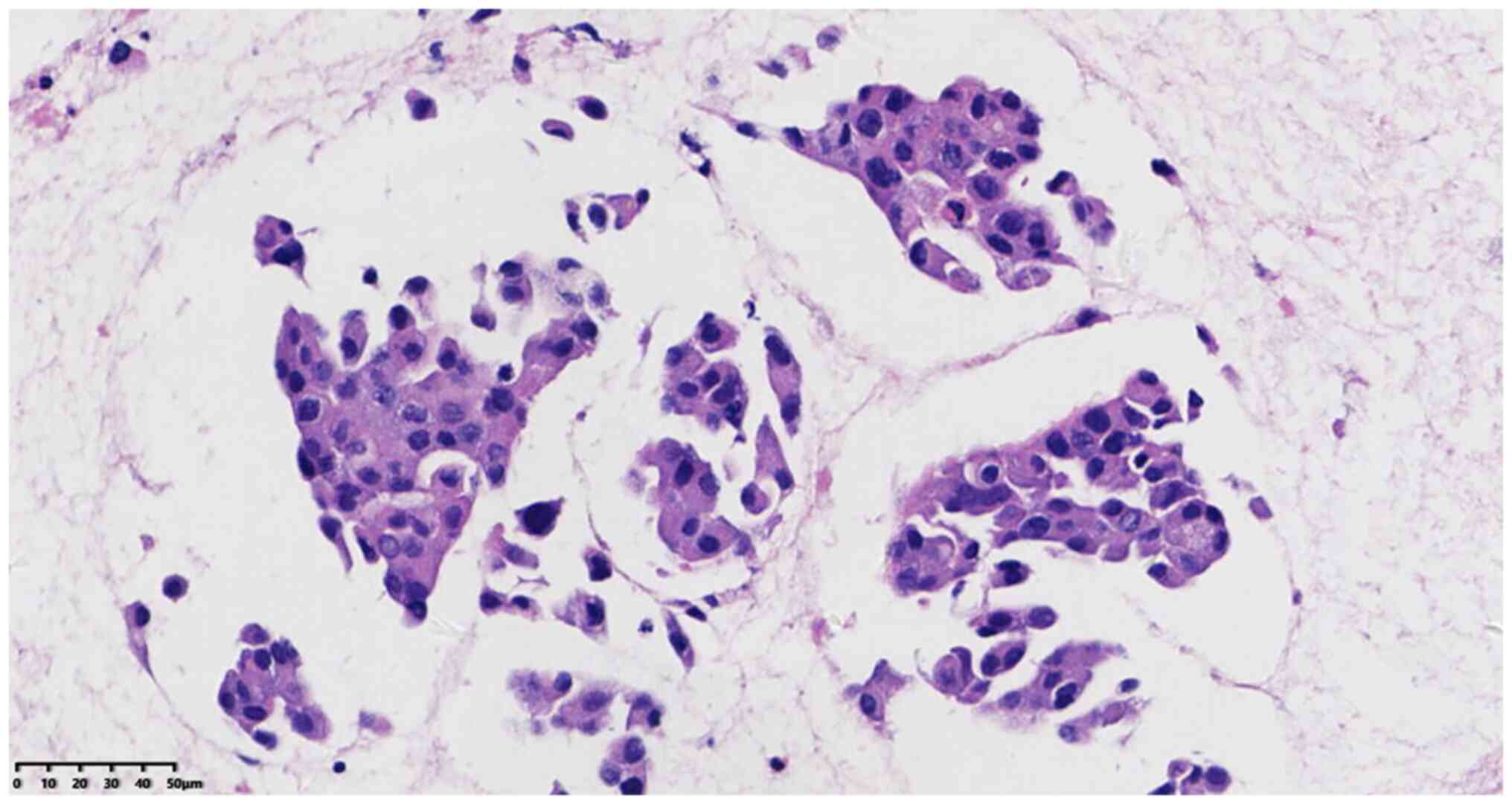
(Fig. 3).
IHC was performed using an EnVision IHC kit (polymer
method; cat. no. KIT-0014; Beijing Jinqiao Zhongshan Biological Co.
Ltd.) with antibodies from Beijing Zhongshan Jinqiao Biological
Co., Ltd. to target the following proteins (pre-diluted working
solutions unless otherwise indicated): P40 (cat. no. 2106230815d),
TTF-1 (cat. no. 2011260599c7), cytokeratin (CK)7 (cat. no.
21050820), napsin A (cat. no. 21110130), Ki-67 (1:200 dilution;
cat. no. 21030436), P63 (cat. no. 21063006), CD56 (cat. no.
