Introduction
Epithelial ovarian carcinoma is a common cause of
cancer death in women worldwide. Most cases are diagnosed at
advanced stage due to absence of symptoms during the early stages
and the lack of useful screening methods (1). Primary surgical tumor debulking and
consecutive platinum-based chemotherapy is the standard treatment
strategy for advanced stage ovarian carcinoma patients. Even though
the initial standard treatment is effective, most patients
experience recurrence (1). The
most frequent site of metastasis is the peritoneum. Cerebral and
meningeal metastases are considered rare (2).
Multifocal dissemination of cancer cells from the
primary tumor sites to the subarachnoid, pia mater, and
cerebrospinal fluid (CSF) of the brain and spinal cord causes
carcinomatous meningitis (CM). This condition is also referred to
as ‘leptomeningeal carcinomatosis’, ‘leptomeningeal meningitis’,
‘leptomeningeal metastasis’, or ‘neoplastic meningitis' (3). The term ‘carcinomatous meningitis’
was first coined by Beerman in 1912 when describing a condition
where in cancer cells metastasized to the meninges without visible
invasion of the brain (4). A wide
variety of symptoms are induced depending on the site of
metastasis. This entity occurs in the advanced stages of any solid
cancer and hematological cancer when cancer cells seed through the
CSF and deposit in the meninges (3). Imaging modalities including computed
tomography (CT) and magnetic resonance imaging (MRI) along with CSF
analysis are useful to diagnose this entity; however, an early
diagnosis is difficult. Radiotherapy and chemotherapy are employed
as treatment options; however, the prognosis remains poor (1).
The present report describes the case of a
59-year-old female patient who developed CM as recurrence of
ovarian cancer stage IIIC after presenting with headache and
decreased level of consciousness.
Case report
A 59-year-old Japanese nulligravid underwent
