Introduction
Penile squamous cell carcinoma (SCC) has a low
incidence rate, accounting for 0.6% of all malignant tumors in
males in the US, and may reach up to 10% in males in certain parts
of Africa, South America and Asia (1,2). Of
note, penile SCC has a high mortality rate (2).
The 5-year survival rate of patients with stage III
disease without surgery is 0% and the average overall survival time
of patients with stage IV disease is only 11.2 months (3). However, only 2.3% of patients with
penile cancer have distant metastasis (4) and cachexia is not a common phenomenon
in penile cancer. It has been reported that a high proportion (17%)
of patients are <40 years old and the survival rate for this
group is lower than for those aged 40–60 years (5). In clinical practice, numerous patients
with penile cancer are relatively young (6), are in otherwise good physical health
and present without any visceral metastasis (5). It remains elusive why their survival
rates are so poor and further exploration of the life-threatening
factors in patients with penile cancer may thus be necessary.
The surgical management of penile cancer has shifted
from radical ablative surgery to organ-preserving techniques, with
closer surgical margins, which provide good oncological,
psychological and functional outcomes (7,8).
Traditionally, the tumour-free margin of partial penectomy is
recommended to be at least 20 mm (7,9).
Agrawal et al (10)
suggested a 10-mm surgical margin for grade 1 or 2 and a 15-mm
surgical margin for grade 3 penile cancer. Hoffman et al
(11) and Minhas et al
(12) suggested a surgical margin
of 10 mm or less, while Sri et al (13) concluded that a >1 mm surgical
margin has a low risk of local recurrence in organ-preserving
surgery. Therefore, no precise and unified clinical data of
appropriate surgical margins have been reported to date (14,15)
and the cause of this issue remains elusive.
The present study reported the recurrence of
multiple carcinomatous foci in the stump after partial penectomy or
positive margins in penile cancer. Subsequently, the metastatic
pathways of penile cancer were summarized according to the current
study and a literature review. The coagulation parameters, such as
prothrombin time (PT), fibrinogen (FIB) and D-dimer (D-D), were
also analysed to evaluate their relationship with T stage and
metastasis of penile cancer.
Patients and methods
Patients with penile SCC
A retrospective review was performed including 94
cases of penile SCC encountered between November 2002 and August
2020 at the WuMing Hospital and Cancer Hospital of Guangxi Medical
University (Nanning, China). The inclusion criteria were that both
the clinical and the pathologic data were integrated. Patients
lacking reliable pathologic data were excluded from the present
analysis.
The study was in accordance with the guidelines of
the Declaration of Helsinki and approved by the Ethics Committee of
WuMing Hospital of Guangxi Medical University (Nanning, China). All
cases were diagnosed as penile SCC by surgical pathology, as
reviewed by two pathologists. Clinical information was acquired
from the patients' clinical charts. The included patients were
diagnosed from specimens of partial or radical penectomy. TNM
staging was performed according to the Eighth Edition TNM Penile
Staging System (16).
The related coagulation lab detection results,
including plasma PT, thrombin coagulation time (TT), FIB and D-D,
were also analysed (Table I).
 | Table I.Characteristics of patients with
penile cancer. |
Table I.
Characteristics of patients with
penile cancer.
| Variable | Cases | Value | P-value |
|---|
| Age, years | 94 | 53.3±1.4 |
|
| Operation |
|
|
|
| Partial
penectomy | 77 | 81.9% |
|
| Radical
penectomy | 17 | 18.1% |
|
| Inguinal lymph node
operation |
|
|
|
| Yes | 70 | 74.5% |
|
| No | 24 | 25.5% |
|
| Lymph node operation
method |
|
|
|
| Inguinal
lymph node dissection | 63 | 90% |
|
| Inguinal
lymph node biopsy | 7 | 10% |
|
| Coagulation function
(grouping by invasion of cavernous body) |
|
|
|
|
Prothrombin time, sec (normal
range, 9–13 sec) |
|
| 0.048a |
|
Invasion of
corpora cavernosum | 45 | 11.8±1.2 |
|
|
No invasion | 24 | 12.4±1.2 |
|
| APTT,
sec (normal range, 20–40 sec) |
|
| 0.677a |
|
Invasion of
corpora cavernosum | 45 | 30.0±4.4 |
|
|
No invasion | 24 | 30.6±6.12 |
|
|
D-dimer, mg/l (normal range,
0–0.5 mg/l) |
|
| 0.131b |
|
Invasion of
corpora cavernosum | 35 | 0.26
(0.12-0.39) |
|
|
No invasion | 19 | 0.32
(0.19-1.20) |
|
|
Fibrinogen, g/l (normal range,
2–4 g/l) |
|
| 0.304a |
|
Invasion of
corpora cavenosum | 45 | 3.6±1.2 |
|
|
Non-invasion | 24 | 3.3±1.0 |
|
| Coagulation
function (grouping by pelvic lymph node metastasis)c |
|
|
|
|
Prothrombin time, sec |
|
| 0.043b |
|
Pelvic lymph node
metastasis | 3 | 9.7 |
|
|
No metastasis | 46 | 11.9
(11.17-12.63) |
|
| APTT,
sec |
|
| 0.677b |
|
Pelvic lymph node
metastasis | 3 | 28.9 |
|
|
No metastasis | 46 | 29.7
(11.18-33.70) |
|
|
D-dimer, mg/l |
|
| 0.637b |
|
Pelvic lymph node
metastasis | 3 | 0.12 |
|
|
No metastasis | 32 | 0.22
(0.12-0.57) |
|
|
Fibrinogen, g/l |
|
| 0.550b |
|
Pelvic lymph node
metastasis | 3 | 3.33 |
|
|
No metastasis | 46 | 3.1
(2.80-3.71) |
|
Statistical analysis
Figures were generated using GraphPad Prism 9
software (GraphPad Software; Dotmatics). An unpaired Student's
t-test was performed to compare the PT, activated partial
thromboplastin time and fibrinogen levels of patients between the
corpora cavernosum invasion and no invasion groups. Mann-Whitney
U-test was used to compare coagulation parameters between the lymph
metastasis group and no metastasis group, and D-dimer levels
between the corpora cavernosum cavernous invasion and no invasion
group. The relationships between coagulation parameters, cavernous
invasion and pelvic lymph node metastasis were evaluated using
Spearman's correlation coefficient. Statistical analyses were
performed using SPSS software (version 21; IBM Corporation).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 94 patients were included in the present
study; the patients' age ranged from 25 to 95 years (mean ±
standard deviation, 53.3±1.4 years). A total of 77 patients (81.9%)
underwent partial penectomy and 17 (18.1%) underwent radical
penectomy. Furthermore, 71 patients (75.5%) underwent open or
laparoscopic inguinal lymph node dissection or biopsy, while 23
patients (24.5%) did not undergo inguinal lymph node dissection.
