Introduction
Prostate cancer (PCa) is one of the most common
cancers and the fifth leading cause of cancer death in men
(1). In 2022, the American Cancer
Society estimated 268,490 new cases of prostate cancer and 34,500
mortalities (2).
Multiple sclerosis (MS) is an immune-related disease
of the central nervous system (3),
with a high rate of teratogenicity (4) and mortality (5) and its associated complications are the
leading causes of mortality, including infections, respiratory
failure, and cardiovascular diseases. Previous studies have shown a
strong relationship between immune-related disorders and cancer
(6,7). While some studies show an increase
risk of cancer in individuals with MS, others show the opposite or
no association at all (8,9).
Results on the association between MS and PCa are
conflicting. For instance, Bosco-Lévy et al (10) suggested that MS was associated with
an increased risk of PCa [hazard ratio (HR)=2.08; 95% CI:
1.68-2.58], while Kingwell et al (11) showed that MS was not associated with
the risk of PCa [standardized incidence ratio (SIR)=0.91; 95% CI:
0.64-1.27]. Marrie et al (12) showed different results. To address
this problem, a meta-analysis was performed to clarify the
relationship between MS and risk of PCa.
Materials and methods
The present study followed the PRISMA statement
(13). A systematic review and
meta-analysis was conducted.
Search strategy
PubMed (http://www.ncbi.nlm.nih.gov/pubmed), EMBASE
(http://www.embase.com), Web of Science
(https://www.webofscience.com/) and
Cochrane Library databases (https://www.cochranelibrary.com/) were searched for
related studies that investigated the PCa risk in patients with MS
up to September 2022. The following search terms were used
(multiple sclerosis) OR (Sclerosis, Multiple) OR (Sclerosis,
Disseminated) OR (Disseminated Sclerosis) OR (MS (Multiple
Sclerosis) OR (Multiple Sclerosis, Acute Fulminating) OR (Multiple
Sclerosis, Relapsing-Remitting) OR (Multiple Sclerosis, Chronic
Progressive) AND (Prostatic Neoplasms) OR (Prostate Cancer) OR
(Prostatic Cancer) OR (cancer).
Data extraction
The titles and abstracts of all articles retrieved
from the initial search were screened to determine their relevance
and all relevant articles were further evaluated to determine their
qualifications for inclusion in the meta-analysis. Two authors
independently extracted data according to the standardized process,
including the author's name, year of publication, country,
follow-up time, number of patients and adjusted confounding
factors, Any differences arising during the study were resolved
through discussion with the third Examiner (Jiawu Wang).
Quality assessment
Two authors evaluated the quality of the included
studies according to the Newcastle Ottawa Scale (14), Based on the different quality
scores, each study could obtain up to nine points, with total
scores ranging from 0 to 9, including high quality (8–9 points),
medium quality (6–7 points), and low quality (≤5 points). Third
party reviewers resolved differences.
Statistical analysis
The data on all outcomes of interest were analyzed
using Stata software version 12.0 (StataCorp LLC). Consistency
indication, the incidence rate ratios, the odds ratios, the SIRs
and the HRs, were directly considered as RRs in the meta-analysis.
Heterogeneity was given by I2, When I2≥ 50%,
it indicates that the heterogeneity is high, and the random effect
model should be used; otherwise, a fixed effect model should be
used. Subgroup analyses was stratified by country and confounding
factors. Sensitivity analysis was used to evaluate the stability
and consistency of the results. The Egger test and Begg test was
used to determine publication deviations. P<0.05 was considered
to indicate a statistically significant difference.
