Introduction
Ovarian cancer (OC) is one of the most common
malignant tumors in gynecology, second only to cervical and uterine
cancer, with the worst prognosis and the highest mortality rate
worldwide (1,2). It is estimated that 39,306 people
will die of OC in China in 2022 (3). OC directly and indirectly adds a high
economic burden on society (4).
The main reason for the high mortality rate is the insidious onset
of OC and the lack of effective screening tools; consequently,
>70% of patients are already at an advanced stage at the time of
diagnosis (5). OC treatment is
based on aggressive cytoreductive surgery combined with platinum
chemotherapy. However, the prognosis of OC remains unsatisfactory,
despite the emergence of new chemotherapeutic and targeted agents
in recent years (6). Therefore,
there is an urgent need to identify simple methods to reduce the
risk of OC development, and improve the prognosis and quality of
life of patients with OC. In previous years, it has been found that
obesity and hyperlipidemia can increase the risk of OC, and lead to
a poor prognosis (7). Statins,
which are widely used in clinical practice, are the most commonly
used lipid-lowering drugs. The effects of statins have been found
to be multi-functional, including not only the lowering of blood
lipids, but also the suppression of tumor proliferation and the
promotion of cell apoptosis (8–10).
Some studies have reported that statins are associated with a
reduced risk of developing OC (11,12).
However, relatively few studies have been conducted on the
association between the use of statins and the prognosis of OC.
Obesity and hyperlipidemia are risk factors, as well
as prognostic factors, for OC, which directly affect the survival
rate of patients (13). Some
previous studies showed that statin treatment improved the
prognosis of OC; however, other studies did not reach similar
conclusions (14–16). Therefore, the current evidence on
the prognostic effects of lipid-lowering drugs on OC is
inconsistent and is insufficient to form a reliable conclusion to
provide a scientific basis for clinical treatment. Moreover, to the
best of our knowledge, there is no meta-analysis considering the
heterogeneous effects of the type of statin, mode of use,
pathological type and clinical stage of OC. Therefore, a
comprehensive updated meta-analysis was performed to guide the
clinical application of statins in OC.
Materials and methods
Search methods and study selection
criteria
A comprehensive literature search of articles was
performed using the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase
(http://www.embase.com) and Cochrane Library
(https://www.cochranelibrary.com).
Case-control trials and cohort studies on statin therapy for OC
that had been conducted were included. The search timeframe was
from database creation to September 1, 2022. The search was
performed using the following subject terms in combination with
free terms: i) ‘Statins’ OR ‘3-hydroxy-3-methylglutaryl CoA
reductase inhibitor’ OR ‘anticholesteremic’ OR ‘simvastatin’ OR
‘atorvastatin’ OR ‘fluvastatin’ OR ‘lovastatin’ OR ‘rosuvastatin’
OR ‘pravastatin’ OR ‘pitavastatin’; ii) ‘ovarian cancer’ OR
‘ovarian neoplasms’ OR ‘ovarian carcinoma’; and iii) ‘survival’ OR
‘prognosis’ OR ‘mortality’ OR ‘death’ OR ‘recurrence’ OR ‘outcome’.
All search terms were restricted to studies involving human
subjects in the English language.
Inclusion and exclusion criteria
Studies included in the present meta-analysis met
the following inclusion criteria: i) The diagnosis of OC was
pathologically confirmed; ii) association between statin use and
overall survival (OS), progression-free survival (PFS) and/or
OC-specific survival (OVS) were reported; iii) studies were
designed as cohort studies or case-control studies; and iv)
adjusted hazard ratios (HRs) and their corresponding 95% confidence
intervals (CIs) were available. The following studies were
excluded: i) Abstracts, editorials, posters, newsletters,
preclinical studies, case reports, reviews, meta-analyses or
non-clinical studies; ii) studies not in the English language; iii)
studies with insufficient data to estimate the HRs and 95% CIs; iv)
studies with duplicate data or repeated analyses; and v) in
vitro studies.
Data extraction
Two researchers independently screened the
literature, extracted information and assessed the study quality
based on the predetermined inclusion criteria. Articles that could
not be classified by screening the title and abstract were assessed
by searching the entire text. Any inconsistencies were resolved by
consultation with the corresponding author. The extracted
information mainly included: i) Basic information of the studies,
including the first author, year of publication, country, study
design and study type; ii) baseline characteristics of the study
population, including the characteristics of the patients, sample
size, mean age, type of statin use, follow-up duration and
definition of statin use; and iii) information on the
interventions, outcome indicators and risk of bias assessment. The
quality of each study was evaluated using the Newcastle-Ottawa
Scale (17), shown in Table I. This scale ranges from 1 to 9
stars and judges the quality of each study based on three aspects:
i) Selection of the study groups; ii) comparability of the groups;
and iii) ascertainment of the outcome of interest. NOS scores of ≥6
were assigned as high-quality studies, while scores of <6 were
considered low-quality studies.
 | Table I.Details of study quality evaluation
via the NOS. |
Table I.
Details of study quality evaluation
via the NOS.
|
|
NOS
score |
|
|---|
|
|
|
|
|---|
| First author,
year | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Demonstration that
outcome of interest was not present at start of study | Control for
age | Control for other
confounding factors | Assessment of
outcome | Was follow-up long
enough for outcomes to occur? | Adequacy of
follow-up of cohorts | Total | (Refs.) |
|---|
| Elmore et
al, 2008 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | (20) |
| Urpilainen et
al, 2018 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | (21) |
| Habis et al,
2014 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | (22) |
| Vogel et al,
2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (23) |
| Verdoodt et
al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (24) |
| Majidi et
al, 2021 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | (25) |
| Couttenier et
al, 2017 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | (26) |
| Bar et al,
2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (27) |
| Harding et
al, 2019 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | (28) |
| Feng et al,
2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (29) |
| Hanley et
al, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (30) |
| Kim et al,
2022 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 8 | (31) |
| Chen et al,
2016 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 | (32) |
| Lavie et al,
2013 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | (12) |
| Nielsen et
al, 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | (33) |
| Wang et al,
2016 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 7 | (34) |
Statistical analysis
HRs and 95% CIs were directly obtained from each
study or estimated according to the methods described by Parmar
et al (18). An HR >1
indicated a worse prognosis for patients with OC. Cochran's Q test
and Higgins I-squared statistics were used to assess the
heterogeneity among the included studies, and a value of <0.10
was used to indicate heterogeneity. The choice between
fixed-effects and random-effects meta-analyses should not be based
on statistical tests of heterogeneity, as recommended in the
Cochrane Handbook for Systematic Reviews of Interventions
(https://training.cochrane.org).
Heterogeneity in intervention effects between multiple studies from
different groups and geographical locations will always occur.
Therefore, all forest plots in the present study used a
random-effects model to account for this. Publication bias was
assessed using Begg's funnel plot (19) and Egger's regression test. All
P-values were two-sided. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using RevMan 5.3 software (Nordic Centre) and STATA 15.0
(StataCorp LLC).
Results
Description of included studies
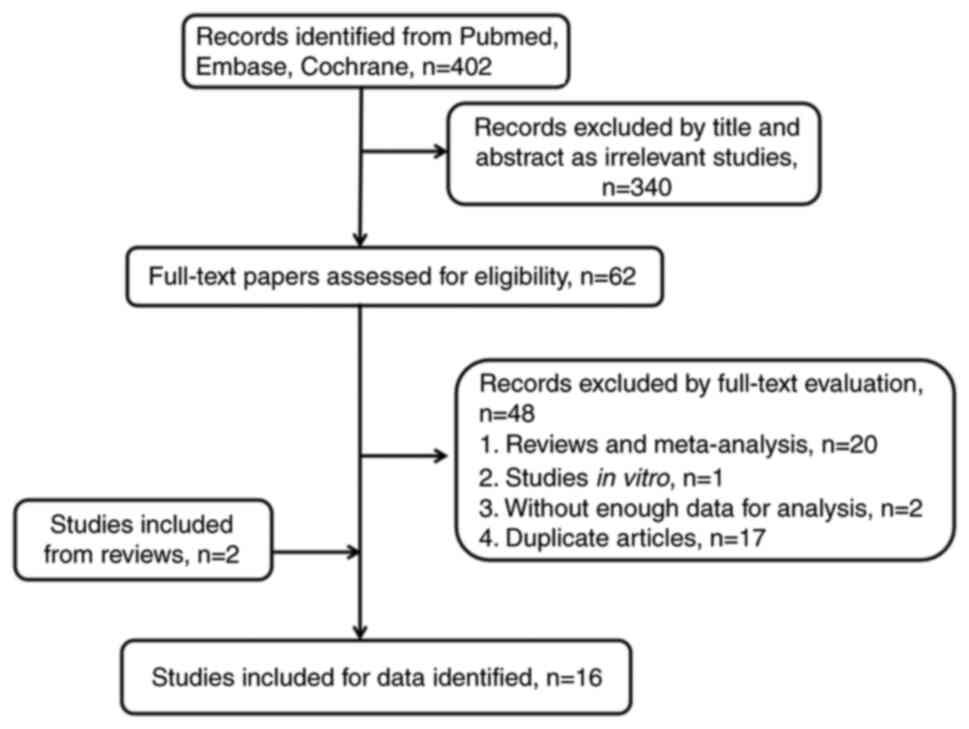
The initial search strategy retrieved 402 studies,
and after careful review, 16 were ultimately included (12,20–34).
These 16 studies were published from 2008–2022 and contained a
total of 37,660 patients with OC, of whom 11,296 were statin users.
The study selection process is summarized in the flow chart in
Fig. 1. Of the included studies, 4
were conducted on Asian participants (12,27,31,32)
and 12 on non-Asian participants (20–26,28–30,33,34).
In total, 5 studies were from the United States of America
(20,22,23,28,34),
2 were conducted each in Australia (25,29),
Denmark (24,33) and Israel (12,27),
and 1 study was conducted each in Korea (31), China (32), Canada (30), Finland (21) and Belgium (26). All studies directly reported HRs
and 95% CIs, and 4 studies enrolled <200 patients with OC
(12,20,27,32).