omentectomy and was diagnosed with ovarian high-grade serous
carcinoma stage IIIC according to International Federation of
Gynecologists and Obstetricians staging system. The gross findings
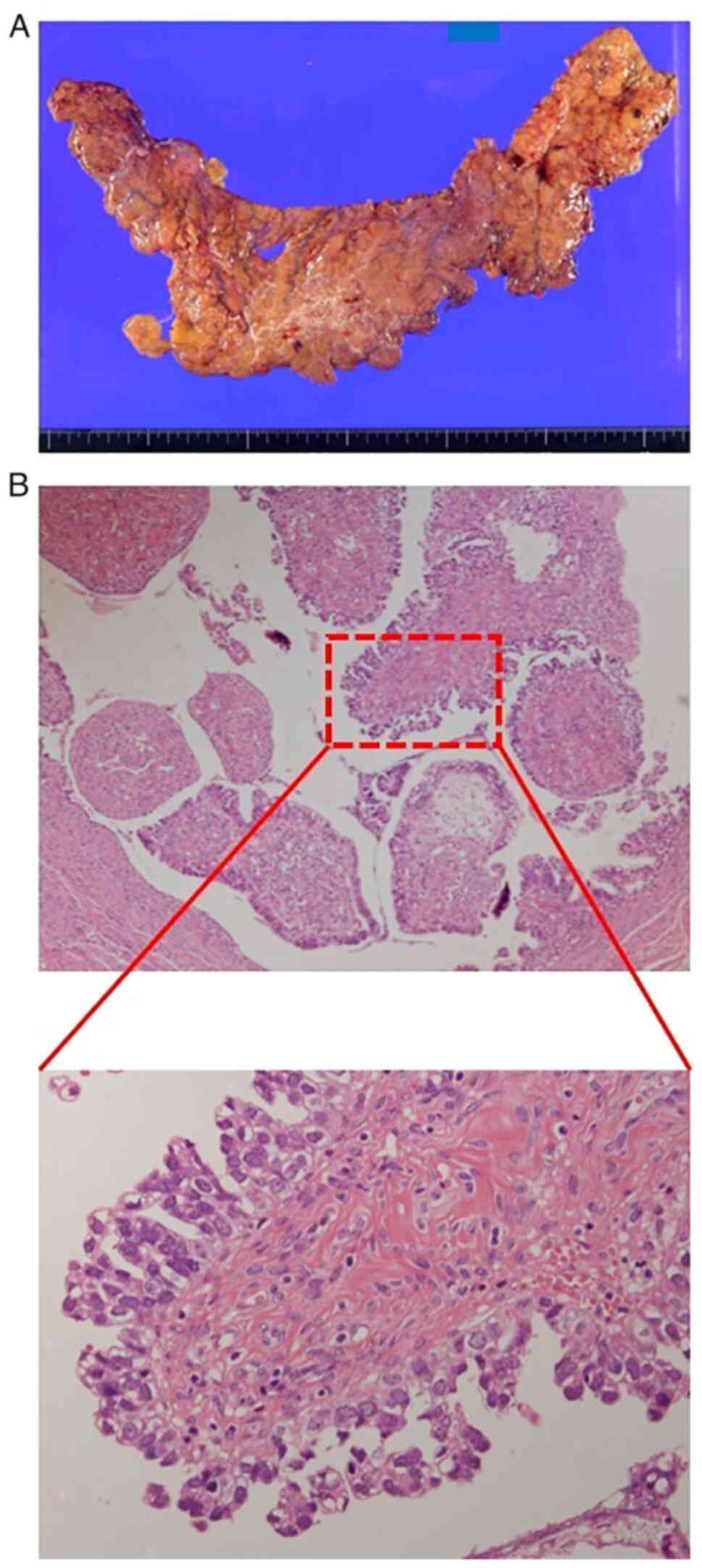
of the resected omentum and the pathological specimens are shown in
Fig. 1. The macroscopic image
shows the omentum with disseminated tumors forming omental cake and
the microscopic images reveals high-grade serous carcinoma with
solid, papillary and glandular structure with nuclear atypia, large
nucleoli, high nuclear to cytoplasmic ratio, and slit-like space.
Hematoxylin and eosin staining was performed in an automated
staining instrument (Tissue-Tek® Prisma™ Plus,
Sakura-finetek) according to the manufacturer's instructions using
Gill's hematoxylin V solution (Muto Pure Chemical Co., Ltd.) and
pure eosin (Muto Pure Chemical Co., Ltd.) at the pathological
department in our hospital. After six cycles of neoadjuvant
chemotherapy combining carboplatin [area under the curve (AUC)=6
mg/ml/min], paclitaxel (175 mg/m2), and bevacizumab (15
mg/kg) administered every 3 weeks, she underwent interval surgical
debulking consisting of a total abdominal hysterectomy with
bilateral salpingo-oophorectomy, pelvic lymphadenectomy, paraaortic
lymphadenectomy and omentectomy without residual tumors. After the
surgery, six cycles of adjuvant chemotherapy combining carboplatin
(AUC=6 mg/ml/min), paclitaxel (175 mg/m2), and
bevacizumab (15 mg/kg) was administered every 3 weeks.
Subsequently, maintenance therapy with bevacizumab (15 mg/kg) was
initiated. After 10 cycles of bevacizumab as maintenance therapy,
she began to develop nausea and vomiting. Despite receiving
infusion at her family physician's clinic, her symptoms fluctuated
but were generally persistent. A month after symptom onset, there
was a noted decline in her consciousness which fluctuated but were
generally persistent also. This prompted admission to our hospital
for further examination.