Results
Study selection process
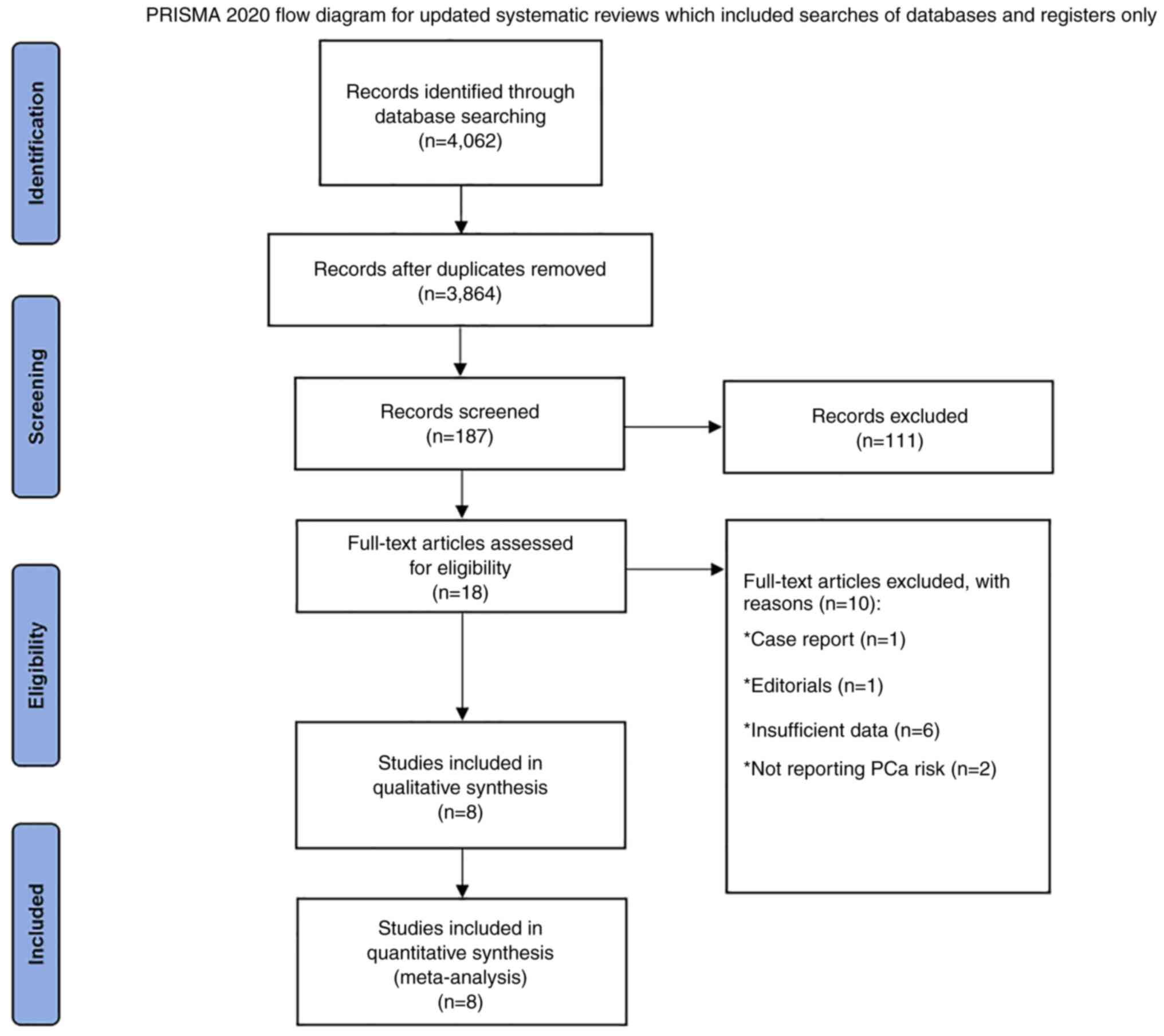
Based on the search strategy, the database search
yielded 4,062 results. After excluding duplicate results and
preliminary screening, 187 studies remained. After excluding
non-relevant articles again, eight articles were included in the
meta-analysis. The screening process is shown in the PRISMA flow
chart (Fig. 1).
