Introduction
Oral cavity squamous cell carcinoma (OCSCC) is a
malignancy that accounts for 2–3% of all malignancies (1–5). In
addition, 90% of all oral cancers arising from the oral mucosa are
squamous cell carcinoma (SCC) in origin (6,7). The
mainstay of the clinical assessment of oral lesions is a
histological diagnosis. Treatment decisions should be based on a
microscopic diagnosis instead of clinical presentation, since the
prediction of which lesions will progress to invasive carcinoma and
which will remain stable with an indolent clinical course is
challenging.
Numerous variables have been identified as potential
prognostic factors in oral carcinoma, and these can be mainly
categorized as tumor-, patient- and treatment-related factors
(8). The Tumor, Node, Metastasis
(TNM) stage, histological grade and tumor thickness are widely
recognized as prognostic factors; however, the prognostic value of
other clinicopathological factors is often uncertain and
controversial (9).
Multiparametric histological risk assessment has
been reported to predict the survival of patients and differentiate
between high- and low-risk patients. Several parameters have been
used to predict the outcome of malignant disease in OCSCC,
including lymphovascular invasion (LVI), perineural invasion (PNI),
worst pattern of invasion (WPOI), surgical margin depth of invasion
(DOI) and extracapsular extension, which are widely used as
indicators of aggressive behavior (10–12).
These have been reported to be adverse prognostic factors in OCSCC,
associated with the risk of local recurrence (LR) and lymph node
metastasis (13,14). The present study aimed to determine
the efficacy of WPOI and other histopathological features as
prognostic factors for OCSCC and analyze the impact of resection
margin status and histopathological as prognostic factors for LR
and the overall survival (OS) of patients with OCSCC in a study
population.
Materials and methods
Study design
A retrospective cohort study was designed to
determine the efficacy of WPOI and other histopathological features
as prognostic factors in oral cancer.
Patients and setting
All patients diagnosed with OCSCC, treated and
followed up at King Abdulaziz University Hospital (KAUH; Jeddah,
Saudi Arabia) between January 2012 and December 2019 were included
in the study. All patients who underwent surgical resection of the
primary tumor, with or without radiation therapy or
chemoradiotherapy were included. A chart review of these patients
was conducted to determine the following parameters: Age, sex, risk
factors (tobacco and alcohol), lesion site, TNM staging,
histopathological parameters, treatment protocol, treatment
response and outcome. The medical records of all the included
patients were reviewed and carefully studied. Patients who had only
a biopsy of the primary tumor with insufficient follow-up data were
excluded from the study.
Medical records
A datasheet was created to include all the
demographic data of the patients, including medical record number,
age at the time of diagnosis, sex and risk factors. The datasheet
also included the disease parameters, namely the diagnosis, date of
diagnosis and site of the primary lesion. The type of surgical
intervention, whether reconstruction was performed, neck
dissection, date of the intervention, and the administration of
radiation or chemotherapy were also included. In addition,
histopathological factors [tumor grade (differentiation), DOI
measured from the tumor surface to the deepest point of invasion,
TNM stage, lymphatic invasion, blood vessel invasion, WPOI and
PNI], regional control, disease-free survival (DFS), date of
recurrence, OS and date of death or last follow-up were also
recorded.
An author and an experienced pathologist at King
Abdulaziz University Hospital performed a histopathological review
of the obtained records. After retrospective data collection, all
the specimens were re-examined to evaluate and examine the WPOI,
which was determined via the assessment of hematoxylin and
eosin-stained and pan-cytokeratin-immunostained sections.
Differentiation was classified according to the World Health
Organization grade (15). Staging
was classified according to the eighth edition of the American
Joint Commission on Cancer/Union for International Cancer Control
TNM classification (16). All
information obtained during the study was kept confidential,
including the identities of the subjects, who were assigned
anonymous identification numbers.
Statistical analysis
Statistical analysis was conducted using IBM SPSS
statistics version 20.0 (IBM Corp.). Basic descriptive statistics
were used to compare patient characteristics, including
socioeconomic, clinical and treatment characteristics (surgery
alone vs. surgery with radiation therapy or chemoradiotherapy),
histopathological features (tumor size, DOI, WPOI, PNI, LVI,
extracapsular extension and surgical margins), tumor
characteristics (tumor site, stage and type of histopathological
differentiation), patient outcome and OS. Univariate and
multivariate binary logistic regression analyses were used to
evaluate the effects of specific risk factors on outcome and
recurrence rate. Kaplan-Meier survival analysis was used to
evaluate the influence on LR, OS and DFS of various factors,
including the histopathological pattern of invasion (POI). DFS was
analyzed based on the recurrence rate for different WPOI
categories.
Results
Patient sociodemographic
characteristics
A total of 63 patients were included in the study,
of which 34 (54%) were men and 29 were women, with a median age of
61 years (range, 31–87 years). Patient age was categorized into two
groups: ≤60 years and >60 years. The median follow-up period was
473 days (range, 3–2,422 days). Eight patients (12.7%) were
smokers, 4 patients had never smoked and the smoking status of the
remainder of the patients (n=51; 81%) was unknown. All patients
denied a history of current or previous alcohol consumption.
Clinical characteristics
Of the 63 patients, 61.9% had SCC of the tongue
(n=39), 27% of the buccal mucosa (n=17), 3.2% each of the hard
palate (n=2), inferior alveolar ridge (n=2) and floor of the mouth
(n=2) and 1.6% of the superior alveolar ridge (n=1). According to
the TNM staging classification, 15 (23.8%), 16 (25.4%), 4 (6.3%),
23 (36.5%) and 5 (7.9%) patients were classified as having TI, T2,
T3, T4A and T4B disease, respectively. Concerning lymph node
staging, a high proportion of the patients (41.3%; n=26) had no
regional lymph node involvement, but 4 (6.3%), 17 (27%), 11
(17.5%), 3 (4.8%) and 2 (3.2%) patients were classified as having
N1, N2a, N2b, N2c and N3a disease, respectively. Among the
different treatment modalities, 11 (17.5%) of patients received
surgery alone, 52 (82.5%) underwent surgery followed by adjuvant
radiotherapy and 16 (25%) underwent surgery with adjuvant
chemoradiotherapy. Overall, 41.3% of patients experienced tumor
recurrence, while 58.7% had no recurrence, were lost to follow-up
or did not develop the outcome by the end of the study. Death was
reported for 18 patients, and the others were alive, lost to
follow-up or had an undocumented death status.
WPOI
In regard to the WPOI, the most frequently observed
classification was grade 3 in 24 patients (38.1%), and 10 (15.9%),
6 (9.5%), 13 (20.6%) and 10 (15.9%) cases were classified as grades
1, 2, 4 and 4, respectively. WPOI was re-categorized into
‘aggressive’ and ‘non-aggressive’ patterns, where aggressive
included grade 4 and 5 tumors and non-aggressive included grade 1–3
tumors.
Other histopathological factors
PNI was observed in 49.2% of the patients. The
relationship of PNI to the tumor was intramural in 62.9% and
intratemporal in 37% of cases. Regarding the size of the nerve, of
the 31 cases with PNI, 9 tumors affected large nerves and 18 tumors
affected small nerves. The remaining cases could not be categorized
due to lack of documentation. LVI was detected in only 19% of the
patients. Regarding the surgical margins, most of the patients had
a tumor-free surgical margin (87.3%; n=55), 9.5% (n=6) had close
margins and only 3.2% (n=2) had positive margins. According to the
histopathological differentiation of SCC, well-differentiated SCC
was the most reported type (49.2%). By comparison, poorly
differentiated SCC was the least reported type (7.9%) and
moderately differentiated was of intermediate incidence (42.9%).