Among the included studies, 13 were cohort studies (21–31,33,34)
with a total sample size of 37,324, while 3 were case-control
studies (12,20,32)
with a total sample size of 336. Overall, 3 prospective studies
(25,33,34)
and 13 retrospective studies (12,20–24,26–32)
were included. All the studies reported a correlation between
statin use and OC prognosis. The characteristics of the included
studies are shown in Table
II.
 | Table II.Characteristics of the included
studies. |
Table II.
Characteristics of the included
studies.
| First author,
year | Country | Patients
ethnicity | Type of study | Study design | Study period, year
range | Patients, n | Patients prescribed
statins, n | Statin
exposure | Statins prescribed
pre- or post-diagnosis | HR (95% CI) | Follow-up time | (Refs.) |
|---|
| Elmore et
al, 2008 | USA | Non-Asian | Case-control | Retrospective | 1996-2001 | 126 | 17 | Lipophilic and
hydrophilic | Post | 0.45
(0.23–0.88) | 4.5 years
(median) | (20) |
| Urpilainen et
al, 2018 | Finland | Non-Asian | Cohort | Retrospective | 1998-2011 | 421 | 186 | Lipophilic | Pre | 0.72
(0.56–0.93) | 2.2
yearsa | (21) |
| Habis et al,
2014 | USA | Non-Asian | Cohort | Retrospective | 1992-2013 | 442 | 68 | Lipophilic and
hydrophilic | Post | 0.80
(0.50–1.29) | 3.5
yearsa | (22) |
| Vogel et al,
2017 | USA | Non-Asian | Cohort | Retrospective | 2007-2009 | 1,431 | 609 | Lipophilic and
hydrophilic | Post | 0.62
(0.50–0.77) | 2.6 years
(median) | (23) |
| Verdoodt et
al, 2017 | Denmark | Non-Asian | Cohort | Retrospective | 2000-2013 | 4,419 | 476 | Lipophilic and
hydrophilic | Post | 0.90
(0.78–1.04) | 2.4 years
(median) | (24) |
| Majidi et
al, 2021 | Australia | Non-Asian | Cohort | Prospective | 2012-2015 | 955 | 199 | Lipophilic and
hydrophilic | Both | 0.73
(0.52–1.03) | 5.0–8.0
yearsa | (25) |
| Couttenier et
al, 2017 | Belgium | Non-Asian | Cohort | Retrospective | 2004-2012 | 5,416 | 1,255 | Lipophilic and
hydrophilic | Both | 0.81
(0.72–0.90) | 0.5 to 3.0
yearsa | (26) |
| Bar et al,
2016 | Israel | Asian | Cohort | Retrospective | 2000-2012 | 143 | 43 | NA | Post | 0.69
(0.41–1.17) | 4.1 years
(median) | (27) |
| Harding et
al, 2019 | USA | Non-Asian | Cohort | Retrospective | 2007-2012 | 2,195 | 489 | Lipophilic and
hydrophilic | Post | 0.74
(0.61–0.91) | 2.2
yearsa | (28) |
| Feng et al,
2021 | Australia | Non-Asian | Cohort | Retrospective | 2003-2013 | 8,629 | 1,897 | Lipophilic and
hydrophilic | Both | 0.87
(0.82–0.94) | 19.0
yearsa | (29) |
| Hanley et
al, 2021 | Canada | Non-Asian | Cohort | Retrospective | 1997-2015 | 4,207 | 535 | Lipophilic and
hydrophilic | Both | 0.80
(0.69–0.93) | 3.0
yearsa | (30) |
| Kim et al,
2022 | Korea | Asian | Cohort | Retrospective | 2005-2013 | 677 | 160 | Lipophilic and
hydrophilic | Post | 0.70
(0.40–1.21) | 7.6
yearsa | (31) |
| Chen et al,
2016 | China | Asian | Case-control | Retrospective | 2009-2013 | 60 | 35 | NA | Post | 0.57
(0.21–1.51) | 2.5 years
(median) | (32) |
| Lavie et al,
2013 | Israel | Asian | Case-control | Retrospective | 2003-2010 | 150 | 67 | NA | Post | 0.24
(0.06–0.78) | 9.0 years
(median) | (12) |
| Nielsen et
al, 2012 | Denmark | Non-Asian | Cohort | Prospective | 1995-2007 | 8,159 | 5,213 | Lipophilic and
hydrophilic | Pre | 0.93
(0.81–1.08) | 2.6 years
median | (33) |
| Wang et al,
2016 | USA | Non-Asian | Cohort | Prospective | 1993-1998 | 230 | 47 | Lipophilic and
hydrophilic | Pre | 0.58
(0.40–0.85) | 14.6 years
median | (34) |
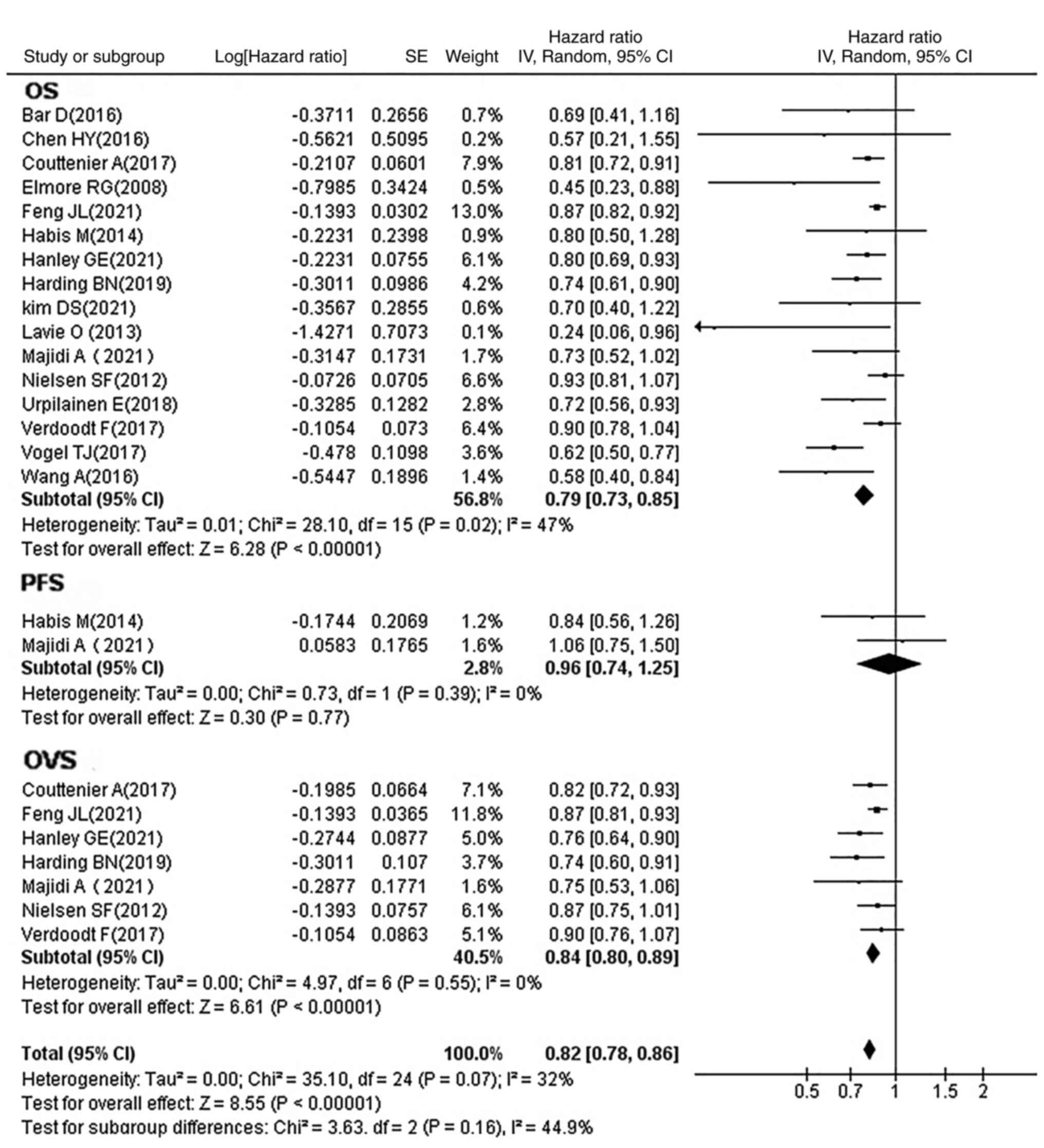
Association between statin use and
prognosis of OC
All included studies reported HRs and their
respective 95% CIs, and OS time, while some also reported OVS
and/or PFS. The studies were therefore divided into three
categories according to the study endpoint (Fig. 2). It was determined that the use of
statins significantly prolonged the OS time (HR, 0.79; 95% CI,
0.73–0.85; P<0.00001) and markedly increased the OVS time (HR,
0.84; 95% CI, 0.80–0.89; P<0.00001) of patients with OC; no
statistical difference was observed in the PFS time (HR, 0.96; 95%
CI, 0.74–1.25; P=0.77).