Study characteristics and
methodological quality
The main characteristics of the included studies are
summarized in Table I. These
studies included seven cohorts studies (10–12,15–18)
and one case-control study (19),
summing 210,943 patients with MS. A total of two studies (16,17)
from Sweden, two (11,12) from Canada, one (15) from Denmark, one (18) from Norway, one (19) from Finland and one (10) from France were included. Among these
studies included in the meta-analysis, three studies were of high
quality, and five were of moderate quality.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| First author,
year | Country | Study source | Study design | Population
(MS/control) | PCa cases
(MS/control) | Follow up
duration | PCa risk (95%
CI) | Adjusted confounding
factors | (Refs.) |
|---|
| Nielsen, 2006 | Denmark | Population-based | CS | 11,817/NA | 20/37.67 | / | SIR (95% CI): 0.53
(0.34-0.82) | NA | (15) |
| Bahmanyar, 2009 | Sweden | Population-based | CS | 20,276/203,951 | 159/2,923 | 35 years | HR (95% CI): 0.80
(0.69-0.94) | Age, region of
residence and socioeconomic index | (16) |
| Liu, 2013 | Sweden | Population-based | CS | 14,616/NA | 86/NA | 44 years | SIR (95% CI): 0.73
(0.58-0.90) | Obesity, chronic
obstructive pulmonary disease | (17) |
| Kingwell, 2012 | Canada |
Population-based | CS | 6,820/NA | 35/NA | / | SIR (95% CI): 0.91
(0.64-1.27) | NA | (11) |
| Hongell, 2019 | Finland | Hospital-based | CCS | 1,074/10,740 | 2/86 | / | OR (95% CI): 0.2
(0.1-0.8) | NA | (19) |