Comparing the histopathological differentiation of recurrent cases,
among the 31 cases diagnosed as well-differentiated 13 (41.9%) were
recurrent, and among the 27 cases diagnosed as moderately
differentiated and the 5 cases diagnosed as poorly differentiated,
11 (40.7%) and 2 (40.0%), respectively, were recurrent. Tumor size
was categorized according to the T staging; in the majority of the
patients (54%) the tumor size was >2 cm but ≤4 cm. Regarding the
DOI, one-third (33.3%) of SCCs were ≤5 mm in depth, 25.4 % were
between 5 and 10 mm in depth, and 41.3% were >10 mm in depth.
Extracapsular extension was present in 18 patients (28.6%).
Univariate analysis
Univariate logistic regression analysis was used to
evaluate which risk factors, if any, influenced the recurrence rate
of OCSCC. No significant association was detected with age,
histopathological differentiation, T stage, DOI, LVI or surgical
margin. However, a significant influence of WPOI [74.9%, odds ratio
(OR)=198, P<0.0005] was observed, with recurrence significantly
more likely in subjects with the aggressive pattern than in those
with the non-aggressive pattern. Patients with PNI were also
significantly more likely to experience recurrence (60.2%,
OR=51.429, P<0.0005; Table
I).
 | Table I.Univariate logistic regression
analysis of risk factors affecting recurrence rate. |
Table I.
Univariate logistic regression
analysis of risk factors affecting recurrence rate.
|
| Recurrence |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Risk factor | No | Yes | Odds ratio | Nagelkerke
R2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
≤60 | 20 | 15 | (Ref.) |
|
|
|
>60 | 17 | 11 | 0.863 | 0.002 | 0.775 |
| Histopathological
differentiation grade |
|
|
|
|
|
|
Well-differentiated | 18 | 13 | (Ref.) |
| 0.994 |
|
Moderately differentiated | 16 | 11 | 0.952 |
| 0.927 |
| Poorly
differentiated | 3 | 2 | 0.923 | 0.000 | 0.935 |
| Staging |
|
|
|
|
|
|
Early-stage oral cancer
pattern | 19 | 12 | (Ref.) |
|
|
|
Advanced stage oral cancer
pattern | 18 | 14 | 1.231 | 0.004 | 0.685 |
| DOI (mm) |
|
|
|
|
|
| ≤5 | 14 | 7 | (Ref.) |
| 0.659 |
| >5
and ≤10 | 9 | 7 | 1.556 |
| 0.518 |
|
>10 | 14 | 12 | 1.714 | 0.018 | 0.375 |
| WPOI |
|
|
|
|
|
|
Non-aggressive pattern | 36 | 4 | (Ref.) |
|
|
|
Aggressive pattern | 1 | 22 | 198.000 | 0.749 | <0.0005 |
| LVI |
|
|
|
|
|
|
Absent | 32 | 19 | (Ref.) |
|
|
|
Present | 5 | 7 | 2.358 | 0.037 | 0.189 |
| PNI |
|
|
|
|
|
|
Absent | 30 | 2 | (Ref.) |
|
|
|
Present | 7 | 24 | 51.429 | 0.602 | <0.0005 |
| Surgical
margins |
|
|
|
|
|
| Free
(≥5 mm) | 35 | 20 | (Ref.) |
| 0.388 |
| Close
(<5 mm) | 2 | 4 | 3.500 | 0.116 | 0.169 |
|
Positive | 0 | 2 |
|
| 0.999 |
Univariate logistic regression analysis was also
used to evaluate which risk factors, if any, influenced OS. No
significant association with age, staging, WPOI or PNI was
detected. However, a significant effect of the presence of LVI
(12.8%, OR=5.091, P=0.016) and margins (10.6%, OR=1.060, P=0.043)
was observed when comparing close (<5 mm) with free (≥5 mm).
Other factors identified as being significant influences on OS
included DOI (14.5%, OR=5.029, P=0.026) when comparing 6–10 mm with
≤5 mm, histopathological grade (15.3%, OR=16.667, P=0.020) when
comparing poorly differentiated to well-differentiated, and size of
the nerve (23.4%, OR=7.000, P=0.032) when comparing large (>1
mm) to small (<1 mm; Table
II)
 | Table II.Univariate logistic regression
analysis of risk factors affecting death status. |
Table II.
Univariate logistic regression
analysis of risk factors affecting death status.
|
| Death |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Risk factor | No | Yes | Odds ratio | Nagelkerke
R2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
≤60 | 27 | 8 | (Ref.) |
|
|
|
>60 | 18 | 10 | 1.875 | 0.028 | 0.265 |
| Histopathological
differentiation grade |
|
|
|
|
|
|
Well-differentiated | 25 | 6 | (Ref.) |
| 0.064 |
|
Moderately differentiated | 19 | 8 | (Ref.) |
| 0.365 |
| Poorly
differentiated | 1 | 4 | 16.667 | 0.153 | 0.020 |
| Staging |
|
|
|
|
|
|
Early-stage oral cancer
pattern | 25 | 6 | (Ref.) |
|
|
|
Advanced stage oral cancer
pattern | 20 | 12 | 2.500 | 0.057 | 0.116 |
| DOI (mm) |
|
|
|
|
|
| ≤5 | 19 | 2 | 7.389 |
| 0.077 |
| >5
and ≤10 | 9 | 7 | 5.029 | 0.145 | 0.026 |
|
>10 | 17 | 9 | (Ref.) |
| 0.057 |
| WPOI |
|
|
|
|
|
|
Non-aggressive pattern | 32 | 8 | (Ref.) |
|
|
|
Aggressive pattern | 13 | 10 | 3.077 | 0.085 | 0.052 |
| LVI |
|
|
|
|
|
|
Absent | 40 | 11 | (Ref.) |
|
|
|
Present | 5 | 7 | 5.091 | 0.128 | 0.016 |
| PNI |
|
|
|
|
|
|
Absent | 26 | 6 | (Ref.) |
|
|
|
Present | 19 | 12 | 2.737 | 0.069 | 0.085 |
| Size of the
nerve |
|
|
|
|
|
|
Small | 14 | 4 | (Ref.) |
|
|
|
Large | 3 | 6 | 7.000 | 0.234 | 0.032 |
| Surgical
margins |
|
|
|
|
|
| Free
(≥5 mm) | 42 | 13 | (Ref.) |
| 0.104 |
| Close
(<5 mm) | 2 | 4 | 1.060 | 0.106 | 0.043 |
|
Positive | 1 | 1 | 3.231 |
| 0.418 |
Multivariate analysis
Multivariate binary logistic regression analysis was
used to test the risk factors for recurrence. Only two variables
were entered into the model, namely WPOI and PNI, as they were the
only variables with P<0.1 in the univariate analysis of LR. As a
result, the model was accurate (R2=0.768). However, only
WPOI was found to be a significant risk factor with P=0.001
(OR=66), indicating that those with the aggressive pattern were
more likely to experience a recurrence (Table III).
 | Table III.Multivariate binary logistic
regression analysis of risk factors affecting the recurrence
rate. |
Table III.
Multivariate binary logistic
regression analysis of risk factors affecting the recurrence
rate.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Risk factor | P-value | Odds ratio | Lower | Upper |
|---|
| WPOI
(non-aggressive vs. aggressive) | 0.001 | 66.000 | 5.079 | 857.679 |
| PNI (absent vs.
present) | 0.142 | 5.000 | 0.584 | 42.797 |
Multivariate binary logistic regression analysis was
also used to test the risk factors for mortality. The variables
shown in Table IV were entered
into the model, which were the variables with P<0.1 in the
univariate analysis. The model was accurate (R2=0.614),
but only the size of the nerve was found to have a significant
effect (OR=26.364, P=0.038) when comparing large (>1 mm) with
small (<1 mm).
 | Table IV.Multivariate binary logistic
regression analysis of risk factors affecting mortality status. |
Table IV.
