Introduction
Oral squamous cell carcinoma (OSCC) is the eighth
most common type of cancer in the world and >177,000 individuals
die from oral carcinoma annually (1,2). In
Europe, this malignancy ranks 11th in terms of mortality rate, with
a cumulative annual incidence of 18.2 in men and 4.9 in women OSCC
has a high recurrence rate and is prone to metastasis (3). Treatment of OSCC include surgical
treatment, chemotherapy and radiotherapy. Other treatment
modalities are considered as immunotherapy and biological therapy
(4). The 5-year overall survival
rate of OSCC is estimated to be ~54.5% (3). The 5-year overall survival rate in
earlier stage is 55–60% while in patients with advanced disease is
30–40% (4). Predicting the clinical
course of patients with OSCC at the early stage of the disease is
difficult due to the possibility of metastasis. Therefore,
identifying novel prognostic factors associated with survival in
these patients is of importance. Pathological tumor volume (PTV) is
quantitative factor of OSCC which could be interesting for survival
prediction. The cut-off value of PTV can indicate significance od
this factor in survival patients with OSCC. The
tumor-node-metastasis (TNM) system is the most common system of
tumor classification used to estimate prognosis (1,5). This
staging system refers to the superficial tumor dimension and depth
of tumor invasion (1,5). Several clinical and pathological
prognostic factors have been investigated to develop a prognostic
model of survival for patients with oral cancer. For example, the
8th edition of the American Join Community for cancer included
depth of primary tumor invasion and extracapsular extension as
prognostic criteria (1,5). The maximal diameter of tumor which is
used in this staging is not volumetric measure and therefore it
does not determine the tridimensional extend of tumor. Tumor volume
can be determined by different methods using imaging scans or by
measuring the surgical specimen. Pathological tumor volume (PTV), a
quantitative prognostic factor that refers to the 3D nature of the
tumor, is associated with patient survival (6). PTV is determined for pathological
tissue samples derived from surgically resected primary tumors by
measuring the tumor diameter (6).
Although the association between PTV and survival of patients with
tongue carcinoma has been previously reported, the association
between PTV and other clinical outcomes (occurrence of recurrence
and local and regional metastasis) in such patients remains elusive
(7,8).
Therefore, the present study aimed to investigate
the association between PTV and overall survival in patients with
OSCC regardless of cervical nodal status and the cut-off values of
PTV.
Materials and methods
Patients
The prospective study included 65 consecutive male
(n=53) and female (n=12) patients with age range 38–83 years, who
were surgically treated for oral cancer between January 2013 and
December 2015 at the Clinic for maxillofacial surgery University
Clinical Center in Novi Sad Vojvodina, Serbia. The follow-up period
for each patient was 5 years from the date of surgery. The present
study was conducted in accordance with the guidelines of the
Declaration of Helsinki and was approved by the Ethics Committee of
the Medicine University of Novi Sad (Novi Sad, Serbia). All
patients provided written informed consent for all examinations and
treatments. Oral cancer was diagnosed based on anamnesis, physical
examination and tumor biopsy. The inclusion criteria were as
follows: i) Newly pathohistologically diagnosed patients of any sex
with untreated resectable OSCC; ii) >18 years of age and iii) no
radiologically diagnosed distant metastasis. The exclusion criteria
were as follows: i) Patients with a history of any malignancy other
than basal cell carcinoma of the skin; ii) patients with recurrent
oral carcinoma; iii) prior completion of one or more courses of
therapeutic irradiation; vi) patients with autoimmune disease or
HIV infection and v) patients with distant metastasis. All patients
included in the study were also HPV-negative. Tumor size and TNM
stage were determined via clinical examination, biopsy and head,
neck and thorax computed tomography (CT). The patients were treated
according to their TNM status determined by the clinical findings
and CT results. Treatment approaches included radical tumor
resection and neck dissection.
Surgically resected tumors were fixed on a styrofoam
surface and marked according to localization in the mouth or neck.
Following tumor diameter measuring, the tissue was fixed with 10%
formalin and embedded in paraffin, followed by staining with
hematoxylin and eosin (H&E) solution. Fixation of the specimen
started as soon as the resection operation finished. The bottle
containing the specimen was kept at room temperature at all times.
The specimen was fixed for 6–24 h. After cutting the tissue,
specimens were moved to the automated procession procedure in the
Epredia™ Excelsior™ AS Tissue Processor (Thermo Fisher Scientific,
Inc.) following the recommended procedure. Staining with H&E
lasted for 90 min at 21°C. The tissue sectioning was performed
using an Accu-Cut SRM 200 Rotary Microtome (Sakura Finetek USA,
Inc.) or a Microtome HM355S (Thermo Fisher Scientific, Inc.). The
thickness of the microscopic sections was 5–7 µm. The primary
antibodies used were as follows: anti-GAPDH (1:1,000; cat. no.
48245; Thermo Fisher Scientific, Inc.), FLEX Monoclonal Mouse
Anti-Human p63 Protein Clone DAK-p63 R, Ready-to-Use (cat. no. IR
662; Dako; Agilent Technologies, Inc.), FLEX Monoclonal Mouse
Anti-Human Ki-67 Clone MIB-1, Ready-to-Use (cat. no. IR626; Dako;
Agilent Technologies, Inc.). Epredia™ Dewax and HIER Buffer L (cat.
no. TA-999-DHBM; X15; Thermo Fisher Scientific, Inc.) was used as
the blocking reagent at 65°C then heated to 98°C for 10 min before
being allowed to cool to 65°C using the LAB Vision™ PT Module
(Thermo Fisher Scientific, Inc.). An Espedia Autostainer 360
(Thermo Fisher Scientific, Inc.) and Bench Mark GX (Roche Tissue
Diagnostics) were used for staining. Incubation with primary
antibodies was at room temperature for 10 min. The following
reagents were used: Epredia™ DAB Quanto Detection System (cat. no.