21082702), chromograninA (CgA: cat. no. 2108052), synaptophysin
(Syn; cat. no. 2105130742c), CK5/6 (cat. no. 21030108), paired box
8 (Pax8; cat. no. 21012350), thyroglobulin (TG; cat. no. 20030932)
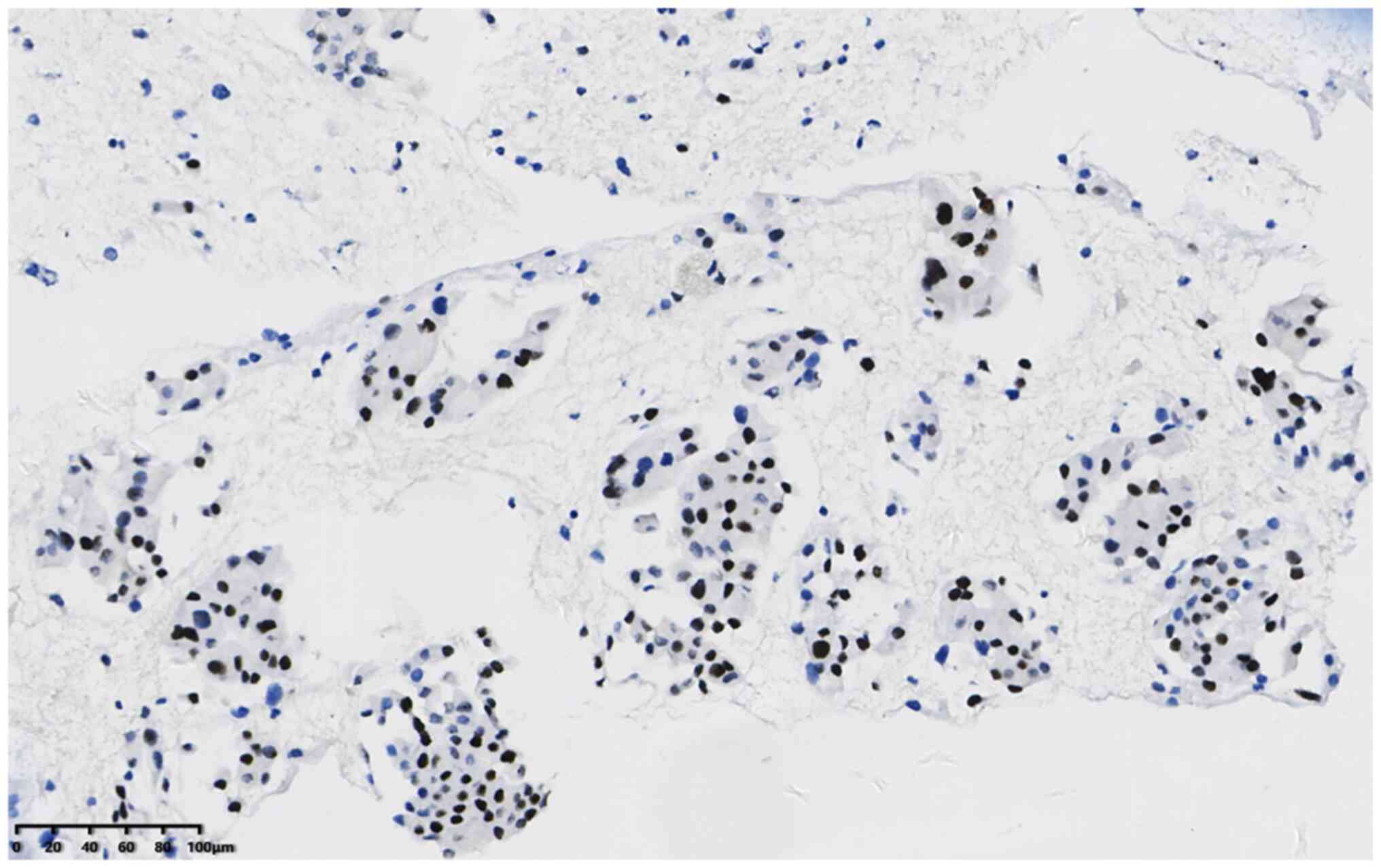
and Tp53 (cat. no. 20082125). The results were as follows: P40
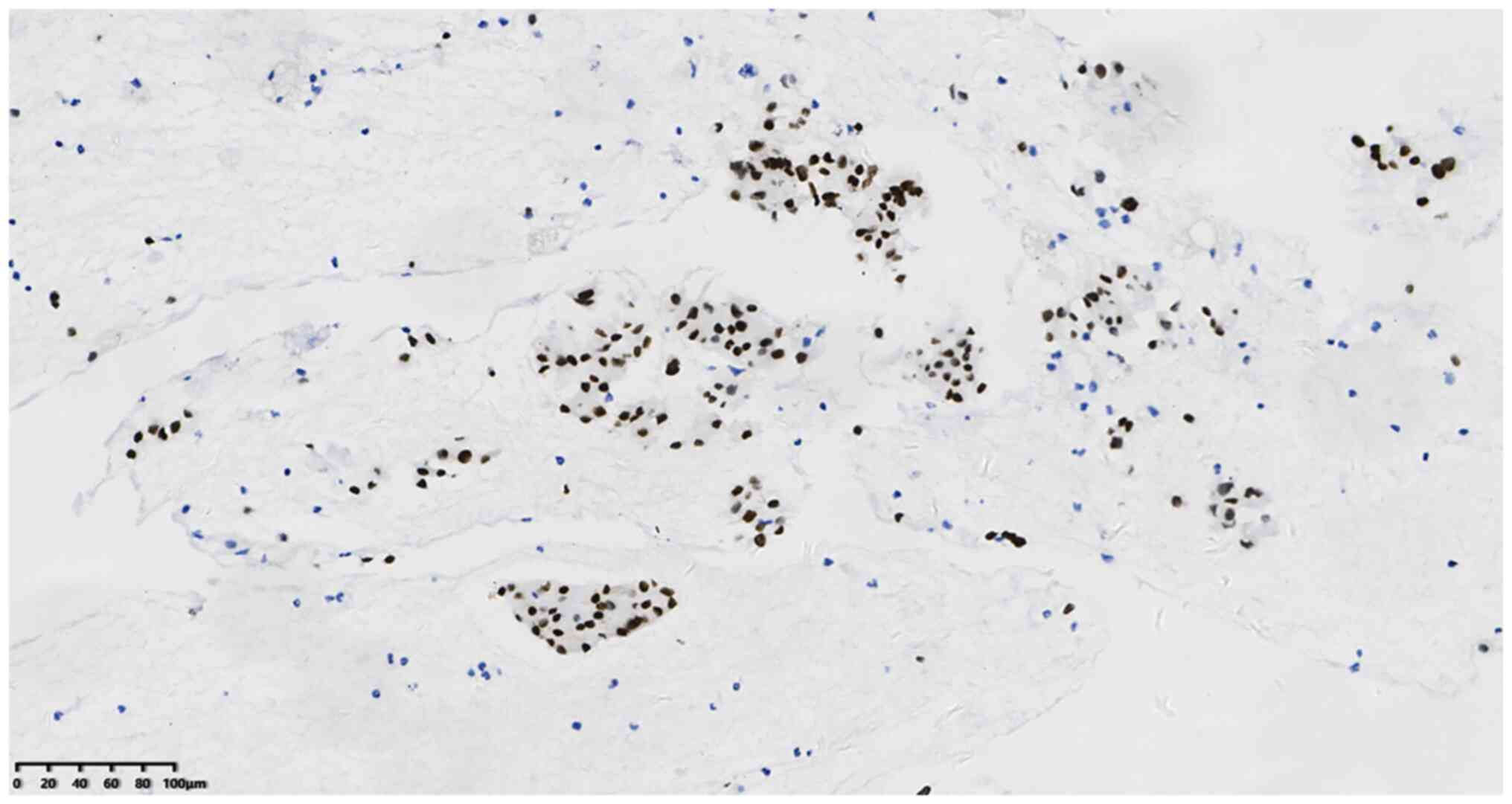
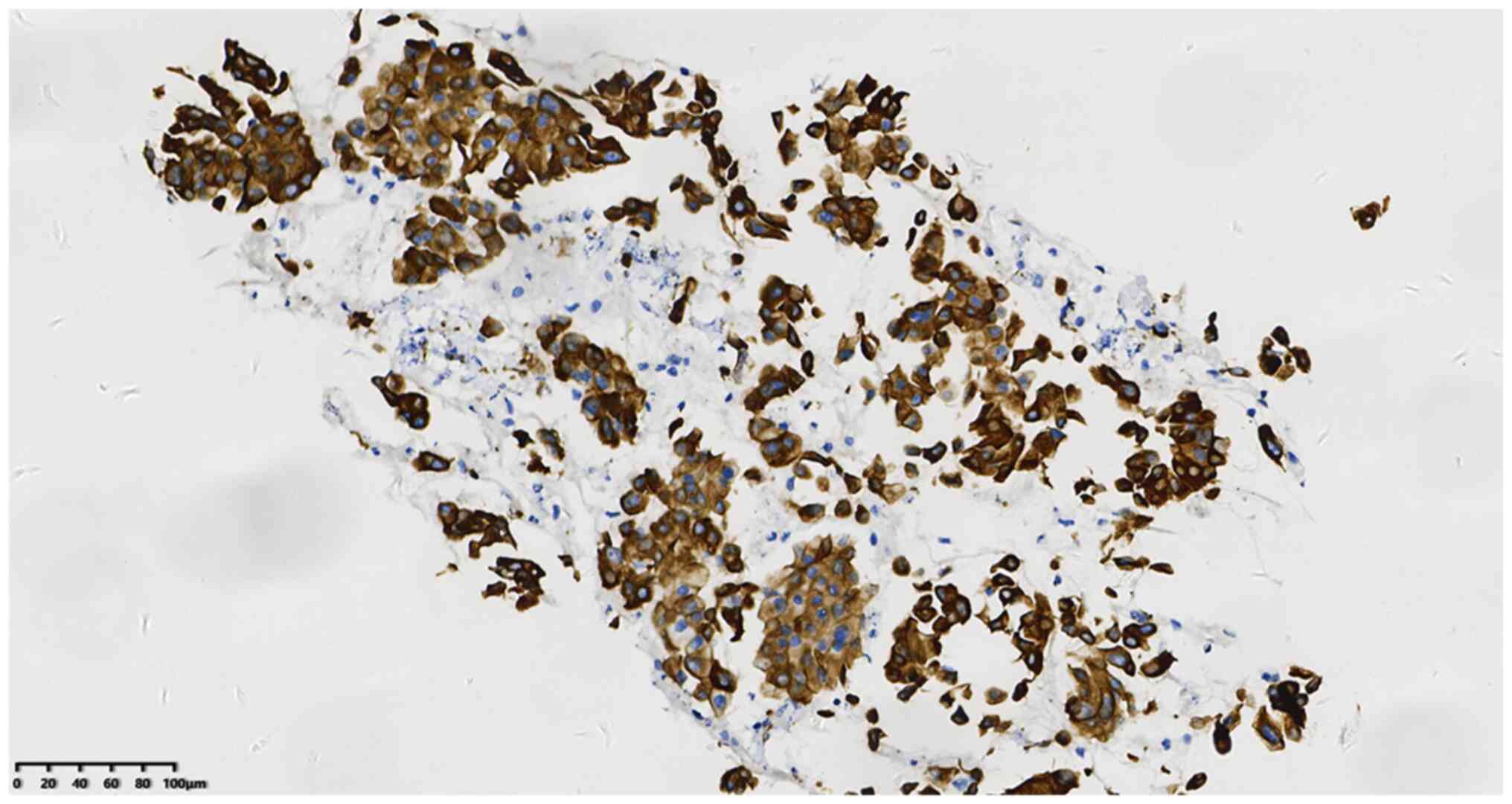
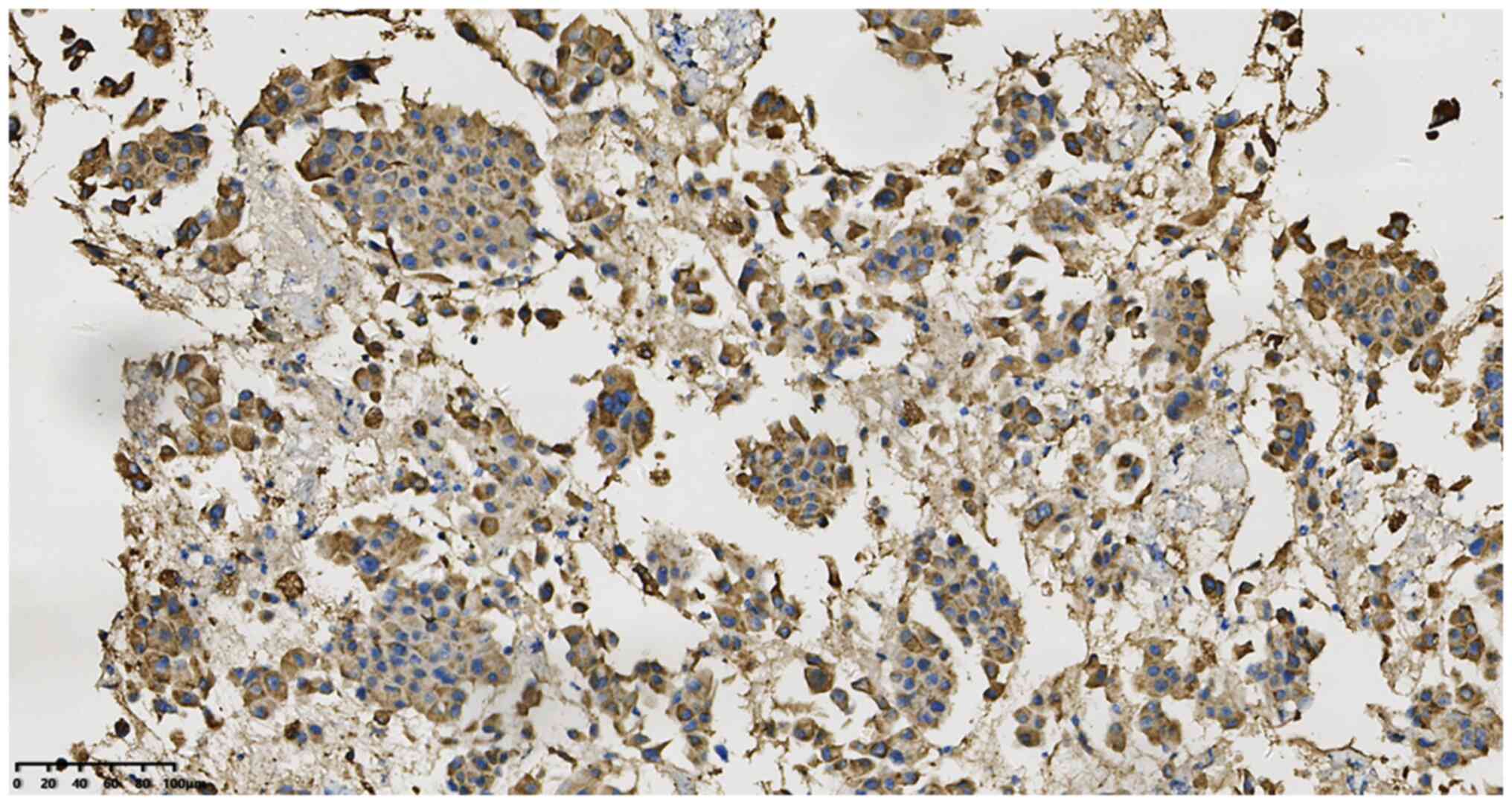
50–60%+ (Fig. 4), TTF-1+ (Fig. 5), CK7+ (Fig. 6) and napsin A+ (Fig. 7). In addition, the Ki-67
proliferation index was 10% positive, P63, CD56, CgA, Syn, CK5/6,
Pax8 and TG staining were negative; and TP53 exhibited no
mutations.
Molecular pathology
For this analysis, 15 paraffin sections were
extracted and library construction and probe capture were performed
(AmoyDx Essential NGS Panel; cat. no. 8.0627401X024I; Amoy
