Introduction
Ca2+ signaling is an important regulator
of pathways involved in the regulation of cancer progression
through oncogenic activation, including cell growth, metastasis and
chemotherapy resistance (1). The
involvement of cell signaling pathways, such as MAPK and PI3K/AKT,
in the promotion of malignancy in cancer cells is Ca2+
dependent (2,3). Ca2+-dependent activation of
Ca2+/calmodulin-dependent protein kinase II (CaMKII)
contributes to the phosphorylation of AKT and ERK, and the
Ca2+/CaMKII/AKT and Ca2+/CaMKII/ERK axes may
be involved in governing cancer progression (4).
Recently, transient receptor potential (TRP)
channels were reported to be associated with the development and
progression of cancer (5).
Ca2+ signaling from nociceptive TRP channels controls
cancer pathophysiology (6) and the
therapeutic targeting of TRP channels provides a novel approach for
the treatment of cancer. A previous study also reported that TRP
canonical 7 (TRPC7) mediates aging-associated tumorigenesis
(7). Activation of the
TRPC7-induced DNA damage response (DDR) triggers the senescence
inflammation response and senescence-associated secretory phenotype
activation, and genomic instability results in the activation of
oncogenes and the dysfunction of tumor suppressor genes (7). Ca2+ signaling from TRPC7 is
therefore involved in the initiation of tumorigenesis, but the role
of TRPC7 in the regulation of cancer progression and malignancy is
still unclear.
According to the results of our previous study, the
pathologic features of TRPC7 were associated with tumor size in
patients with lung adenocarcinoma (7). Lung adenocarcinoma falls under the
umbrella of non-small cell lung cancer (NSCLC), which is the most
common type of lung cancer (accounting for ~85% of lung cancer
cases) and the majority of patients with lung adenocarcinoma
exhibit metastatic disease or local recurrence after surgery
(8). The expression of TRPCs,
especially TRPC1, 3, 4 and 6, have been reported to be associated
with lung cancer differentiation (9) and TRPC7 may be involved in the
regulation of lung cancer growth. Therefore, it could be
hypothesized that TRPC7 promotes cancer progression via
Ca2+-mediated CaMKII, MAPK and AKT signaling pathways.
The present study evaluated the role of TRPC7 in lung cancer
progression. The association of TRPC7 with the overall survival
rate of patients with lung adenocarcinoma was evaluated and the
functional role of TRPC7 in regulating cancer malignancy was
assessed.
Materials and methods
Patients and tumor specimens
Between January 2013- December 2016 there were 521
newly diagnosed lung adenocarcinoma in Chi-Mei Medical Center,
Liouying. A total of 93 patients received curative thoracic surgery
for tumor eradication. A total of 43 patients were excluded from
the present study due to coexisting malignancy, previous
neoadjuvant therapy, death within one month after operation and
those who did not want to participate in this study. Finally, the
remaining 50 patients were enrolled into the present study. Because
seven patients were lost to follow up after surgery, only 43
patients had complete information for calculation of their 5-year
survival rate. A total of 12 patients were randomly selected for
examination of the protein expression levels of TRPC7 in their
paired lung adenocarcinoma tissues and non-tumor tissues. Written
informed consent was obtained from each patient and tissue
specimens were processed according to protocols approved by the
Institutional Review Board/Ethics Committee of Chi-Mei Medical
Center (approval no. 10405-L01) for the study ‘A potential oncogene
gene, TRPC7 in NSCLS study’. The mean age of patients was 68 years
(range, 38–88 years). The overall survival time after tumor removal
was 55 months (range, 2–177 months). Specimens from these patients
were received from the Department of Surgery at Chi-Mei Medical
Center, and paired lung tumor and adjacent normal tissue were used
in the present study were collected from these patient specimens.
The clinicopathological characteristics of the patients were
assessed according to the American Joint Committee on Cancer 7th
edition lung cancer staging system (10).
Immunohistochemistry staining
Paraffin sections (3 µm) from the samples were
incubated in a Thermotank set at 60°C, for 20 min. Tissue sections
were then immersed in 100% xylene for 10 min, and then in a graded
(100, 90 and 75%) alcohol solution, each for 1–3 min. The sections
were finally immersed in distilled water for 3 min. The
high-pressure antigen retrieval was in sodium citrate buffer (pH 6)
for 2 min at 100°C. Bovine serum albumin (BSA; 5%; Sigma-Aldrich;
Merck KGaA) in phosphate buffered saline (PBS; Sigma-Aldrich; Merck
KGaA) was added to block the activity of the endogenous peroxidase.
The samples were then incubated at room temperature for 1 h. An
antibody against TRPC7 (antibody information and dilution ratio are
presented in Table S1) was used to
assess the expression of target molecule and the samples were
incubated overnight at 4°C. Immunoreactivity was visualized after
incubation using the 3,3′-diaminobenzidine substrate-chromogen
system (Dako Omnis; Agilent Technologies, Inc.) according to the
manufacturer's protocol. The samples were imaged using an Axi-oPlan
2 bright field microscope (Zeiss AG). The staining intensity of
TRPC7 was assessed using the ImageJ IHC Profiler plugin (11) for ImageJ (National Institutes of
Health). After analysis of each image using the software, the image
was automatically assigned a score as positive (++), low positive
(+) or negative (−), which was dependent on its color deconvolution
and computerized pixel profiling (11). Depending on the score, TRPC7
expression was classified as high expression (++) or low expression
(+ and -).
Cell culture and plasmid
transfection
The IMR-90, BEAS-2B, BES-1A1.6, H1299, A549 and H520
cell lines were purchased from the American Type Culture
Collection. H125 and the primary normal human epidermal
keratinocytes (cat. no. C-12005) were purchased from Creative
Biolabs, Inc. and PromoCell GmbH., respectively. The IMR-90 human
lung fibroblast, BEAS-2B human bronchial epithelium, BES-1A1.6
human bronchial epithelium and H125 human lung squamous cell
carcinoma cell lines were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), and the H1299 human lung adenocarcinoma, A549
human lung adenocarcinoma and H520 human lung squamous cell
carcinoma cells were cultured in RPMI 1640 (Gibco; Thermo Fisher
Scientific, Inc.). All cell lines were maintained in RPMI 1640 or
DMEM supplemented with 1% penicillin/streptomycin and 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The
primary normal human epidermal keratinocytes were cultured in
serum-free keratinocyte growth medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 0.005 µg/ml recombinant human
epidermal growth factor, 0.05 mg/ml bovine pituitary extract and 5
µg/ml recombinant human insulin (all Gibco; Thermo Fisher
Scientific, Inc.). All cells were incubated at 37°C in a humidified
incubator with 5% CO2.
The pCMV3-TRPC7-GFPSpark plasmid encoding
N-GFPSpark-tagged human TRPC7 (cat. no. HG15975-ACG) was purchased
from Sino Biological, Inc. The TRPC7 sequence was cloned into the
pCMV3-N-GFPSpark vector to generate the expression vector
pCMV3-TRPC7-GFPSpark, which expressed a GFP-tagged TRPC7 fusion
protein. The BEAS-2B cells were seeded into six-well plates at
4.5×105 cells/well. The cells were then transfected with
1 µg pCMV3-TRPC7-GFPSpark using TransIT-X2® Dynamic
Delivery System (Mirus Bio, Inc.). After 48 h incubation at 37°C in
a humidified incubator with 5% CO2, cells were collected
for use in cell cycle analysis, invasion assay and calcium response
assay. For immunoblot analysis, TRPC7-overexpressing BEAS-2B (or
H1299) cells were further treated with TRPC7 inhibitors, 5 µM
SKF96365 (Sigma-Aldrich; Merck KGaA), 20 µM 2-aminoethyl
diphenylborinate (2-APB, Sigma-Aldrich; Merck KGaA) or DMSO control
for 24 h. The empty pCMV3-GFPSpark vector was used as a
transfection control.