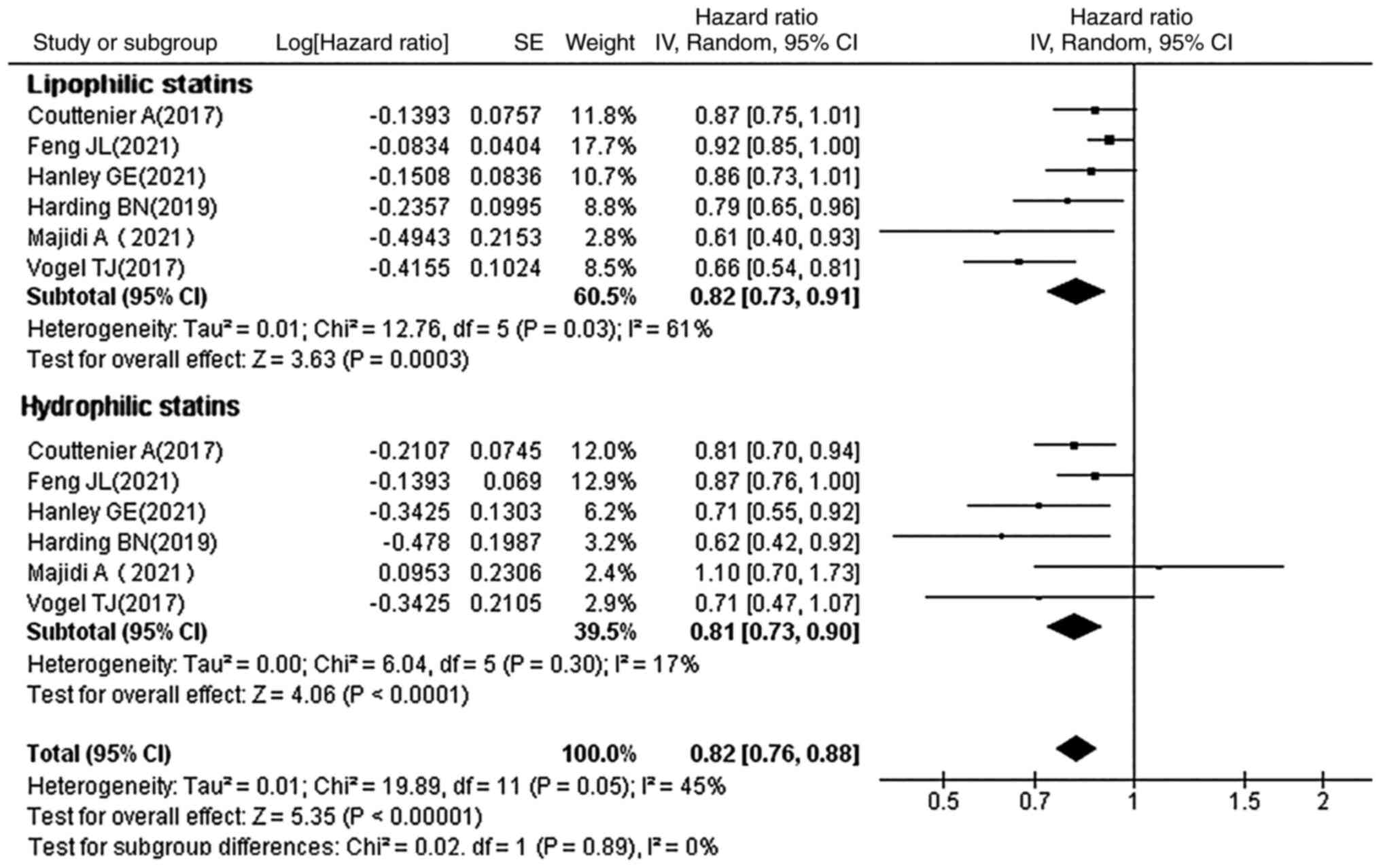
Subgroup analysis by type of
statin
Statins can be divided into two categories based on
their solubility: Hydrophilic statins (pravastatin and
rosuvastatin) and lipophilic statins (simvastatin, lovastatin,
fluvastatin and atorvastatin). The HRs and 95% CIs for OC mortality
with lipophilic and hydrophilic statins, were each reported in 6
studies. It was observed that the type of statin used had a
statistically significant effect on the prognosis of OC (lipophilic
statins: HR, 0.82; 95% CI, 0.73–0.91; P=0.0003; hydrophobic
statins: HR, 0.81; 95% CI, 0.73–0.90; P<0.0001; Fig. 3). A statistically significant
effect on the prognosis of OC regardless of the type of statins
used was therefore observed.
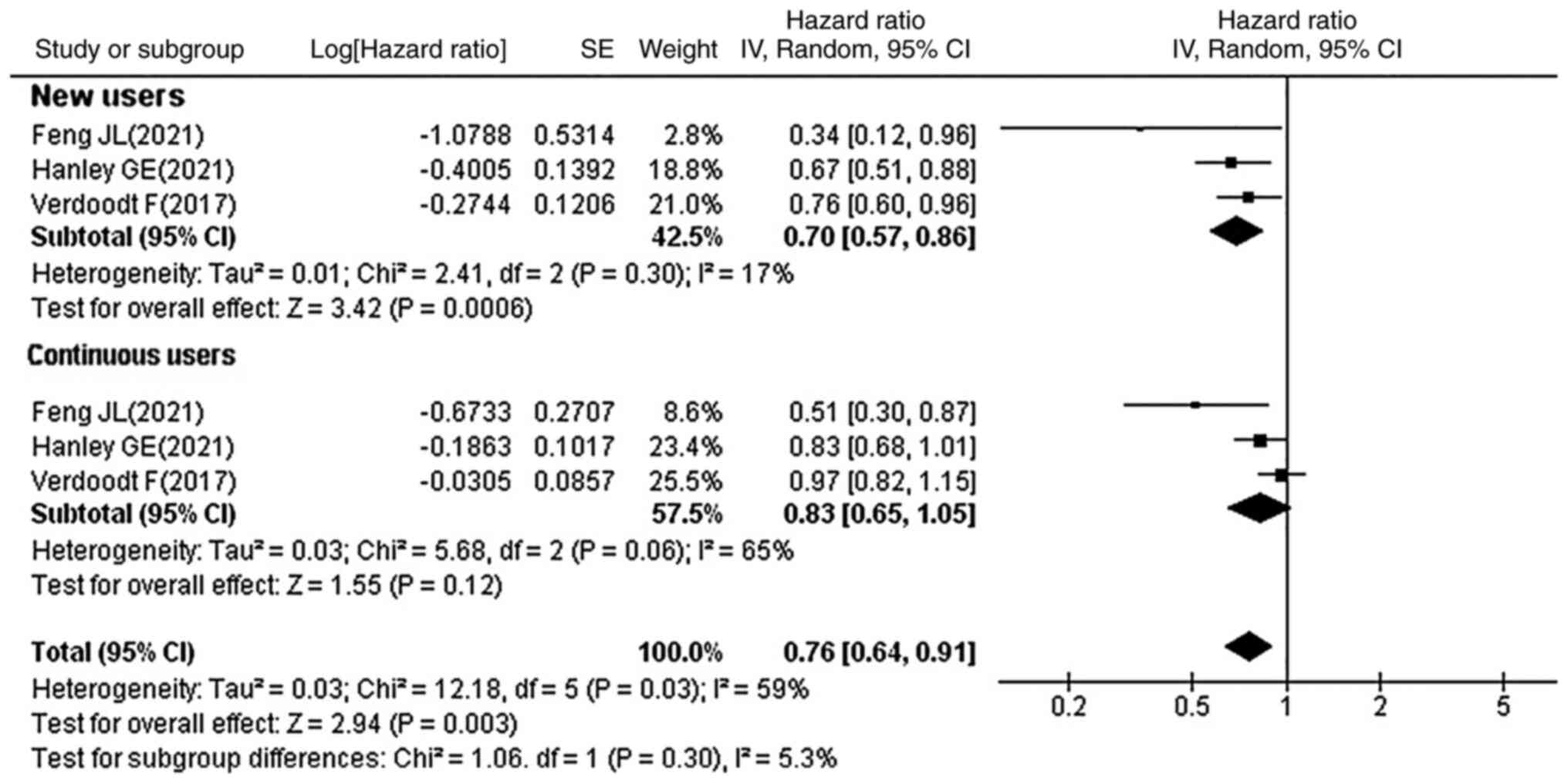
Subgroup analysis by usage of
statins
Of the 16 studies, 3 reported the significance of
new and continuous statin use on OC prognosis. It was observed that
new statin users were associated with reduced OC mortality (HR,
0.70; 95% CI, 0.57–0.86; P=0.0006), whereas no significant
association with OC prognosis was observed in continuous statin
users (HR, 0.83; 95% CI, 0.65–1.05; P=0.12) (Fig. 4). A total of 13 studies reported
the association between post-diagnostic statin use and OC
prognosis, showing that post-diagnostic statin use improved OC
prognosis (HR, 0.79; 95% CI, 0.73–0.85; P<0.00001), while no
statistically significant association was observed with
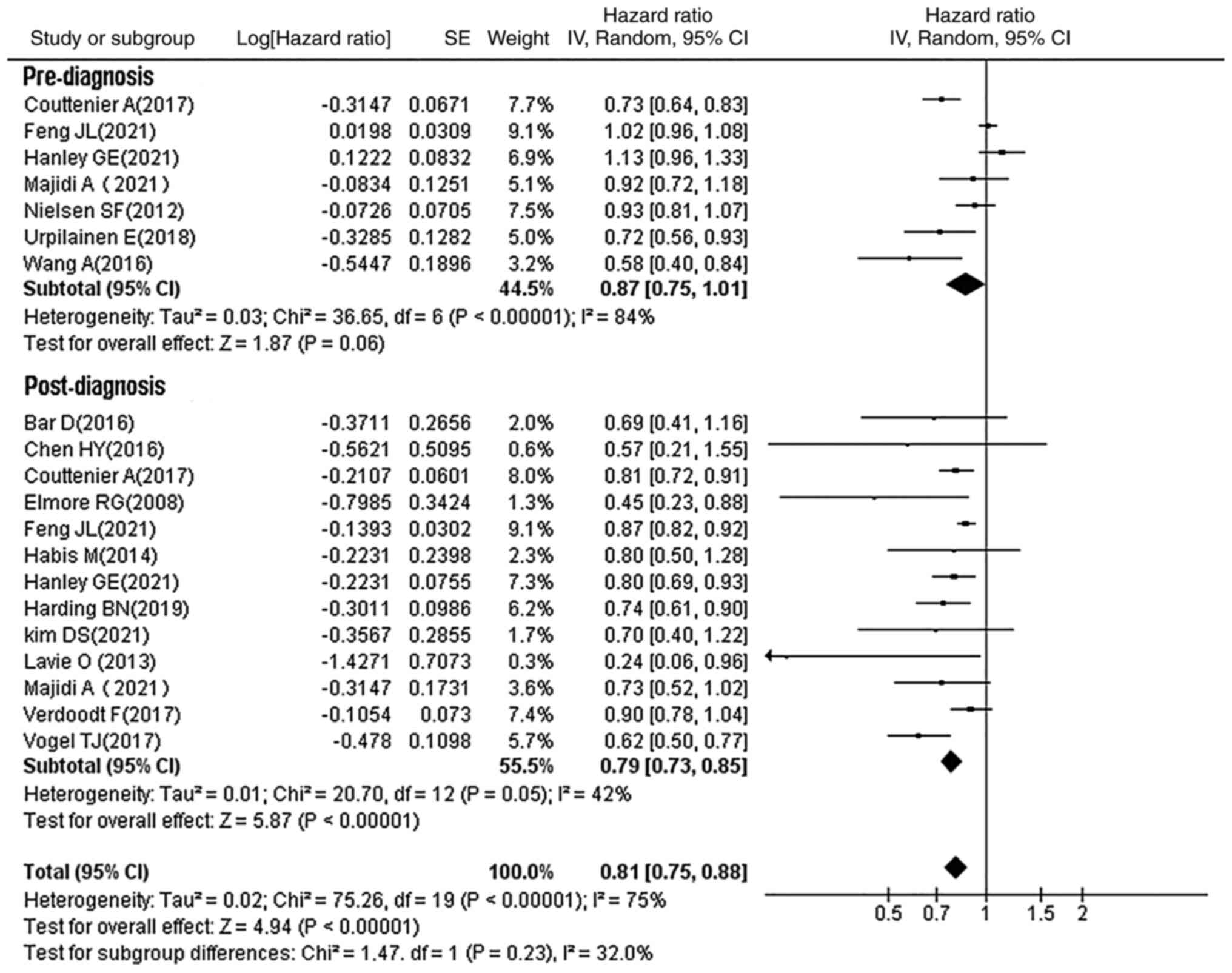
pre-diagnostic statin use (HR, 0.87; 95% CI, 0.75–1.01; P=0.06;
Fig. 5).