12674017; Fisher Scientific; Thermo Fisher Scientific. Inc.), Ultra
view Universal DAB Detection Kit (cat. no. 760-500; Roche Tissue
Diagnostics; Roche Diagnostics, Ltd.) and Erpedia™ Ultra Vision
Detection System HRP (cat. no. 12684017; Fisher Scientific; Thermo
Fisher Scientific, Inc.). Both primary antibodies were conjugated
with the Epredia™ Primary Antibody Amplifier Quanto and Epredia™
HRP Polymer Quanto supplied with the aforementioned Epredia™ DAB
Quanto Detection System.
The postoperative pathological examination was
performed by an experienced pathologist. The light microscope Zeiss
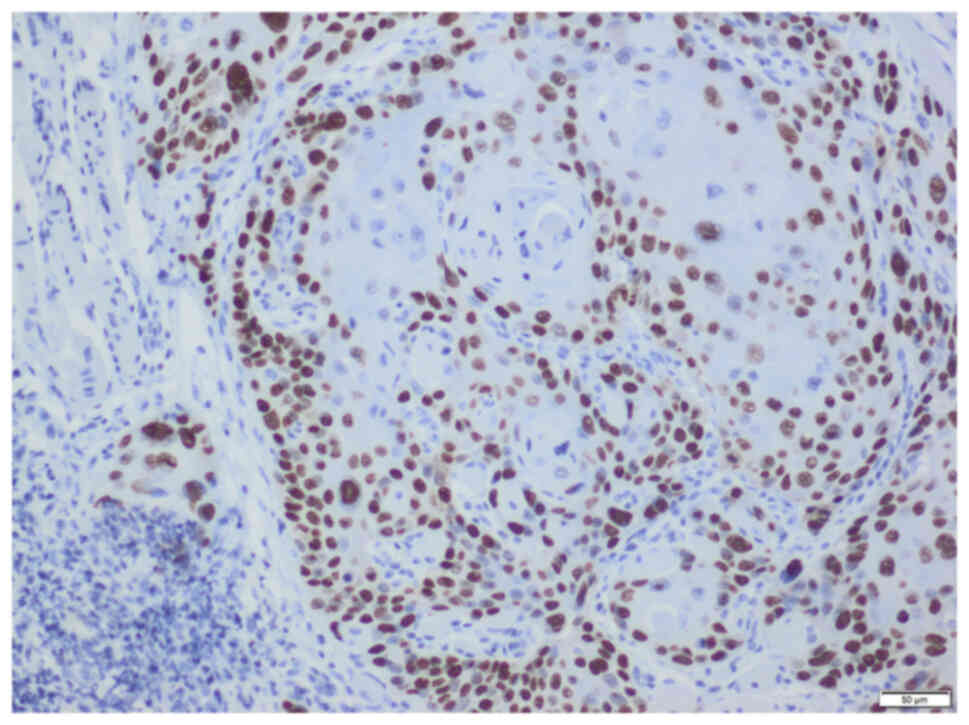
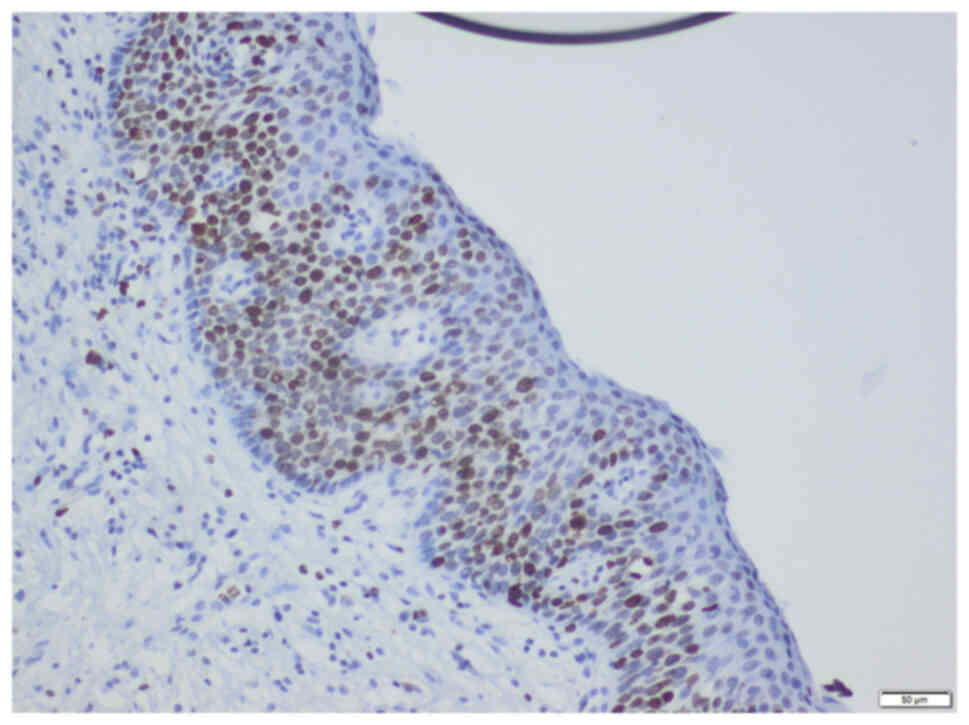
Axio Scope A1 was used (representative images are shown in Figs. 1 and 2).