TRPC7 knockdown in lung cancer
cells
For TRPC7 knockdown experiments using small
interfering RNAs (siRNAs), H1299 cells were transfected with si
TRPC7 (sc-106641; Santa Cruz Biotechnology, Inc.) or si control
(sc-37007; Santa Cruz Biotechnology, Inc) constructs using GenMute™
siRNA Transfection Reagent (SignaGen Laboratories), as previously
described (7). After transfection,
cells were cultured for 48 h at 37°C in a humidified incubator with
5% CO2 before undergoing further analyses. The
expression of target genes was validated using immunoblot
analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
After transfection with the siRNAs (si control and
si TRPC7) or the plasmids (pCMV3-GFPSpark and pCMV3-TRPC7-GFPSpark)
for 24 h, total RNA was extracted from cells utilizing TRIzol™
reagent (Thermo Fisher Scientific, Inc.). Complementary DNA was
synthesized from RNA (1 µg) utilizing a RevertAid RT Reverse
Transcription Kit (Thermo Fisher Scientific, Inc.). Incubation
conditions were 10 min at 25°C, 120 min at 37°C, and 5 min at 85°C.
The resulting complementary DNAs were used to identify the
expression of TRPC7 and GAPDH by performing qPCR with
a TaqMan™ Gene Expression Assay kit (Thermo Fisher Scientific,
Inc.) and the following assay IDs, Hs00220638_m1 (TRPC7) and
Hs02758991_g1 (GAPDH). According to the manufacturer's
protocol, incubation conditions were 2 min at 50°C, 10 min at 95°C,
and or 40 cycles for 15 sec at 95°C and 1 min at 60°C using a 7500
Fast Real-Time PCR System (Thermo Fisher Scientific; Inc.). The
mRNA expression level of TRPC7 was measured by the cycle
threshold value quantification and normalization, using the
2−ΔΔCq method and compared with the GAPDH (12).
Immunoblot analysis
Ground tissues or cells were lysed by incubation on
ice for 30 min in M-PER® Mammalian Protein Extraction
Reagent (Thermo Fisher Scientific, Inc.) containing proteinase
(cat. no. S8830-20TAB; Sigma-Aldrich; Merck KGaA) and phosphatase
inhibitors (cat. no. 5870S; Cell Signaling Technology, Inc.). Cell
debris were removed by centrifugation at 14,000 × g for 30 min at
4°C. The protein concentration of cell lysates was determined using
the Bradford method using a Quick Start Bradford Protein Assay Kit
1 (Bio-Rad Laboratories, Inc.). Proteins lysates (20 µg/lane) which
were denatured using SDS-PAGE sample loading buffer (Bio-Rad
Laboratories, Inc.) and were resolved by SDS-PAGE on a 10% gel and
then transferred to a Hybond-P polyvinylidene fluoride membrane
(Amersham; Cytiva). After blocking with 5% BSA (Sigma-Aldrich;
Merck KGaA) in PBS solution at room temperature for 1 h, the
membrane was first incubated with primary antibodies against TRPC7,
β-actin, GFP, phosphorylated (p)-Thr286-CaMKII (p-CaMKII), CaMKII,
p-Ser473-AKT (p-AKT), AKT, ERK, p-Thr202/Tyr204-ERK (p-ERK) or
GAPDH at 4°C overnight. Antibody information and the dilution
ratios were described in Table SI.
The membrane was then incubated with horseradish
peroxidase-conjugated secondary antibodies at room temperature for
1 h. Immunoreactive proteins were visualized using enhanced
chemiluminescence reagents (Amersham; Cytiva). The
semi-quantification of protein expression levels was performed
using ImageJ2 (National Institutes of Health).
Liquid chromatography mass
spectrometry analysis
Cell lysates were collected to analyze the
components by utilizing a Waters Xevo G2 qT of mass spectrometer
(Waters Corporation) as previously described (13). In brief, the tryptic peptide sample
was chromatographically separated on an M-class UPLC separations
module (Waters Corporation) incorporating 50 femtomole tryptic
digested BSA as the internally spiked protein quantification
standard. Peptide was eluted through a 75 µm × 25 cm BEH C-18
column (Waters Corporation) under gradient conditions at a flow
rate of 300 nl/min over 70 min at 40°C. The mobile phase was
composed of acetonitrile as the organic modifier and formic acid
(0.1% v/v) for molecule protonation. Mass spectrometry was
performed on a Xevo G2 qT of (Waters Corporation) instrument
equipped with a nanoflow electrospray ionization interface and
operated in the data-independent collection mode. Parallel ion
fragmentation was switched between low (4 eV) and high (15–45 eV)
energies in the collision cell and data was collected from 300 to
2,000 m/z utilizing glu-fibrinopeptide B (m/z 785.8426;
Sigma-Aldrich; Merck KGaA) as the separate data channel lock mass
calibrant. Data were processed using ProteinLynx GlobalServer v3.0
(Water Corporation) for qualification and Progenesis QI for
proteomics (Waters Corporation) for relative quantification.
Deisotoped results were evaluated for protein association and
modification using the Uniprot (www.uniprot.org) human protein database.
Cell cycle analysis
TRPC7 knockdown H1299 and TRPC7-overexpressing
BEAS-2B cells were collected and fixed overnight at 4°C using 70%
ethanol. After fixation, the ethanol was removed from the fixed
cells by centrifugation at 800 × g for 5 min at 4°C. The fixed
cells were then incubated with 1 ml of propidium iodide
(eBioscience; Thermo Fisher, Inc.) staining solution for 30 min at
room temperature in the dark. The cell cycle phase distribution was
determined using flow cytometry (LSR II; BD Biosciences), and
quantified using BD FlowJo™ v10 software (BD Biosciences).
Invasion assay
Cell invasion assays were performed using a
Transwell system (Falcon; Corning Life Sciences) as previously
described (14). Briefly, Matrigel
inserts containing an 8 µm pore size membrane with a thin layer of
Matrigel Basement Membrane Matrix were pre-incubated with
serum-free media for 2 h at 37°C. A total of 105 cells
were then plated in the top chamber which contained a Matrigel (250
µg/ml)-coated membrane. In the assay, cells were plated in medium
without serum or growth factor, and medium supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) was placed in the lower
chamber as a chemoattractant. After incubation for 36 h at 37°C in
a humidified incubator with 5% CO2. The cells of the
membrane were fixed using 3.7% formaldehyde and stained using 0.1%
crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for
30 min. Cells that had not infiltrated the membrane pores were
removed with a cotton swab. Images of invaded cells on the lower
surface of the membrane were observed using a Ti-U bright field
microscope (Nikon Corporation). In each condition, 5 independent
fields were quantified using ImageJ2 (National Institutes of
Health) and the average of these fields considered as the mean
number of invasive cells per condition.
Calcium response assay
Intracellular Ca2+ responses in cells
were assessed by applying 1 µM thapsigargin (TG; Sigma-Aldrich;
Merck KGaA) or 100 µM 1-oleoyl-2-acetyl-sn-glycerol (OAG;
Sigma-Aldrich; Merck KGaA), as previously described (7). Prior to these experiments, cells were
loaded with 1 µM Fluo-4-AM (Molecular Probes; Thermo Fisher
Scientific, Inc.) and incubated at 37°C for 20 min. Intracellular
Ca2+ concentration [Ca2+]i was
calculated from the ratio of fluorescence intensities
(excitation/emission wavelength, 488/525 nm) using an Olympus
Cell^R IX81 living cell real-time long-term fluorescence
micro-imaging system (Olympus Corporation). The intracellular
Ca2+ concentration was determined based on calibration
curves as follows. A Ca2+ calibration curve was created
using a Ca2+ Calibration Buffer kit (Molecular Probes;
Thermo Fisher Scientific, Inc.). Intracellular Ca2+
([Ca2+]i) was estimated from Fluo-4 excited
at 488 nm and imaged using an Olympus CellˆR IX81 fluorescence
microscope and UPLanApo 10× objective lens at 20°C. Fluo-4 signals
were calibrated by measuring the fluorescence intensity from
microcuvettes containing 10 mM K2-EGTA (pH 7.20)
buffered to numerous [Ca2+] levels. Ca2+
concentration was analyzed using the following formula:
[Ca2+]i=KD × (F-Fmin/Fmax-F). Plotting the
fluorescence intensity versus [Ca2+] yielded the
calibration curve with the formula of:
[Ca2+]i=KD × (F-Fmin/Fmax-F), where KD=345
nM, F=Fluo-4 intensity, Fmax=640 and Fmin=21.7 for Fluo-4.