Upon admission, her vital signs were as follows:
Blood pressure 162/107 mmHg, body temperature 37.4°C, and pulse
rate 84 bpm. Her Glasgow Coma Scale was 15 (E4V5M6). Her laboratory
data showed a normal white blood cell count (7,900/µl) and, an
elevated C-reactive protein (CRP) level (3.35 mg/dl) (normal range:
<0.3 mg/dl), a decreased Na/K/Cl (128/3.3/93 mEq/l) (normal
range: 138 mEq/l< Na <145 mEq/l, 3.6 mEq/l< K <4.8
mEq/l, 101 mEq/l< Cl <108 mEq/l) and an elevated CA125 (114
U/ml) (normal range: <35.0 U/ml). The post-contrast
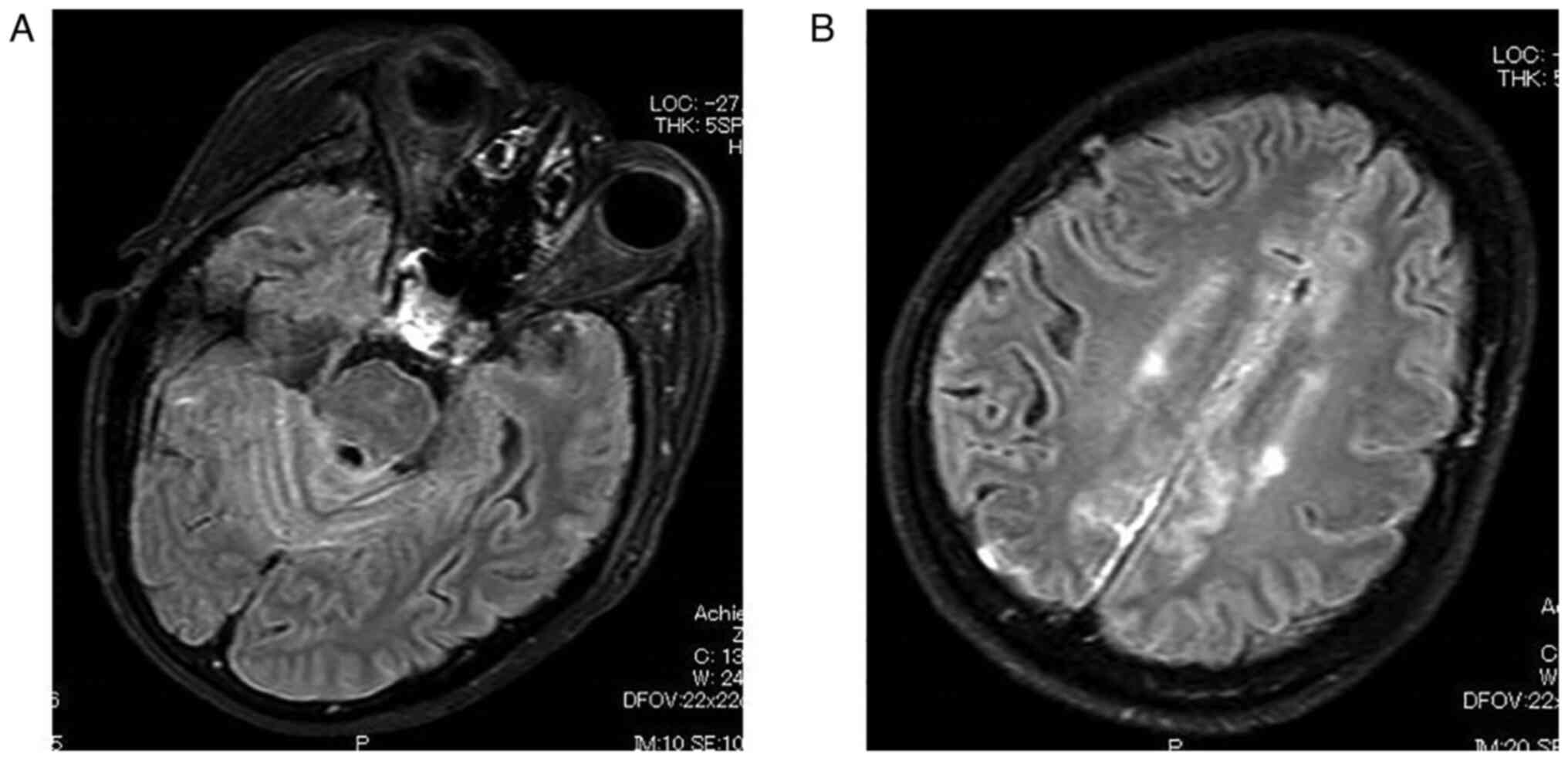
fluid-attenuated inversion-recovery (FLAIR) MRI, which is a MRI
technique that provides strong T2-weighted, CSF signal suppression
and minimized gray matter-to-white matter contrast, showed
leptomeningeal enhancement over all sulci especially around falx
cerebri and cerebellar hemisphere (Philips Ingenia 3.0 was used to
generate the images) (Fig. 2).