Multivariate binary logistic
regression analysis of risk factors affecting mortality status.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Risk factor | P-value | Odds ratio | Lower | Upper |
|---|
| Age | 0.455 | 3.566 | 0.452 | 54.453 |
| Grade | 0.210 | 5.321 | 0.391 | 72.455 |
| Staging | 0.999 | 0.000 | 0.000 | 33.478 |
| DOI | 0.688 | 2.685 | 0.432 | 60.486 |
| WPOI | 0.709 | 2.151 | 0.039 | 119.432 |
| LVI | 0.707 | 0.496 | 0.013 | 19.124 |
| PNI | 0.547 | 2.185 | 0.172 | 27.780 |
| Size of the
nerve | 0.038 | 26.364 | 1.198 | 580.189 |
| Margin | 0.865 | 0.000 | 0.000 |
|
Survival analysis
OS
OS was calculated from the time (in days) of initial
surgical management to the date of the event (death), or to the
censoring time in patients who did not develop the event by the end
of the study or whose death status was not reported.
Kaplan-Meier estimates were calculated according to
the stage of oral cancer by creating two groups: Those with stages
I and II (early stage) and those with stage III, IVa, and IVb
(advanced stage) oral cancer. According to the Kaplan-Meier
survival estimates for the entire cohort, assuming a 0.05 level of
significance and using the log-rank test for equality of survivor
functions, a statistically significant difference in time-to-death
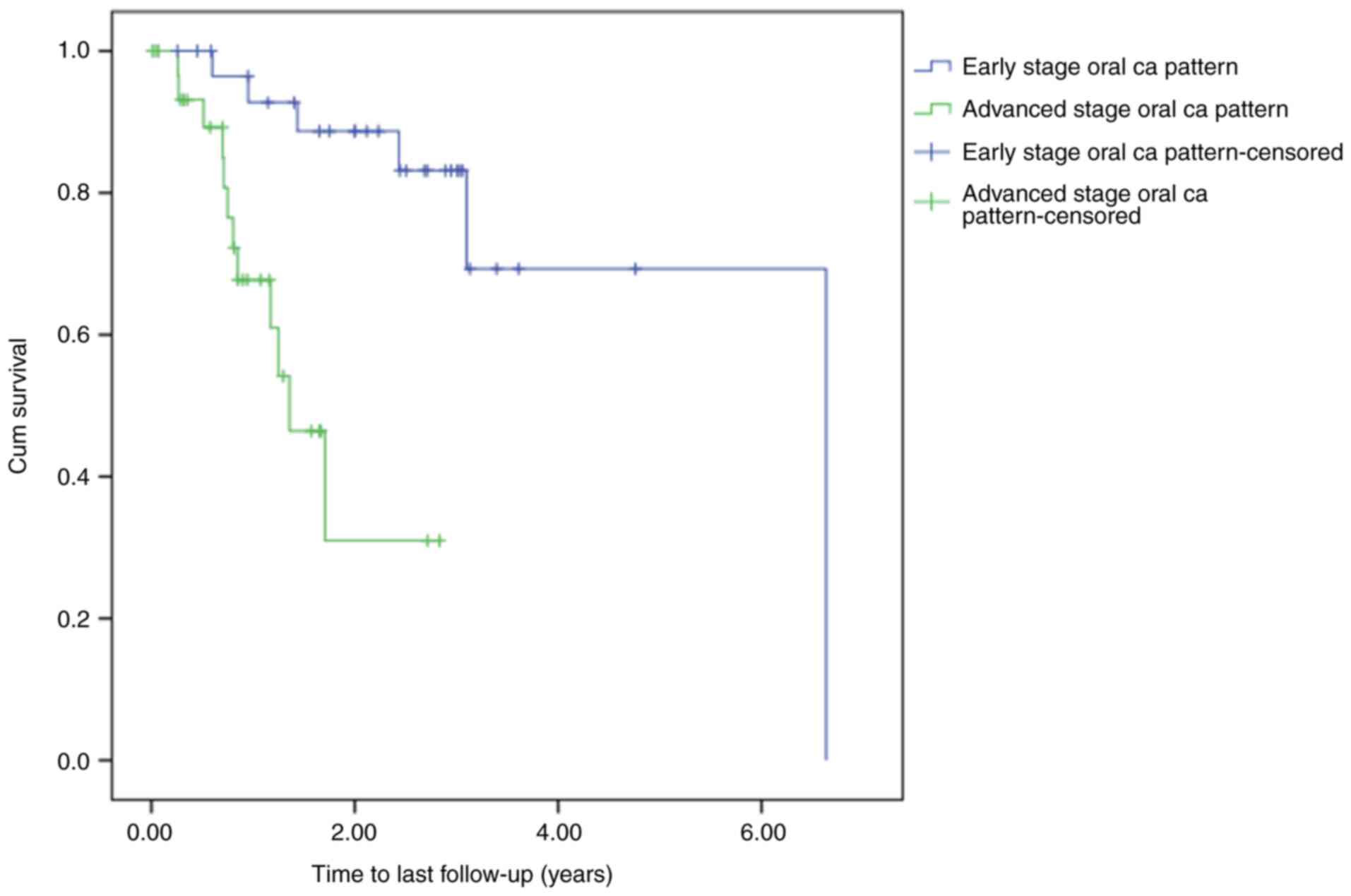
was observed between the groups (P<0.0005; Fig. 1).
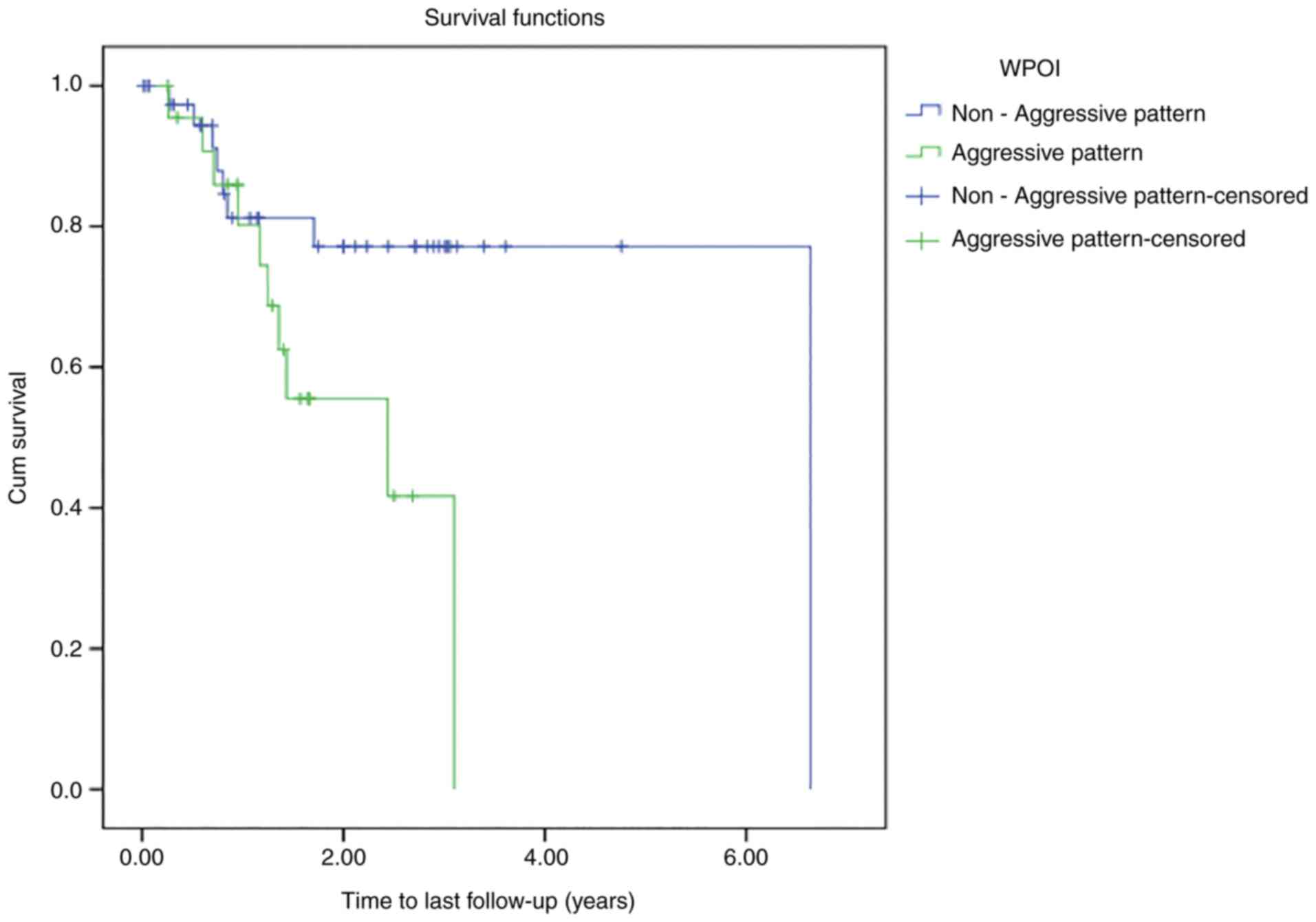
Kaplan-Meier estimates were also calculated
according to WPOI by creating two new groups: Those with grade 4
and 5 tumors (aggressive pattern) and those with grade 1–3 tumors
(non-aggressive pattern). According to the Kaplan-Meier survival
estimates for the entire cohort, assuming a 0.05 level of
significance and using the log-rank test for equality of survivor
functions, a statistically significant difference in time-to-death
was detected between the groups (P=0.036; data not shown).