Cell viability
Cell viability was determined by replacing the
medium from TRPC7 knockdown H1299 and TRPC7-overexpressing BEAS-2B
cells on day 0, 1, 2 and 3, with MTT (Sigma-Aldrich; Merck KGaA) at
a final concentration of 500 g/ml in Phenol-Red-free medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.). Cells were then incubated in the dark at
37°C for 4 h. After dissolving the resulting formazan crystals
through incubation with 100% DMSO at 37°C for 5 min, the solution
was transferred to a 96-well ELISA plate and quantified at 570 nm
using an XS2 ELISA reader (Biotex, Inc.).
Gene Expression Omnibus (GEO) Database
Analysis
GEO is a gene expression profiling database
(https://www.ncbi.nlm.nih.gov/geo)
(15). The GSE149507 dataset was
used during analysis of the expression of TRPC7, CaMKII, AKT,
ERK1 and ERK2 in 18 pairs of small cell lung cancer
tumors and adjacent lung tissues which were obtained from surgical
resection. Gene expression profiling was performed by
microarray.
Ingenuity Pathway Analysis (IPA)
QIAGEN IPA software (QIAGEN IPA Winter Release,
December 2021; Ingenuity Systems, Inc.) includes a large database
with thousands of biological, chemical, and medical studies. IPA
also enables identification of related signaling pathways, upstream
regulators, molecular interactions, disease processes and candidate
biomarkers. Thus, TRPC7, CaMKII, AKT and MAPK were searched in the
IPA database to clarify their relationship in signaling pathways
and related diseases or functions (16).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.;
Dotmatics) was utilized to generate bar charts with error bars
indicating standard deviations. One-way ANOVA followed by
Bonferroni's post-hoc test and two-tailed, paired or unpaired
Student's t-test were used to compare the differences between
groups. Furthermore, overall patient survival was calculated from
the time of surgery to the time of death or to the time of the last
follow-up, at which point the data were censored. The Kaplan-Meier
method was used to evaluate the difference between subgroups with
high and low TRPC7 expression levels, and overall survival curves
were generated. P<0.05 was considered to indicate a
statistically significant difference.
Results
TRPC7 overexpression correlated with a
lower survival rate in patients with lung adenocarcinoma
To evaluate the role of TRPC7 in the regulation of
lung cancer progression, the expression of TRPC7 in paired biopsy
specimens from patients with lung adenocarcinoma was first
examined. The protein expression level of TRPC7 was significantly
increased in tumor tissues compared with non-tumor tissues
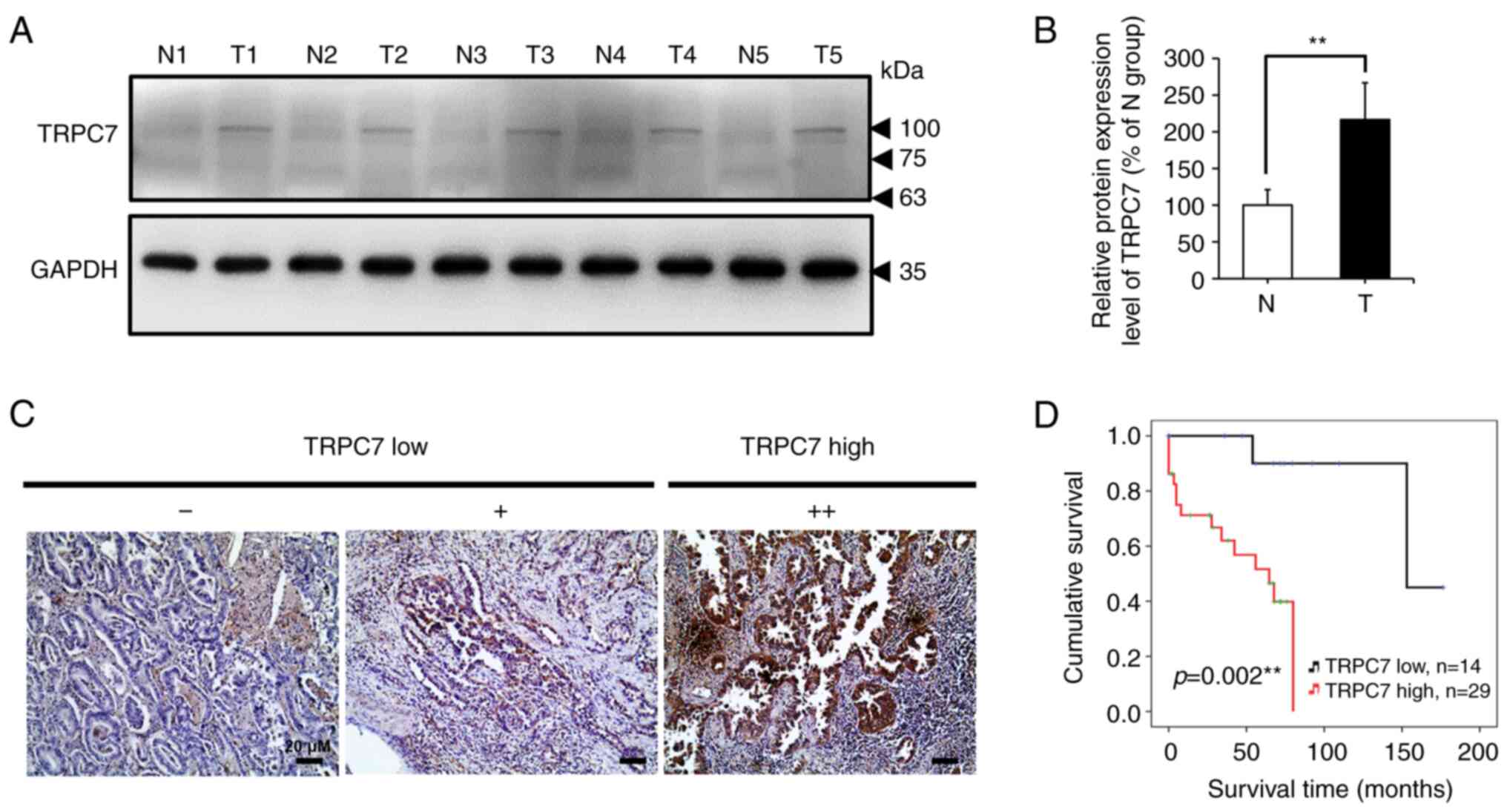
(Fig. 1A and B). This indicated
that TRPC7 may serve a role in the promotion of tumor malignancy.
Therefore, the clinicopathological role of TRPC7 in adenocarcinoma
progression was evaluated, and the results from the TRPC7 high and
low expression groups were presented (Fig. 1C). Kaplan-Meier analysis according
to TRPC7 expression level indicated that patients with lung
adenocarcinoma with high TRPC7 expression levels had a
significantly decreased overall survival compared to those patients
with low TRPC7 expression levels (P=0.002) (Fig. 1D). These results suggested that
TRPC7 upregulation was associated with a worse prognosis for
patients with lung adenocarcinoma.
Role of TRPC7 in the regulation of
cell proliferation and cell cycle progression in lung
adenocarcinoma
To evaluate the mechanism of TRPC7-mediated
malignancy in lung cancer, the protein expression levels of TRPC7
in NSCLC cell lines were evaluated. The results demonstrated that
there were markedly higher protein expression levels of TRPC7 in
NSCLC cell lines, especially in the adenocarcinoma H1299 and A549
cells compared with the lung cell lines, BEAS-2B, BES-1A1.6 and
IMR-90 (Fig. 2A). A similar result
was demonstrated by the results of the proteomic analysis (Fig. 2B). As pathological features of TRPC7
are associated with tumor size in patients with lung adenocarcinoma
(7), cell growth and cell cycle
progression after knockdown or overexpression of TRPC7 in lung
cancer cells were explored. TRPC7 was knocked down in
TRPC7-overexpressing H1299 cells using TRPC7 siRNA (Fig. 2E), which markedly reduced cell
proliferation (Fig. 2C). This was
due to most H1299 cells being in the G0/G1 phase during TRPC7
knockdown, which indicated increased cell cycle arrest and
decreased cell cycle progression in these cells (Fig. 2G). A significant decrease in the
proportion of cells in the G2/M phase, compared with the si control
group was also demonstrated following TRPC7 knockdown. However,
overexpression of TRPC7 in BEAS-2B cells which is a lung cell line
with slight TRPC7 expression, markedly increased cell proliferation
(Fig. 2D) and promoted cell cycle
progression with a significantly increased population of cells in
the G2/M phase (Fig. 2H), compared
with the control. These results supported the hypothesis that the
functional role of TRPC7 in adenocarcinoma was to modulate cell
growth through effects on cell cycle progression.