These findings led to the consideration of meningitis, specifically
CM, considering the existing ovarian carcinoma. Lumbar puncture was
thus, performed and an opening pressure was 30 cmH2O was
noted. Biochemical analysis of CSF revealed increased number of
cells (14/µl), protein concentration (78 mg/dl) (normal range:
10–52.6 mg/dl), and lactate dehydrogenase concentration (204 IU/l)
(normal range: 0–50 mg/dl), and a decreased glucose concentration
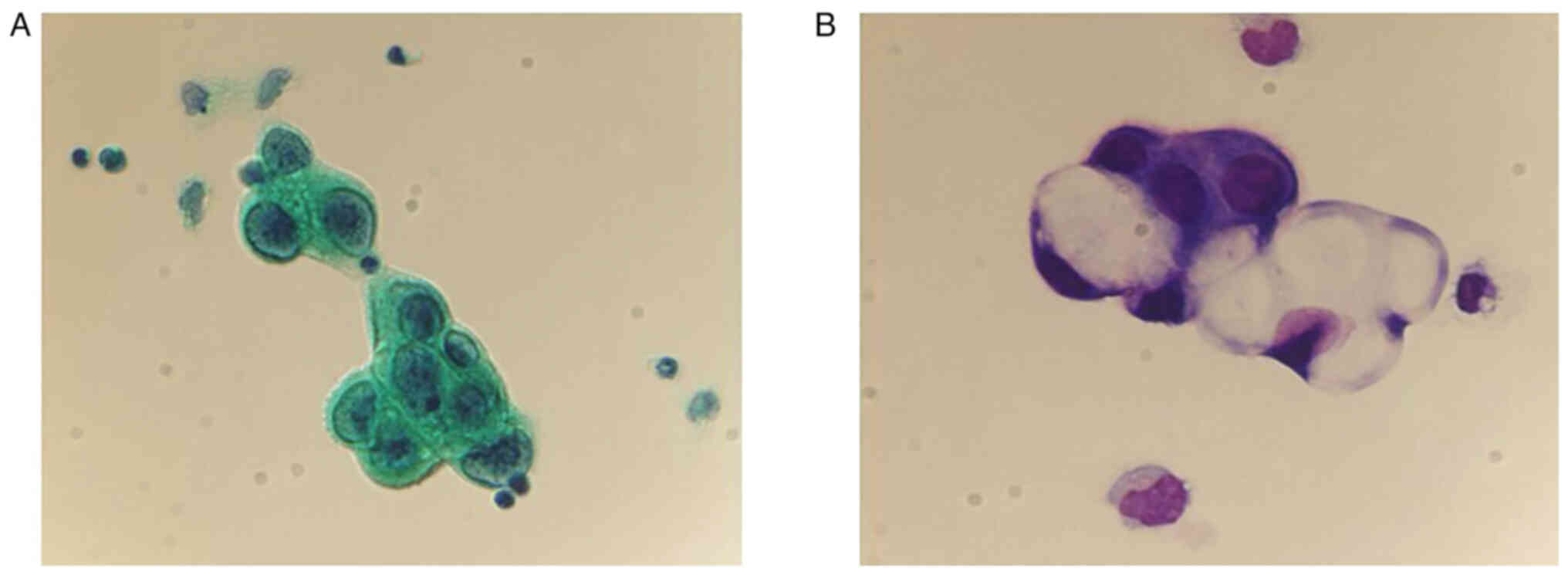
(4 mg/dl) (normal range: 50–80 mg/dl). Cytology of CSF revealed
large tumor cells with increased nucleocytoplasmic ratio, prominent
nucleoli, and centrally placed hyperchromatic nuclei which is
consistent with adenocarcinoma cells leading to the diagnosis of CM
(Fig. 3). Conventional
Papanicolaou staining was performed in an automated staining
instrument (HistoCore Spectra ST, Leica Biosystems) according to
the manufacturer's instructions using OG-6 (Muto Pure Chemical Co.,
Ltd.) and EA-50 (Muto Pure Chemical Co., Ltd.) at the pathological
department in our hospital. Giemsa staining was performed as
fallows at the pathological department in our hospital; i) The
slides were placed in May-Grünwald (Merck) for 3 min. ii) The
slides were placed in phosphate buffer for 1 min. iii) the slides
were placed in dilute giemsa solution (Merck) for 15 min. iv) the
slides were rinsed in deionized water. v) the slides were air dried
and evaluated. After the diagnosis, we proposed treatment with
radiation therapy or intrathecal chemotherapy despite the lack of
evidence of efficacy. However, the patient and her family opted for
palliative care and she expired 30 days after the diagnosis.
Discussion
CM is caused by multifocal dissemination of cancer
cells from the primary tumor sites to the subarachnoid, pia mater,
and CSF in the brain and spinal cord. The incidence rate of CM in
patients with solid tumors is reported to range from 3 to 5%
(5). Melanoma, lung and breast
cancers are the most common solid tumors known to cause CM. CM has
been reported in 23, 9 to 25 and 5% of melanoma, lung cancer, and
breast cancer patients, respectively. Nonetheless, CM may develop
in all types of malignant tumors (5). Although CM may be caused by any
malignant tumor, it is rarely observed in the patients with
gynecological cancers. One study reported that only 0.06, 0.03, and