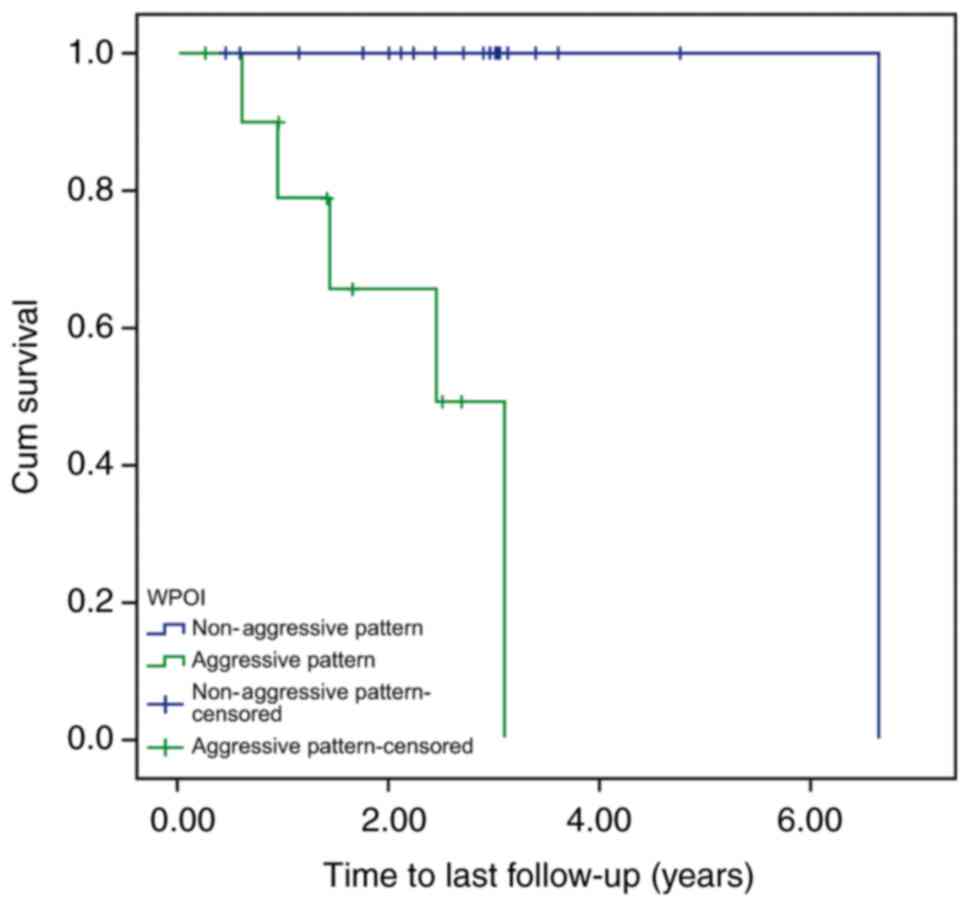
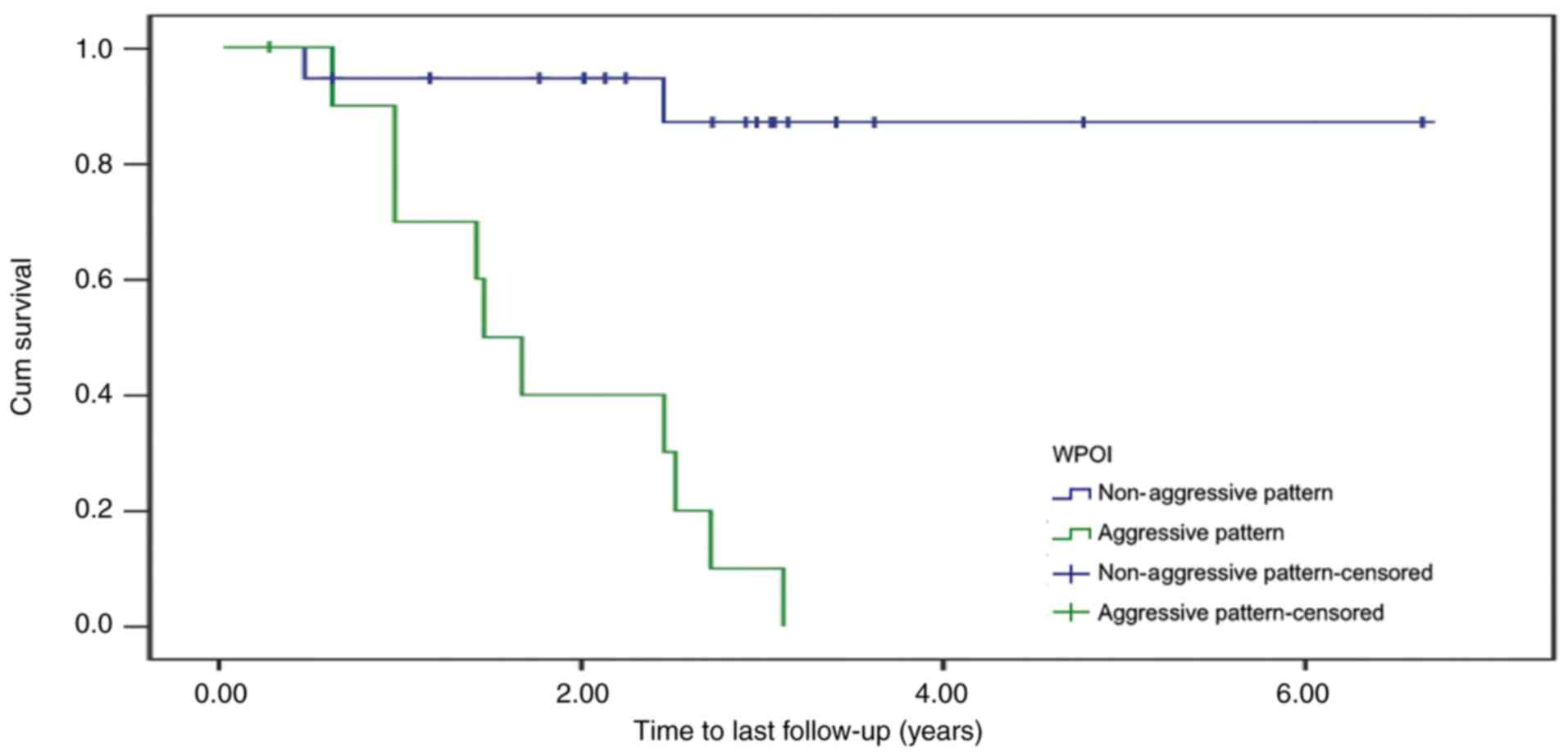
Furthermore, when focusing on the patients with early-stage oral
cancer, a statistically significant difference in time-to-death
between the aggressive and non-aggressive groups was identified