Among these former 71 cases, 63 (90%) were subjected to inguinal
lymph node dissection, while 7 (10%) underwent inguinal lymph node
biopsy.
A total of six patients presented with embolism or
typical multiple lesions were observed (Fig. 1, Fig.
2, Fig. 3, Fig. 4, S1
and S2). Table I presents the laboratory parameters
of cases 1, 2, 4. A total of two patients who died of inguinal
blood vessel rupture were also not presented in detail (their data
were included in the study but not included in the evaluation of
coagulation function. These cases were not among the 6 cases
mentioned at the start of this paragraph and not included in the
case reports). Furthermore, one case of secondary penile carcinoma
that occurred via a different metastatic pathway was noted, and
presented as case 4 in the case reports (this case was not included
in the SCC group and not included in the statistical analysis).
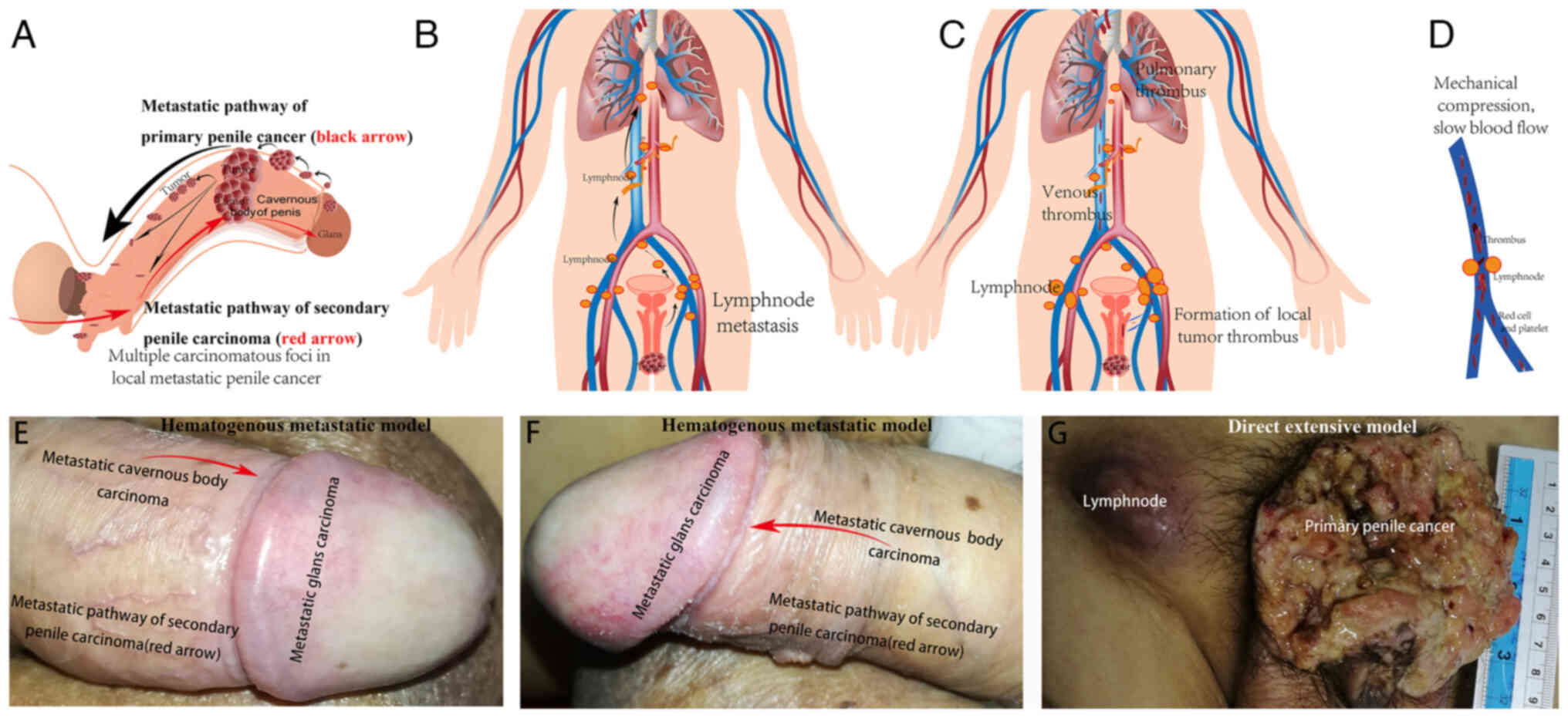 | Figure 4.(A) Different model of metastasis of
primary and secondary penile carcinoma. Primary penile cancer is
mainly located under the albuginea and may spread from the distant
to the proximal region of the penis (black arrow). However, in
secondary penile carcinoma, the metastatic lesion diffuses from the
proximal part of the penis to the distal region (red arrow), which
was supported by the findings in (E and F) for case 4. (B) The
inguinal lymph node is the most common site of lymph node
metastasis. Lymph node metastasis may extend from the pelvic
vessels and abdominal aorta to the mediastinum. (C) The local small
tumour thrombus of penile cancer may flow along the vein. (D) When
an enlarged metastatic inguinal lymph node compresses the vein, the
local blood flow will slow down, which increases the risk of
thrombosis. Venous metastasis may be life-threatening and may
result in pulmonary embolisms. (E) In case 4 of primary urothelial
carcinoma of bladder, the penis was invaded and rapidly
metastasized from the penile body to the glans, and then the whole
penile cavernous body was hardened. The metastatic pathway was
indicated by the erythema in the glans, as was observed in case 4.