Diagnostics Co., Ltd.). High-throughput NGS was performed to detect
gene mutations associated with drug sensitivity in the solid tumor
sections. The reagent used was a 10-gene kit procured from Amoy
Diagnostics Co., Ltd. A sequencing platform from Illumina, Inc. was
used with a cancer gene mutation information analysis system
developed by Amoy Diagnostics Co., Ltd., as the analytical
software.
Molecular pathology results and drug
sensitivity
The mutation rate was 24.77% for the fusion gene
between exon 6 of EML4 and exon 20 of ALK (ALK transcript
NM_004304.4 and EML4 transcript NM_019063.4; indel fusion), and
according to US Food and Drug Association/National Comprehensive
Cancer Network guidelines, this mutation indicates that the tumor
is sensitive to crizotinib (www.nccn.org/patients). The mutation rate of the
PIK3CA gene was 4.32% (exon10 c.1658 G>C p.S553t, transcript
NM_0062182, mutation type single nucleotide variant; indel),
suggesting a lack of drug sensitivity, and resistance to gefitinib,
erlotinib, afatinib and icotinib. No mutations were observed in
EGFR, BRAF, HER2, KRAS, MET, ROS1, RET and NRAS genes.
Pathological diagnosis
The patient presented with lymph node metastatic
poorly differentiated carcinoma, combined with a clinical diagnosis
of NSCLC with P40 and TTF-1 expression and EML4-ALK and PIK3CA gene
mutations. Fine needle aspiration cytology of the right
supraclavicular lymph node revealed poorly differentiated cancer
cells. The clinical stage was cT3N3M1C-stage IVB.
Treatment and follow-up
In July 2019, 850 mg pemetrexed combined with 600 mg
carboplatin was started daily as intravenous chemotherapy. Due to
abnormal liver function, the patient stopped chemotherapy
immediately after the first chemotherapy cycle, and then began
single-agent targeted therapy with 250-mg oral crizotinib capsules
8 days after starting intravenous chemotherapy. The tumor shrank by
~50% within 1 month on CT (Fig.
8). After 3 months, the lung tumor had shrunk further on CT
(Fig. 9). The patient was
subsequently lost to follow-up.
Discussion
In the GLOBOCAN 2020 estimates reported by Sung
et al (7) for 36 types of
cancer in 185 countries, the incidence and mortality of lung cancer
ranked first and second worldwide, respectively. The section on
thoracic neoplasms in the fifth edition of the WHO Classification
of Tumors (6) recommends the use
of an IHC package containing TTF-1 and P40 as specific markers to
diagnose adenocarcinoma and squamous cell carcinoma, respectively,
in NSCLC biopsy tissues without distinct morphological
differentiation of adenocarcinoma and squamous cell carcinoma.