| Grytten, 2020 | Norway |
Population-based | CS | 6,883/37,919 | 66/493 | 65 years | HR (95% CI): 0.80
(0.62-1.03) | Age, residence, and
attained educational level | (18) |
| Marrie, 2021 | Canada |
Population-based | CS | 53,983/269,915 | NA/NA | 10 years | IRR (95% CI): 0.62
(0.52-0.75) | Age, region, SES
and comorbidity | (12) |
| Bosco-Lévy,
2022 | France |
Population-based | CS | 95,474/95,474 | 253/122 | / | HR (95% CI): 2.08
(1.68-2.58) | NA | (10) |
MS and PCa risk
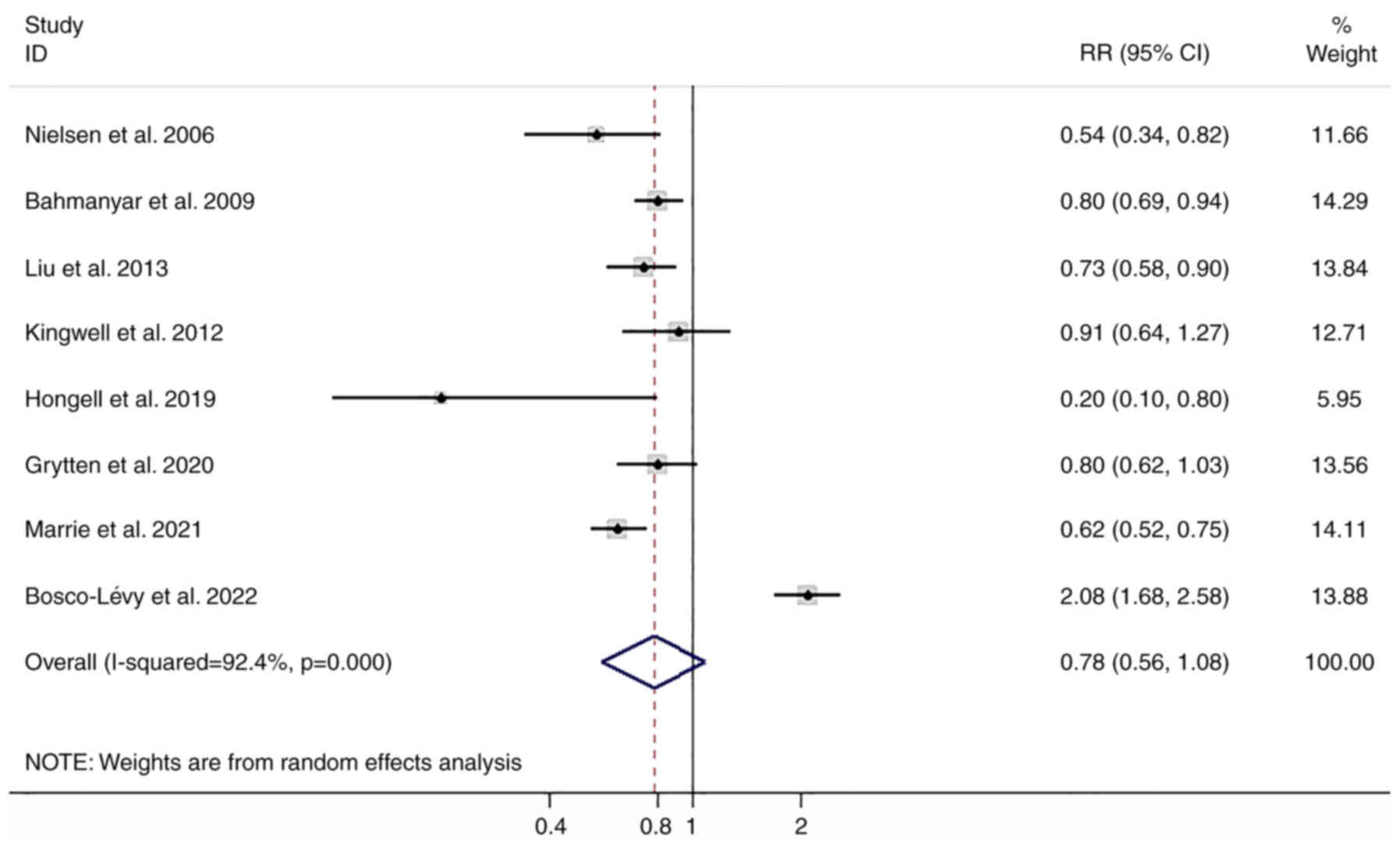
Random-effects meta-analysis showed MS was not
associated with the risk of PCa (RR=0.78; 95% CI: 0.56-1.08), with
substantial heterogeneity (I2=92.4%; P<0.001;
Fig. 2). The present study was
unable to perform a subgroup analysis based on study design because
of limited data. A subgroup analysis based on the distinct regions
showed that RR of 0.78 (95% CI: 0.51-1.19) among European countries
and the RR of 0.73 (95% CI: 0.50-1.06) among other countries
(Table II). The studies that
adjusted for potential confounders gave a RR of 0.73 (95% CI:
0.64-0.83), while the RR of other unadjusted studies was 0.75 (95%
CI: 0.33-1.67) (Table II).
 | Table II.PCa risk in patients with multiple
sclerosis. |
Table II.
PCa risk in patients with multiple
sclerosis.
|
| PCa risk |
|---|
|
|
|
|---|
| Group | n | RR (95% CI) | Model |
|---|
| Overall | 8 | 0.78
(0.56-1.08) | Random |
| Country |
|
|
|
|
European | 6 | 0.78
(0.51-1.19) | Random |
|
Other | 2 | 0.73
(0.50-1.56) | Random |
| Adjustment for
other factors |
|
|
|
|