The metastatic lesion originated from the proximal penile body, and
then spread to the distal penile glans, which was visible to the
naked eye (red arrow; top view of case 4). (F) Metastatic pathway
of secondary penile carcinoma (red arrow; side view of case 4). (G)
In the early and middle stage, local invasion and lymphatic
metastasis are the main mode of metastasis in primary penile
cancer. However, unlike the secondary metastatic model, the direct
extensive model of primary local advanced penile cancer involves
direct infiltration and lymphatic metastasis, and the carcinoma
diffuses from the glans and distal penis to proximal penis. Parts
of the figure were drawn by using pictures from Servier Medical
Art. Servier Medical Art by Servier is licensed under a Creative
Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). |
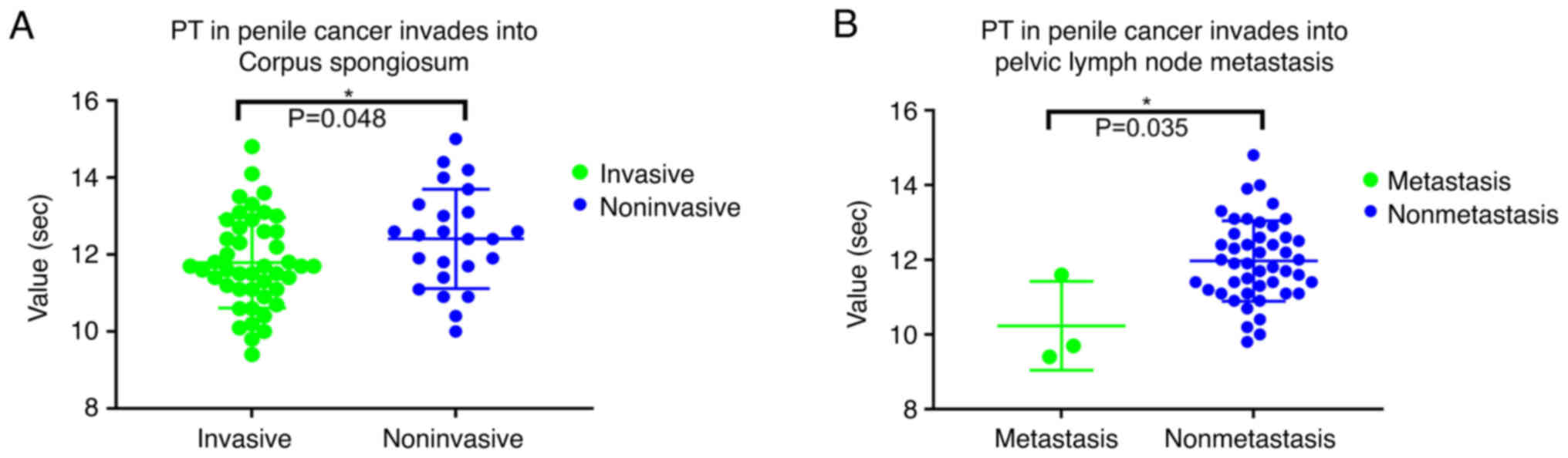
PT in patients with and without
invasion of the corpora cavenosum
The PT (normal range, 9–13 sec) exhibited
differences between the groups of patients with and without
invasion of the corpora cavernosum (Fig. 1A; Table
II; P=0.048). The PT was also different between patients with
or without pelvic lymph node metastasis (Fig. 1B; P=0.006). The PT was negatively
correlated with pelvic lymph node metastasis (ρ=−0.366;
P=0.009).
 | Table II.Clinicopathological features of
typical cases of primary and secondary penile carcinoma in the
present study. |
Table II.
Clinicopathological features of
typical cases of primary and secondary penile carcinoma in the
present study.