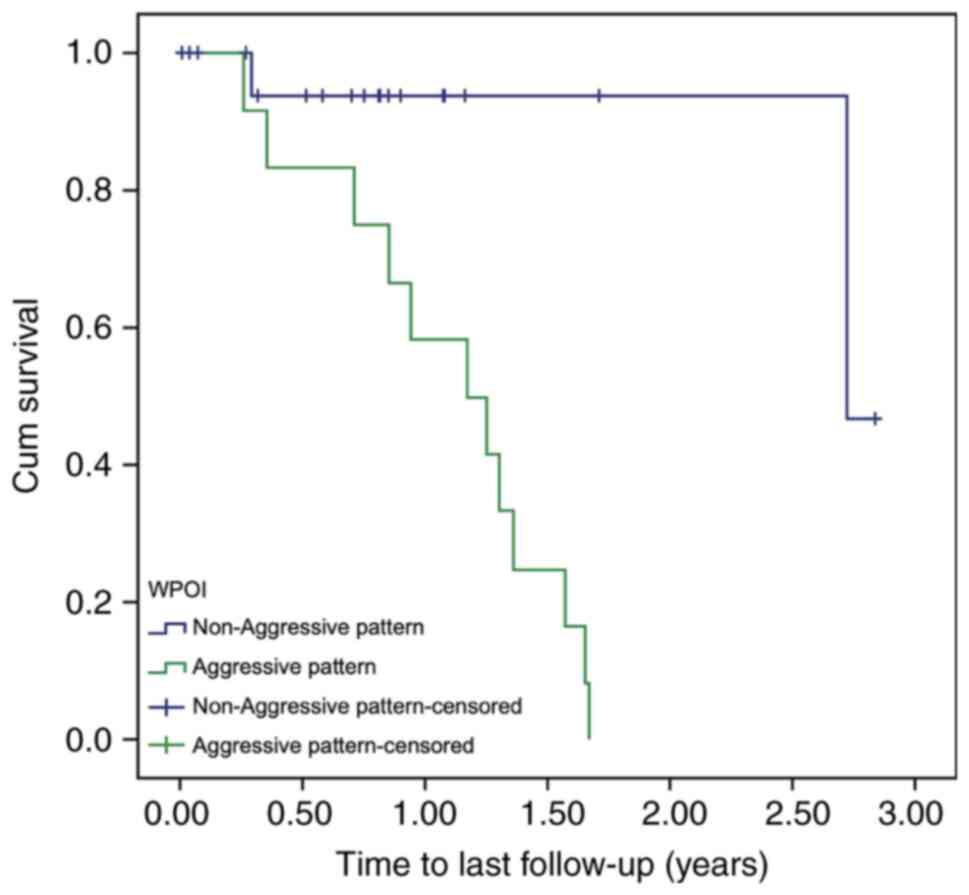
(P<0.0005; Fig. 2). However, no
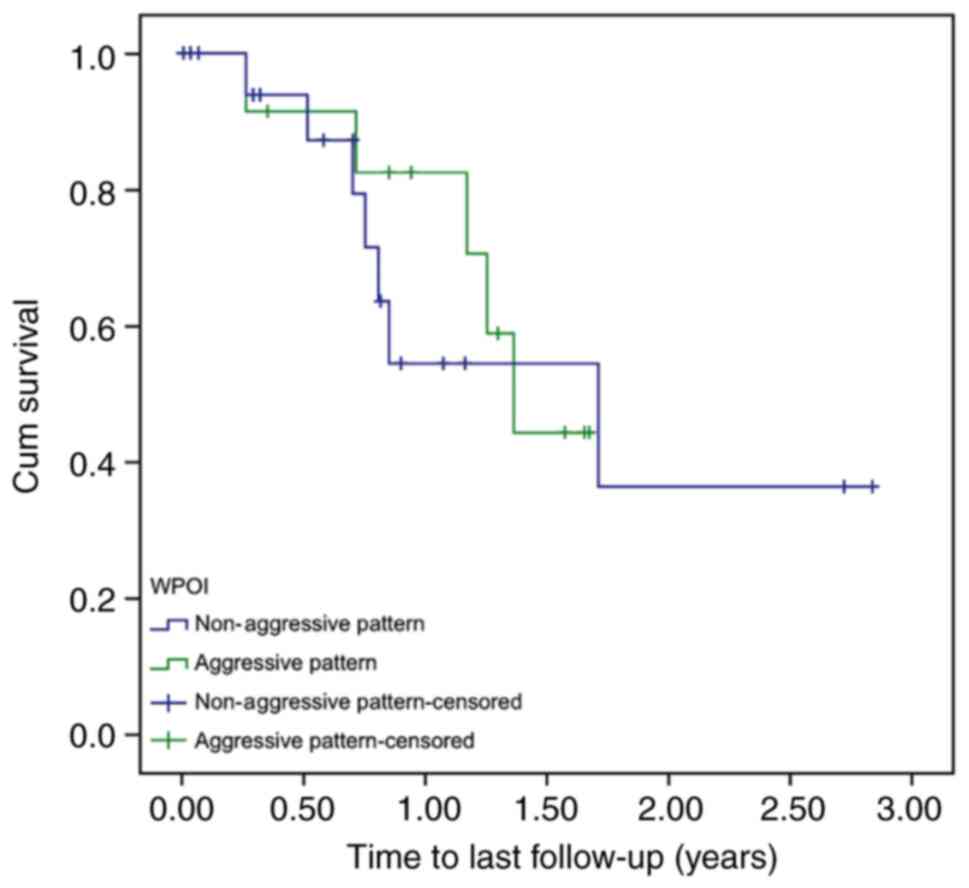
significant difference was found between the aggressive and
non-aggressive groups for the patients with advanced-stage oral
cancer (P=0.679; Fig. 3).