Subgroup analysis by type of OC
Clinically, OC is a heterogeneous disease with four
distinct histological subtypes: Serous, endometrioid, clear cell
and mucinous OC, each with its own unique clinical, genetic and
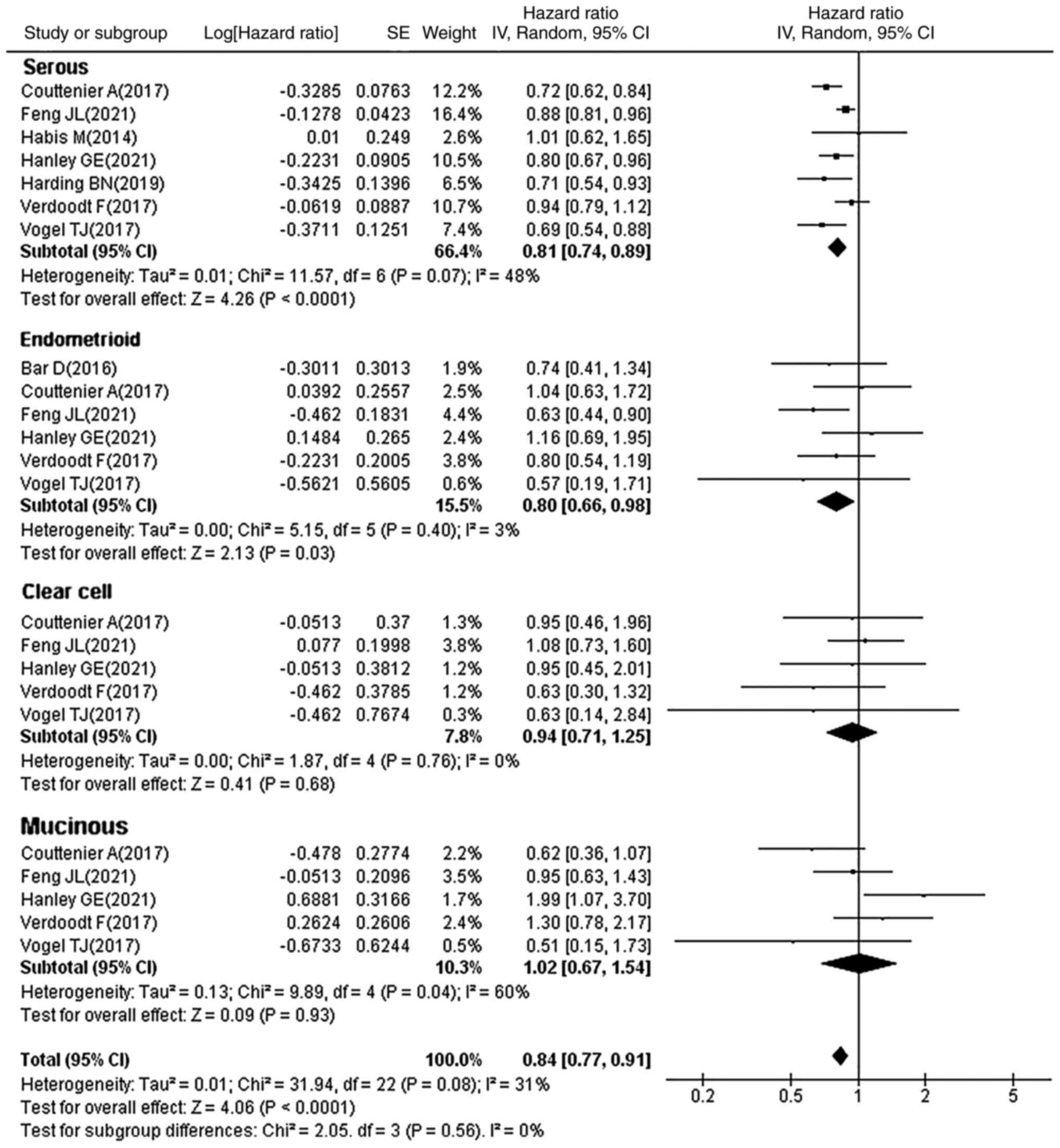
molecular features. Subgroup analysis (Fig. 6) showed that statin use
significantly improved the survival in patients with serous (HR,
0.81; 95% CI, 0.74–0.89; P<0.0001) and endometrioid (HR, 0.80;
95% CI, 0.66–0.98; P=0.03) OC, whereas no significant association
was observed in patients with clear cell (HR, 0.94; 95% CI,
0.71–1.25; P=0.68) and mucinous (HR, 1.02; 95% CI, 0.67–1.54;
P=0.93) OC. This may be related to the low prevalence of these two
pathological types, which resulted in a small number of enrolled
cases. It is evident that the protective effect of statins in
improving OC survival may be limited to specific OC subtypes.
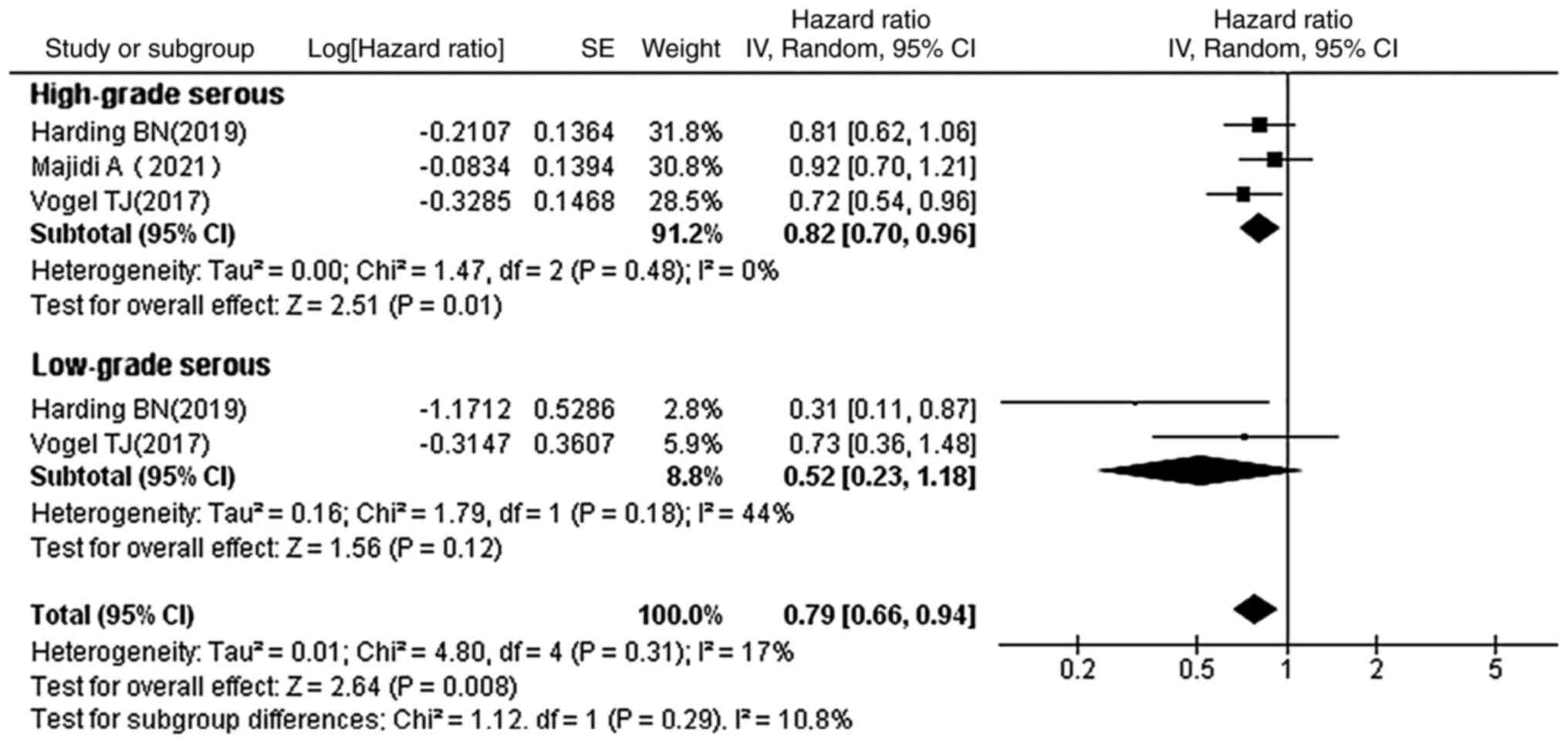
Subgroup analysis by grades of serous
OC
The relationship between statin use and OS with
regard to the histological subtypes of serous OC was further
evaluated in order to explore whether statin use was associated
with an improved prognosis in different grades of serous OC
(Fig. 7). The results showed that
statins prolonged the OS in patients with high-grade serous OC (HR,
0.82; 95% CI, 0.70–0.96; P=0.01), but statin use did not show a
statistical difference in patients with low-grade serous OC
subtypes (HR, 0.52; 95% CI, 0.23–1.18; P=0.12).