Based on the histopathological findings, patients
were treated with the appropriate chemotherapy and radiotherapy
regimen <6 weeks following surgical resection. Low-risk patients
were treated with external radiotherapy of 60–70 Gy in 30–35
fractions for 6–7 weeks. High-risk patients with positive margins,
>2 positive nodes or extracapsular spreading received concurrent
chemotherapy with intravenous bolus of 100 mg/m3
cisplatin for 3 weeks combined with radiotherapy of 60–70 Gy in
30–35 fractions for 6–7 weeks. At the end of the
chemotherapy/radiotherapy cycles, the patients were monitored every
2 months for 2 years, every 6 months for the next 2 years and then
every year after that. CT of the head, neck and chest were
routinely performed to detect any recurrence or distant
metastasis.
Determination of pathological
parameters
The pathological parameters determined for surgical
specimens were as follows: i) Largest (diameter A) and smallest
transverse diameter (diameter B) of the tumor were measured on an
unfixed macroscopic sample; ii) tumor thickness (vertical distance
from the surface of the tumor to the point of its deepest invasion,
cm) was measured via microscopic examination with an accuracy of
0.1 mm; iii) the invasion depth (vertical distance between basal
membranes of the closest intact mucosa and the deepest point of
tumor invasion) was also determined via microscopic examination
with an accuracy of 0.1 mm and was expressed in mm; iv)
pathological volume of the primary tumor was calculated using the
following formula: PTV (cm3)=π/6 × diameter А × diameter
B × tumor thickness; v) radiological tumor volume (RTV) was
determined by measuring tumor dimensions from the CT scan using the
following formula: RTV (cm3)=π/6 × diameter А × diameter
B × tumor thickness (8) and vi)
presence of perineural, perivascular and perinodular spread,
dysplasia and positive resection margins determined by
pathologist.
Statistical analysis
Data were analyzed using SPSS 25.0 software (IBM
Corp.). Data are presented as the mean and standard deviation. The
association between PTV, according to the pathohistological
findings, and the clinicopathological features of patients,
including sex, tumor site, pathological tumor (T) and node (N)
stage status, dysplasia, margins of resection, perineural and
perivascular spreading and metastasis, were analyzed by
Mann-Whitney U or Kruskal-Wallis test. Mann-Whitney test was used
to detect statistically significant differences between two
independent samples. Kruskal-Wallis was used to detect differences
in medians between more than two independent samples. Spearman
correlation was used to asses the relationship between variables
that do not follow normal distribution. To determine the optimal
cut-off value of tumor volume, to divide cases into groups,
receiver operating characteristic (ROC) curve analysis was
performed using the Youden index (9). The Kaplan-Meier method and log-rank
test were performed to evaluate differences in the survival
distribution for the calculated cut-off values. χ2 was
used to compare differences in the survival between the two cut-off
groups. Two multiple Cox proportional hazards regression models
were constructed to explore the association between the
tumor-dependent variables and survival time. Omnibus test was
performed to assess the validity of the models. The regression
analysis results are expressed as a P-value, hazard ratio and
confidence interval (CI) for hazard ratio [95 CI for Exp(B)].
Survival time was defined as the period between surgery and a
target event (such as death) or last contact. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic and tumor-associated
features
A total of 65 patients with a mean age of 59.6±9.4
years were included in the study. 81.5% of patients were males. Of
all patients, 53.8% had no metastases. With regards to localization
of the tumor in the oral cavity, a primary tumor was located on the
tongue of 32 patients, on the floor of the mouth in 22 patients, on
the hard palate in 4 patients, on the gingiva in 4 patients and on
the buccal mucosa in 3 patients (Table
I).
 | Table I.Demographic data and tumor-associated
characteristics of 65 patients. |
Table I.
Demographic data and tumor-associated
characteristics of 65 patients.
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 59.65±9.42 |
| Male, n (%) | 53 (81.50) |
| Without metastases, n
(%) | 35 (53.80) |
| Censored (patient
died), n (%) | 28 (43.10) |
| Median life
expectancy following surgery, months; IQ | 41;64 |
| Dysplasia present, n
(%) | 26 (40.00) |
| Positive margins of
resection, n (%) | 6 (9.20) |
| Tumor location, n
(%) |
|
|
Tongue | 32 (49.20) |
| Floor of
the mouth | 22 (33.80) |
| Hard
palate | 4 (6.20) |
|
Gingiva | 4 (6.20) |
| Buccal
mucosa | 3 (4.60) |
| RTV mean ± SD,
cm3 | 6.71±5.23 |
| PTV mean ± SD,
cm3 | 6.67±7.77 |
| PT diameter A mean ±
SD, cm | 3.19±1.42 |
| PT diameter B mean ±
SD, cm | 2.23±1.00 |
| PT thickness mean ±
SD, cm | 1.34±0.51 |
| PT depth of tumor
invasion | 9.17±5.46 |
| DOI, mean ± SD,
mm |
|
| T stagea, n (%) |
|
| T1 | 8 (12.30) |
| T2 | 29 (44.60) |
| T3 | 27 (41.50) |
| T4 | 1 (1.50) |
| N
stagea, n (%) |
|
| N0 | 35 (53.80) |
| N1 | 7 (10.80) |
| N2 | 15 (21.10) |
| N3 | 8 (12.30) |
| Perineural
spreading, n (%) | 23 (35.40) |
| Perivascular
spreading, n (%) | 10 (15.40) |
| Perinodalar
spreading, n (%) | 11 (16.90) |
Association between PTV and
clinicopathological features of patients
The association between PTV and clinicopathological
characteristics of patients with OSCC is presented in Table II. PTV was significantly higher in
patients with metastasis and those with higher pathological T and N
status. However, PTV was significantly decreased in patients who
survived compared with those who died. No significant association
was observed between PTV and the other parameters examined. There
was no significant difference between RTV calculated using CT
examination and PTV calculated on the surgically resected specimen
which indicate that pretreatment tumor could be determinated using
CT. These two quantities are positively correlated (ρ=0.4), meaning
that an increase in one volume shows an increment in the other
volume.
 | Table II.Association between pathological
tumor volume and clinicopathological parameters. |
Table II.