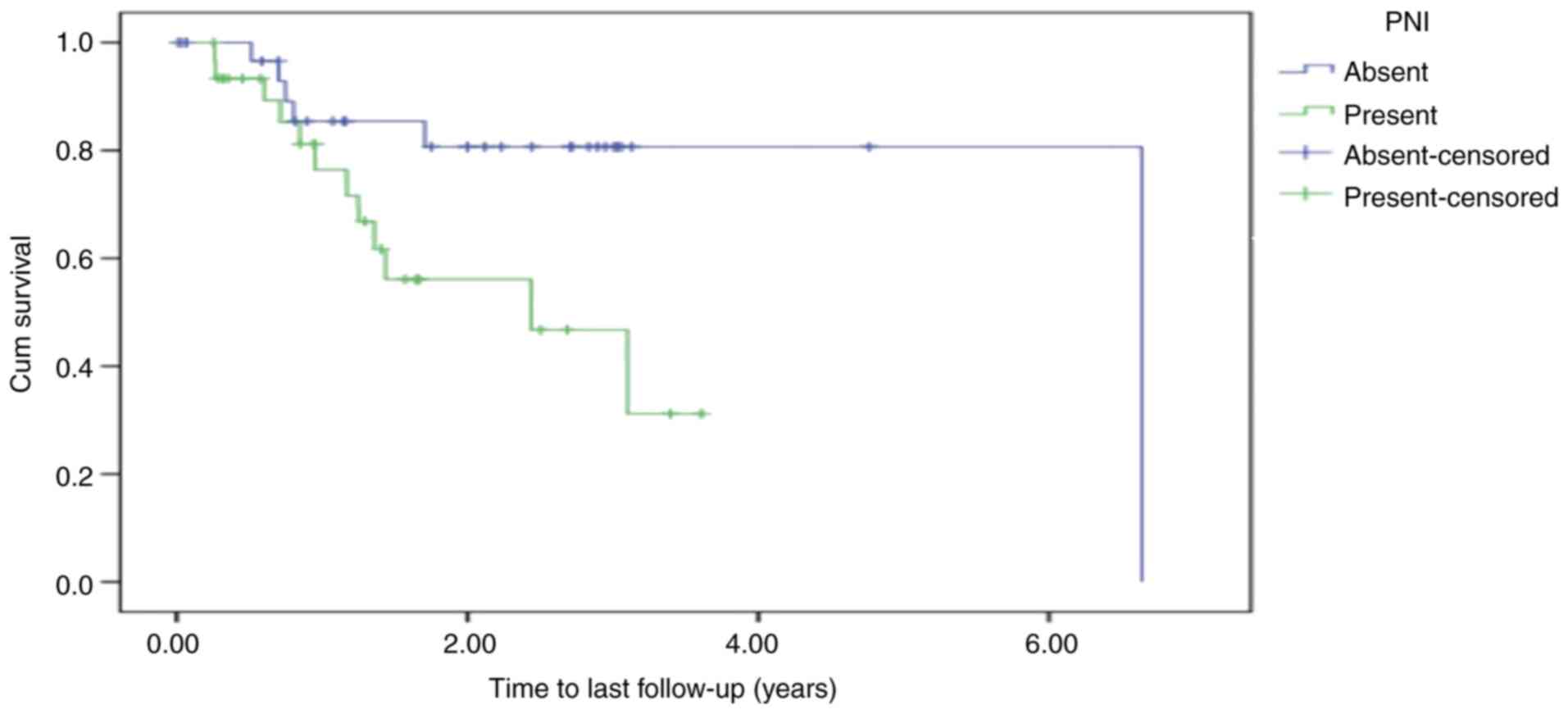
Kaplan-Meier estimates were calculated for OS in
relation to PNI by creating two groups: Those with PNI and those
without. According to the Kaplan-Meier survival estimates for the
entire cohort, assuming a 0.05 level of significance and using the
log-rank test for equality of survivor functions, a statistically
significant difference in time-to-death was detected between the
independent PNI groups (P=0.027; Fig.
4).
DFS
DFS was calculated from the time (in days) of
initial surgical management to the date of the event (time to the
last follow-up, in years), or to the censoring time of patients who
did not develop an event by the end of the study or whose follow-up
notes were not available. The same WPOI groups (aggressive and
non-aggressive) were used when calculating DFS.
According to the Kaplan-Meier survival estimates for
the entire cohort, assuming a 0.05 level of significance and using
the log-rank test for equality of survivor functions, a
statistically significant difference was identified in time to last
follow-up between the aggressive and non-aggressive groups
(P<0.0001; Fig. 5). In addition,
a statistically significant difference in time to last follow-up
between the aggressive and non-aggressive groups was also detected
in patients with early-stage oral cancer (P<0.0001; Fig. 6) and those with advanced-stage oral
cancer (P=0.002; Fig. 7).
Discussion
The global prevalence of oral malignant disorders is
estimated to range from 1 to 5% (8), although much higher rates have been
reported in Southeast Asia (17).
Nearly 274,300 new oral cancer cases occur worldwide each year
(18). It has been shown that the
tongue is the most common intraoral site for cancer. SCC
constitutes the vast majority (95%) of lingual malignancies and is
also the most prevalent type of cancer at other oral sites
(19).
Surgery alone is the usual treatment modality for
patients with early-stage oral SCC. Unfortunately, LR and/or
regional lymph node metastasis develop in certain patients, and
disease-related mortality may also occur. However, the prognosis of
the disease depends on numerous factors. A multi-parametric
histological risk model assessment was initially proposed in 2005,
which was reported to predict survival and differentiate between
high- and low-risk patients and was validated in a different
patient cohort in 2010 (13). This
risk model is a modified extension of prior multivariable
histological models (20–23). The current study tested the
hypothesis that a risk model has prognostic value for early and
advanced stage oral cancer patients.
The current study evaluated the efficacy of
different histopathological parameters in predicting the outcome of
patients with OCSCC. The patients were grouped into a high-risk
category (advanced stage oral cancer, stage III–IVb) that would
benefit from multimodal treatments, and a low-risk category
(early-stage oral cancer, stages I and II) in which local surgical
treatment would be adequate.
A well-established association between cancers of
the oral cavity and tobacco use has been studied in the literature.
In the present study, no association between smoking status and
oral cancer recurrence was observed, and no effect of smoking on
survival rate was detected (data not shown). These findings can be
explained by the smoking status of most patients being unknown.
Alcohol intake has been identified as a significant
risk factor for cancers of the aerodigestive tract. In studies
where smoking has been controlled for, moderate-to-heavy drinkers
have been shown to have a 3-9-fold increased risk of developing
oral cancer (24–27). However, none of the patients in the
present study admitted to drinking alcohol.
POI was first described by Anneroth et al
(21) in 1987, who recommended that
the tumor structure should be considered as a separate parameter
from the tumor cell population. The infiltrative characteristics of
the tumor were proposed to be expressed through the POI,
categorized into four grades: Grade 1, neoplasm with pressing,
well-defined infiltrating border; grade 2, invasion by solid cords
and strands of neoplastic cells; grade 3, invasion by small groups
of cells or cords (n>15); and grade 4, broad front invasion by
single cells or small groups of cells (n<15) (19).
A retrospective study by Bryne et al
(22) compared Broders' grading
method with a modified version of the malignancy grading system
proposed by Anneroth et al (21) where the latter was performed only
within the histologically most invasive tumor areas. Using Cox's
multivariate survival analyses, this grading of the invasive sites
was found to be of significant prognostic value. On this basis, it
was hypothesized that the histologically invasive areas are
essentially responsible for the clinical behavior of the tumor,
which may be of relevance when selecting the therapy for OCSCC.
Bryne et al (22) described
the grading system in terms of five morphological features: Degree
of keratinization, nuclear polymorphism, number of mitoses, mode of
invasion and plasma-lymphocytic infiltration, each scored from 1 to
4 according to definitions proposed by Anneroth et al
(21). In this system, only cells
at the deep invasive margins of the tumors are graded, and the
scores for each morphological feature are added to yield a total
malignancy score.
In another study by Bryne et al (23), all 96 cases of SCCs in the floor of
the mouth registered with the Cancer Registry of Norway between
1963 and 1972 were retrospectively analyzed. The study concluded
that invasive cell grading is potentially valuable for planning the
treatment of oral cancers, and suggested that the deep, invasive
parts of oral and other cancers require further study to improve
the understanding of tumor cell invasion and metastasis.
Regarding the predictive value of WPOI at the tumor
interface, Brandwein-Gensler et al (28) conducted a study to examine the
effect of surgical margin status and histological prognosticators
on LR and OS for patients with OCSCC. The traditional Bryne WPOI
was expanded by the addition of pattern 5, defined as tumor
satellites (regardless of size) dispersed ≥1 mm from the closest
intervening tumor island. In this study, Brandwein-Gensler
described POIs as: Grade 1, pushing border; grade 2, finger-like
growth; grade 3, large separate islands, >15 cells/island; grade
4, small tumor islands, ≤15 cells/island; and grade 5, tumor
satellites, ≥1 mm from the main tumor or next closest satellite.
They also validated the process of considering only the WPOI by
comparing predominant POI (PPOI) with the WPOI at the tumor/host
interface. WPOI 4 and WPOI 5 were found to be high-risk patterns
significantly associated with OS compared with WPOI 1–3 (28).