However, no clear definition or explanation has been provided for
the double expression of P40 and TTF-1 markers in NSCLC. Following
the first case reported by Pelosi et al (1), only six additional cases have been
reported, including the current case (2–5). To
the best of our knowledge, the present study reports the first-ever
case of NSCLC with EML4-ALK and PIK3CA gene mutations. The
clinicopathological and molecular characteristics of this disease
were analyzed using methods documented in the literature. The
clinical features of all the reported cases are presented in
Table I. A total of seven cases
have been investigated. The tumor has mostly affected males (male
to female ratio, 5:2). The age range of the patients is 38–77
(median, 62; mean, 59.6) years, and six of the seven cases reported
a history of smoking. A symptomatic cough and expectoration were
present in one case, and a persistent headache was reported in one
case in which the lung cancer was accompanied by brain metastasis.
Lung tumors were found by CT examination in 3 patients. The
symptoms of the remaining two cases remains unknown. The cancer was
located in the left lobe in four cases and in the right lobe in
three cases. The maximum diameter of the tumor was 1.9-8.5 cm. The
thorax, liver, bone, brain and other sites were associated with
pleural effusion in one case. CT scanning revealed no particular
difference between these lung tumors and other cancers.
 | Table I.Clinicopathologic information of the
seven known cases of P40 and TTF-1 expression in non-small cell
lung cancer. |
Table I.
Clinicopathologic information of the
seven known cases of P40 and TTF-1 expression in non-small cell
lung cancer.
| First author/s,
year | Sex/age (years) | Smoker (Y/N) | Site | Maximum diameter
(cm) | Pleural effusion
(Y/N) | IHC findings | Electron microscopy
findings | Tumor stage | Therapy | Clinical outcome | (Refs.) |
|---|
| Pelosi et al,
2015 | M/77 | Y | Left lung, hilar
mass | 8.5 | Y | P40+ and TTF-1+in
most tumor cells | Characteristics of
glandular and squamous differentiation | c-IVA | None | DOD 1.5 months | (1) |
| Hayashi et al,
2018 | M/73 | Y | Left lung lobe | 1.9 | N | P40+ and TTF-1+in
most tumor cells, CD56 focal+, napsin-, CK5/6-, Syn-, CgA- and
Ki-67 (40%) | None | NA | LC | NA | (2) |
| Spinelli et
al, 2019 | M/51 | Y | Right upper lobe | 3.1 | N | P40+ and TTF-1+in
most tumor cells | None | c-IVB | RT and CT | Fast progress, no
specific details | (3) |
| Pelosi et al,
2021 | W/62 | Y | Upper right lobe | 4.5 | N | P40+ and TTF-1+in
most tumor cells | Characteristics of
glandular and squamous differentiation | IIIB | LC + CT+ TT | DOD 48 months | (4) |
| Pelosi et al,
2021 | M/62 | Y | Left lower lobe | 4.7 | N | P40+ and TTF-1+in
most tumor cells | Characteristics of
glandular and squamous differentiation | yIIIA | LC + CT + TT | DOD 3 months | (4) |
| Chen and Cheng,
2022 | F/54 | Y | Center of lower left
lobe | 2.2 | N | P40+, TTF-1+, | None CK7-, napsin A-,
CK5/6+, P63+ and Ki-67 (70%) | NA | None | 9 mo Lost to
follow-up | (5) |
| Present study | M/38 | N | Right
mediastinum | 4.5 | N | P40 (50–60%), TTF-1+,
P53-, P63-, CK7+, CD56-, napsin A+, CK5/6-, Syn-, CgA- and Ki-67
(10%) | None | c-IVB | CT + TT | 3 mo Lost to
follow-up | - |
Several specimens were obtained for the analysis of
pathological features, including bronchial biopsy, lung tumor
puncture, lymph node puncture and lobectomy specimens. The findings
of the microscopic examination were not unusual compared with those
of other lung cancer biopsies, punctures and surgical specimens. On
microscopic observation, the high-grade tumor cells showed a solid
nested patchy distribution, conspicuous atypia, high nucleoplasmic
ratio and mitotic figures in all cells, and necrosis was present in
some cases. These features were sometimes accompanied by squamous
differentiation, glandular differentiation and peripheral
palisading.
Pelosi et al (1,4)
performed an electron microscopic examination of three cases, which
revealed adenoid and squamous differentiation concurrently in the
same tumor cells, which included abundant perinuclear stress
fibers, cytoplasmic dense keratin fiber bundles and desmosomal
junctions. The features of adenoid differentiation included the
formation of extracellular lumen, villous cytoplasmic processes and
mucous granules. The IHC results revealed that the same tumor cells
co-expressed TTF-1 and P40 in all cases.
With regard to molecular genetic characteristics, no
specific pattern in gene mutations was observed in these cases. Six
cases had a TP53 mutation or polymorphism, but no TP53 mutation was
detected in the current case. EGFR, KRAS, PTEN and NF1 mutations,
and FGFR1 amplification with PD-L1 expression were also detected in
certain cases. EML4-ALK and PIK3CA gene mutations were concurrently
observed in the present case. ALK is a powerful tumor driver gene.