Association between pathological
tumor volume and clinicopathological parameters.
| Parameter | Median | Interquartile
range | P-value |
|---|
| Sex |
|
| 0.859 |
|
Male | 3.930 | 43.86 |
|
|
Female | 3.210 | 10.24 |
|
| Dysplasia |
|
| 0.904 |
|
Absent | 3.925 | 33.39 |
|
|
Present | 3.310 | 42.78 |
|
| Margins of
resection |
|
| 0.196 |
|
Negative | 3.271 | 43.86 |
|
|
Positive | 8.308 | 14.95 |
|
| Perineural
spreading |
|
| 0.105 |
|
Absent | 2.826 | 23.18 |
|
|
Present | 4.710 | 43.86 |
|
| Perivascular
spreading |
|
| 0.964 |
|
Absent | 3.533 | 3.533 |
|
|
Present | 3.925 | 33.39 |
|
| Metastasis |
|
| 0.006 |
|
Absent | 2.355 | 23.45 |
|
|
Present | 6.018 | 43.02 |
|
| Survival |
|
| 0.005 |
|
Survived | 2.944 | 23.45 |
|
|
Censoreda | 6.411 | 43.02 |
|
| Tumor site |
|
| 0.341 |
|
Tongue | 4.710 | 33.39 |
|
| Floor
of the mouth | 3.336 | 43.21 |
|
|
Other | 3.140 | 12.19 |
|
| Pathological tumor
classificationb |
|
| 0.001 |
| T1 | 0.994 | 1.47 |
|
| T2 | 2.512 | 11.78 |
|
| T3 | 8.373 | 42.78 |
|
| T4 | 16.485 | 0.00 |
|
| Pathological node
classificationb |
|
| 0.005 |
| N0 | 2.355 | 23.45 |
|
| N1 | 3.140 | 5.23 |
|
| N2 | 6.280 | 14.76 |
|
| N3 | 12.560 | 42.39 |
|
The association between survival rate of patients
and their clinicopathological parameters is shown in Table III. Statistically significant
associations were observed between survival time and tumor
metastasis, as well as with the pathological T and N status.
 | Table III.Association between pathological
tumor volume and clinicopathological parameters. |
Table III.
Association between pathological
tumor volume and clinicopathological parameters.
| Parameter | Survived, % |
Censoreda, % | P-value |
|---|
| Sex |
|
| 0.913 |
|
Male | 56.6 | 43.4 |
|
|
Female | 58.3 | 41.7 |
|
| Dysplasia |
|
| 0.919 |
|
Absent | 56.4 | 43.6 |
|
|
Present | 57.7 | 42.3 |
|
| Margins of
resection |
|
| 0.719 |
|
Negative | 57.6 | 42.4 |
|
|
Positive | 50.0 | 50.0 |
|
| Perineural
spreading |
|
| 0.567 |
|
Absent | 59.5 | 40.5 |
|
|
Present | 52.2 | 47.8 |
|
| Perivascular
spreading |
|
| 0.631 |
|
Absent | 58.2 | 41.8 |
|
|
Present | 50.0 | 50.0 |
|
| Metastasis |
|
| 0.011 |
|
Absent | 67.6 | 32.4 |
|
|
Present | 35.7 | 64.3 |
|
| Tumor site |
|
| 0.334 |
|
Tongue | 65.6 | 34.4 |
|
| Floor
of the mouth | 45.5 | 54.5 |
|
|
Other | 54.5 | 45.5 |
|
| Pathological tumor
classificationb |
|
| 0.002 |
| T1 | 75.0 | 25.0 |
|
| T2 | 69.0 | 31.0 |
|
| T3 | 40.7 | 59.3 |
|
| T4 | 0.0 | 100.0 |
|
| Pathological node
classificationb |
|
| 0.002 |
| N0 | 71.4 | 28.6 |
|
| N1 | 71.4 | 28.6 |
|
| N2 | 46.7 | 53.3 |
|
| N3 | 0.0 | 100.0 |
|
Cox model survival analysis
Two Cox proportional hazard regression models were
constructed to identify potential predictors of survival in
patients with OSCC (Table IV).
There were no associations between risk factors for oral cancer
analyzed. In the first model, four predictors were analyzed, RTV,
PTV, perineural spreading and perivascular spreading. In the second
model, RTV, perineural spreading and perivascular spreading were
also used as predictors. However, in the second model, tumor
dimensions were used instead of PTV. Omnibus tests of model
coefficients revealed that both models were statistically
significant [model 1, χ2(4)=13.617; model 2, χ2(7)=19.070]. However, the only significant
variable in model 1 was PTV, indicating that the higher the PTV,
the less likely the patient was to survive. In the second model,
none of the indicators were statistically significant.
 | Table IV.Variables in the Cox proportional
hazard regression models. |
Table IV.