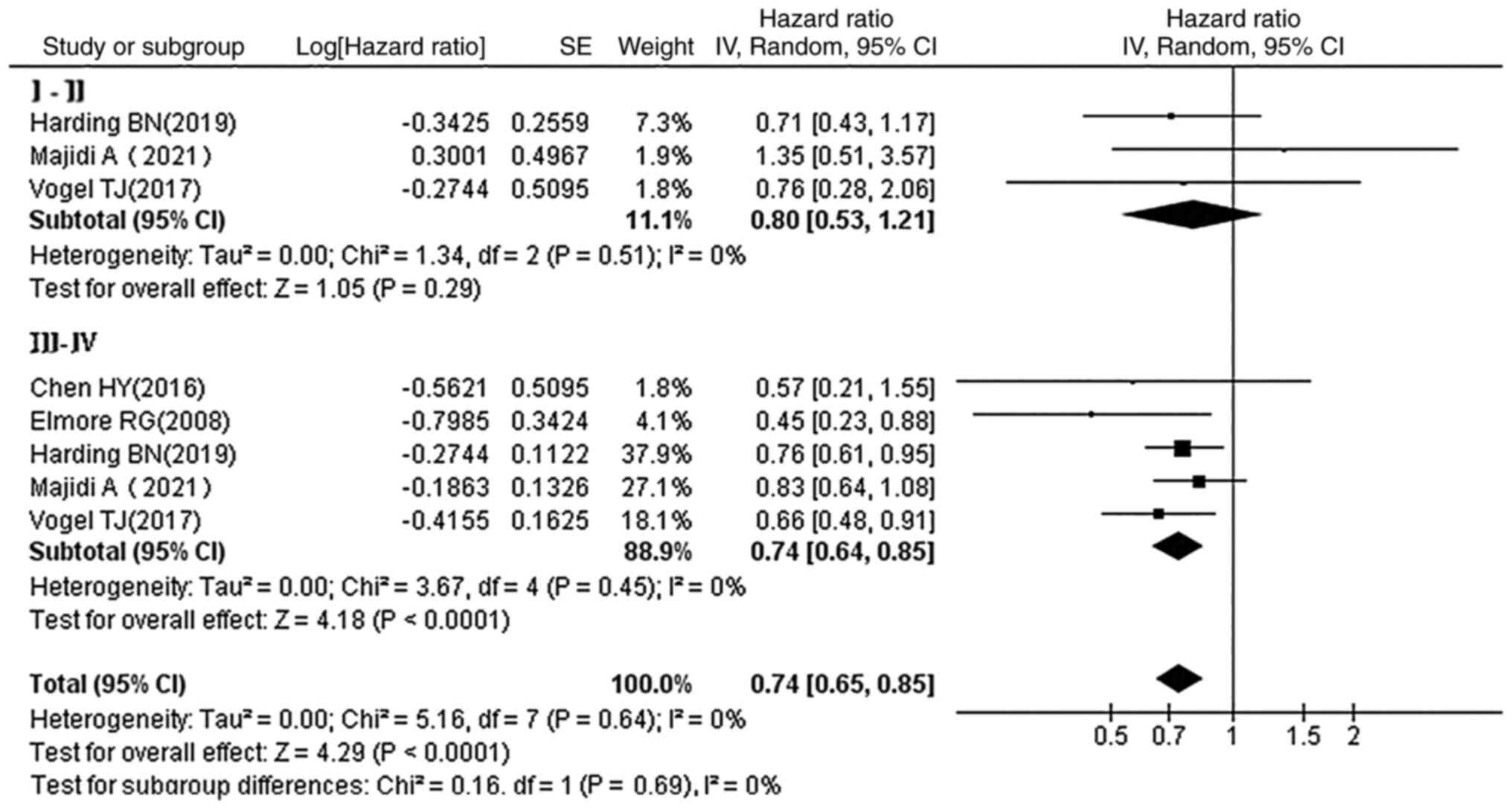
Subgroup analysis by stages of OC
In a subgroup analysis of staging at diagnosis,
statins reduced the mortality in patients with stage III–IV OC (HR,
0.74; 95% CI, 0.64–0.85; P<0.0001), whereas no statistical
association was observed between statin use and prognosis in
patients with stage I–II OC (HR, 0.80; 95% CI, 0.53–1.21; P=0.29)
(Fig. 8).
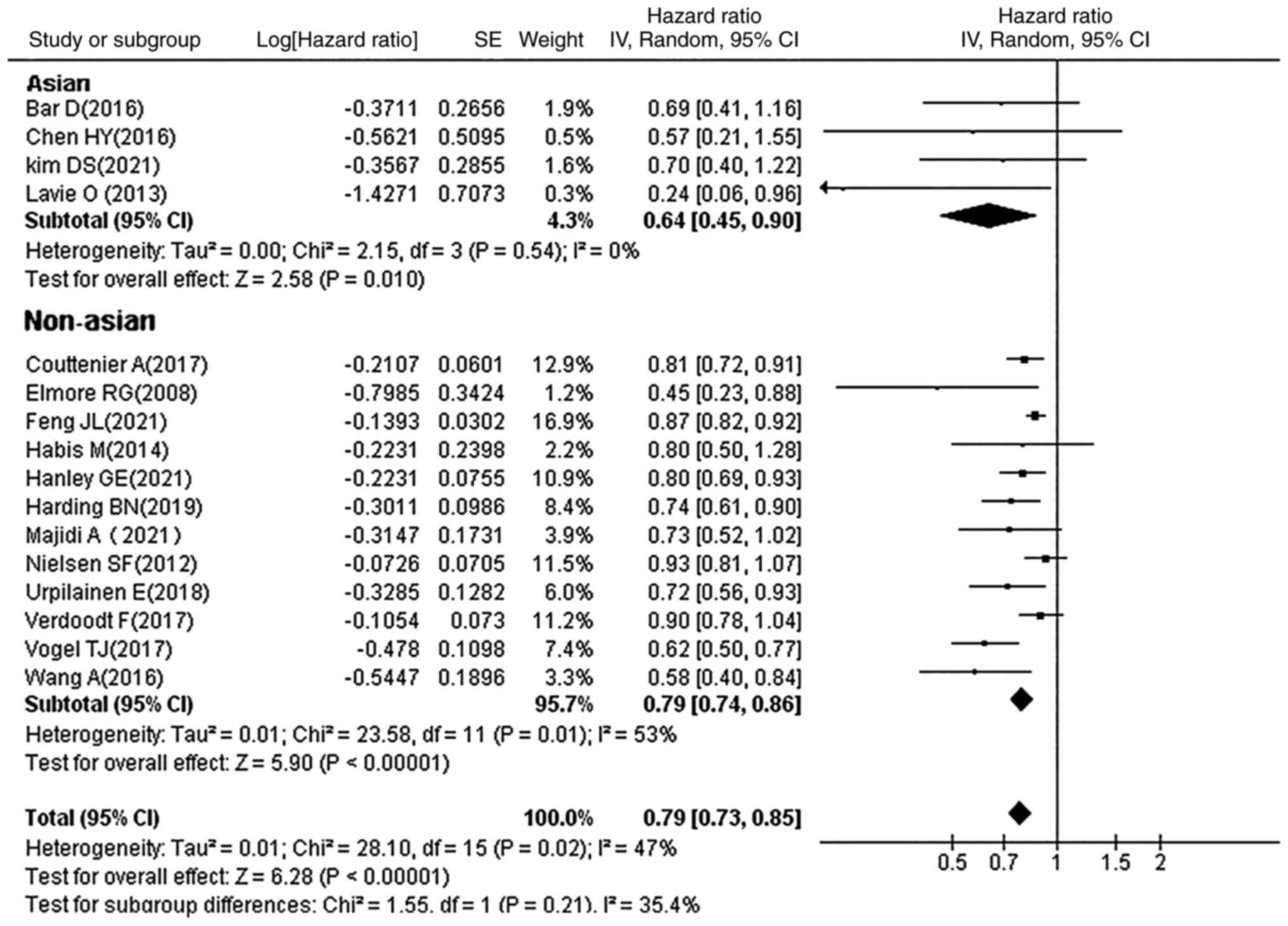
Subgroup analysis by ethnicity
Patients in the 16 studies were divided into Asian
and non-Asian groups, according to ethnicity. Statins were found to
improve the prognosis of OC in patients of Asian and other
ethnicities (HR, 0.79; 95% CI, 0.73–0.85; P<0.00001; Fig. 9).