|
| Primary penile
carcinoma | Secondary penile
carcinoma |
|---|
|
|
|
|
|---|
|
| Case 1 (66
years) | Case 2 (55
years) | Case 4 (49
years) | Normal reference
range |
|---|
|
|
|
|
|
|---|
| Item | Radical
penectomy | First partial
penectomy | Second radical
penectomy | First
cystectomy | Penile
metastasis |
|---|
| Stage |
|
|
|
|
|
|
| AJCC
2017 | IV | IV | IV | ND | ND | - |
|
TNM | T3N3M0 | T3N3M1 | TxN3M1 | T3bN0M0 | TxN2M1 | - |
| Blood
parameters |
|
|
|
|
|
|
| Total
prostate-specific antigen, ng/ml | ND | 0.98 | 0.88 | 2.82 | ND | 0-4 |
| Free
prostate-specific antigen, ng/ml | ND | 0.26 | 0.32 | 0.86 | ND | 0-1.3 |
| CRP,
mg/l | ND | 22.34a | 4.55 | ND | ND | 0-10 |
| hs-CRP,
mg/l | ND | 7.01a | 0.92 | ND | ND | 0-3 |
| IgM,
g/l | 0.59 | 0.56 | 0.7 | 0.79 | 1.04 | 0.5-2.2 |
| IgG,
g/l | 14.47 | 8.92 | 9.13 | 17.97 | 17.05 | 8-16 |
| Albumin
to globulin ratio | 0.99 | 1.14 | 1.38 | 0.72 | 1.24 | 1-2.5 |
| Alexin
C3, g/l | 1.69 | 1.51 | 1.09 | 0.9 | 1.2 | 0.9-1.5 |
| Alexin
C4, g/l | 0.36 | 0.31 | 0.22 | 0.28 | 0.4 | 0.2-0.4 |
|
Creatinine, µmol/l | 73 | 55 | 43 | 350a | 336a | 53-123 |
|
Platelets,
×109/l | 322 | 288 | 249 | 421a | 292 | 100-300 |
|
Alkaline phosphatase, g/l | 87 | 158a | 87 | 74 | 88 | 25-135 |
| Lactate
dehydrogenase, g/l | 207 | 176 | 135 | 148 | 198 | 114-240 |
| Serum
ferritin, µg/l | 703a | 114 | 121 | 109 | 91 | 20-300 |
|
D-dimer, mg/l | 2.01a | ND | 0.17 | 0.43 | 2.96a | 0-0.5 |
| White
blood cell count, ×109/l | 9.89 | 11.57a | 7.75 | 10.33a | 9.86 | 3.97-9.15 |
|
Neutrophil ratio, % | 73.30 | 67.30 | 53.70 | 71.60 | 68 | 45-77 |
|
Lymphocyte ratio, % | 14.60 | 18.20 | 26.10 | 18.20 | 17.4 | 20-40 |
|
Neutrophils,
×109/l | 7.26 | 7.78 | 4.17 | 7.39 | 6.71 | 2-7.7 |
|
Lymphocytes,
×109/l | 1.44 | 2.11 | 2.02 | 1.88 | 1.72 | 0.8-4 |
|
Neutrophil to lymphocyte
ratio | 5.04 | 3.69 | 2.06 | 3.93 | 3.90 |
|
|
Cytokeratin 19 fragment,
ng/ml | 3.79a | 3.66a | ND | ND | 50.59a | 0-3.3 |
|
Squamous cell
carcinoma-associated antigen, ng/ml | 43.4a | 4.9a | ND | ND | 51.50a | 0-1.5 |
|
Carcinoembryonic antigen,
ng/ml | 13.48a | 5.83a | ND | ND | 71.16a | <5 |
| Pathology |
|
|
|
|
|
|
| Size of
lesion, cm | 3.9 | 7 | 3 | ND | Total penis | - |
| G
stage | ND | G1 | G3 | ND | ND | - |
|
Albuginea infiltrated | ND | Yes | No | ND | ND | - |
|
Surgical margin | Positive | Negative | Negative | ND | ND | - |
| Local
abscess formation | Yes | Yes | Yes | ND | ND | - |
| Tumor
thrombi | Yes | Yes | 0 | ND | ND | - |
| Nerve
invasion | Yes | ND | Yes | ND | ND | - |
Coagulation parameters in penile
lesions
D-D was correlated with T stage (ρ=−0.287; P=0.048)
and with cholinesterase (ρ=−0.380; P=0.009; Fig. S2A and B). FIB was correlated with
neutrophils (ρ=0.337; P=0.004; Fig.
S2D) and lymphocytes (ρ=−0.241; P=0.041; Fig. S2E). A relationship between FIB and
carcinoembryonic antigen was also demonstrated (ρ=0.643; P=0.018;
Fig. S2C).
Presentation of typical cases
General
Cases 1–3 had multiple penile lesions, Case 4 had
secondary penile carcinoma and Cases 5–7 had embolisms.