Variables in the Cox proportional
hazard regression models.
| A, Model 1 |
|---|
|
|---|
|
|
|
| 95% CI for
Exp(B) |
|---|
|
|
|
|
|
|---|
| Variable | P-value | Hazard ratio | Lower | Upper |
|---|
| RTV | 0.284 | 1.039 | 0.969 | 1.114 |
| PTV | 0.013 | 1.057 | 1.012 | 1.105 |
| Perineural
spreading | 0.941 | 1.035 | 0.422 | 2.534 |
| Perivascular
spreading | 0.977 | 0.984 | 0.313 | 3.095 |
|
| B, Model
2 |
|
|
|
|
| 95% CI for
Exp(B) |
|
|
|
|
|
|
Variable | P-value | Hazard
ratio | Lower | Upper |
|
| RTV | 0.294 | 1.041 | 0.966 | 1.122 |
| PH tumor size
A | 0.233 | 1.261 | 0.861 | 1.845 |
| PH tumor size
B | 0.223 | 1.470 | 0.790 | 2.735 |
| PH tumor
thickness | 0.421 | 0.643 | 0.220 | 1.884 |
| PH depth of tumor
invasion | 0.804 | 1.014 | 0.908 | 1.133 |
| Perineural
spreading | 0.709 | 1.200 | 0.460 | 3.129 |
| Perivascular
spreading | 0.688 | 0.770 | 0.215 | 2.759 |
ROC survival analysis
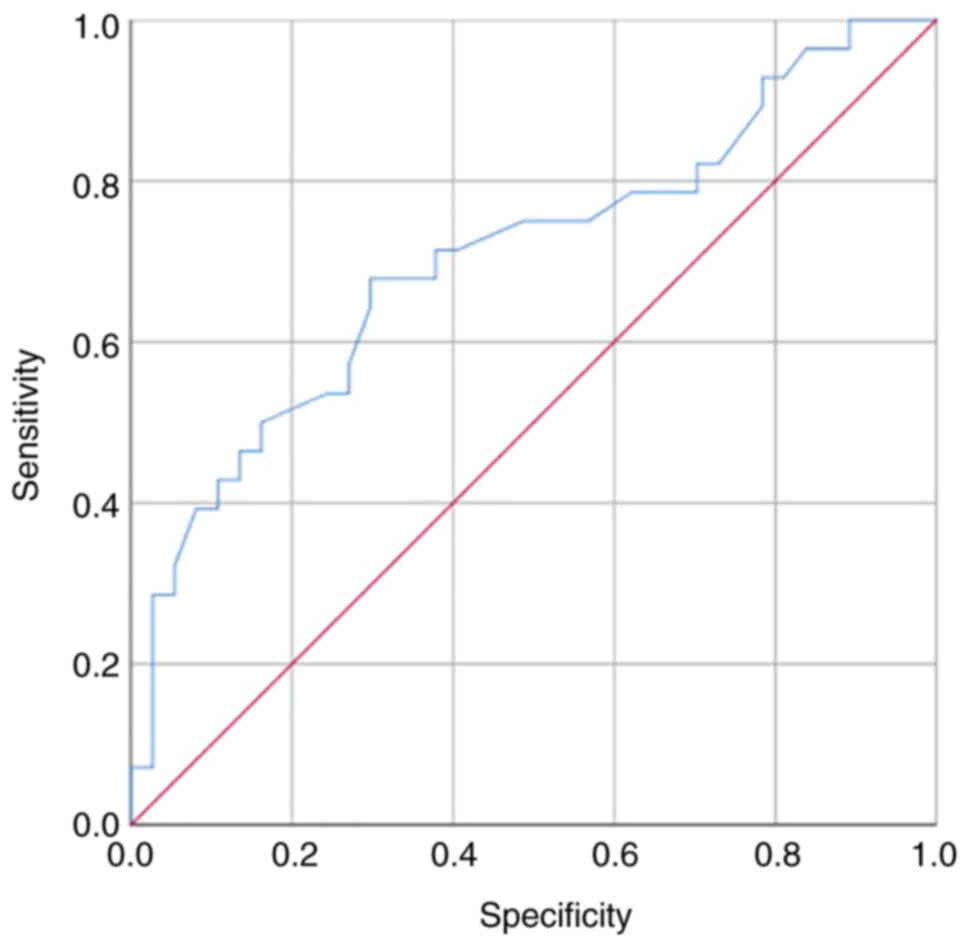
ROC curve analysis was performed to evaluate the
significant predictors in the Cox model and to determine the
optimal cut-off volume for identifying pathological tumors. PTV
exhibited a good capacity to discriminate pathological from
non-pathological tumors (area under the curve=0.70608; Fig. 3). The performance of PTV was
determined by calculating sensitivity, specificity and positive and
negative predictive values (Table
V). Sensitivity and specificity values of PTV were 68 and 70%,
respectively, while the negative and positive predictive values
were 63 and 74%, respectively. Furthermore, ROC curve analysis
(Fig. 3) and Youden index showed
that the optimal cut-off volume of PTV was 4.24 cm3
(Youden index, 0.3812).
 | Table V.Pathological tumor volume performance
measures. |
Table V.
Pathological tumor volume performance
measures.