Yes | 4 | 0.73
(0.64-0.83) | Random |
| No | 4 | 0.75
(0.33-1.67) | Random |
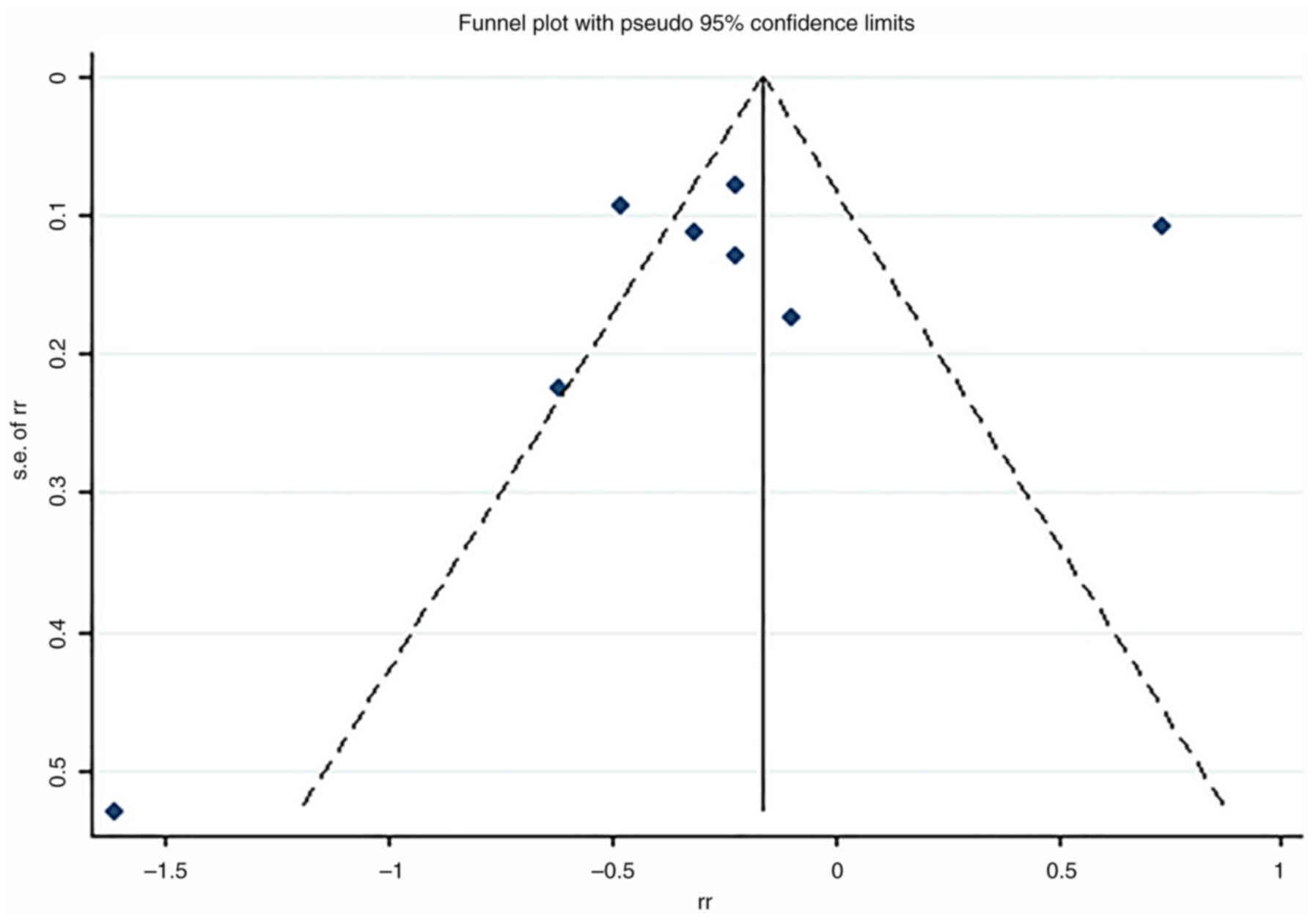
The Begg and Egger tests and funnel plot (Fig. 3) was used to determine publication
bias, the results of Begg and Egger tests (Pb=0.711 and Pe=0.612)
and almost symmetrical funnel plots showed there was no publication
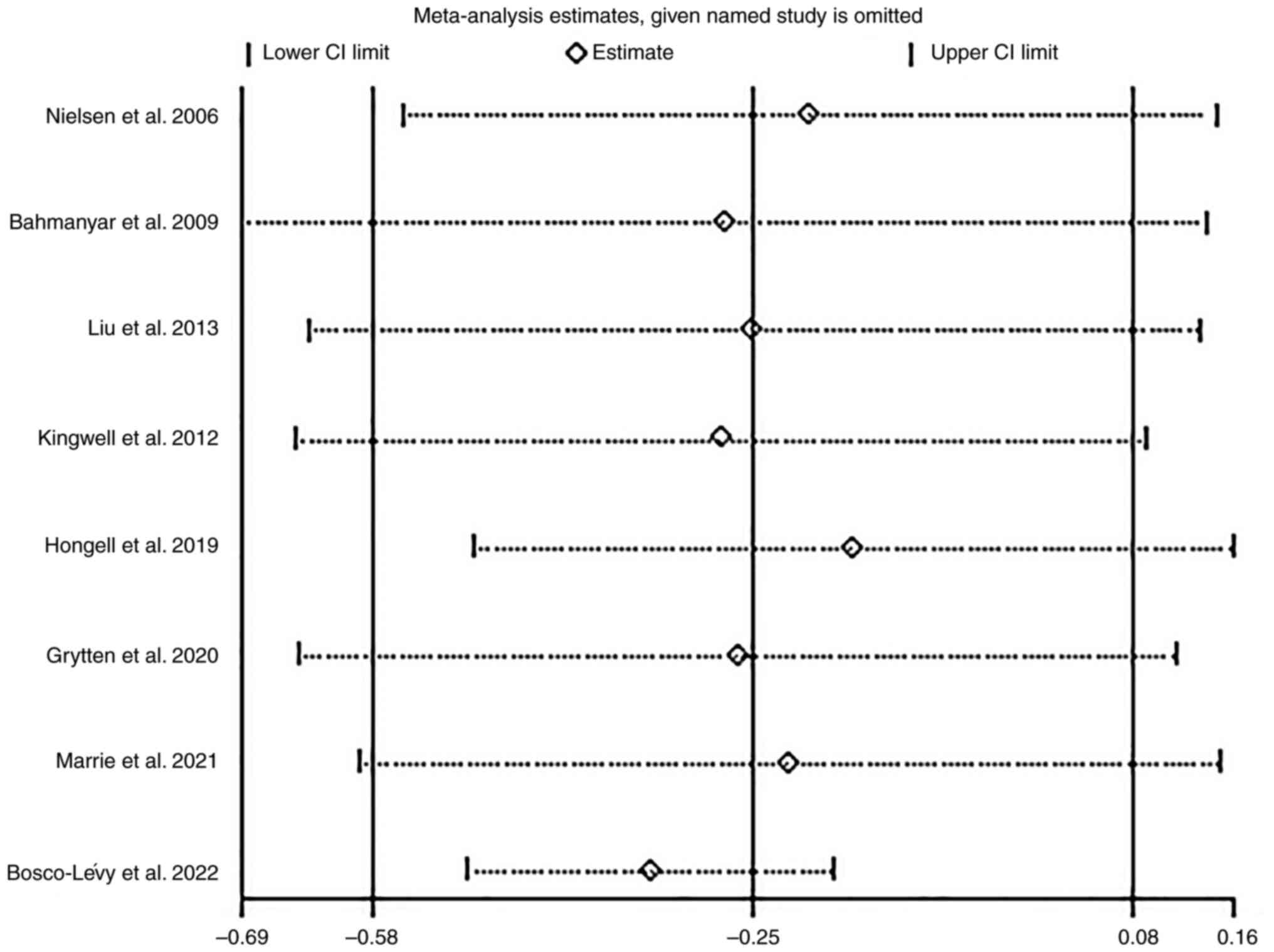
bias. Sensitivity analysis (Fig. 4)
found that no individual study would significantly change the
pooled RRs after removal, indicating that the results were
reliable.
Discussion
The first comprehensive meta-analysis on the
relationship between MS and PCa risk was published in 2015
(20), suggesting that MS reduced
PCa risk. Notably, a study published in Cancer Hematology Review in
2016 also reported MS is associated with the reduction of PCa risk
(21), while a cohort study in 2020
summing up more than 6,800 patients showed that during an average
follow-up of 65 years, MS was not associated with PCa risk.
However, a recent cohort study in 2022 found that MS increased the
risk of PCa (10).
The risk of PCa in patients with MS is unknown and
may be related to genetic and environmental factors, such as
chronic inflammation and infection (22), which can cause tumor growth and
escape by interfering with normal immune surveillance. Previous
studies have shown that regulatory T-cell function is significantly
impaired in patients with MS compared to normal function (23) and that regulatory T-cells can both
promote tumorigenesis and inhibit the growth of some inflammatory
tumors (24). Hormones also play an
important role in patients with MS; studies have shown that
testosterone levels are significantly lower in patients with MS and
due to the significant anti-inflammatory properties of
testosterone, some patients with MS opt for testosterone
supplementation therapy, which may also have a relevant impact on
PCa development due to the complex mechanism of action of
testosterone (25–28). The treatment of MS may lead to the
loss of immune protection against cancer or the activation of the
immune system, making it a primary tumor (29). Moreover, the possible reasons
include that studies conducted in different countries may have
different factors that affect the results. For example, common risk
factors for PCa include being elderly (30), diet (31) and independent protective factors
including regular screening for PCa.
The present meta-analysis builds on previous
meta-studies. Ghajarzadeh et al (32) summarized studies up to September
2019 and calculated a pooled RR estimate of 0.79 for cancer in
patients with MS, thus they concluded that patients with MS would
have a reduced risk of cancer. Nevertheless, due to the small
number of studies, they did not summarize the PCa risk data, and no
subgroup analysis was performed. Therefore the present study became
necessary and it found a significant negative association between
MS and PCa risk in subgroup analysis adjusted for confounders,
suggesting that some of the confounders may also confound the
results.