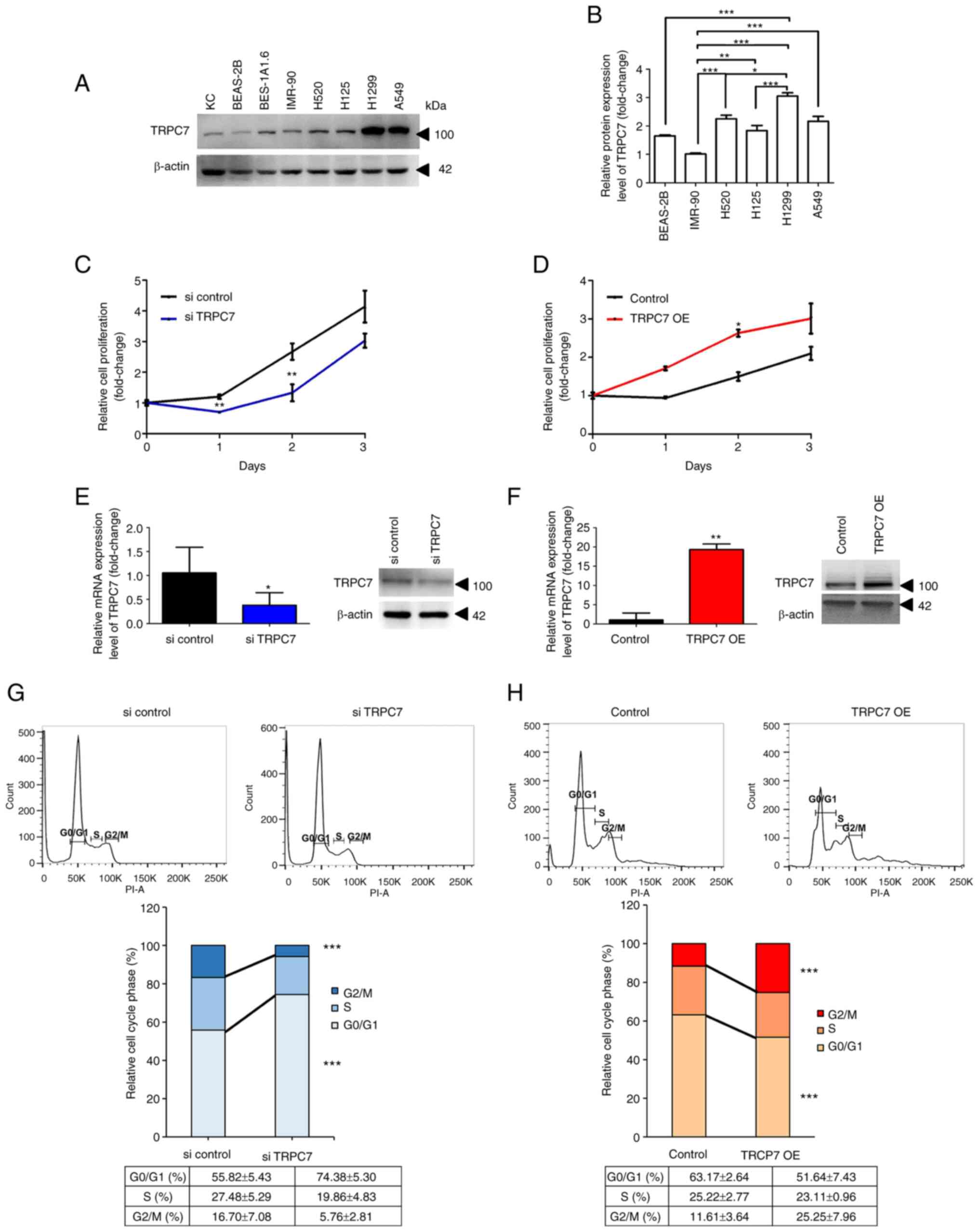 | Figure 2.Effect of TRPC7 on cell proliferation
in lung adenocarcinoma. (A) Immunoblot detection of the protein
expression level of TRPC7 in lung cell lines. Normal lung cell
lines were BEAS-2B (human bronchial epithelium), BES-1A1.6 (human
bronchial epithelium) and IMR-90 (human lung fibroblast). Squamous
cell carcinoma cell lines were H125 and H520. Adenocarcinoma cell
lines were A549 and H1299. KC were used as TRPC7-positive control
cells. (B) Further comparison of endogenous TRPC7 protein
expression levels among normal lung, squamous cell carcinoma and
adenocarcinoma cell lines using LC/MS. Quantification of the TRPC7
expression analyzed using unpaired Student's t-test. Data were
presented as mean ± SD. Effect of (C) TRPC7 knockdown and (D) TRPC7
overexpression on H1299 and BEAS-2B cell proliferation (analyzed
using unpaired t-test), respectively. Reverse
transcription-quantitative PCR and immunoblotting analysis
demonstrated that TRPC7 mRNA and protein expression levels were (E)
reduced in H1299 and (F) overexpressed in BEAS-2B cells (analyzed
using unpaired t-test). The percentage of (G) TRPC7 knockdown H1299
and (H) TRPC7-overexpressing BEAS-2B cells in the G0/G1, S and G2/M
phases (analyzed using unpaired Student's t-test; n=3). Data were
presented as mean ± SD for all experiments. *P<0.05, **P<0.01
and ***P<0.001. KC, primary normal human epidermal
keratinocytes; LC/MS, liquid chromatography tandem mass
spectrometry; OE, overexpression; si, small interfering RNA; TRPC7,
transient receptor potential canonical 7. |
TRPC7 regulates cell migration in lung
adenocarcinoma
TRPC7 upregulation is closely associated with a poor
prognosis in patients with lung adenocarcinoma; the metastatic
tendency of cancer cells is related to malignancy and this could
explain the poor prognosis of patients (17). The capacity of TRPC7 to promote
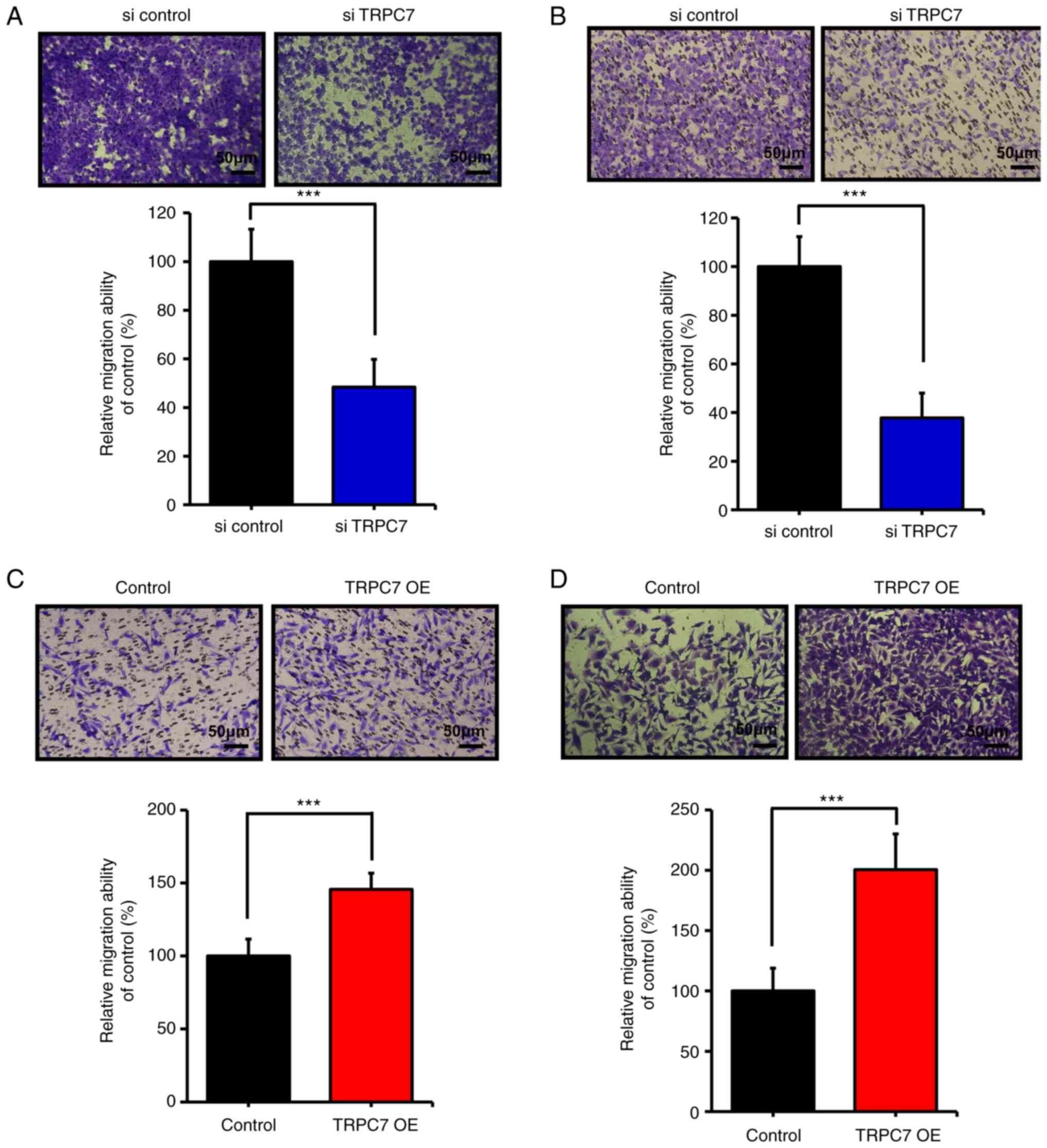
cancer cell migration was assessed. TRPC7 downregulation by siRNA
in H1299 cells significantly restrained cell migration (Fig. 3A) and invasion (Fig. 3B), and TRPC7 upregulation in normal
lung cells significantly promoted cell migration (Fig. 3C) and invasion (Fig 3D). Consequently, the results
demonstrated that TRPC7 overexpression contributed to a significant
increase in cell migration in lung adenocarcinoma.