Case 1: Multiple carcinomatous foci
confirmed by radical penectomy
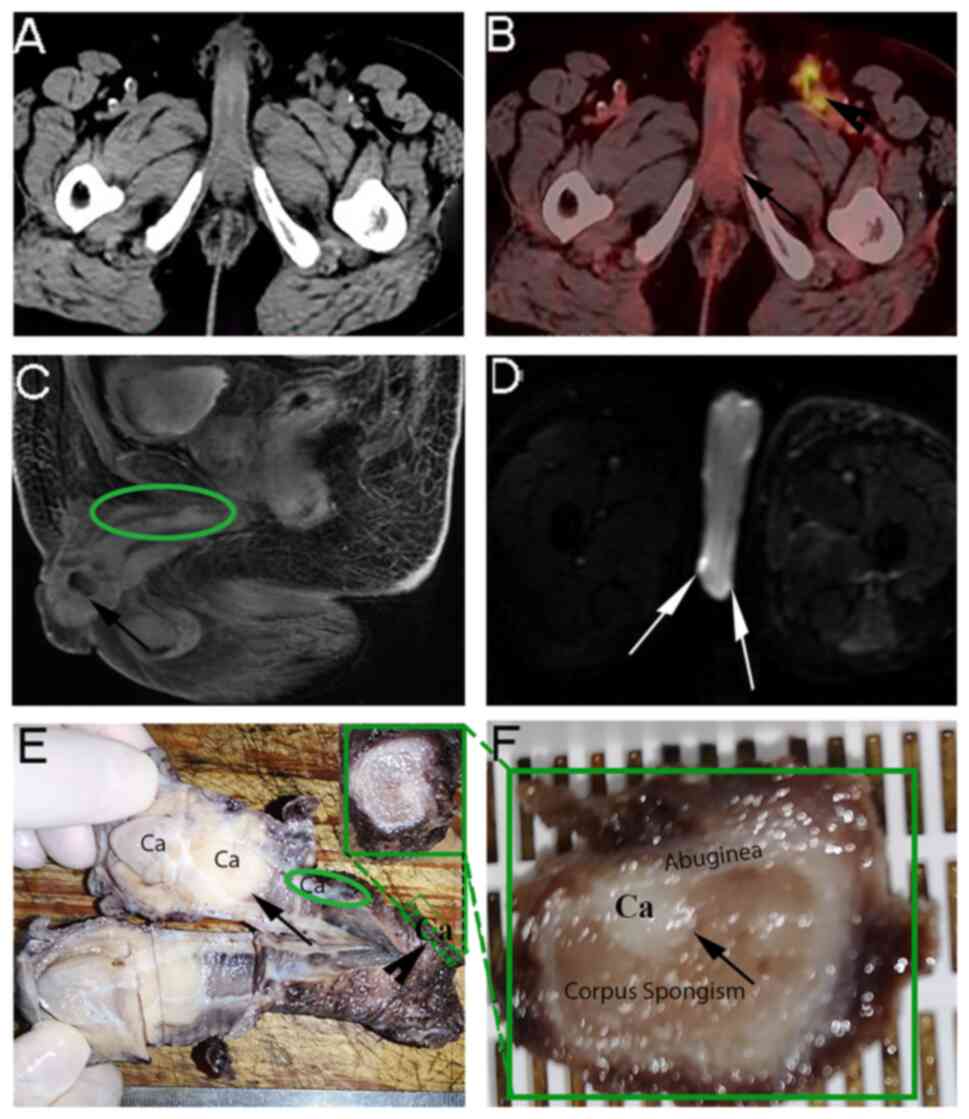
A 66-year-old male complained of a penile tumour for
2 months in July 2020 (Fig. 2A).
The positron emission tomography (PET)/CT results indicated
multiple metastatic lymph nodes in the lower thoracic spine,
abdominal aorta, bilateral iliac vessels and bilateral inguinal
regions (Fig. 2B). Radical
penectomy was performed after neoadjuvant chemotherapy. However,
the postoperative pathology suggested that the margin of the
corpora cavernosum was positive. Multiple carcinomatous foci were
confirmed by MRI (Fig. 2C and D),
and histological and pathological examination (Fig. 2E and F; Table I).
Case 2: Two penile operations within 3
months due to recurrence and multiple penile lesions
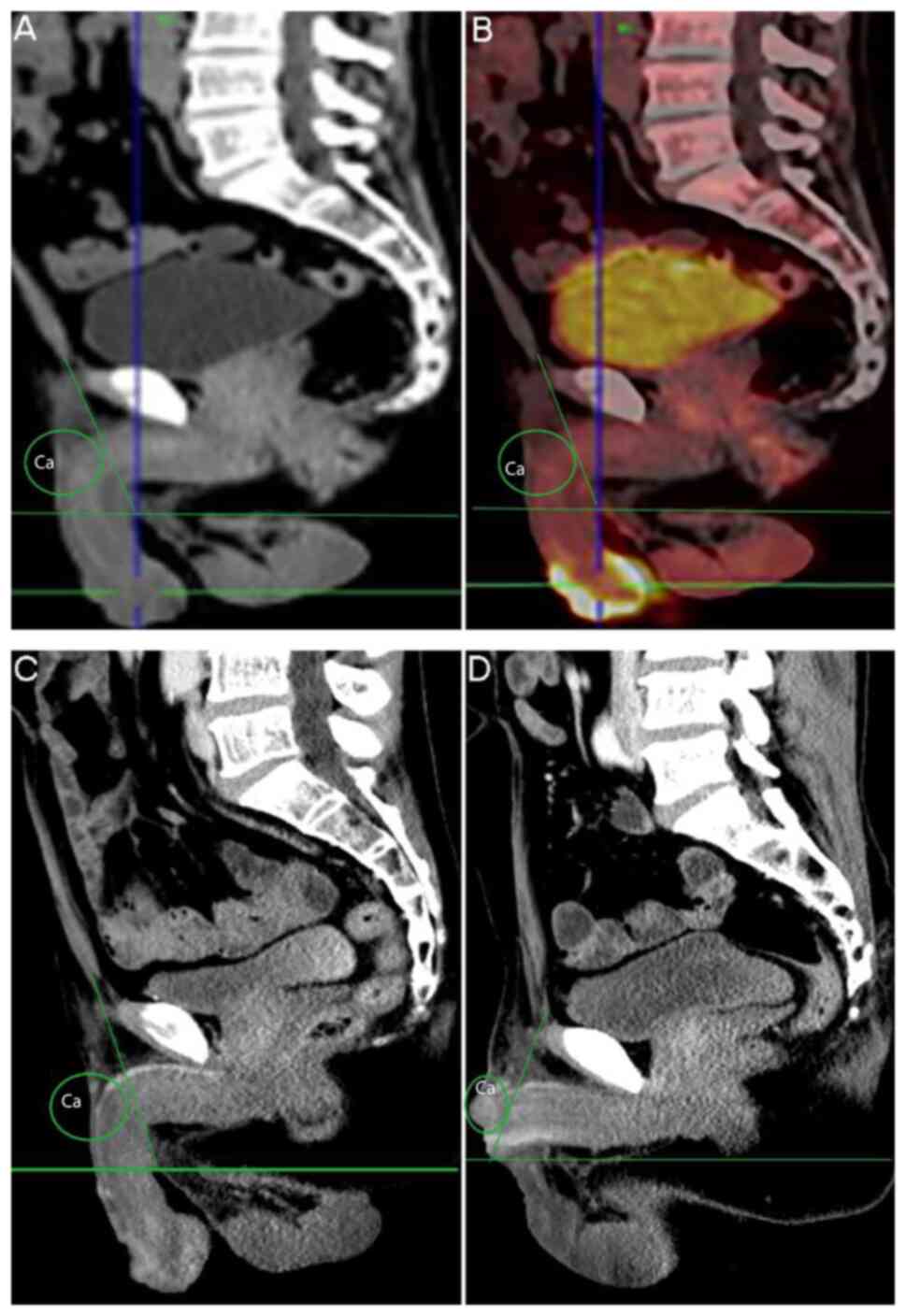
A 55-year-old male complained of a penile tumour
that had recurred for one month in August 2018. The neoplasms had
originally been resected 20 years previously. CT and PET/CT
indicated metastasis of the bilateral inguinal and the left rib
(Fig. 3A-D). SCC was confirmed
after partial penectomy with unilateral inguinal lymph node
dissection. The margin was negative (Table I).