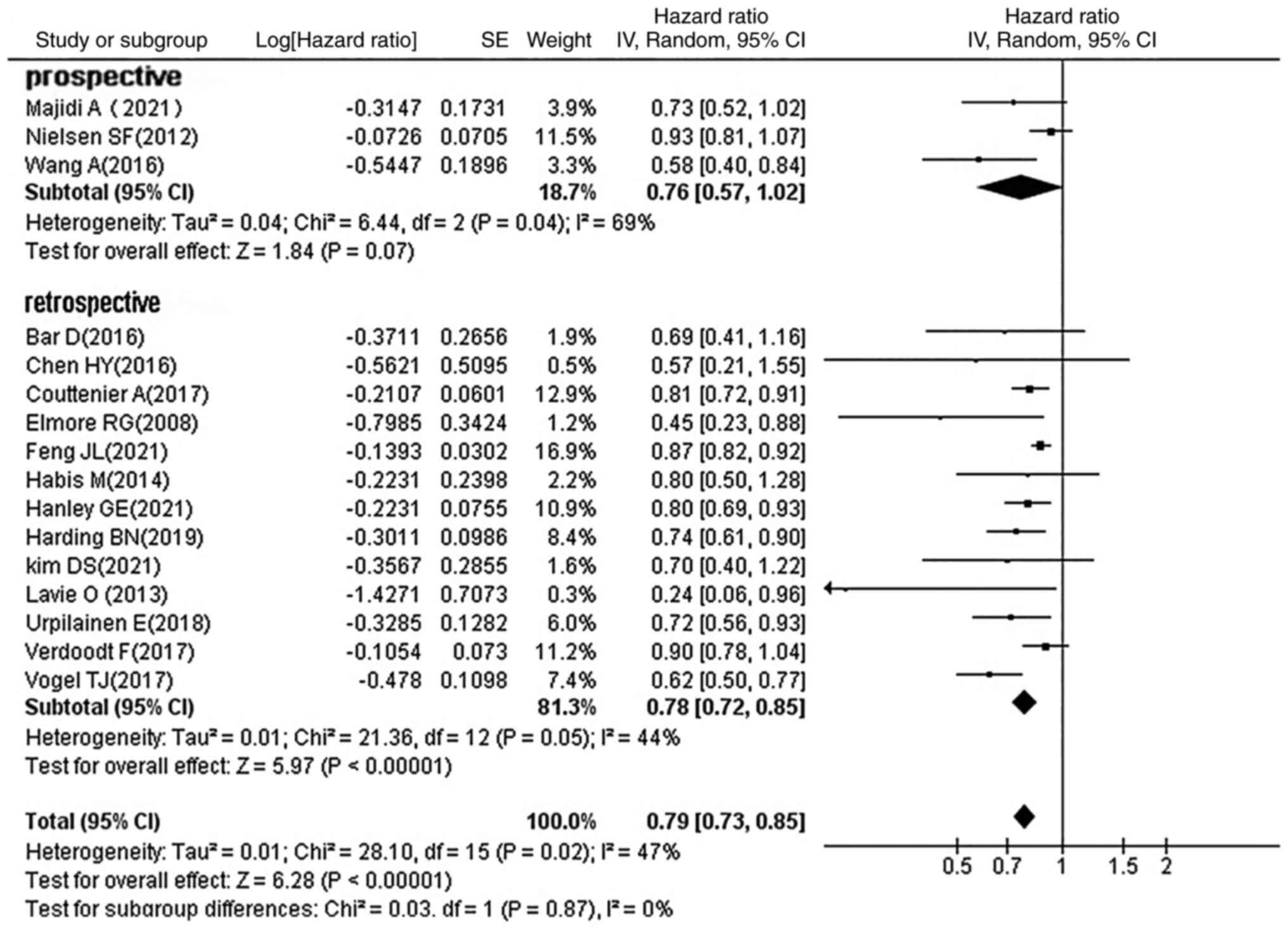
Subgroup analysis by study design
Of the 16 studies included, 3 were prospective
studies and the remainder were retrospective studies. The results
of the subgroup analysis (HR, 0.79; 95% CI, 0.73–0.85;
P<0.00001; Fig. 10) showed
that both prospective and retrospective studies found that statins
improved OC prognosis.
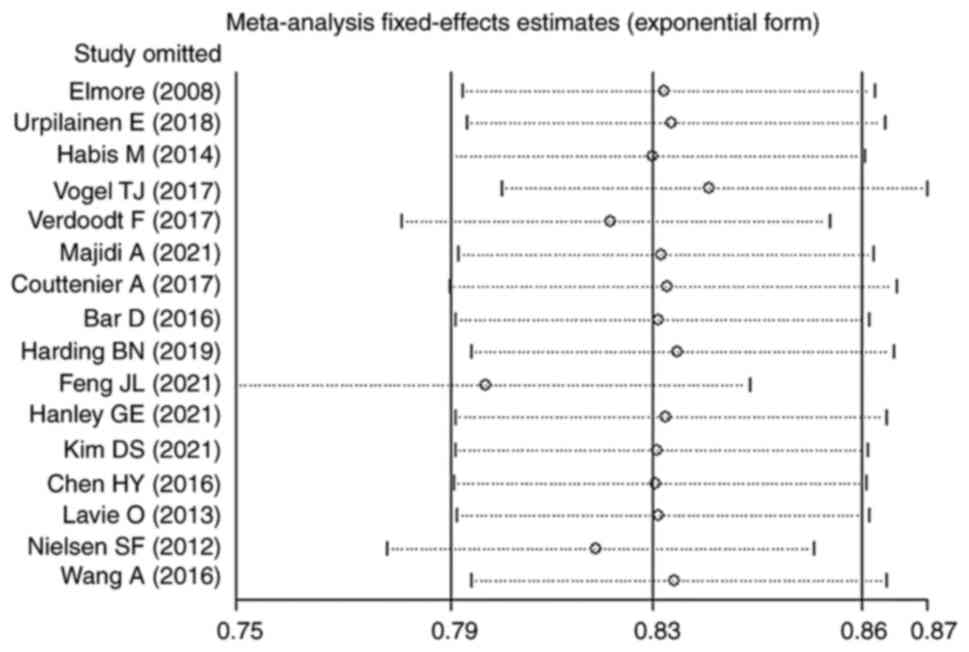
Heterogeneity and sensitivity
analyses
Except for the results of the subgroup analyses on
patients with OC taking continuous statins (I2, 65%;
P=0.06), those taking lipophilic statins (I2, 61%;
P=0.03), those taking statins before OC diagnosis (I2,
84%; P<0.00001), patients of non-Asian ethnicity taking statins
(I2, 53%; P=0.01), prospective studies (I2,
69%; P=0.04) and mucinous patients taking statins (I2,
60%; P=0.04), heterogeneity was not significant in most of the
studies analyzed. These values all indicate heterogeneity in
subgroup analysis results. After careful reading of the literature
and a sensitivity analysis, it was found that Feng et al
(29) may be the source of
heterogeneity. The pooled HRs calculated by random effects models
for these heavily heterogeneous subgroup analyses were not
significantly associated and the pooled results were stable in
sensitivity analyses. Sensitivity analyses showed no change in the
direction of effect when omitting one study at a time, and the
pooled results were similar to the overall results (HR, 0.83; 95%
CI, 0.79–0.86; Fig. 11). Notably,
the results of most studies in the subgroup analysis were
statistically significant and consistent with the primary
results.
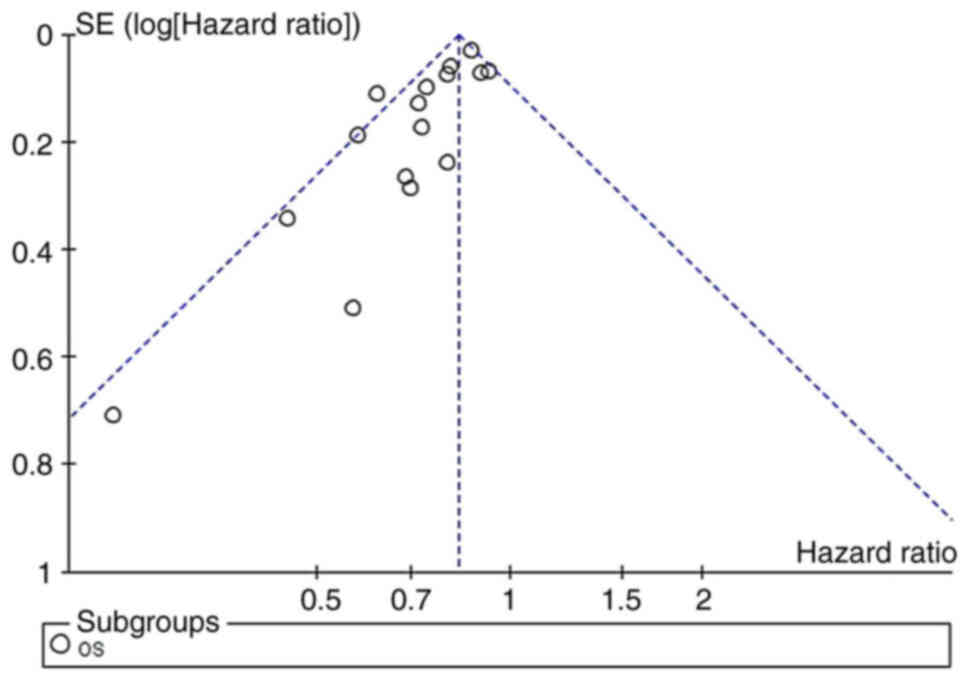
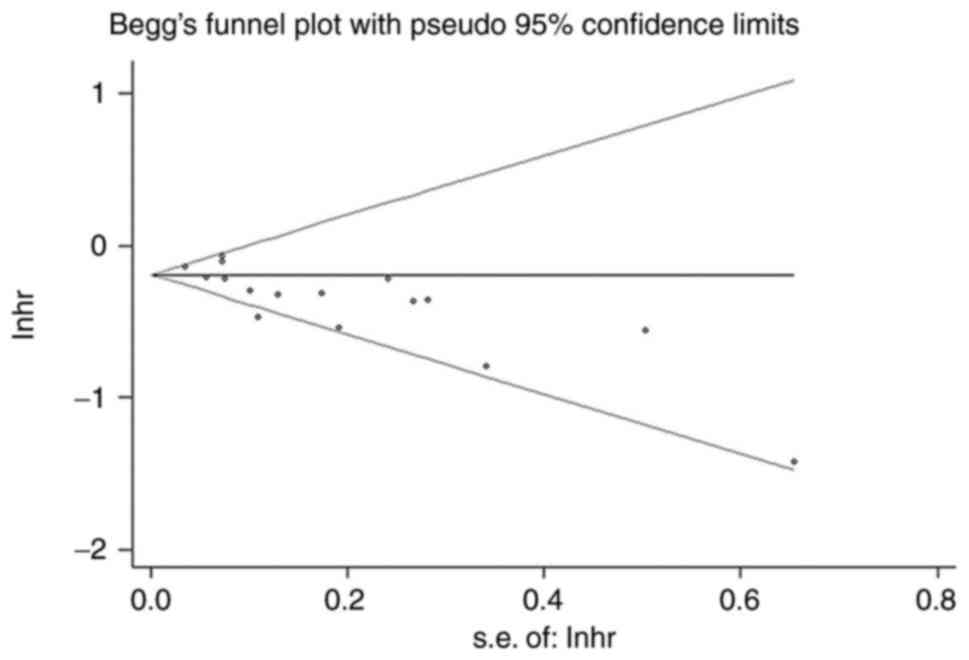
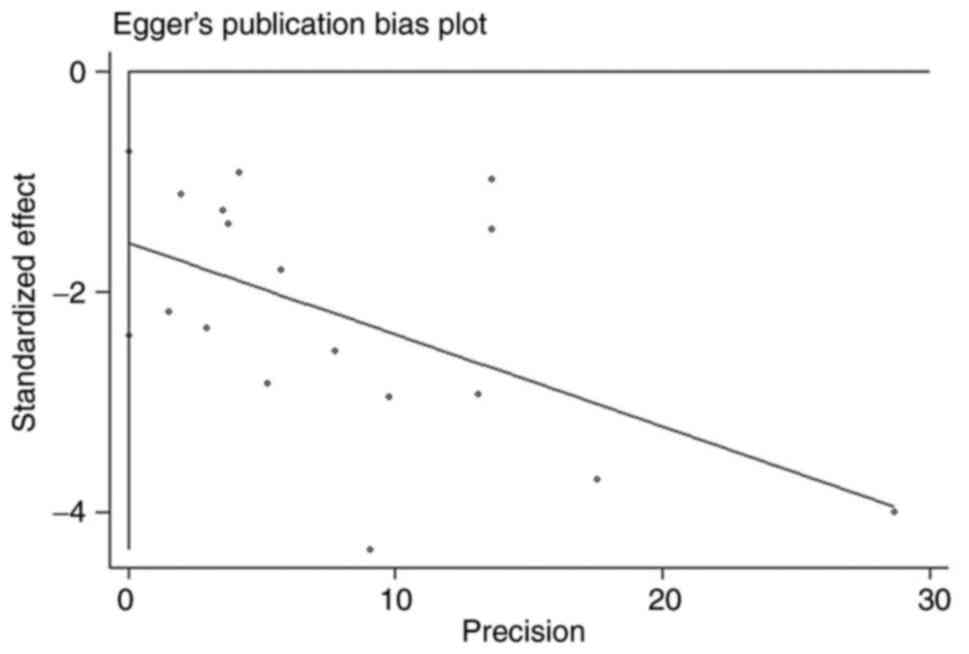
Publication bias
Using funnel plots (Fig. 12), Begg's test (Fig. 13) and Egger's regression test
(Fig. 14) to assess for
publication bias, the Begg's (P=0.027) and Egger tests (P=0.001)
did detect publication bias, and examination of funnel plots showed
visual asymmetry. In similar studies, statistically significant
findings were more likely to be published than non-statistically
significant findings, which may lead to publication bias.
Discussion
Since OC starts insidiously and lacks effective
detection methods in the early stages of the disease, most patients
are already in an advanced stage at the time of diagnosis. Although
there have been rapid developments in chemotherapy, targeted
therapy and immunotherapy for OC, the 5-year OS rate of patients
with OC is <50% (35), and
there is recurrent relapse, which is not easily curable. There is
an urgent need to improve the prognosis of patients with OC or to
achieve an improved synergy with therapy. Therefore, the present
study focused on improving prognosis and prolonging the survival
time of patients with OC.
In the present study, the relationship between
statin use and the prognosis of patients with OC was investigated
by analyzing the results of 11,296 patients with OC taking statins,
with data from 16 individual studies. The use of statins
significantly prolonged the OS time of patients with OC by
improving lipid metabolism, suggesting that statins and other
lipid-lowering drugs may improve the prognosis of patients with OC
by improving lipid metabolism disorders, such as hyperlipidemia.
For example, Habis et al (22) reported that lowering plasma lipid
levels improved the prognosis of patients with OC. Similarly, other
studies showed that the clinical application of statins reduced the
risk of recurrence and metastasis of breast cancer by improving
lipid metabolism disorders (36,37).
Four reasons for this improvement were considered, one of which is
that lipids are the basic building blocks of the membrane
structure, and rapidly dividing cancer cells need more lipids to
synthesize this cell membrane (38). In our preliminary study, it was
found that inhibiting the exogenous lipid uptake by inhibiting CD36
can significantly inhibit the proliferation and migration of breast
cancer cells (39). Statins can
reduce the availability of exogenous lipids to cancer cells and
thus reduce the uptake of exogenous lipids by cancer cells, thus
inhibiting the division and proliferation of tumor cells and
playing an antitumor role (35).
Secondly, lipids can be metabolized through β-oxidation in a more
efficient and effective way to provide more energy to the rapidly
proliferating tumor cells. Camarda et al (40) showed that inhibition of fatty acid
oxidation produced significant antitumor effects. Thirdly, lipids
act as signaling molecules and mediate several pro-cancer signaling
pathways. Liu et al (41)
reported that statins induce the apoptosis of OC cells by
activating JNK and enhancing Bim expression. Another study by Niemi
et al (42) suggested that
OC is associated with lipid metabolism disorders, and statins