| Performance
measure | Value | Lower limit | Upper limit |
|---|
| Sensitivity | 0.679 | 0.476 | 0.841 |
| Specificity | 0.703 | 0.530 | 0.841 |
| Positive predictive
value | 0.633 | 0.452 | 0.813 |
| Negative predictive
value | 0.743 | 0.555 | 0.866 |
| Positive likelihood
ratio | 2.282 | 1.308 | 3.984 |
| Negative likelihood
ratio | 0.457 | 0.257 | 0.815 |
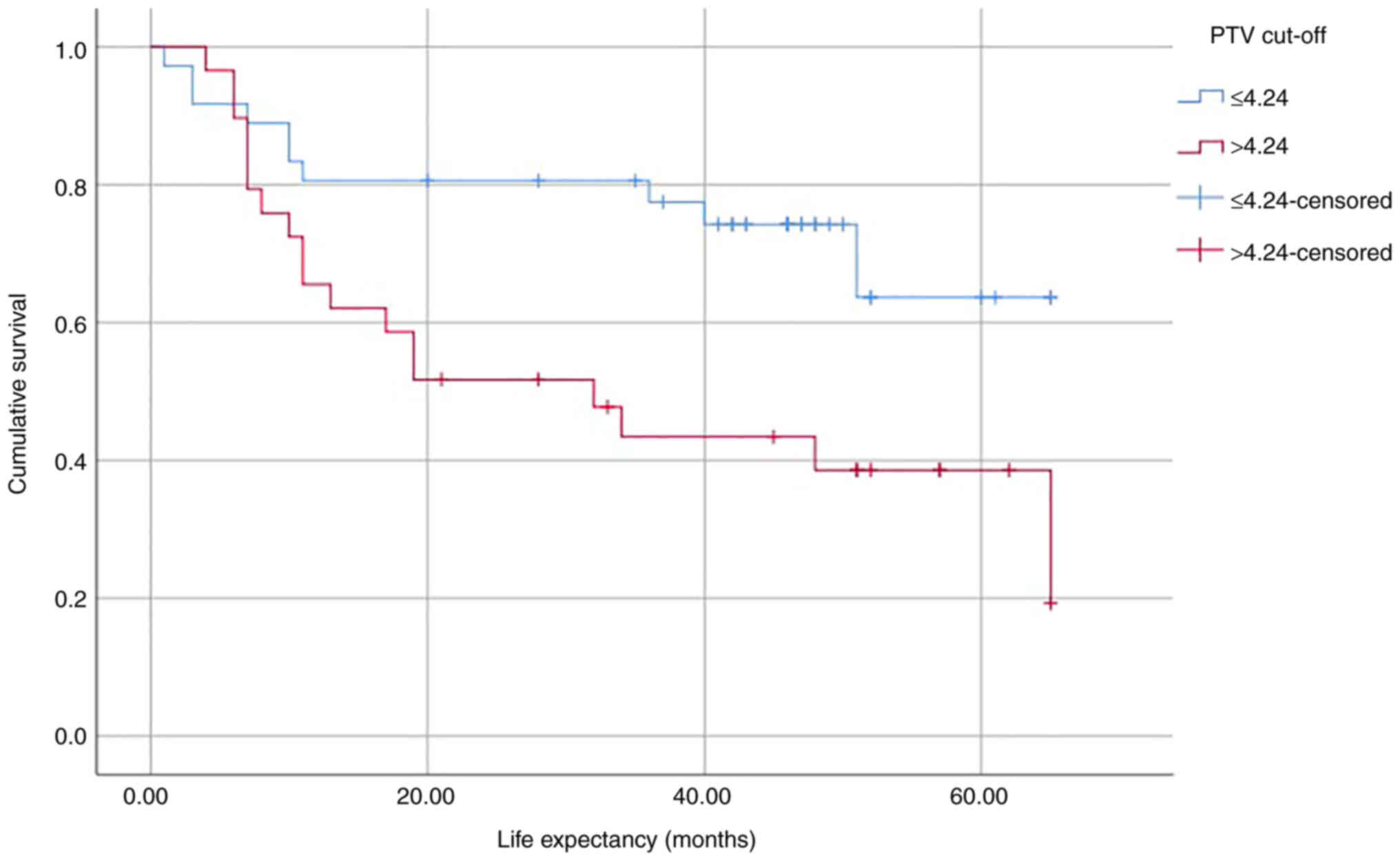
Kaplan-Meier survival analysis
Log-rank test was performed to determine the
differences in the survival distribution for different PTV cut-off
values (Table VI). The analysis
showed that differences in survival distribution between the two
groups (PTV ≤4.24 and PTV >4.24) were statistically significant
[χ2(1)=6.318]. Patients
with PTV >4.24 cm3 exhibited a significantly shorter
survival time compared with those with PTV ≤4.24 cm3.
Kaplan-Meier survival curves were plotted based on the PTV cut-off
(Fig. 4).
 | Table VI.Calculated means for the survival
time. |
Table VI.
Calculated means for the survival
time.
|
|
|
| 95% Confidence
interval |
|---|
|
|
|
|
|
|---|
| PTV | Mean estimate | Standard error | Lower bound | Upper bound |
|---|
| ≤4.24 | 50.421 | 4.012 | 42.558 | 58.285 |
| >4.24 | 35.142 | 4.976 | 25.390 | 44.895 |
| Overall | 43.796 | 3.274 | 37.378 | 50.214 |
Discussion
The results of the present study showed that PTV
>4.24 cm3 was significantly associated with shorter
overall survival time in patients with OSCC. Two models were
constructed using Cox proportional hazard regression models. In the
first model, PTV was used but in the second model,
pathohistological parameters used to calculate PTV (such as tumor
dimensions and thickness) were used. The first model exhibited an
improved prognostic value compared with the second model. In
addition, PTV was higher in patients with metastatic OSCC and in
those with higher pathological T and N status. Additionally, PTV
was notably decreased in patients who survived compared with those
who died. However, no significant differences were observed between
PTV and the other parameters examined. Of patients included in the
present study, 43.1% died. This was consistent with a previous
study demonstrating that the average 5-year mortality rate in
patients with oral cancer is 45.5% (3).
Several pathological parameters serve a key role in
the prognosis of OSCC. The significant association between tumor
invasion depth and thickness with cancer prognosis has been
reported in several studies, suggesting that tumor invasion depth
could be used in TNM staging of OSCC (6,7).
Additionally, tumor size, grade, thickness and invasion depth as
well as perineural and perivascular spreading, bone infiltration
and surgical margins status are significant factors associated with
overall survival in patients with oral cancer (8). Therefore, these parameters have been
incorporated into guidelines for the treatment of oral carcinoma
(10,11). Nevertheless, investigating these
parameters is of great importance as prognostic stratification
serves a critical role in treatment planning.