In the present study, WPOI was categorized into
aggressive and non-aggressive patterns, where aggressive included
grades 4 and 5, and non-aggressive included grades 1–3. Comparative
univariate analysis showed a significant association between
aggressive patterns and recurrence rate. The WPOI effect was 74.9%
(OR=198, P<0.0005) when the aggressive pattern was compared with
the non-aggressive pattern. Multivariate binary logistic regression
analysis modeling indicated that WPOI was significantly associated
with LR (P=0.001). The model was significant (R2=0.768,
OR=66) when the aggressive pattern was compared with the
non-aggressive pattern; thus, patients with an aggressive pattern
were more likely to develop recurrence.
In North India, a retrospective validation study of
the Brandwein-Gensler risk model in 149 patients with OCSCC
conducted by Chaturvedi et al (29) showed that aggressive type WPOI was
significantly associated with LR (P=0.016). Almangush et al
(30) conducted a retrospective
study in 479 patients from three countries, who were treated for
early-stage OCSCC between 1979 and 2012. Comparing the invasive
pattern to the cohesive pattern, the authors found that in
early-stage OCSCC, WPOI was a strong pathological predictor for
locoregional recurrence and death.
With regard to WPOI and survival, the aforementioned
study conducted by Brandwein-Gensler et al (28) used Cox regression analyses to
examine the effect of surgical margin status and histological
prognostic indicators on LR and OS for patients with OCSCC. The
study concluded that WPOI 4 (P=0.004) and 5 (P=0.001) tumor types
were significantly associated with OS in comparison with WPOI 1–3
tumor types. In addition, as WPOI and PPOI were both predictive of
OS, the authors suggested that it is valid to use WPOI as a
variable in place of PPOI since WPOI was found to associated with
LR, but PPOI was not.
A study that included only patients with stage I
disease was performed by Bundgaard et al (31) with the aim of confirming that not
all histological parameters are equally important predictors of
malignancy. A total of 78 patients with stage I (T1N0M0) oral SCC
from two different ear, nose and throat departments were included
in the study. POI was found to be the only significant prognostic
parameter for disease-specific survival (P=0.04). Hori et al
(32) conducted a retrospective
study of 62 patients with early-stage OCSCC, defining grades 4 and
5 as the WPOI. Univariate analysis identified WPOI (P<0.001) to
be a predictive factor for DFS, and multivariate analysis
identified WPOI (hazard ratio=3.84, 95% CI=1.30-11.34, P<0.05)
to be an independent histopathological risk factor for DFS.
A retrospective study of 340 patients with
early-stage tongue SCC evaluated various histopathological
prognostic indicators. In the study, WPOI was divided into a
two-tiered system in which score 0, representing grades 1–3, was
considered low and scores 1 and 3, representing grades 4 and 5,
respectively, were considered high, and a statistically significant
association of WPOI with mortality from oral tongue SCC was
identified. The patients with a high WPOI score (defined as <15
cells in an invasive island, single cells, or satellite tumor
cells) were associated with higher mortality compared to those with
a low WPOI score (defined as pushing borders, finger-like and
cohesive invasion) (30).
In the current study, other factors that could be
relevant to LR and DFS were evaluated, including age, sex, PNI,
surgical margin status, LVI, T and N stage, DOI and
histopathological differentiation. Patient age was categorized into
two groups, ≤60 and >60 years, with a median age of 61 years
(range, 31–87 years). The results of the present study did not show
any influence of age on prognosis. Whilst the disease itself was
more commonly found in males, the sex of a patient was not observed
to have a significant effect on the LR. In univariate analyses, age
and sex did not significantly influence LR or DFS.
PNI is a well-recognized prognostic factor for
survival and LR. In the present study, univariate logistic
regression analysis was used to test if the presence of PNI
affected the recurrence rate. The effect of PNI was 60.2%
(OR=51.429, P<0.0005) when the presence and absence of PNI were
compared. Kaplan-Meier estimates were calculated in patients with
and without PNI. The Kaplan-Meier survival analysis of the entire
cohort revealed a statistically significant difference in
time-to-death between the independent PNI groups (P=0.027). In a
retrospective study on patients with OCSCC conducted by Chaturvedi
et al (29), 53 of the 149
specimens included in the study (35.5%) were found to have PNI.
Additionally, PNI was observed in 10 of the 17 patients with
recurrence (58.8%) and exhibited a statistically significant
association with recurrence (P=0.03). However, it was not found to
be associated with disease-specific survival (P=0.39).
The status of the surgical margins was not
predictive of LR or survival rates in the present study. However, a
strong association between disease-free margin and higher survival
rates, with delayed time to recurrence was shown in studies by
Guerra et al (33) and
Woolgar et al (34). The
results of the present study are consistent with those of
Brandwein-Gensler et al (28), which found no association between
margin status and LR (P=0.2) or OS (P=0.8). Previous studies have
found LVI to be highly prognostic; Jones et al (35) found a significant association
between LVI and survival (P=0.015), while Liu et al
(36) found LVI to be an
independent predictor of DFS. In the current study, LVI was present
in only 19% of the patients and was not found to be associated with
recurrence or disease-specific mortality in the multivariate
analysis.
The present study showed no statistically
significant association of the T stage with recurrence rate. The
recurrence rate was not found to be affected by T staging
(P=0.685). However, a statistically significant association between
early-stage and DFS was identified, which may be explained by
patients with advanced-stage cancer being lost to follow-up or
dying before experiencing a recurrence. N stage did not demonstrate
an influence on LR or DFS.
With regard to the effect of histological
differentiation on prognosis, differentiation was not found to
influence either the risk of recurrence or the DFS in the present
study, probably as most of the cases were of well-differentiated
SCC.
There were several limitations to the present study.
First, because the sample size was small, stratifying various oral
cancer stages with no appropriate control group of data could have
resulted in misleading associations. In future studies, this can be
minimized by controlling or matching factors that could produce
such associations, especially with the differences in the treatment
modalities used, risk factors, recurrence rate, and OS. A larger
sample size with a larger number of events may produce different
conclusions. Second, given the retrospective nature of the study,
it may be subject to selection bias, since 18 patients were
excluded from the analysis due to incomplete data. Third, again
because this study was retrospective, interpretation of the data is
highly dependent on what was documented at the time of surgery and
at the time of follow-up in the outpatient clinic. Finally, the
study was performed in a single center and, therefore, the results
require external validation to support widespread changes in
practice.
In conclusion, according to the data in the present
study, those patients with OCSCC who had an aggressive POI or the
presence of PNI had worse clinical outcomes. Moreover, WPOI and PNI
were found to be significant independent prognostic indicators for
local tumor control and DFS. Therefore, follow-up plans for
patients, especially those with early-stage OCSCC, should consider
these pathological invasion patterns on surgical specimens. In
addition, multimodal treatment is likely to benefit patients with
early-stage oral SCC in whom aggressive high-risk disease is found
by evaluating these factors. Based on the present findings, a
multicentric analysis of pooled data is recommended for better
clarity on this issue.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZM was the primary contributor to manuscript
preparation, created and designed the study, made major
contributions to writing the manuscript and revised the manuscript.
AFB analyzed the data, made major contributions to writing the
manuscript, and was responsible for all other aspects of the
submission, including data collection, interpretation of data,
analysis and manuscript preparation. RMAl and YRA collected the
data by reviewing the patient hospital records, prepared the
manuscript and reviewed the literature. DAA and RMAb assessed
histopathology slides and reviewed the literature. MAH and SK
verified the analytical methods and reviewed the results. RAW, MM
and HAM aided the interpretation of the results and worked on the
manuscript. All authors were involved in manuscript preparation.
HZM and AFB confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval
The study protocol was reviewed and approved by an
institutional review board committee at KAUH, Jeddah, Saudi Arabia.
Ethical approval for this study was obtained from the Research
Ethics Committee at KAUH (ref. no. 662-19).