The present study found strong heterogeneity.
Possible reasons for this are: First, the number of studies was
relatively small (eight studies). Second, the sample sizes were
different across the studies. Finally, the reasons may be related
to geographic region, with no reports of PCa risk in Asia. In
addition, different types of studies, including cohort studies and
controlled case studies, and differences in study populations may
be other sources of heterogeneity.
Overall, the present study was the most recent and
comprehensive study on the association between MS and PCa risk. Its
conclusions are meaningful, and the results of the subgroup and
sensitivity analyses further validate the reasonableness and
reliability of the findings.
However, the present study had several limitations.
First, the number of studies was relatively small. Second,
different study methods and social factors of different study
populations may also cause heterogeneity. Finally, observational
studies themselves may be subject to information bias. Therefore,
future high-quality studies should address these issues
comprehensively.
In summary, the present study demonstrated there was
no significant association between MS and PCa risk, and the
correlation between treatment modalities and PCa needs to be
further explored in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QJ contributed to the study conception and design.
Data collection and analysis were performed by ZH, YF, JW and YL.
The first draft of the manuscript was written by ZH and all authors
commented on previous versions of the manuscript. All authors read
and approved the final manuscript. ZH and YF confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CS
|
cohort study
|
|
CCS
|
control-case study
|
|
NA
|
not applicable
|
|
MS
|
multiple sclerosis
|
|
PCa
|
prostate cancer
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Browne P, Chandraratna D, Angood C,
Tremlett H, Baker C, Taylor BV and Thompson AJ: Atlas of multiple
sclerosis 2013: A growing global problem with widespread inequity.
Neurology. 83:1022–1024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghajarzadeh M, Jalilian R, Eskandari G,
Ali Sahraian M and Reza Azimi A: Validity and reliability of
persian version of modified fatigue impact scale (MFIS)
questionnaire in Iranian patients with multiple sclerosis. Disabil
Rehabil. 35:1509–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lalmohamed A, Bazelier MT, Van Staa TP,
Uitdehaag BM, Leufkens HG, De Boer A and De Vries F: Causes of
death in patients with multiple sclerosis and matched referent
subjects: A population-based cohort study. Eur J Neurol.
19:1007–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasunaga M: Antibody therapeutics and
immunoregulation in cancer and autoimmune disease. Semin Cancer
Biol. 64:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Liu H, Yang Y, Zhou J, Zhao L,
Chen H, Fei Y, Zhang W, Li M, Zhao Y, et al: The five major
autoimmune diseases increase the risk of cancer: Epidemiological
data from a large-scale cohort study in China. Cancer Commun
(Lond). 42:435–446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jick SS, Li L, Falcone GJ, Vassilev ZP and
Wallander MA: Mortality of patients with multiple sclerosis: A
cohort study in UK primary care. J Neurol. 261:1508–1517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moisset X, Perié M, Pereira B, Dumont E,
Lebrun-Frenay C, Lesage FX, Dutheil F, Taithe F and Clavelou P:
Decreased prevalence of cancer in patients with multiple sclerosis:
A case-control study. PLoS One. 12:e01881202017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosco-Lévy P, Foch C, Grelaud A, Sabidó M,
Lacueille C, Jové J, Boutmy E and Blin P: Incidence and risk of
cancer among multiple sclerosis patients: A matched
population-based cohort study. Eur J Neurol. 29:1091–1099. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kingwell E, Bajdik C, Phillips N, Zhu F,
Oger J, Hashimoto S and Tremlett H: Cancer risk in multiple
sclerosis: Findings from British Columbia, Canada. Brain.
135:2973–2979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marrie RA, Maxwell C, Mahar A, Ekuma O,
McClintock C, Seitz D, Webber C and Groome PA: Cancer incidence and
mortality rates in multiple sclerosis: A matched cohort study.
Neurology. 96:e501–e512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Rev Esp Cardiol (Engl Ed).