0% of ovarian, cervical, and endometrial cancer patients, developed
CM, respectively (6). However, the
development of CM in patients with ovarian cancer will likely
increase due to the increase in overall survival resulting from the
improvement of tumor control by more effective therapies.
The mechanisms of leptomeningeal invasion of tumor
cells are thought to involve (1)
hematogenous spread through the arterial or venous circulation,
which is probably the most common route; (2) direct seeding from the existing brain
or spinal parenchymal metastases in contact with the CSF; (3) direct extension from subdural or
extradural tumor; or (4) direct
extension from sites outside of, but adjacent to the central
nervous system (7). For pelvic
tumors, hematogenous spread from the pelvic venous plexus to the
vertebral venous system, also known as the Batson's plexus, is
considered one of the routes of spread to the leptomeninges
(8).
The most common locations affected by leptomeningeal
seeding are the posterior fossa, basal cisterns, and cauda equina,
because of the slower CSF flow and the gravitational effects
(9). The most common symptoms
described in CM patients with solid tumors were headache (39%),
nausea and vomiting (25%), leg weakness (21%), cerebellar
dysfunction (17%), altered mental status (16%), diplopia (14%), and
facial weakness (13%) (10).
Headache, the most common symptom, is caused by raised intracranial
pressure (ICP) or meningeal irritation. In relation to the elevated
ICP, nausea and vomiting accompany the headache, which is noted to
be worse in the morning. As a result of meningeal irritation,
headaches are correlated with nuchal rigidity that is worsened by
leg flexion (Kernig sign) (3).
However, nuchal rigidity is observed in only 15% of cases (9). Involvement of the spinal cord and its
nerve roots in CM causes symptoms in the anatomically associated
regions. Segmental numbness, pain, dysesthesia, and lower motor
neuron pattern limb weakness are symptoms caused by spinal nerve
cord involvement. Bladder and bowel dysfunction result from sacral
nerve root involvement. Although the symptoms mentioned above are
common, clinical signs and symptoms may still be absent in 25% of
cases at the time of diagnosis (11). In the current case, the patient
experienced headache, nausea, and vomiting due to raised ICP.