Patient consent for publication
Informed consent for publication was waived due to
the retrospective nature of the study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOI
|
depth of invasion
|
|
LR
|
local recurrence
|
|
LVI
|
lymphovascular invasion
|
|
PNI
|
perineural invasion
|
|
WPOI
|
worst pattern of invasion
|
|
PPOI
|
predominant pattern of invasion
|
References
|
1
|
Silverman S Jr: Demographics and
occurrence of oral and pharyngeal cancers. The outcomes, the
trends, the challenge. J Am Dent Assoc. 132 (Suppl):7S–11S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swango PA: Cancers of the oral cavity and
pharynx in the United States: An epidemiologic overview. J Public
Health Dent. 56:309–318. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen JK, Katz RV and Krutchkoff DJ:
Intraoral squamous cell carcinoma. Epidemiologic patterns in
Connecticut from 1935 to 1985. Cancer. 66:1288–1296. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silverman S Jr and Gorsky M: Epidemiologic
and demographic update in oral cancer: California and national
data-1973 to 1985. J Am Dent Assoc. 120:495–499. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llewellyn CD, Johnson NW and
Warnakulasuriya KA: Risk factors for squamous cell carcinoma of the
oral cavity in young people-a comprehensive literature review. Oral
Oncol. 37:401–418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YK, Huang HC, Lin LM and Lin CC:
Primary oral squamous cell carcinoma: An analysis of 703 cases in
southern Taiwan. Oral Oncol. 35:173–179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silverman S Jr: Epidemiology. Silverman S
Jr: Oral Cancer. 4th edition. Hamilton, ON, Canada: B C Decker
Inc.; pp. 1–6. 1998
|
|
8
|
Johnson NW, Jayasekara P and Amarasinghe
AA: Squamous cell carcinoma and precursor lesions of the oral
cavity: Epidemiology and aetiology. Periodontol. 2000.57:19–37.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones KR, Lodge-Rigal D, Reddick RL, Tudor
GE and Shockley WW: Prognostic factors in the recurrence of stage I
and II squamous cell cancer of the oral cavity. Arch Otolaryngol
Head Neck Surg. 118:483–485. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woolgar JA: Histopathological
prognosticators in oral and oropharyngeal squamous cell carcinoma.
Oral Oncol. 42:229–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massano J, Regateiro FS, Januário G and
Ferreira A: Oral squamous cell carcinoma: Review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Binmadi NO and Basile JR: Perineural
invasion in oral squamous cell carcinoma: A discussion of
significance and review of the literature. Oral Oncol.
47:1005–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandwein-Gensler M, Smith RV, Wang B,
Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta
C, et al: Validation of the histologic risk model in a new cohort
of patients with head and neck squamous cell carcinoma. Am J Surg
Pathol. 34:676–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sessions DG, Spector GJ, Lenox J, Haughey
B, Chao C and Marks J: Analysis of treatment results for oral
tongue cancer. Laryngoscope. 112:616–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
World Health Organization (WHO)
classification, . (4th edition). 2017.
|
|
16
|
O'Sullivan B, Huang SH, Su J, Garden AS,
Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA, et al:
Development and validation of a staging system for HPV-related
oropharyngeal cancer by the International Collaboration on
Oropharyngeal cancer Network for Staging (ICON-S): A multicentre
cohort study. Lancet Oncol. 17:440–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maleki S, Schlecht NF, Keller C, Diaz J,
Moss J, Prystowsky MB, Macian F and Brandwein-Gensler M:
Lymphocytic host response to oral squamous cell carcinoma: An
adaptive T-cell response at the tumor interface. Head Neck Pathol.
5:117–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morse DE, Psoter WJ, Cleveland D, Cohen D,
Mohit-Tabatabai M, Kosis DL and Eisenberg E: Smoking and drinking
in relation to oral cancer and oral epithelial dysplasia. Cancer
Causes Control. 18:919–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krishna Rao SV, Mejia G, Roberts-Thomson K
and Logan R: Epidemiology of oral cancer in Asia in the past
decade-an update (2000–2012). Asian Pac J Cancer Prev.
14:5567–5577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jakobsson PA, Eneroth CM, Killander D,
Killander D, Moberger G and Mårtensson B: Histologic classification
and grading of malignancy in carcinoma of the larynx. Acta Radiol
Ther Phys Biol. 12:1–8. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anneroth G, Batsakis J and Luna M: Review
of the literature and a recommended system of malignancy grading in
oral squamous cell carcinomas. Scand J Dent Res. 95:229–249.
1987.PubMed/NCBI
|
|
22
|
Bryne M, Koppang HS, Lilleng R, Stene T,
Bang G and Dabelsteen E: New malignancy grading is a better
prognostic indicator than Broders' grading in oral squamous cell
carcinomas. J Oral Pathol Med. 18:432–437. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bryne M, Jenssen N and Boysen M:
Histologic grading in the deep invasive front of T1 and T2 glottic
squamous cell carcinomas has high prognostic value. Virchows Arch.
427:277–281. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mashberg A, Boffetta P, Winkelman R and
Garfinkel L: Tobacco smoking, alcohol drinking, and cancer of the
oral cavity and oropharynx among U.S. veterans. Cancer.
72:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jovanovic A, Schulten EA, Kostense PJ,
Snow GB and van der Waal I: Tobacco and alcohol-related to the
anatomical site of oral squamous cell carcinoma. J Oral Pathol Med.
22:459–462. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blot WJ, McLaughlin JK, Winn DM, Austin
DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB,
Stemhagen A and Fraumeni JF Jr: Smoking and drinking in relation to
oral and pharyngeal cancer. Cancer Res. 48:3282–3287.
1998.PubMed/NCBI
|
|
27
|
Lewin F, Norell SE, Johansson H,
Gustavsson P, Wennerberg J, Biörklund A and Rutqvist LE: Smoking
tobacco, oral snuff, and alcohol in the etiology of squamous cell
carcinoma of the head and neck: A population-based case-referent
study in Sweden. Cancer. 82:1367–1375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brandwein-Gensler M, Teixeira MS, Lewis
CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML and Wang BY:
Oral squamous cell carcinoma: Histologic risk assessment, but not
margin status, is strongly predictive of local disease-free and
overall survival. Am J Surg Pathol. 29:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaturvedi A, Husain N, Misra S, Kumar V,
Gupta S, Akhtar N, Lakshmanan M, Garg S, Arora A and Jain K:
Validation of the Brandwein Gensler risk model in patients of oral
cavity squamous cell carcinoma in North India. Head Neck Pathol.
14:616–622. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Almangush A, Bello IO, Keski-Säntti H,
Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola
S, Nieminen O, et al: Depth of invasion, tumor budding, and worst
pattern of invasion: Prognostic indicators in early-stage oral
tongue cancer. Head Neck. 36:811–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bundgaard T, Rossen K, Henriksen SD,
Charabi S, Søgaard H and Grau C: Histopathologic parameters in the
evaluation of T1 squamous cell carcinomas of the oral cavity. Head
Neck. 24:656–660. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hori Y, Kubota A, Yokose T, Furukawa M,
Matsushita T and Oridate N: Association between pathological
invasion patterns and late lymph node metastases in patients with
surgically treated clinical No early oral tongue carcinoma. Head
Neck. 42:238–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guerra MFM, Gias LN, Campo FR and Perez
JS: Marginal and segmental mandibulectomy in patients with oral
cancer: A statistical analysis of 106 cases. J Oral Maxillofac
Surg. 61:1289–1296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woolgar JA, Rogers SN, Lowe D, Brown JS
and Vaughan ED: Cervical lymph node metastasis in oral cancer: The
importance of even microscopic extracapsular spread. Oral Oncol.
39:130–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jones HB, Sykes A, Bayman N, Sloan P,
Swindell R, Patel M and Musgrove B: The impact of lymphovascular
invasion on survival in oral carcinoma. Oral Oncol. 45:10–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu SA, Wang CC, Jiang RS, Lee FY, Lin WJ
and Lin JC: Pathological features and their prognostic impacts on
oral cavity cancer patients among different subsites-A singe
institute's experience in Taiwan. Sci Rep. 7:74512017. View Article : Google Scholar : PubMed/NCBI
|