Introduction
Myelodysplastic syndromes (MDSs) are a heterogeneous
group of blood disorders characterized by peripheral blood
cytopenia due to ineffective hematopoiesis, dysplasia in ≥1
hematopoietic cell lineages and increased risk of transformation to
acute myeloid leukemia (AML). MDSs are hypothesized to be clonal
stem cell disorders arising from accumulation of multiple gene
abnormalities, such as somatic point mutations, copy-number
alterations and chromosomal aberrations (1). Genomic and chromosomal instability and
variable molecular mechanisms contribute to pathogenesis and
prognosis of MDS (2).
Accumulation of bone marrow (BM) blasts is a key
feature and one of the main risk criterion and prognostic factors
in patients with MDS (3). However,
the majority of patients with MDS die from causes intrinsic to the
disease, such as infection, pneumonia, sepsis and bleeding, which
are not associated with leukemic transformation (4). This is particularly relevant to
patients with lower risk MDS, which is defined as low or
intermediate-1-risk according to the International Prognostic
Scoring System (IPSS) (5).
VEGFs and their receptors, VEGFRs, are involved in
regulation of proliferation, migration, invasion and
differentiation of normal and cancer cells (6–9). VEGFs
exert their effects by binding to receptors of the VEGFR family
that consists of tyrosine kinase receptors, VEGFR1, VEGFR2 and
VEGFR3 (10). The role of
VEGF-A/VEGFR1 and VEGF-A/VEGFR2 signaling pathways has been
evaluated in certain hematological malignancies, such as multiple
myeloma, lymphoma and myeloproliferative neoplasms (11–13).
However, a potential role of VEGF-A-dependent signaling in MDS
pathogenesis has been poorly studied and the results are
contradictory. Studies have demonstrated that BM blasts express
VEGF-A and modulate VEGF-A-dependent autocrine loop signaling in
MDS (14,15). However, Aguayo et al
(16) found no prognostic
significance of plasma VEGF-A levels in patients with MDS but
suggested that VEGF-A plays a role as a prognostic factor in
patients with AML. Verstovsek et al (17) showed that increased VEGF-A
expression in BM specimens is inversely associated with survival
time in patients with MDS or AML, whereas VEGFR1 and VEGFR2
expression levels have no prognostic impact.
To the best of our knowledge, there is no published
data on the role of VEGF-C, VEGF-D ligands and VEGFR3 in MDS
pathogenesis. However, a previous study revealed that activation of
the VEGF-C/VEGFR3 pathway promotes cancer cell mobility to induce
metastasis and an increase in VEGF-C and/or VEGFR3 expression may
be associated with a shorter survival in numerous types of
malignancy (18). The aim of the
present study was to evaluate VEGF and VEGFRs
expression as putative prognostic markers for MDS.
Patients and methods
Patients and controls
The study group consisted of 51 patients with
verified MDS (31 female and 20 male) with a median age of 69.8
years (range, 59–77 years) who were diagnosed between January 1,
2011 and August 31, 2018 and treated at A.S. Loginov Moscow
Clinical Scientific Center (Moscow, Russia). The diagnosis of MDS
was based on cytological examination of peripheral blood cells. The
patients were followed-up from MDS diagnosis until May 31, 2019.
The transformation to AML or the death of patients was considered,
if they occurred during the follow-up period. The control group
consisted of 15 volunteers (8 females and 7 male) free of neoplasms
or any other abnormality. The median age and range were 65.3 and
61–68 years, respectively. Clinical and hematological variables
(hemoglobin content and platelet and leukocyte count) in the
control group were within normal ranges.
Laboratory procedures
Hemoglobin concentration, as well as platelet and
leukocyte counts were measured using the automated hematology
analyzer ADVIA 2120i according to the manufacturer's
recommendations (Siemens Healthineers AG).
Inclusion and exclusion criteria
Inclusion criteria were de novo female and
male patients with MDS aged ≥18 years old or patients with MDS who
only received prior supportive care (such as red blood cell and/or
platelet transfusions for severe anemia and severe thrombocytopenia
improvement). Patients treated with erythropoiesis-stimulating
agents (in patients with chromosome 5q deletion) were also
included. Patients with BM blast cells=5% were excluded. Patients
previously treated with hypomethylating agents and/or who received
immunosuppressive therapy were excluded. Patients with the
hypoplastic variant of MDS as well as patients that refused to
participate were not included.