To diagnose this entity, obtaining a comprehensive
history and physical exam including a neurologic exam is an
essential first step. The appropriate neurologic exam and
proficient knowledge of this entity leads physicians to suspect CM
(3). Gadolinium-enhanced MRI is a
useful modality to diagnose CM in which the sensitivity is 76%
(12). Both focal and diffuse
leptomeningeal enhancement of the brain in the T1-weighted image
with contrast are typical findings (11). Although conventional
gadolinium-enhanced T1-weighted images are largely employed to
diagnose CM, there are cases where no enhancement is seen, such as
in the current case. For these cases, FLAIR sequences with contrast
have been reported to show better sensitivity in detecting CM
(13). The common areas that show
enhancement are the basilar cisterns, cerebral convexities,
cerebellar folia, and ventricular ependymal regions (2). In the current case, FLAIR sequences
were useful to arrive at the diagnosis.
Enhancement on MRI is observed in both CM and
inflammatory meningitis; therefore, it is crucial to differentiate
the two through CSF analysis (3).
CSF analysis is crucial in diagnosing CM. CSF abnormalities have
been reported in >90% of CM patients. These irregularities may
present as the following: i) high CSF pressure >25
cmH2O, detected in ~50% of cases (14); ii) elevated CSF protein levels, in
~80% of cases (15); iii)
decreased CSF glucose level (hypoglycorrhachia), in ~25–40% of
cases (14); iv) pleocytosis, in
~33–79% of cases (16); and v) a
positive CSF tumor cytology, the most important and gold standard
test to diagnose CM, detected in 45–55, 80, and 90% of cases at a
first, second, and third lumbar puncture (7). Lumbar puncture just before
gadolinium-enhanced MRI should be avoided because it may cause
artificial contrast enhancement of the leptomeninges from
persistent lumber CSF leakage and intracranial hypotension
associated with venous vasodilation (5). In the current case, adenocarcinoma
was detected on the first lumbar puncture and CSF characteristics
showed an elevated opening pressure and protein level, and a
decreased glucose level. These findings helped clinch the
diagnosis.
The differential diagnoses that should be taken into
consideration are as follows: i) Intraparenchymal primary brain
lesions; ii) chronic or recurrent meningitis caused by a variety of
bacterial, fungal, viral, or protozoal organisms; iii) meningitis
caused by an autoimmune disease or drugs; and iv) paraneoplastic
syndromes, including Lambert Eaton syndrome, Myasthenic crises,
cerebellar degeneration, encephalomyelitis, neuropathies, and
limbic encephalitis (3).
Because of its rarity, there is no standard
treatment for this entity because there are currently no clinical
trials to establish standard treatment for this disease. Most
patients underwent intrathecal chemotherapy with or without
radiation therapy. The efficacy of most systemic chemotherapy
agents are limited due to the blood-brain barrier; therefore,
intrathecal chemotherapy remains the mainstay of treatment for CM
(3). The most common drugs for the
intrathecal route are methotrexate, cytarabine, and less commonly,
thiotepa (3). Radiation therapy
for CM consists of diffuse radiation therapy to linear
leptomeningeal contrast-enhanced lesions or focal radiation to
nodular plaque-like meningeal deposits of malignant cells (3).
CM is usually caused by advanced stage disease;
therefore, the prognosis of this entity is poor with a median
survival time of 2 to 4 months even with treatment (17). Low CSF protein, normal CSF glucose
level, preserved cognitive function, and controlled systemic
disease are reported to be associated with better survival
(6). In the current case, the
patient demised 30 days after diagnosis without treatment.
Although CM is rare, clinicians should consider this
unusual complication whenever patients with malignancies experience
neurological symptoms including headache, nausea, and vomiting that
cannot be easily explained or treated. With improved locoregional
control and survival, CM will likely become more prevalent.
Awareness of this condition should help clinicians maintain a high
index of suspicion for CM and lead them to an accurate diagnosis.