TRPC7 regulates cell growth and
migration due to the increased level of Ca2+ influx
As Ca2+ signaling is crucial in the
regulation of cancer cell growth and metastasis, TRPC7 channel
activity was further evaluated. According to a previous study,
TRPC7 can be activated by both receptor- and store-operated modes
(18). Diacylglycerol (DAG) is a
TRPC7 agonist that interacts with the TRPC7 receptor to induce
Ca2+ influx; TG, an inhibitor of sarcoplasmic and
endoplasmic reticular Ca2+ ATPase pumps, is utilized to
trigger store-operated Ca2+ entry (SOCE). Therefore, the
DAG analog, OAG, and TG were used to evaluate TRPC7 channel
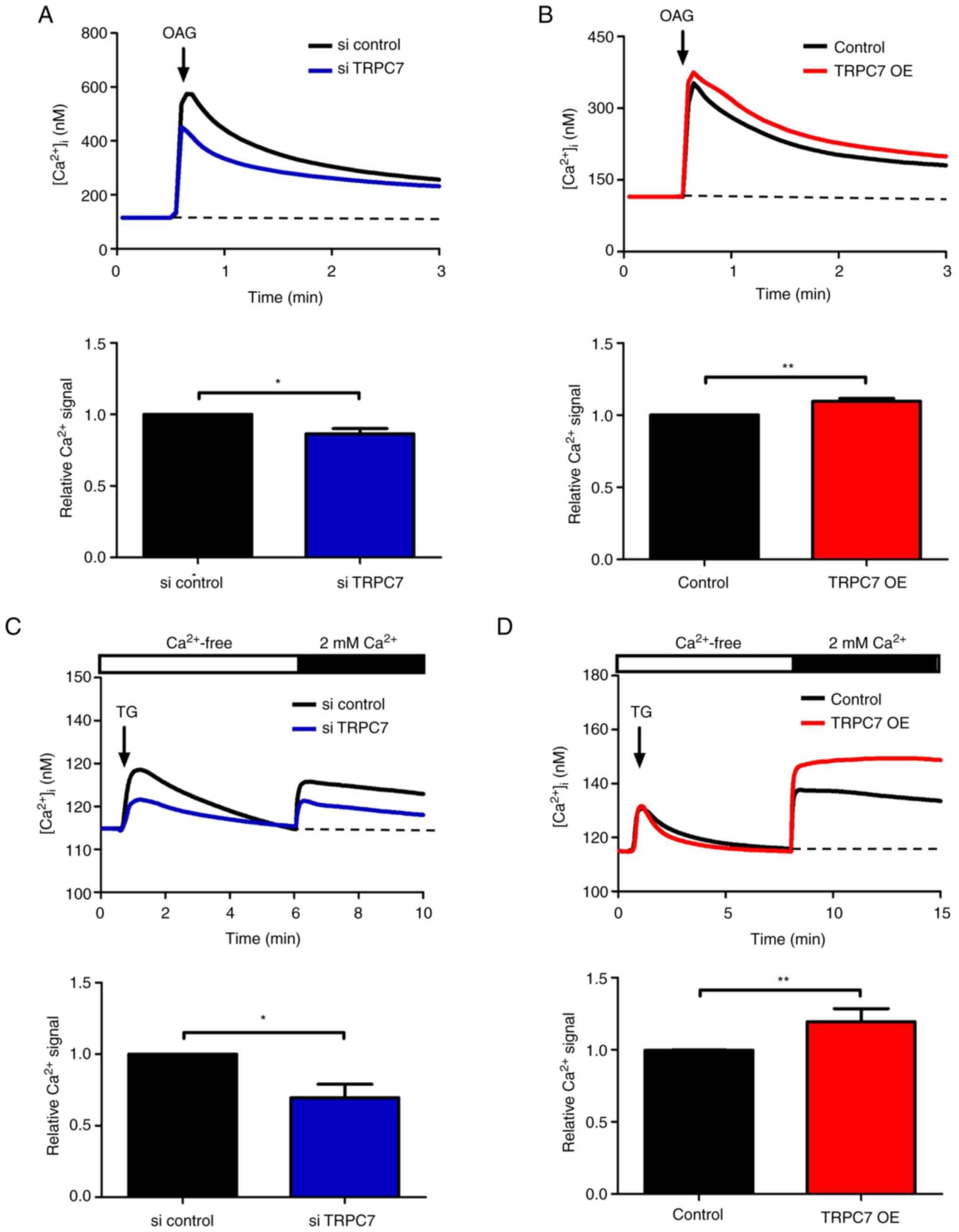
activity in lung cancer cells. OAG-induced Ca2+ influx
was significantly attenuated in TRPC7 knockdown H1299 cells but was
significantly promoted in TRPC7-overexpressing BEAS-2B cells
compared with control cells (Fig. 4A
and B and, S1A and B).
Similar results were also demonstrated for
TG-induced SOCE activity. TRPC7 knockdown in H1299 cells
significantly inhibited TG-induced SOCE activity compared with that
in the si control group (Fig. 4C
and S1C). As TRPC7 overexpression
in BEAS-2B cells significantly increased SOCE activity following TG
stimulation (Fig. 4D and S1D), which indicated that TRPC7
potentially regulated TG-induced SOCE activity. These data
demonstrated that TRPC7-mediated cell growth and migration were
Ca2+ signaling dependent.
CaMKII, AKT and MAPK signaling
pathways are involved in TRPC7-mediated Ca2+
signaling
The involvement of MAPK and AKT signaling pathways
in the enhancement of cell growth and migration, that leads to
malignancy in cancer cells, is Ca2+ signaling dependent
(2). IPA demonstrated that
TRPC7-mediated Ca2+/CaMKII, AKT and MAPK signaling
pathways were associated with several diseases and cell functions,
such as organismal injury and abnormalities, cancer development,
post-translation modification, cellular growth, cell cycle and cell
morphology (Table SII).
Ca2+-dependent activation of CaMKII induces the
phosphorylation of AKT and ERK, which promotes cancer cell
proliferation and migration (4).