induce apoptosis by being involved in signaling pathways, such as
Ras/AMP-activated kinase, Janus kinase/stress-activated protein
kinase, PI3K/AKT and NF-κB, which inhibit the mevalonate pathway to
lower lipid levels and inhibit tumor growth (43–46).
Finally, estrogen produced by adipose tissue-derived aromatase is
the main source of estrogen in postmenopausal women, and elevated
estrogen is associated with the etiology of OC (47). Therefore, obesity is closely
related to the occurrence and prognosis of hormone-sensitive OC.
Furthermore, obesity is linked to the predisposition to lipid
metabolism disorders, such as hyperlipidemia, which indicates that
lipid metabolism disorders are risk factors for OC. Therefore, the
prognosis of patients with OC improves after successful treatment
of lipid metabolism disorders, which was also confirmed in the
present study. All the aforementioned mechanisms suggest that
statins can affect the prognosis of OC by improving lipid
metabolism disorders.
Although the results of the present meta-analysis
revealed that statins significantly improved the OS time in
patients with OC, the current findings did not yield an association
between statin use and the PFS time of patients with OC, and this
is likely due to the small sample size studied. Only 2 studies
(22,25) analyzed the PFS time. Therefore,
more studies are required with PFS as an endpoint.
A subgroup analysis to analyze the association
between statin use and the prognosis of different pathological
types of OC was performed, which found a significant benefit in
patients with serous and endometrioid OC with statin use.
Considering that both these pathological types of OC are associated
with hormone sensitivity, the result corroborates the fourth
mechanism aforementioned. However, no statistical association was
observed for the prognosis of patients with mucinous or clear cell
OC. This may be related to the low prevalence of these two
pathological types, which resulted in a small number of enrolled
cases.
There is no clear recommendation on what type of
statin to use and how to use them clinically. Therefore, a subgroup
analysis on the type and usage of statins was performed in the
present study. It was found that the use of statins after OC
diagnosis significantly prolonged the survival of patients with OC,
while no survival benefit was seen in patients who had been taking
such drugs consistently since before diagnosis. It is possible that
as medications to improve hyperlipidemia were being consistently
used before the diagnosis of OC, the lipid metabolism disorder in
such patients was already corrected to some extent at the time of
inclusion in the study, which may have led to the absence of an
association between lipid-lowering medications and the prognosis of
OC. This also suggests that OC is the result of a combination of
multiple factors. Others have argued that cancer that develops in
the presence of a statin is then ‘resistant’ to statin use after
the diagnosis of OC (29). Based
on the results in the present study, it cannot be argued that there
is no survival benefit for patients with OC who had been using
statins consistently before the OC diagnosis. Regarding the type of
statin used, the subgroup analysis revealed that both lipophilic
and hydrophilic statins improved the prognosis of patients with
OC.
To date, 3 studies have been published on the
association between statin use and OC prognosis (14–16),
but none of them have considered the heterogeneous effects of the
type of statin, mode of use, and the pathological type and clinical
stage of OC. The present study incorporates the most recent studies
with detailed subgroup analyses to provide more specific scientific
evidence for optimal clinical decision-making.
Several limitations of the present meta-analysis
should be considered. Firstly, a number of included studies
evaluated multiple endpoints, resulting in the same study being
evaluated more than once in a single analysis. Secondly, although
the HR data after multifactorial adjustment were combined, numerous
confounding factors affecting the prognostic relevance of statin
use in OC remained. Thirdly, the duration of statin use and
exposure varied across the included studies. The present study did
not allow for a specific analysis and conclusion on the duration of
statin use, and further large clinical trials are needed to draw
any conclusions here. Finally, the present meta-analysis was
limited to studies published in English. Therefore, publication
bias cannot be excluded.
In conclusion, the use of statins significantly
improved the prognosis of patients with OC, especially those with
serous and endometrial OC. It is recommended that statins should be
prescribed as early as possible after the diagnosis of OC to
improve lipid metabolism and prolong patient survival.
Acknowledgements
Not applicable.