Therefore, owing to its rarity, case reports such as the one
presented, are essential in facilitating the dialogue needed to
spread awareness of this clinical entity.
Acknowledgements
Not applicable.
Funding
This study was funded by The Osaka Medical Research Foundation
for Intractable Diseases (grant no. 27-2-4).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EU, TF and TS conceived and designed the study. EU,
TF, KI, MY, MK, TI, YK and TY acquired, analyzed and interpreted
the data. UE, TF and TS drafted and revised the manuscript. TF and
TS confirm the authenticity of all the raw data. YK reviewed the
pathological specimens. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toyoshima M, Tsuji K, Shigeta S, Tokunaga
H, Ito K, Watanabe Y, Yoshinaga K, Otsuki T, Niikura H and Yaegashi
N: Leptomeningeal metastasis from gynecologic cancers diagnosed by
brain MRI. Clin Imaging. 41:42–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anwar A, Gudlavalleti A and Ramadas P:
Carcinomatous meningitis. StatPearls. StatPearls Publishing
Copyright © 2021. StatPearls Publishing LLC.; Treasure Island (FL):
2021
|
|
4
|
Delle Grottaglie B, Girotti F, Ghisolfi A,
Tafi A and Pescia M: A case of carcinomatous meningitis with
papilledema as the only symptom: Fvorable response to intrathecal
chemotherapy. Ital J Neurol Sci. 4:95–97. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gleissner B and Chamberlain MC: Neoplastic
meningitis. Lancet Neurol. 5:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yust-Katz S, Mathis S and Groves MD:
Leptomeningeal metastases from genitourinary cancer: The University
of Texas MD Anderson cancer center experience. Med Oncol.
30:4292013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Rhun E, Taillibert S and Chamberlain
MC: Carcinomatous meningitis: Leptomeningeal metastases in solid
tumors. Surg Neurol Int. 4 (Suppl 4):S265–S288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batson OV: The function of the vertebral
veins and their role in the spread of metastases. 1940. Clin Orthop
Relat Res. 4–9. 1995.PubMed/NCBI
|
|
9
|
Chamberlain MC: Neoplastic meningitis.
Oncologist. 13:967–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke JL: Leptomeningeal metastasis from
systemic cancer. Continuum (Minneap Minn). 18:328–342.
2012.PubMed/NCBI
|
|
11
|
Beauchesne P: Intrathecal chemotherapy for
treatment of leptomeningeal dissemination of metastatic tumours.
Lancet Oncol. 11:871–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ko Y, Gwak HS, Park EY, Joo J, Lee YJ, Lee
SH, Kwon JW, Shin SH and Yoo H: Association of MRI findings with
clinical characteristics and prognosis in patients with
leptomeningeal carcinomatosis from non-small cell lung cancer. J
Neurooncol. 143:553–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kremer S, Abu Eid M, Bierry G, Bogorin A,
Koob M, Dietemann JL and Fruehlich S: Accuracy of delayed
post-contrast FLAIR MR imaging for the diagnosis of leptomeningeal
infectious or tumoral diseases. J Neuroradiol. 33:285–291. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taillibert S, Laigle-Donadey F,
Chodkiewicz C, Sanson M, Hoang-Xuan K and Delattre JY:
Leptomeningeal metastases from solid malignancy: A review. J
Neurooncol. 75:85–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foo CT, Burrell LM and Johnson DF: An
unusual presentation of carcinomatous meningitis. Oxf Med Case
Reports. 2016:omw0682016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nayar G, Ejikeme T, Chongsathidkiet P,
Elsamadicy AA, Blackwell KL, Clarke JM, Lad SP and Fecci PE:
Leptomeningeal disease: Current diagnostic and therapeutic
strategies. Oncotarget. 8:73312–73328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thakkar JP, Kumthekar P, Dixit KS, Stupp R
and Lukas RV: Leptomeningeal metastasis from solid tumors. J Neurol
Sci. 411:1167062020. View Article : Google Scholar : PubMed/NCBI
|