Therefore, the TRPC7-mediated Ca2+/CaMKII/AKT and
Ca2+/CaMKII/ERK axes may be involved in the control of
cancer progression. A similar result was also demonstrated in small
cell lung cancer according to the Gene Expression Omnibus database
analysis. The expression levels of TRPC7, CaMKII, AKT, ERK1
and ERK2 were significantly upregulated in tumor tissues
compared to non-tumor tissues (Fig.
S2). The effect of TRPC7-mediated Ca2+ signaling on
the CaMKII, AKT and MAPK pathways was explored. The data indicated
that blockade of TRPC7 expression in lung adenocarcinoma cells
using siRNA and the TRPC7 inhibitors, SKF96365 and 2-APB,
significantly suppressed the activation of CaMKII, AKT and ERK as
indicated by the decreased expression levels of their
phosphorylated proteins, but did not influence the expression of
total CaMKII, AKT and ERK (Figs. 5A,
B and S3A). Furthermore,
SKF96365 and 2-APB both markedly reduced the protein expression
levels of p-CaMKII, p-AKT and p-ERK but not total AKT and ERK in
TRPC7-overexpressing BEAS-2B cells compared with the control
(Figs. 5C, D and S3B). The ratio of p-CaMKII/CaMKII,
p-AKT/AKT and p-ERK/ERK was also decreased by the inhibition of
TRPC7 expression (Figs. 5B and D).
These results demonstrated that the activation of the AKT and MAPK
signaling pathways could be dependent on TRPC7-mediated
Ca2+/CaMKII signaling.
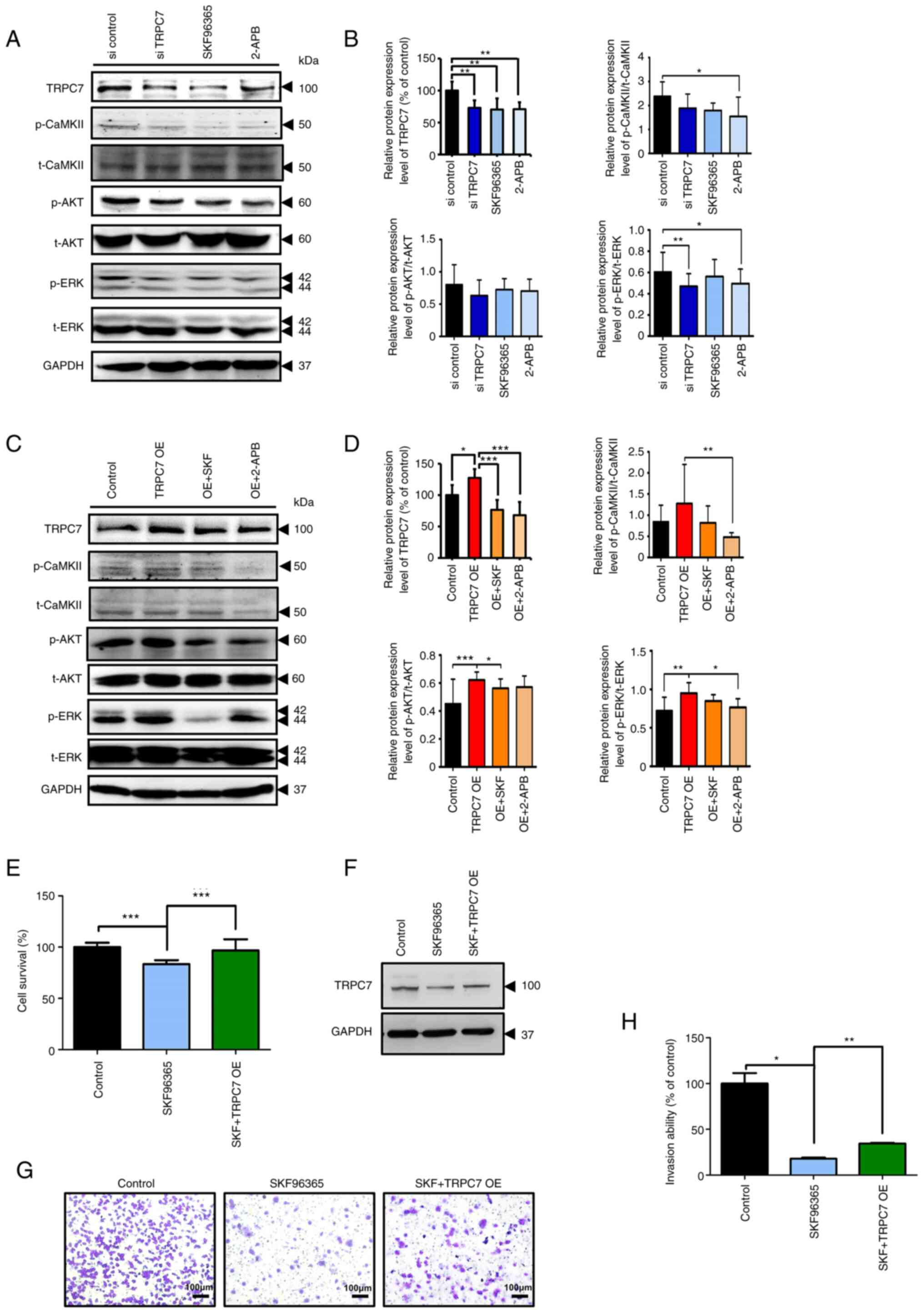 | Figure 5.TRPC7 accelerates the progression of
adenocarcinoma by regulating the AKT and MAPK signaling pathways.
Immunoblotting analysis results demonstrated the protein expression
levels of TRPC7, p-CaMKII, t-CaMKII, p-AKT, t-AKT, p-ERK, t-ERK and
GAPDH in (A) TRPC7 knockdown H1299 and (C) TRPC7-overexpressing
BEAS-2B cells with or without treatment with the TRPC7 inhibitors,
SKF96365 and 2-APB. (B) and (D) Semi-quantification of the protein
expression levels of TRPC7 and modulation of pCaMKII/CaMKII,
pAKT/AKT and pERK/ERK ratios. Data were normalized to the protein
expression of GAPDH as the internal control. Semi-quantification of
the protein expression levels analyzed using unpaired Student's
t-test. n=7, data were presented as mean ± SD. SKF96365-induced
reduction of (E) cell viability, and (G) invasion ability were
rescued by overexpression of TRPC7 in H1299 cells (magnification,
×100). (F) Immunoblotting analysis demonstrated TRPC7 expression in
H1299 cells. (H) Quantification of the invading cells, analyzed
using unpaired Student's t-test. n=6, data were presented as mean ±
SD. *P<0.05, **P<0.01 and ***P<0.001. 2-APB, 2-aminoethyl
diphenylborinate; CaMKII, Ca2+/calmodulin-dependent
protein kinase II; OE, overexpression; p, phosphorylated (protein);
si, small interfering RNA; SKF, SKF96365; t, total (protein);
TRPC7, transient receptor potential canonical 7. |
To further demonstrate that TRPC7-mediated
Ca2+ signaling was important in the regulation of cancer
progression, cancer cell growth and invasion, H1299 cells where
TRPC7 expression was inhibited by SKF96365, or in combination with
overexpressed-TRPC7 for rescue, were evaluated. SKF96365-induced
TRPC7 downregulation in H1299 cells significantly attenuated cancer
cell viability and invasion compared with the control; however,
overexpression of TRPC7 in H1299 cells which were pretreated with
SKF96365 caused recovery (Figs.
5E-H). These findings indicated that the decrease in
TRPC7-mediated Ca2+ signaling restrained lung
adenocarcinoma cell growth and migration via interruption of the
CaMKII, AKT and MAPK signaling pathways.
Discussion
A number of TRP channels have been identified using
their characteristics and functions in cancers and, the role of
TRPC7 in cancer progression has been independently reported;
however, the clinicopathological functions of TRPC7 required
further exploration. Clinical data in the present study, indicated
that TRPC7 overexpression in patients with lung adenocarcinoma was
associated with worse prognosis (Fig.