PTV, calculated by 3D measurement of the primary
tumor, is a significant pathological parameter in several types of
cancer (laryngeal carcinoma, tumors of pancreas, liver tumors)
(10). Tumors of higher T status
exhibit larger tumor volume. However, this is not always
applicable. The lower tumor volume observed in some cases of T3 and
T4 OSCC could be due to the amorphic nature and irregular shape of
the tumor (6). Tumor volume has
been also associated with survival time (11). Mucoyama et al (8) demonstrated that PTV >18
cm3 is significantly associated with a shorter survival
time and suggested that PTV, calculated using the same formula used
in the present study, serves as an important pathological parameter
of OSCC localization. In addition, Mücke et al (12) showed that increased PTV is notably
associated with shorter survival time in patients with tongue
squamous cell carcinoma. Therefore, the present study hypothesized
that PTV may be significantly associated with overall survival in
patients with OSCC, which includes patients with squamous cell
carcinoma of the entire oral cavity. The results showed that in a
clinical sample of 65 patients, PTV value >4.24 cm3
was significantly associated with shorter overall survival time.
This finding indicated that patients with PTV >4.24
cm3 should undergo more frequent postoperative
examinations and CT scans to improve overall survival rate.
The multivariate Cox regression analysis results in
the present study suggested that PTV may be considered as a
significant prognostic factor. It has also been reported that PTV
is associated with pathological T and N status, thus indicating
that it may be significantly associated with the survival of
patients with OSCC (6). Therefore,
in addition to tumor invasion depth and tumor thickness, PTV may be
used in OSCC staging (13,14). However, in order to obtain a more
optimal prognostic model, it should be investigated whether the
prognostic value of PTV improves when combined with that of other
prognostic factors, such as those associated with patient and tumor
characteristics as well as treatment.
The present study had certain limitations. Firstly,
the study size was relatively small. Therefore, a larger
multicenter study with a larger sample size should be performed in
the future to verify the significant prognostic value of PTV. The
results of a multicenter study could further support the role of
PTV as a promising prognostic parameter in OSCC staging. Secondly,
in the present study PTV values were determined based on data
obtained from postoperative samples. These values may differ from
those obtained during an in vivo assessment due to tissue
shrinkage during the sample drying procedure. Advances in imaging
technology facilitate preoperative determination of tumor volume,
particularly in patients unwilling to undergo surgery. Therefore,
F-fludeoxyglucose positron emission tomography in combination with
CT may be a good basis for diagnostic assessment of patients with
head and neck tumors (15). In
addition, RTV of OSCC can be measured before surgical treatment,
thus helping surgeons in planning the resection surgery (16,17).
Tumor volume in OSCC is an important tumor metric, since it is
associated with OSCC outcome as well as tumor size, TNM stage,
marginal status and perineural spreading (17). Programmed death-ligand 1 (PD-L1)
expression calculated using tumor proportion score was not
determined in the present study as this is not a standard for oral
cancer diagnosis in Serbia. Due to the data type in the present
study, differences in PTV between surviving and deceased patients
were assessed. Survival status was binary; therefore, correlation
was not a measure of dependence.
Disease staging in patients with OSCC is of
importance since it allows the classification of patients into
prognostic groups and the application of appropriate therapies,
while also facilitating communication between physicians involved
in treatment and the familiarization of patients with the prognosis
(18,19). TNM classification plays an important
role in planning treatment of patients with cancer, predicting
survival and establishing treatment protocols (1,5). The
system is serviceable and practical. However, it leaves room for
expansion of additional variables. In OSCC, T stage is based on the
maximum size of tumor and invasion depth. However, other parameters
such as tumor thickness and PTV could also be included in T status,
thus providing a more accurate determination of the 3D image of the
tumor (20,21). The head and neck regions contain
several anatomical structures that can affect the clinical picture
of the disease. Nodal status is also considered a significant
parameter associated with patient survival. However, other
pathological and radiological parameters could also exhibit
prognostic value and could therefore be applied in OSCC staging
(6). The significance of several
simple, quantitative prognostic factors such as tumor thickness and
invasion depth has been already reported (13,20).
However, applying additional quantitative and qualitative factors,
could make OSCC staging easier and more precise for clinicians,
inferring a need for further research in this field (21). The present study suggested that PTV
contributed to improved disease staging and survival prognosis.
Moreover, the improved survival of patients with low PTV
highlighted the importance of detecting OSCC at an earlier stage of
the disease when tumor volume is decreased. Tumor volume has a
notable effect on overall survival of patients with oral cancer, so
it may be considered an essential factor in the selection of
appropriate treatment options for each patient (7).
In conclusion, the present study demonstrated that
PTV, as a 3D tumor measure, was a significant factor associated
with survival time of patients with OSCC. PTV value >4.24
cm3 was significantly associated with shorter overall
survival time in patients with OSCC, suggesting that PTV exerted a
significant prognostic value in OSCC, which could be applied in
disease staging. 3D analysis of the tumor using PTV may complement
the T staging system, while tumor sphericity determination may lead
to an improved determination of OSCC in the future. In the future,
it would be beneficial to fit the Cox proportional hazard model on
a dataset with a larger sample size and extended set of prognosis
predictors (such as PD-L1 expression, Epidermal Growth Factor
Receptor gene polymorphism or ZEB-1 expression).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IM, SM, AK, MPI and NV contributed to conception and
design of the study. IM, SM, AK and MPI were involved in the
surgical treatment of patients. IM was involved in the statistical
analysis of data and writing the manuscript. AK, NV, JN and AT made
critical revisions to the manuscript. NV performed
pathohistological examination. AS, JN and AT contributed to data
collection, analysis and interpretation of data. AS performed the
radiological measurements. AK and NV confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Faculty of Medicine University of Novi Sad (Novi
Sad, Serbia; approval no. 01-39/112/1) and all patients provided
written informed consent for all the examinations and
treatments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PTV
|
pathological tumor volume
|
|
RTV
|
radiological tumor volume
|
|
PD-L1
|
Programmed death-ligand 1
|
References
|
1
|
Amit M, Tam S, Takahashi H, Choi KY,
Zafereo M, Bell D and Weber RS: Prognostic performance of the
American joint committee on cancer 8th edition of the TNM staging
system in patients with early oral tongue cancer. Head Neck.