Funding
This study was supported by The Key Project Plan of Hebei
Province Medical Science Research in 2018 (grant no. 20180013), and
the 2020 Hebei Provincial Science and Technology Plan Project
(grant no. 20377749D).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZZ and JZ received funding, conceived and designed
the study, and resolved all differences through discussion. HH, QZ,
XW, SC, ZZ and QW performed data extraction, analysis,
interpretation and literature review. QW, ZZ and SC performed
literature collection, statistical analysis. QW wrote the first
draft of the manuscript. JZ revised important intellectual content
manuscript. All authors read and approved the final manuscript. QW
and ZZ confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Viale PH: The American cancer society's
facts & figures: 2020 Edition. J Adv Pract Oncol. 11:135–136.
2020.PubMed/NCBI
|
|
3
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delgado-Ortega L, González-Domínguez A,
Borrás JM, Oliva-Moreno J, González-Haba E, Menjón S, Pérez P,
Vicente D, Cordero L, Jiménez M, et al: The economic burden of
disease of epithelial ovarian cancer in Spain: The OvarCost study.
Eur J Health Econ. 20:135–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orr B and Edwards RP: Diagnosis and
treatment of ovarian cancer. Hematol Oncol Clin North Am.
32:943–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Purdie DM, Bain CJ, Webb PM, Whiteman DC,
Pirozzo S and Green AC: Body size and ovarian cancer: Case-control
study and systematic review (Australia). Cancer Causes Control.
12:855–863. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YC, Park MJ, Ye SK, Kim CW and Kim YN:
Elevated levels of cholesterol-rich lipid rafts in cancer cells are
correlated with apoptosis sensitivity induced by
cholesterol-depleting agents. Am J Pathol. 168:1107–1118.
1404–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dulak J and Józkowicz A: Anti-angiogenic
and anti-inflammatory effects of statins: Relevance to anti-cancer
therapy. Curr Cancer Drug Targets. 5:579–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakobisiak M and Golab J: Potential
antitumor effects of statins (Review). Int J Oncol. 23:1055–1069.
2003.PubMed/NCBI
|
|
11
|
Irvin S, Clarke MA, Trabert B and
Wentzensen N: Systematic review and meta-analysis of studies
assessing the relationship between statin use and risk of ovarian
cancer. Cancer Causes Control. 31:869–879. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lavie O, Pinchev M, Rennert HS, Segev Y
and Rennert G: The effect of statins on risk and survival of
gynecological malignancies. Gynecol Oncol. 130:615–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olsen CM, Green AC, Whiteman DC, Sadeghi
S, Kolahdooz F and Webb PM: Obesity and the risk of epithelial
ovarian cancer: A systematic review and meta-analysis. Eur J
Cancer. 43:690–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohammadian-Hafshejani A, Sherwin CMT and
Heidari-Soureshjani S: Do statins play any role in reducing the
incidence and mortality of ovarian cancer? A systematic review and
meta-analysis. J Prev Med Hyg. 61:E331–E339. 2020.PubMed/NCBI
|
|
15
|
Majidi A, Na R, Dixon-Suen S, Jordan SJ
and Webb PM: Common medications and survival in women with ovarian
cancer: A systematic review and meta-analysis. Gynecol Oncol.
157:678–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X and Zhou J: Impact of postdiagnostic
statin use on ovarian cancer mortality: A systematic review and
meta-analysis of observational studies. Br J Clin Pharmacol.
84:1109–1120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2010.Available online at:. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspSeptember
10–2022
|
|
18
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irwig L, Macaskill P, Berry G and Glasziou
P: Bias in meta-analysis detected by a simple, graphical test.
Graphical test is itself biased. BMJ. 316:470–471. 1998.PubMed/NCBI
|
|
20
|
Elmore RG, Ioffe Y, Scoles DR, Karlan BY
and Li AJ: Impact of statin therapy on survival in epithelial
ovarian cancer. Gynecol Oncol. 111:102–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Urpilainen E, Marttila M, Hautakoski A,
Arffman M, Sund R, Ilanne-Parikka P, Arima R, Kangaskokko J,
Puistola U, Hinkula M and Läärä E: Prognosis of ovarian cancer in
women with type 2 diabetes using metformin and other forms of
antidiabetic medication or statins: A retrospective cohort study.
BMC Cancer. 18:7672018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Habis M, Wroblewski K, Bradaric M, Ismail
N, Yamada SD, Litchfield L, Lengyel E and Romero IL: Statin therapy
is associated with improved survival in patients with
non-serous-papillary epithelial ovarian cancer: A retrospective
cohort analysis. PLoS One. 9:e1045212014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogel TJ, Goodman MT, Li AJ and Jeon CY:
Statin treatment is associated with survival in a nationally
representative population of elderly women with epithelial ovarian
cancer. Gynecol Oncol. 146:340–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verdoodt F, Kjaer Hansen M, Kjaer SK,
Pottegård A, Friis S and Dehlendorff C: Statin use and mortality
among ovarian cancer patients: A population-based cohort study. Int
J Cancer. 141:279–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majidi A, Na R, Jordan SJ, De Fazio A and
Webb PM; OPAL Study Group, : Statin use and survival following a
diagnosis of ovarian cancer: A prospective observational study. Int
J Cancer. 148:1608–1615. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Couttenier A, Lacroix O, Vaes E, Cardwell
CR, De Schutter H and Robert A: Statin use is associated with
improved survival in ovarian cancer: A retrospective
population-based study. PLoS One. 12:e01892332017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bar D, Lavie O, Stein N, Feferkorn I and
Shai A: The effect of metabolic comorbidities and commonly used
drugs on the prognosis of patients with ovarian cancer. Eur J
Obstet Gynecol Reprod Biol. 207:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harding BN, Delaney JA, Urban RR and Weiss
NS: Use of statin medications following diagnosis in relation to
survival among women with ovarian cancer. Cancer Epidemiol
Biomarkers Prev. 28:1127–1133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng JL, Dixon-Suen SC, Jordan SJ and Webb
PM: Statin use and survival among women with ovarian cancer: An
Australian national data-linkage study. Br J Cancer. 125:766–771.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanley GE, Kaur P, Berchuck A, Chase A,
Grout B, Deurloo CM, Pike M, Richardson J, Terry KL, Webb PM and
Pearce CL: Cardiovascular medications and survival in people with
ovarian cancer: A population-based cohort study from British
Columbia, Canada. Gynecol Oncol. 162:461–468. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DS, Ahn HS and Kim HJ: Statin use and
incidence and mortality of breast and gynecology cancer: A cohort
study using the national health insurance claims database. Int J
Cancer. 150:1156–1165. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HY, Wang Q, Xu QH, Yan L, Gao XF, Lu
YH and Wang L: Statin as a combined therapy for advanced-stage
ovarian cancer: A propensity score matched analysis. Biomed Res
Int. 2016:91252382016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang A, Aragaki AK, Tang JY, Kurian AW,
Manson JE, Chlebowski RT, Simon M, Desai P, Wassertheil-Smoller S,
Liu S, et al: Statin use and all-cancer survival: Prospective
results from the women's health initiative. Br J Cancer.
115:129–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Surveillance Epidemiology and End Results
Program, Ovary Cancer Survival Statistics, . http://seer.cancer.gov/statfacts/html/ovary.html2021April
21–2021
|
|
36
|
Kwan ML, Habel LA, Flick ED, Quesenberry
CP and Caan B: Post-diagnosis statin use and breast cancer
recurrence in a prospective cohort study of early stage breast
cancer survivors. Breast Cancer Res Treat. 109:573–579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahern TP, Pedersen L, Tarp M,
Cronin-Fenton DP, Garne JP, Silliman RA, Sørensen HT and Lash TL:
Statin prescriptions and breast cancer recurrence risk: A Danish
nationwide prospective cohort study. J Natl Cancer Inst.
103:1461–1468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cruz PMR, Mo H, McConathy WJ, Sabnis N and
Lacko AG: The role of cholesterol metabolism and cholesterol
transport in carcinogenesis: A review of scientific findings,
relevant to future cancer therapeutics. Front Pharmacol. 4:1192013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao J, Zhi Z, Wang C, Xing H, Song G, Yu
X, Zhu Y, Wang X, Zhang X and Di Y: Exogenous lipids promote the
growth of breast cancer cells via CD36. Oncol Rep. 38:2105–2115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Camarda R, Zhou AY, Kohnz RA, Balakrishnan
S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G, et
al: Inhibition of fatty acid oxidation as a therapy for
MYC-overexpressing triple-negative breast cancer. Nat Med.
22:427–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Liang SL, Kumar S, Weyman CM, Liu W
and Zhou A: Statins induce apoptosis in ovarian cancer cells
through activation of JNK and enhancement of Bim expression. Cancer
Chemother Pharmacol. 63:997–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niemi RJ, Braicu EI, Kulbe H, Koistinen
KM, Sehouli J, Puistola U, Mäenpää JU and Hilvo M: Ovarian tumours
of different histologic type and clinical stage induce similar
changes in lipid metabolism. Br J Cancer. 119:847–854. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park YH, Jung HH, Ahn JS and Im YH: Statin
induces inhibition of triple negative breast cancer (TNBC) cells
via PI3K pathway. Biochem Biophys Res Commun. 439:275–279. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sassano A and Platanias LC: Statins in
tumor suppression. Cancer Lett. 260:11–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang P, Mukthavaram R, Chao Y, Nomura N,
Bharati IS, Fogal V, Pastorino S, Teng D, Cong X, Pingle SC, et al:
In vitro and in vivo anticancer effects of mevalonate pathway
modulation on human cancer cells. Br J Cancer. 111:1562–1571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Campbell MJ, Esserman LJ, Zhou Y,
Shoemaker M, Lobo M, Borman E, Baehner F, Kumar AS, Adduci K, Marx
C, et al: Breast cancer growth prevention by statins. Cancer Res.
66:8707–8714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Risch HA: Hormonal etiology of epithelial
ovarian cancer, with a hypothesis concerning the role of androgens
and progesterone. J Natl Cancer Inst. 90:1774–1786. 1998.
View Article : Google Scholar : PubMed/NCBI
|