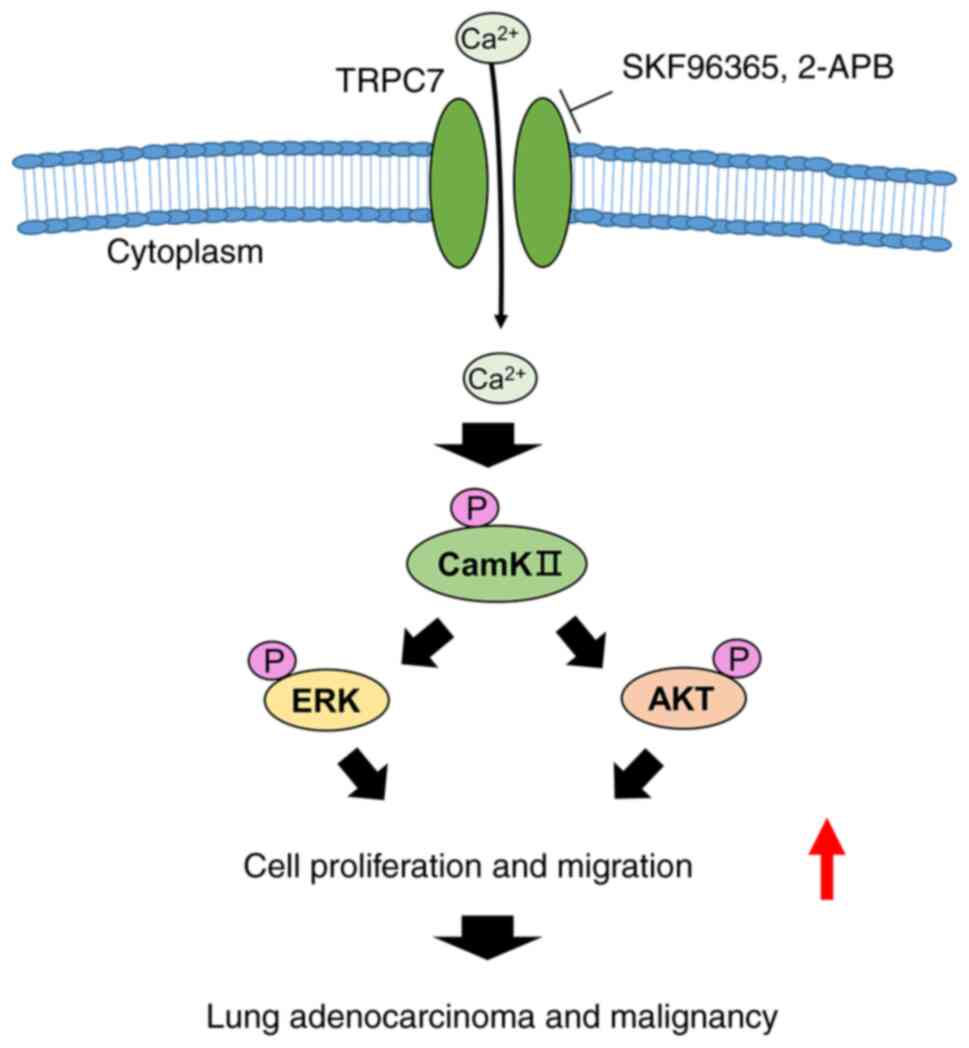
1). The present study also investigated the role of TRPC7 in
the regulation of lung adenocarcinoma cell growth and migration via
the TRPC7-mediated Ca2+ signaling-dependent
Ca2+/CaMKII/AKT and Ca2+/CaMKII/ERK axes
(Fig. 6). It was demonstrated that
TRPC7 overexpression promoted cell cycle progression and cell
migration in a lung adenocarcinoma cell line, and blockade of
TRPC7-mediated Ca2+ signaling inhibited cancer
malignancy by restraining the phosphorylation of CaMKII, AKT and
ERK. These results suggested that oncogenic TRPC7 was a potential
prognostic marker in lung adenocarcinoma because TRPC7
overexpression accelerates cancer malignancy. Therefore, the
blocking of TRPC7 activity using SKF96365, 2-APB or TRPC7 siRNA
potentially inhibits the progression of lung adenocarcinoma.
Interestingly, the ratio of p-CaMKII/CaMKII,
p-AKT/AKT and p-ERK/ERK were not all significantly decreased
following treatment with 2-APB or SKF96365 in the present study.
For example, p-CaMKII/t-CaMKII was significantly decreased by 2-APB
but not SKF96365 compared with the transfected control and
p-AKT/t-AKT was significantly reduced by SKF96365 but not 2-APB
compared with untransfected cells (Fig.
5B and D). It could be that SKF96365 or 2-APB may also affect
other Ca2+ channels following effects on the signal
molecules. Calcium signals are central in the mechanisms underlying
cancer cell proliferation, death and migration (1). Therefore, TRPC7 may promote cancer
development and TRPC6 or other TRP channels cannot be excluded from
roles in the regulation of cancer development through
Ca2+/CaMKII/AKT and/or Ca2+/CaMKII/ERK axes
(5). Furthermore, cell
proliferation results of the present study only demonstrated the
significance at Day 2 (Fig. 2C and
D); which indicated that the role of TRPC7 was specific for
cell growth. The cells at Day 1 could maintain their survival when
changed to the new environment for experiments and the cells at Day
3 almost filled the culture dish, which may have affected their
cell division and the increase in the number of cells. It could
therefore by hypothesized that the cells at Day 2 were in the
logarithmic growth phase which was why a significant increase was
observed at Day 2.
TRPC7 is not only a tumor initiator gene
(7) but also accelerates cancer
progression through the regulation of cell cycle progression and
cell migration. The present study provided direct evidence that
there was a relationship between oncogenic TRPC7 and TRPC7-mediated
Ca2+ signaling-dependent CaMKII, AKT and MAPK signaling
pathways in the regulation of lung adenocarcinoma malignancy
(Fig. 6). There are an increasing
number of TRP channels that have been reported to promote cancer
development and Ca2+/CaMKII has emerged as a crucial
signaling pathway in the modulation of cell proliferation, the cell
cycle, invasion and metastasis, and therapy efficacy in cancer
(5). For example, TRPV4 promotes
oral squamous cell carcinoma cell proliferation via activation of
the Ca2+/CaMKII/AKT axis (19) and TRPM7 mediates breast cancer cell
migration and invasion via the CaMKII-dependent MAPK signaling
pathway (20). The
Ca2+/CaMKII/ERK axis is responsible for the
phosphorylation and subsequent proteasomal degradation of
cyclin-dependent kinase inhibitor 1B (p27Kip1), which
contributes to the enhancement of the S-G2/M transition of the cell
cycle in colon adenocarcinoma cells (21). The results of the present study also
demonstrated that overexpression of TRPC7 promoted cell cycle
progression with a significant increase in the population of G2/M
phase cells. p27Kip1 may be involved in TRPC7-mediated
cell cycle progression; however, this hypothesis requires
assessment in future work.
AKT can directly regulate focal adhesion kinase
(FAK) through the AKT-FAK interaction, which causes cancer cell
adhesion and metastasis (22,23).
TRP channels, such as TRP cation channel subfamily M member (TRPM)4
and TRP cation channel subfamily V member 4, enhance cell migration
with activation of FAK and actin cytoskeleton reorganization
(6,24). Therefore, it could be hypothesized
that AKT-FAK participates in the TRPC7-mediated
Ca2+/CaMKII-dependent signaling pathway to enhance
cancer cell mobility. In gastric cancer, TRPM2-mediated
Ca2+/CaMKII signaling also promotes metastasis through
upregulation of NF-κB and AKT-mediated matrix metalloproteinases
(MMPs) (6). The pathological role
of TRPC7-mediated Ca2+ signaling in the regulation of
lung adenocarcinoma malignancy may therefore be related to the
CaMKII/AKT/FAK and CaMKII/AKT/MMP axes.
TRPC7-mediated excess Ca2+ signaling in
primary keratinocytes induces intracellular reactive oxygen species
(ROS) accumulation, DDR and intracellular senescence following cell
death (7). Notably, oncogenes
induce ROS production and ROS have been reported to be mitogenic
signaling molecules which mediate cancer cell hyperproliferation
when DDR is activated (25). It has
also been reported that TRPC7 overexpression in UVB-induced skin
tumorigenesis contributed to intracellular senescence and
p53 family mutation (7).