Recurrent cancer of the penile stump was found only
one month after the second operation. Radical penectomy and
inguinal lymphadenectomy were performed. After one year, the
patient died without having undergone any chemoradiotherapy.
Case 3: A negative margin was
confirmed, but recurrence with multiple penile lesions was
observed
A 49-year-old male complained of a penile tumour in
January 2016. An SCC with a negative margin was confirmed by frozen
and routine pathology. However, the recurrence of SCC with multiple
penile lesions was confirmed by radical penectomy within one year.
The patient died 8 months later.
Case 4: Different metastatic pathways
of secondary penile carcinoma
This case is presented only to illustrate the
metastatic pathway, but it was not included in the SCC group and
not included in the statistical analysis. A 49-year-old male
underwent radical cystectomy because of urothelial carcinoma of the
bladder 1 year prior to presentation in March 2020. A penile nodule
was observed following symptoms of scrotum and left lower limb
swelling for 1 month. At first, the penile lesion was small, but it
rapidly expanded in size (Fig. 4E and
F; Table I). Metastasis from a
bladder urothelial carcinoma was confirmed by pathology. The
patient died 6 months later without chemoradiotherapy and
immunotherapy.
Case 5: Pulmonary embolisms were found
during hospitalization
A 45-year-old male complained of right inguinal
ulceration for one month in March 2020. Partial penectomy had been
performed one year prior. Four cycles of postoperative chemotherapy
were administered. A 4×8 cm inguinal mass was palpated in the right
groin.
Right pulmonary embolism was found while the patient
was hospitalized. Inferior vena cava filter placement and
anti-freezing treatment were performed. Partial penile resection
plus laparoscopic bilateral inguinal lymph node dissection was then
performed. The patient remained alive after a follow-up of 3
months.
Case 6: Femoral vein embolus and
pulmonary embolism
A 48-year-old male underwent partial penile
resection and bilateral inguinal lymph node dissection 4 years
prior in February 2018. Recurrent right inguinal lymph node
metastasis was detected. The metastatic lymph nodes were adjacent
to the right iliac artery and inguinal area. Right femoral vein
embolus and pulmonary embolism were confirmed by CT (Fig. S1). Inferior vena cava filter
implantation, thrombolysis and radiotherapy were performed. The
patient died 4 months later.
Case 7: External iliac vein and
femoral vein embolisms migrated to the popliteal vein
A 53-year-old male complained of inguinal masses for
10 days in August 2015. A penile small mass was noted that had
gradually increased in size for two years. The largest inguinal
lymph node was found on the left and was 50×30 mm in size. External
iliac vein thrombus was found on the same side (left), and the left
femoral vein migrated to the popliteal vein. Inferior vena cava
filter implantation, left iliac vein stent placement and
thrombolysis were performed. The venous thromboses were controlled.
Total penectomy was performed after chemotherapy (5-fluorouracil
and cisplatin). The patient died 10 months later.
Discussion
The present study suggests that venous thrombosis
may be a serious life-threatening complication of advanced penile
cancer. Furthermore, multiple carcinomatous foci were found in
histological images. More importantly, direct clinical evidence for
the different metastatic pathways of primary and secondary penile
carcinoma was provided (Fig.
4A-D).
PT measurement is a screening test to check for
impairment of the function of the extrinsic coagulation system. The
PT exhibited differences between patients with or without pelvic
lymph node metastasis, and between patients with or without
invasion of the corpora cavenosum. Shortened PT indicates
thrombotic disease, disseminated intravascular coagulation (DIC)
hypercoagulability or congenital increase in coagulation factor V
levels. Venous thromboembolism (VTE) is a common complication in
patients with cancer. Direct oral anticoagulants may be an
effective and safe therapeutic choice (17) for patients with advanced penile
cancer.
The coagulation parameters, including FIB and D-D,
have been reported to influence the prognosis of cancer (18). D-D was also associated with T stage
in the present study. D-D has been commonly used for the diagnosis
and evaluation of VTE (19).