In NSCLC, 3–7% of cases have ALK fusion mutations (8), of which EML4-ALK fusion mutations are
the most common, with >20 fusion configurations. EML4 exon
13-ALK exon 20 is the most common EML4-ALK fusion mutation,
constituting ~50% of all mutations (9). Most fusion breakpoints occur within
or upstream of exon 20 in the ALK gene, although a few occur in
exon 19, enabling the fused ALK protein to retain an intact kinase
region, a key site of carcinogenic activity. PIK3CA, a common
proto-oncogene, occurs in 1–3% of lung cancers (10). Most mutations of the PIK3CA gene
occur in exons 10 and 21, which encode the helical and kinase
domains of the protein, respectively. PIK3CA gene mutation can
activate different downstream signaling pathways, including AKT and
mTOR pathways, thereby promoting the occurrence and development of
tumors. As indicated by clinical analyses, PIK3CA mutation is an
important mechanism of secondary resistance to EGFR-tyrosine kinase
drugs in lung cancer (11). In the
present case, the fusion configuration EML4 exon 6-ALK exon 20 had
a mutation proportion of 24.77%. Cancers with this rare mutation
are sensitive to treatment with crizotinib. PIK3CA mutations
(4.32%) were also detected, for which no drug sensitivity is known,
and resistance to gefitinib, erlotinib, afatinib and icotinib is
recorded. EGFR mutation, which is most commonly found in patients
with NSCLC, especially adenocarcinoma, was found in a previous
case. The mutation leads to a change in normal cell biology that
results in cancer (12).
No mutations in BRAF, HER2, KRAS, MET, ROS1, RET and
NRAS genes were detected in the present case. BRAF-encoded RAF
kinase is a key regulator of the MAPK/ERK pathway, and its mutation
can lead to the continuous activation of RAF protein, resulting in
uncontrolled cell growth and proliferation (13). HER2 mutations occur in ~3% of cases
of NSCLC. HER2 is a member of the EGFR family; it is a receptor
tyrosine kinase that is bound to the surface of cell membranes and
regulates various signal transduction pathways to promote cell
growth and differentiation. Mutations in HER2 cause abnormal cell
growth and differentiation (14).
The KRAS gene is mutated in 20–30% of cases of NSCLC, and these
mutations lead to uncontrolled malignant cell proliferation and
division (15). MET is a
proto-oncogene. The MET gene encodes a transmembrane receptor
protein with tyrosine kinase activity, which can affect cell
growth, survival, invasion, metastasis and angiogenesis. The
mutation of MET is known to cause excessive cell proliferation
(16). ROS1 mutation can produce
an ROS1 fusion protein in which the ROS1 tyrosine kinase is
continuously activated and induces downstream signaling, resulting
in excessive cell growth and proliferation (17). The mutation probability of the NRAS
gene in NSCLC is ~1%. The NRAS protein encoded by the NRAS gene
operates within the RAS/MAPK signaling pathway, and its mutation
can lead to continuous activation of the NRAS protein, causing
uncontrolled cell proliferation (18).
Hypotheses for the causes of such lesions have been
disclosed in previous studies. Specifically, Pelosi et al
(1) proposed that the double
expression of P40 and TTF-1 may be caused by stem/progenitor cell
plasticity. In another study, it was suggested that the common
mutation of TP53 and PTEN alleles leads to poorly differentiated
and multiphenotypic tumors (2).
Pelosi et al (4) also
suggested that this tumor may be derived from the
co-differentiation of distal bronchial basal stem cells with the
double-positive expression of adenosquamous markers. The present
case had no TP53 mutations, which supports the third hypothesis.
Such lesions are not distinctly classified in the fifth edition of
the WHO Classification of Tumors. Multiple names for adenosquamous
carcinoma have been proposed, including NSCLC-not otherwise
specified, NSCLC with adenosquamous cell immunophenotype, and NSCLC
with the double expression of adenocarcinoma and squamous cell
carcinoma markers. Based on the observation of the tumor cells
using electron microscopy, which revealed that three cases had
characteristics of glandular and squamous differentiation, and the
detection of seven cases with the concurrent expression of
glandular and squamous markers by IHC, an appropriately named
adenosquamous carcinoma is likely to be accepted. The current
authors propose that the WHO term adenosquamous carcinoma (6) should be renamed as compound
carcinoma, defined as squamous carcinoma and adenocarcinoma, each
≥10%. This suggestion is similar to the concept of compound small
cell carcinoma (6), which is
considered as small cell carcinoma plus any NSCLC, adenocarcinoma,
squamous cell carcinoma or large cell carcinoma.
Treatments used for this cancer include surgery,
radiotherapy, chemotherapy and targeted therapy. Three patients
died during follow-up, and the survival time ranged from 1.5 to
48.0 months (mean, 17.5 months). In the present case, the tumor
size shrank remarkably after 3 months of chemotherapy plus targeted
therapy, but the patient was lost to further follow-up.