Although TRP channels are involved in oncogenic ROS production, DDR
inactivation seems to be the key point for ROS-mediated cancer
malignancy. It can therefore be hypothesized that TRPC7
overexpression in lung adenocarcinoma cells induces intracellular
ROS accumulation through activation of mitogenic signaling, which
fuels the aberrant proliferation of cells with p53 family
dysfunction. Oncogenic TRPC7 promotes skin tumorigenesis and lung
adenocarcinoma development (7).
Accordingly, the mechanism of TRPC7-regulated malignancy does not
belong to a cell-specific response and it may also apply to other
types of cancer.
Furthermore, epigenetic mechanisms have been
reported to promote the expression of TRP channels in cancer cells
(5). Therefore, TRPC7
overexpression in lung adenocarcinoma cells may be involved in
epigenetic mechanisms. As genetic variants can alter epigenetic
features and epigenetic variations can mediate genetic variability
(26), TRPC7 may possess specific
gene polymorphisms which are correlated with lung cancer
development. A study of the identification of TRPC genetic
variants in the Chinese population by Zhang et al (27) reported that TRPC4 rs9547991
and rs978156, and TRPC7 rs11748198 were candidate
susceptibility markers for lung cancer. The association of TRPC7
rs11748198 with lung cancer risks may be due to changes in the
epigenetic features that cause upregulation of TRPC7 expression in
lung cancer. Although the present study demonstrated the mechanism
of TRPC7 in cancer development, it is still unclear whether TRPC7
can affect epigenetic regulation to promote cancer malignancy. The
effect of TRPC7 rs11748198 on lung cancer progression
requires validation in future studies.
Overall, to the best of our knowledge, the present
study was the first to demonstrate the pathological role of TRPC7
in the regulation of cancer progression. High TRPC7 expression was
associated with a worse prognosis for patients with lung
adenocarcinoma and modulated the growth and migration of lung
adenocarcinoma cells through the activation of TRPC7-mediated
Ca2+ signaling-dependent CaMKII, AKT and MAPK pathways.
The present study increased understanding of the precise role
served by TRPC7 in cancer malignancy and could provide a novel
therapeutic molecular target for patients with lung
adenocarcinoma.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank the Center for
Research Resources and Development at Kaohsiung Medical University
(Kaohsiung, Taiwan) for the use of the confocal microscope, the
Olympus Cell-R IX81 fluorescence microscope and the LSR II flow
cytometer.
Funding
The present study was supported by the Ministry of Science and
Technology of Taiwan (grant no. MOST 109-2314-B-037-143 and MOST
110-2314-B-037-035), Kaohsiung Municipal Ta-Tung Hospital,
Kaohsiung Medical University Hospital (grant no. kmtth-110-R003)
and Chi Mei Medical Center (grant no. CLFHR10509 and
CLFHR10709).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLL and WLH were responsible for conceptualization.
JLL, YCH, YFL and WLH were responsible for clinical data curation.
JLL and WLH were responsible for funding acquisition. MHT performed
the liquid chromatography mass spectrometry; YCH and HSL performed
the immunoblot analysis and RT-qPCR; SWC performed the invasion
assay; YYH, LCL, TFT performed the cell cycle analysis and cell
viability assay; YFL performed the immunoblot analysis and GEO
database analysis; WLH performed the calcium response assay and
IPA. MHT, YCH and WLH were responsible for the methodology. HSL and
YFL confirmed the authenticity of the data. JLL and WLH were
responsible for writing the original draft. WLH was responsible for
reviewing and editing the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Chi-Mei Medical Center, (Tainan, Taiwan; approval no.
10405-L01). Written informed consent was obtained from all
individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bong AHL and Monteith GR: Calcium
signaling and the therapeutic targeting of cancer cells. Biochim
Biophys Acta Mol Cell Res. 1865:1786–1794. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Apati A, Janossy J, Brozik A, Bauer PI and
Magocsi M: Calcium induces cell survival and proliferation through
the activation of the MAPK pathway in a human hormone-dependent
leukemia cell line, TF-1. J Biol Chem. 278:9235–9243. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gocher AM, Azabdaftari G, Euscher LM, Dai
S, Karacosta LG, Franke TF and Edelman AM: Akt activation by
Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in
ovarian cancer cells. J Biol Chem. 292:14188–14204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villalobo A and Berchtold MW: The role of
calmodulin in tumor cell migration, invasiveness, and metastasis.
Int J Mol Sci. 21:7652020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu WL, Noda M, Yoshioka T and Ito E: A
novel strategy for treating cancer: Understanding the role of
Ca(2+) signaling from nociceptive TRP channels in regulating cancer
progression. Explor Target Antitumor Ther. 2:401–415.
2021.PubMed/NCBI
|
|
6
|
Yang D and Kim J: Emerging role of
transient receptor potential (TRP) channels in cancer progression.
BMB Rep. 53:125–132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu WL, Tsai MH, Wu CY, Liang JL, Lu JH,
Kahle JS, Yu HS, Yen CJ, Yen CT, Hsieh YC, et al: Nociceptive
transient receptor potential canonical 7 (TRPC7) mediates
aging-associated tumorigenesis induced by ultraviolet B. Aging
Cell. 19:e130752020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang HN, Zeng B, Zhang Y, Daskoulidou N,
Fan H, Qu JM and Xu SZ: Involvement of TRPC channels in lung cancer
cell differentiation and the correlation analysis in human
non-small cell lung cancer. PLoS One. 8:e676372013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: An open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PLoS One. 9:e968012014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CY, Hsu WL, Tsai MH, Liang JL, Lu JH,
Yen CJ, Yu HS, Noda M, Lu CY, Chen CH, et al: Hydrogen gas protects
IP3Rs by reducing disulfide bridges in human keratinocytes under
oxidative stress. Sci Rep. 7:36062017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu WL, Chung PJ, Tsai MH, Chang CL and
Liang CL: A role for Epstein-Barr viral BALF1 in facilitating tumor
formation and metastasis potential. Virus Res. 163:617–627. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clough E and Barrett T: The gene
expression omnibus database. Methods Mol Biol. 1418:93–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas S and Bonchev D: A survey of
current software for network analysis in molecular biology. Hum
Genomics. 4:353–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lievremont JP, Bird GS and Putney JW Jr:
Canonical transient receptor potential TRPC7 can function as both a
receptor- and store-operated channel in HEK-293 cells. Am J Physiol
Cell Physiol. 287:C1709–C1716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii S, Tajiri Y, Hasegawa K, Matsumoto
S, Yoshimoto RU, Wada H, Kishida S, Kido MA, Yoshikawa H, Ozeki S
and Kiyoshima T: The TRPV4-AKT axis promotes oral squamous cell
carcinoma cell proliferation via CaMKII activation. Lab Invest.
100:311–323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhennin-Duthille I, Gautier M, Korichneva
I and Ouadid-Ahidouch H: TRPM7 involvement in cancer: A potential
prognostic factor. Magnes Res. 27:103–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YY, Zhao R and Zhe H: The emerging
role of CaMKII in cancer. Oncotarget. 6:11725–11734. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
More SK, Vomhof-Dekrey EE and Basson MD:
ZINC4085554 inhibits cancer cell adhesion by interfering with the
interaction of Akt1 and FAK. Oncol Lett. 17:5251–5260.
2019.PubMed/NCBI
|
|
23
|
Wang S and Basson MD: Akt directly
regulates focal adhesion kinase through association and serine
phosphorylation: Implication for pressure-induced colon cancer
metastasis. Am J Physiol Cell Physiol. 300:C657–C670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fels B, Bulk E, Petho Z and Schwab A: The
role of TRP channels in the metastatic cascade. Pharmaceuticals
(Basel). 11:482018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ogrunc M, Di Micco R, Liontos M,
Bombardelli L, Mione M, Fumagalli M, Gorgoulis VG and d'Adda di
Fagagna F: Oncogene-induced reactive oxygen species fuel
hyperproliferation and DNA damage response activation. Cell Death
Differ. 21:998–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung A, Schones DE and Natarajan R: Using
epigenetic mechanisms to understand the impact of common disease
causing alleles. Curr Opin Immunol. 24:558–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Wang J, He J, Zeng X, Chen X,
Xiong M, Zhou Q, Guo M, Li D and Lu W: Identification of TRPCs
genetic variants that modify risk for lung cancer based on the
pathway and two-stage study. Meta Gene. 9:191–196. 2016. View Article : Google Scholar : PubMed/NCBI
|