To the best of our knowledge, the present study was
the first to investigate the relationship between venous metastasis
and penile thrombosis, and femoral vein thrombosis and pulmonary
embolism. Clinicians should pay attention to the life-threatening
vein emboli. Additionally, it should be noted that some patients
with penile cancer rarely develop cachexia despite the poor
prognosis, unlike patients with other types of tumour. In the
present study, numerous patients were relatively young (6), were in otherwise good physical health
and had no visceral metastases, but their prognosis was poor
(5). Life-threatening venous
embolisms have an important role in prognosis, as demonstrated by
the current study (Figs. 1,
4 and S1). Considering that three current
patients who required clinical intervention for thrombosis were
found during hospitalization (3.2%; 3/94), the proportion of
patients with urgent thromboembolic events may be higher than
expected. Inferior vena cava filter implantation and thrombolysis
are the treatments for vein embolus. These treatments were
effective, as demonstrated in cases 5–7.
Venous thrombosis may be a life-threatening
complication of penile cancer, particularly in relatively young
males in otherwise good physical condition. The occurrence of a
thromboembolic event may be a life-threatening complication for
numerous cancer patients (20). The
presence of a hypercoagulable state in patients with malignancies
is generally emphasized in clinical practice (19,21),
and it is particularly important to monitor various indicators of
coagulation, evaluate VTE and assess the hypercoagulable state in
high-risk patients with penile cancer.
For venous metastasis, the local small tumour
thrombus of penile cancer may propagate along the vein. When
inguinal lymph node metastases compress the vein, the local blood
flow is slowed, which increases the risk of thrombosis. After the
formation of thrombi, this condition may embolize to vital organs,
such as the lung, which may be life-threatening, as indicated in
cases 5–7 (Fig. 4C and D).
It may be suggested that the multifocal features of
high-stage penile carcinoma is one of the reasons why it is
difficult to develop guidelines regarding an adequate surgical
margin. In case 1, direct histological evidence of multifocal
lesions of penile carcinoma was found. In case 2, the surgical
margin of the patient was negative after partial penectomy, but SCC
recurred on the penile stump only 1 month after surgery, which also
highlights the occurrence of multifocal lesions in advanced-stage
penile cancer.
In the present study, three patients with at least
stage T2 cancer had lymph node metastasis and infiltrated tunica
albuginea (cases 1–3). In addition, local multiple microvascular
tumour thrombi were present and tumour markers, such as
SCC-associated antigen and cytokeratin 19 fragment, were increased.
If these features are present, radical penectomy may be
recommended.
Thus, the present study suggested that for improved
prognosis of patients with a late clinical stage, instead of
partial penectomy, a more reasonable choice of treatment may be
radical amputation. The following signs may indicate the
requirement for radical penectomy: Stage T2 or above and
accompanied by lymph node metastasis; infiltrated tunica albuginea;
formatted local multiple vascular tumour thrombus; increased tumour
markers, such as SCC-associated antigen and cytokeratin 19
fragment; and multiple hypermetabolic masses in the penile shaft as
indicated by PET/CT.
In the early stage, local invasion and lymphatic
metastasis are the main mode of metastasis in primary penile
cancer, while in advanced primary tumors, hematogenous metastasis
may serve a main role.
These multifocal lesions may represent the local
metastatic pathway of primary penile cancer and this metastasis
appears mainly located under the albuginea and may spread to the
distant cavernous body (Fig. 4A,
black arrow). The inguinal lymph node is the most common site of
lymph node metastasis, which may extend from the pelvic vessels and
abdominal aorta to the mediastinum in primary penile cancer
(Fig. 4B).
However, the model of metastasis of secondary penile
carcinoma is different. The local advanced model of primary penile
cancer is direct extension, the lesion diffuses from the glans and
distal penis to proximal region. However, the mode of metastasis of
secondary carcinoma is hematogenous metastasis. In the secondary
model, the lesion diffuses from the proximal penis to the distal
shaft and glans (Fig. 4E-G). As
shown in case 4 with primary urothelial carcinoma of bladder, the
penis was invaded and rapidly metastasized from the penile body to
the glans, and then the whole penile cavernous body was harden
(Fig. 4E-G). The metastatic pathway
can be evidenced by the erythema in the glans as shown in case 4.
The metastatic lesion originated from the proximal penile body, and
then spread to the distal penile glans, which was visible to the
naked eye (Fig. 4A, black and red
arrows; Fig. 4E-G).
Although the prognosis of penile cancer is generally
poor, radical penectomy in high-risk patients with multiple lesions
may reduce the symptoms of dysuria and pain, and the economic
burden of secondary surgery for certain patients who may have
financial difficulties. The combination of chemotherapy,
radiotherapy, ongoing immunotherapy and the results of the
International Penile Advanced Cancer Trial may bring hope to
patients with advanced penile cancer (22).
One of the limitations of the present study is that
thrombus examination was not performed in all patients, such as
deep vein color Doppler ultrasound studies. As another limitation,
there was a lack of survival analysis data. Furthermore, the sample
size of the present study was relatively small.
In conclusion, the present study was the first to
find multiple carcinomatous focus in primary high-grade penile
cancer. Multiple carcinomatous focus should be evaluated in
patients with metastatic penile cancer of T2 stage or above. It may
be suggested that venous thrombosis is one of the life-threatening
complications of advanced penile cancer. PT exhibited differences
between patients with or without pelvic lymph node metastasis, and
with or without invasion of the corpora cavernosum. Inferior vena
cava filter implantation and thrombolysis are treatment choices for
venous thrombosis. Most importantly, to the best of our knowledge,
the present study was the first to report clinical evidence for the
different metastatic pathways of primary and secondary penile
carcinoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the ‘139’ Program Training Project
for the Cultivation of High-level and Key Talent in Guangxi
Medicine, China (grant no. G201903036), the National Natural
Science Fund of China (grant nos. 31860289 and 32260241), Guangxi
Scientific Research and Technology Development Projects of China
(grant no. 1355005-3-11), Guangxi Medical and Health Key Research
Projects of China (grant no. 2012093), Scientific Research and
Technology Development Project, Wuming District, Nanning, China
(grant no. 20180120) and the self-financing research of the Health
Department of Guangxi Autonomous Region (grant no. Z20210631),
which funded the fee for the polishing and publication of the
article.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and WL were involved in the design and
conceptualization of the study. Drafting of the manuscript and the
acquisition, analysis and interpretation of data were performed by
XY, WL, HL and YT. XY and HL confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Wuming Hospital of Guangxi Medical University [Nanning, China; no.