74:790–799. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2014.Available from:. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
|
|
15
|
Nielsen NM, Rostgaard K, Rasmussen S,
Koch-Henriksen N, Storm HH, Melbye M and Hjalgrim H: Cancer risk
among patients with multiple sclerosis: A population-based register
study. Int J Cancer. 118:979–984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bahmanyar S, Montgomery SM, Hillert J,
Ekbom A and Olsson T: Cancer risk among patients with multiple
sclerosis and their parents. Neurology. 72:1170–1177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Ji J, Forsti A, Sundquist K,
Sundquist J and Hemminki K: Autoimmune disease and subsequent
urological cancer. J Urol. 189:2262–2268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grytten N, Myhr KM, Celius EG, Benjaminsen
E, Kampman M, Midgard R, Vatne A, Aarseth JH, Riise T and
Torkildsen Ø: Risk of cancer among multiple sclerosis patients,
siblings, and population controls: A prospective cohort study. Mult
Scler. 26:1569–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hongell K, Kurki S, Sumelahti ML and
Soilu-Hänninen M: Risk of cancer among Finnish multiple sclerosis
patients. Mult Scler Relat Disord. 35:221–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marrie RA, Reider N, Cohen J, Stuve O,
Trojano M, Sorensen PS, Reingold SC and Cutter G: A systematic
review of the incidence and prevalence of cancer in multiple
sclerosis. Mult Scler. 21:294–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kyritsis AP, Boussios S and Pavlidis N:
Cancer specific risk in multiple sclerosis patients. Crit Rev Oncol
Hematol. 98:29–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ngu JH, Gearry RB, Frampton CM and Stedman
CA: Mortality and the risk of malignancy in autoimmune liver
diseases: A population-based study in Canterbury, New Zealand.
Hepatology. 55:522–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shariati M, Shaygannejad V, Abbasirad F,
Hosseininasab F, Kazemi M, Mirmosayyeb O and Esmaeil N: Silymarin
restores regulatory T cells (Tregs) function in multiple sclerosis
(MS) patients in vitro. Inflammation. 42:1203–1214. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oleinika K, Nibbs RJ, Graham GJ and Fraser
AR: Suppression, subversion and escape: The role of regulatory T
cells in cancer progression. Clin Exp Immunol. 171:36–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ysrraelit MC and Correale J: Impact of
andropause on multiple sclerosis. Front Neurol. 12:7663082021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collongues N, Patte-Mensah C, De Seze J,
Mensah-Nyagan AG and Derfuss T: Testosterone and estrogen in
multiple sclerosis: From pathophysiology to therapeutics. Expert
Rev Neurother. 18:515–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watts EL, Perez-Cornago A, Fensom GK,
Smith-Byrne K, Noor U, Andrews CD, Gunter MJ, Holmes MV, Martin RM,
Tsilidis KK, et al: Circulating free testosterone and risk of
aggressive prostate cancer: Prospective and Mendelian randomisation
analyses in international consortia. Int J Cancer. 151:1033–1046.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watts EL, Appleby PN, Perez-Cornago A,
Bueno-de-Mesquita HB, Chan JM, Chen C, Cohn BA, Cook MB, Flicker L,
Freedman ND, et al: Low free testosterone and prostate cancer risk:
A collaborative analysis of 20 prospective studies. Eur Urol.
74:585–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thormann A, Koch-Henriksen N, Laursen B,
Sørensen PS and Magyari M: Inverse comorbidity in multiple
sclerosis: Findings in a complete nationwide cohort. Mult Scler
Relat Disord. 10:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Godtman RA, Kollberg KS, Pihl CG, Månsson
M and Hugosson J: The association between age, prostate cancer
risk, and higher gleason score in a long-term screening program:
Results from the Göteborg-1 Prostate cancer screening trial. Eur
Urol. 82:311–317. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loeb S, Fu BC, Bauer SR, Pernar CH, Chan
JM, Van Blarigan EL, Giovannucci EL, Kenfield SA and Mucci LA:
Association of plant-based diet index with prostate cancer risk. Am
J Clin Nutr. 115:662–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghajarzadeh M, Mohammadi A and Sahraian
MA: Risk of cancer in multiple sclerosis (MS): A systematic review
and meta-analysis. Autoimmun Rev. 19:1026502020. View Article : Google Scholar : PubMed/NCBI
|