41:1270–1276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conway DI, Purkayastha M and Chestnutt IG:
The changing epidemiology of oral cancer: Definitions, trends, and
risk factors. Br Dent J. 225:867–873. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diz P, Meleti M, Diniz-Freitas M, Vescovi
P, Warnakulasuriya S, Johnson NW and Kerr AR: Oral and pharyngeal
cancer in Europe. Transl Res Oral Oncol. 2:20571782017.
|
|
4
|
Wang B, Zhang S, Yue K and Wang DX: The
recurrence and survival of oral squamous cell carcinoma: A report
of 275 cases. Chin J Cancer. 32:614–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mijatov I and Mijatov S: Application of
the eighth edition of the American joint committee on cancer
staging system for oral carcinoma. Med Pregl. 72:165–170. 2019.
View Article : Google Scholar
|
|
6
|
Tarsitano A, Ricotta F, Cercenelli L,
Bortolano B, Battaglia S, Lucci E, Marchetti C and Marceli E:
Pretreatment tumor volume and tumor sphericity as prognostic
factors in patients with oral cavity squamous cell carcinoma. J
Craniomaxillofac Surg. 47:510–515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nixon IJ, Palmer FL, Lakin P, Kattan MM,
Lee NY and Ganly I: Pathologically determined tumor volume vs
pathologic T stage in the prediction of outcome after surgical
treatment of oropharyngeal squamous cell carcinoma. JAMA
Otolaryngol Head Neck Surg. 139:1151–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mucoyama N, Suzuki H, Hanai N, Sone M and
Hasewaga Y: Pathological tumor volume predicts survival outcomes in
oral squamous cell carcinoma. Oncol Lett. 16:2471–2477.
2018.PubMed/NCBI
|
|
9
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panarese I, Aquino G, Ronchi A, Longo F,
Montella M, Cozzolino I, Roccuzzo G, Colella G, Caraglia M and
Franco R: Oral and oropharyngeal squamous cell carcinoma:
Prognostic and predictive parameters in the etiopathogenetic route.
Expert Rev Anticancer Ther. 19:105–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majumdar B, Patil S, Sarode SC, Sarode GS
and Rao RS: Clinico-pathological prognosticators in oral squamous
cell carcinoma: An update. Transl Res Oral Oncol. 2:1–14. 2017.
|
|
12
|
Mücke T, Mitchell DA, Ritschl LM,
Tannapfel A, Wolff KD, Kesting MR, Lofelbein DJ and Kanatas A:
Influence of tumor volume on survival in patients with oral
squamous cell carcinoma. J Cancer Res Clin Oncol. 141:1007–1011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan S, Tang QL, Lin YJ, Chen WL, Li JS,
Huang ZQ, Yang ZH, Wang YY, Zhang DM, Wang HJ, et al: A review of
clinical and histological parameters associated with contralateral
neck metastases in oral squamous cell carcinoma. Int J Oral Sci.
3:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weimar EAM, Huang SH, Lu L, O'Sulivan B,
Perez-Ordonez B, Weinreb I, Hope A, Tong L, Goldstein D, irish J,
et al: Radiologic-pathologic correlation of tumor thickness and its
prognostic importance in squamous cell carcinoma of the oral
cavity: Implications for the eight edition tumor, node, metastasis
classification. AJNR Am J Neuroradiol. 39:1896–1902. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arora A, Husain N, Bansal A, Neyaz A,
Jaiswal R, Jain K, Chaturvedi A, Anand N, Malhotra K and Shukla S:
Development of a new outcome prediction model in early-stage
squamous cell carcinoma of the oral cavity based on histopathologic
parameters with multivariate analysis: The aditi-nuzhat lymph-node
prediction score (ANLPS) system. Am J Surg Pathol. 41:950–60. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Po Wing Yuen A, Lam KY, Lam LK, Ho CM,
Wong A, Chow TL, Yuen WF and Wei WI: Prognostic factors of
clinically stage I and II oral tongue carcinoma-a comparative study
of stage, thickness, shape, growth pattern, invasive front
malignancy grading, martinez-gimeno score, and pathologic features.
Head Neck. 24:513–520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuznetskov S, Yu Q, Spieler B, Hartsoug R,
Zhu X, Murnan E, Hironaka M and Zaid W: Can radiographic tumor
volume of oral squamous cell carcinoma help predict clinical and
pathological tumor features? J Oral Maxillofac Surg. 79:2582–2592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strongin A, Yovino S, Taylor R, Wolf J,
Cullen K, Zimrin A, Strome S, Regine W and Suntharalingam M:
Primary tumor volume is an important predictor of clinical outcomes
among patients with locally advanced squamous cell cancer of the
head and neck treated with definitive chemoradiotherapy. Int J
Radiat Oncol Biol Phys. 82:1823–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montebugnoli L, Gissi DB, Flamminio F,
Gentile L, Dallera V, Leonardi E, Beccarini T and Foschini MP:
Clinicopathologic parameters related to recurrence and locoregional
metastasis in 180 oral squamous cell carcinomas. Int J Surg Pathol.
22:55–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MK, Chen CM, Lee MC, Chen LS and Chen
HC: Primary tumor volume is an independent predictor of outcome
within pT4a-staged tongue carcinoma. Ann Surg Oncol. 18:1447–1452.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin NC, Su IH, Hsu JT, Tsai KY and Chen
MYC: FDG-PET predicts bone invasion and prognosis in patients with
oral squamous cell carcinoma. Sci Rep. 11:151532021. View Article : Google Scholar : PubMed/NCBI
|