Overall, this type of NSCLC with concurrent P40 and
TTF-1 expression presents several unique clinicopathological
features. This cancer is not yet mentioned in the guidelines of the
WHO Classification of Tumors, the International Association for the
Study of Lung Cancer, or other authoritative organizations. For
consideration as an independent subtype, the clinicopathological
characteristics, molecular phenotype, treatment and prognosis of
NSCLC with concurrent P40 and TTF-1 expression warrant further
studies and investigation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and BH conceived the study and drafted the
manuscript. HL and XC were responsible for the collection and
analysis of case data and literature. BH and JY interpreted the
data and revised the manuscript. YC and HL confirm the authenticity
of all the raw data. All authors agreed on the journal to which the
article has been submitted and have agreed to be accountable for
all aspects of the work. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
As the patient was lost to follow-up, a waiver of
patient consent for publication was provided by the Ethics
Committee of The First People's Hospital of Xiaoshan District
(Hangzhou, China).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pelosi G, Fabbri A, Tamborini E, Perrone
F, Testi AM, Settanni G, Busico A, Centonze G, Braidotti P,
Bulfamante G, et al: Challenging lung carcinoma with coexistent
ΔNp63/p40 and thyroid transcription factor-1 labeling within the
same individual tumor cells. J Thorac Oncol. 10:1500–1502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi T, Takamochi K, Yanai Y, Mitani K,
Tomita H, Mogushi K, Suehara Y, Takahashi F, Suzuki K, Saito T and
Yao T: Non-small cell lung carcinoma with diffuse coexpression of
thyroid transcription factor-1 and ΔNp63/p40. Hum Pathol.
78:177–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spinelli M, Khorshad J and Viola P: When
tumor doesn't read textbook. Third case of TTF1 and p40
co-expression in the same tumour cells in a non-small cell
carcinoma. A potential new entity to consider? Pathologica.
111:58–61. 2019.PubMed/NCBI
|
|
4
|
Pelosi G, Bulloni M, Martina Vescio M,
Uccella S, Forest F, Leone G, Barberis M, Rahal D, Bossi P, Finzi
G, et al: Coexpression of ΔNp63/p40 and TTF1 within most of the
same individual cells identifies life threatening NSCLC featuring
squamous and glandular biphenotypic differentiation:
Clinicopathologic correlations. JTO Clin Res Rep.
2:1002222021.PubMed/NCBI
|
|
5
|
Chen B and Cheng N: Coexpression of
thyroid transcription factor 1 and P40 in non-small cell lung
cancer cells: A case report. Chin J Pathol. 51:558–560. 2022.(In
Chinese).
|
|
6
|
WHO Classification of Tumours Editorial
Board, . WHO classification of tumours. Thoracic Tumours. 5th
edition. IARC Press; Lyon: 2021
|
|
7
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Au JSK, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pillai RN and Ramalingam SS: The biology
and clinical features of non-small cell lung cancers with EML4-ALK
translocation. Curr Oncol Rep. 14:105–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parra HS, Cavina R, Latteri F, Zucali PA,
Campagnoli E, Morenghi E, Grimaldi GC, Roncalli M and Santoro A:
Analysis of epidermal growth factor receptor expression as a
predictive factor for response to gefitinib (‘Iressa’, ZD1839) in
non-small-cell lung cancer. Br J Cancer. 91:208–212. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dalle S, Poulalhon N and Thomas L:
Vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med.
365:1448–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri
S, Lau C, Zaidinski M, Paik PK, Zakowski MF, Kris MG and Ladanyi M:
Prevalence, clinicopathologic associations, and molecular spectrum
of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas.
Clin Cancer Res. 18:4910–4918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benvenuti S, Sartore-Bianchi A, Di
Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S and Bardelli
A: Oncogenic activation of the RAS/RAF signaling pathway impairs
the response of metastatic colorectal cancers to anti-epidermal
growth factor receptor antibody therapies. Cancer Res.
67:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwab R, Petak I, Kollar M, Pinter F,
Varkondi E, Kohanka A, Barti-Juhasz H, Schönleber J, Brauswetter D,
Kopper L and Urban L: Major partial response to crizotinib, a dual
MET/ALK inhibitor, in a squamous cell lung (SCC) carcinoma patient
with de novo c-MET amplification in the absence of ALK
rearrangement. Lung Cancer. 83:109–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaw AT and Solomon BJ: Crizotinib in
ROS1-rearranged non-small-cell lung cancer. N Engl J Med.
372:683–684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumaz N: Mechanism of RAF isoform
switching induced by oncogenic RAS in melanoma. Small GTPases.
2:289–292. 2011. View Article : Google Scholar : PubMed/NCBI
|