WM2022(080)].
Patient consent for publication
Written informed consent was obtained from the
patients or the patients' next of kin for the publication of their
case data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SCC
|
squamous cell carcinoma
|
|
PET/CT
|
positron emission-computed
tomography
|
|
PT
|
prothrombin time
|
References
|
1
|
Misra S, Chaturvedi A and Misra NC: Penile
carcinoma: A challenge for the developing world. Lancet Oncol.
5:240–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montella M, Sabetta R, Ronchi A, De Sio M,
Arcaniolo D, De Vita F, Tirino G, Caputo A, D'Antonio A, Fiorentino
F, et al: Immunotherapy in penile squamous cell carcinoma: Present
or future? Multi-target analysis of programmed cell death ligand 1
expression and microsatellite instability. Front Med (Lausanne).
9:8742132022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sirithanaphol W, Sookprasert A,
Rompsaithong U, Kiatsopit P, Wirasorn K and Chindaprasirt J:
Prognostic factors for penile cancer and survival in response to
multimodality therapy. Res Rep Urol. 12:29–34. 2020.PubMed/NCBI
|
|
4
|
Rippentrop JM, Joslyn SA and Konety BR:
Squamous cell carcinoma of the penis: Evaluation of data from the
surveillance, epidemiology, and end results program. Cancer.
101:1357–1363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paiva GR, de Oliveira Araújo IB, Athanazio
DA and de Freitas LAR: Penile cancer: Impact of age at diagnosis on
morphology and prognosis. Int Urol Nephrol. 47:295–299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W, Luo Y, Wang G, Li N, Wang Z, Lei J
and Wang X: Conditional survival after surgery for patients with
penile cancer. Andrology. 8:1744–1752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emmanuel A and Watkin N: Update on organ
preserving surgical strategies for penile cancer. Urol Oncol.
40:179–183. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDougal WS: Phallic preserving surgery in
patients with invasive squamous cell carcinoma of the penis. J
Urol. 174:2218–2220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam JK, Lee DH, Park SW, Kam SC, Lee KS,
Kim TH, Kim TS, Oh CK, Park HJ and Kim TN: Clinicopathologic
characteristics and treatment outcomes of penile cancer. World J
Mens Health. 35:28–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agrawal A, Pai D, Ananthakrishnan N, Smile
SR and Ratnakar C: The histological extent of the local spread of
carcinoma of the penis and its therapeutic implications. BJU Int.
85:299–301. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman MA, Renshaw AA and Loughlin KR:
Squamous cell carcinoma of the penis and microscopic pathologic
margins: How much margin is needed for local cure? Cancer.
85:1565–1568. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minhas S, Kayes O, Hegarty P, Kumar P,
Freeman A and Ralph D: What surgical resection margins are required
to achieve oncological control in men with primary penile cancer?
BJU Int. 96:1040–1043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sri D, Sujenthiran A, Lam W, Minter J,
Tinwell BE, Corbishley CM, Yap T, Sharma DM, Ayres BE and Watkin
NW: A study into the association between local recurrence rates and
surgical resection margins in organ-sparing surgery for penile
squamous cell cancer. BJU Int. 122:576–582. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lindner AK, Schachtner G, Steiner E,
Kroiss A, Uprimny C, Steinkohl F, Horninger W, Heidegger I,
Madersbacher S and Pichler R: Organ-sparing surgery of penile
cancer: Higher rate of local recurrence yet no impact on overall
survival. World J Urol. 38:417–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamoto H: Clinical benefits of frozen
section assessment during urological surgery: Does it contribute to
improving surgical margin status and patient outcomes as previously
thought? Int J Urol. 24:25–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pettaway CA, Srigley JR, Brookland RK,
Choyke PL and Amin MB: Penis. Amin MB, Edge SB, Greene FL, et al:
AJCC cancer staging manual. 8th edition. New York: Springer; pp.
pp7012017
|
|
17
|
Lee AYY: Anticoagulant therapy for venous
thromboembolism in cancer. N Engl J Med. 382:1650–1652. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang FM and Xing NZ: Systemic coagulation
markers especially fibrinogen are closely associated with the
aggressiveness of prostate cancer in patients who underwent
transrectal ultrasound-guided prostate biopsy. Dis Markers.
2021:88999942021.PubMed/NCBI
|
|
19
|
Bates SM, Greer IA, Middeldorp S, Veenstra
DL, Prabulos AM and Vandvik PO: VTE, thrombophilia, antithrombotic
therapy, and pregnancy: Antithrombotic therapy and prevention of
thrombosis, 9th ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 141 (2
Suppl):e691S–e736S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winters J and Garcia D: Cancer-associated
thrombosis. Hematol Oncol Clin North Am. 24695–707. (viii)2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Leary JG, Greenberg CS, Patton HM and
Caldwell SH: AGA Clinical practice update: Coagulation in
cirrhosis. Gastroenterology. 157:34–43.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chahoud J, Kohli M and Spiess PE:
Management of advanced penile cancer. Mayo Clin Proc. 96:720–732.
2021. View Article : Google Scholar : PubMed/NCBI
|