The patient risk stratification according to the
2017 World Health Organization (WHO) classification of tumors of
hematopoietic and lymphoid tissue (19) and IPSS (20), as well as other patient
characteristics are presented in Table
I. Karyotype was classified using the International System for
Human Cytogenetic Nomenclature (20,21).
 | Table I.Clinical variables of 51 patients
with MDS. |
Table I.
Clinical variables of 51 patients
with MDS.
| Clinical
variable | Value |
|---|
| Median age (range),
years | 69.80
(59.00–77.00) |
| Sex, n |
|
|
Female | 31.00 |
|
Male | 20.00 |
| WHO classification,
n |
|
|
MDS-SLD | 8.00 |
|
MDS-RS | 2.00 |
|
MDS-MLD | 11.00 |
|
MDS-EBa | 22.00 |
|
MDS-del(5q) | 8.00 |
| IPSS
classification, n |
|
|
Low | 15.00 |
|
Intermediate-1 | 12.00 |
|
Intermediate-2 | 9.00 |
|
High | 15.00 |
|
Karyotypeb, n |
|
|
Good | 30.00 |
|
Intermediate | 2.00 |
|
Poor | 6.00 |
|
n/d | 13.00 |
| Mean hemoglobin
count ± SEM, g/dl | 6.59±1.60 |
| Mean platelets
count ± SEM, ×109/l | 118.33±60.85 |
| Mean leukocyte
count ± SEM, ×109/l | 4.67±3.53 |
| Bone marrow blasts,
n |
|
|
>5% | 24.00 |
|
<5% | 27.00 |
| AML progression,
n | 14.00 |
| Death, n | 26.00 |
| Survival time ± SD,
months | 24.80±22.68 |
Mononuclear cell preparation and cDNA
synthesis
The peripheral blood specimens (5–10 ml) were
separated using a Ficoll® density gradient (PanEko) and
the obtained mononuclear cell fraction was used for further study.
Total RNA was isolated by TRI Reagent® (Molecular
Research Center). All procedures were as previously described
(22). Briefly, cDNA synthesis
reaction mixture contained 1 µg purified total RNA, 1 µl random 6
primers (Syntol), 2.5 mM dNTP mixture (Thermo Fisher Scientific,
Inc.), 0.4 units RNase inhibitor (Thermo Fisher Scientific, Inc.)
and 2 units M-MuLVplus reverse transcriptase (Thermo Fisher
Scientific, Inc.). The mixture volume was 25 µl. The synthesis was
performed in Terzic’ thermocycler (DNA technology, Russia) at 42°С
for 50 min with pre-incubating for 10 min at 25°С. The reaction was
stopped by heating at 70°С for 10 min.
Quantitative PCR (qPCR)
The amplification of cDNA was performed in a Bio-Rad
CFX (Bio-Rad Laboratories, Inc.) detection system using
EvaGreen® dye (Biotium) and qPCR master mix (Syntol)
according to the manufacture's protocol. PCR conditions for all
genes were 95°С for 5 min followed by 39 cycles of 95°С for 20 sec,
59°С for 25 sec and 72°С for 20 sec. Each sample was measured in
triplicate. For data standardization, the 60S subunit of the
RPL27 gene was used. The relative expression was determined
according to the 2−∆∆Cq equation
[∆Cq=Cq (RPL27)-Cq (test
gene), where Cq is the threshold cycle of the gene in the
exponential phase of the amplification curve] (23). The following primers were used:
VEGF-A forward, 5′-AGGGCAGAATCATCACGAAGT-3′ and reverse,
5′-AGGGCTTCGATTGGATGGCA-3′; VEGF-C forward,
5′-GAGGAGCAGTTACGGTCTGTG-3′ and reverse,
5′-tcctttccttagctgacacttgt-3′; VEGF-D forward,
5′-TCCCATCGGTCCACTAGGTTT-3′ and reverse,
5′-AGGGCTGCACTGAGTTCTTTG-3′; VEGFR1 forward,
5′-TTTGCCTGAAATGGTGAGTAAGG-3′ and reverse,
5′-TGGTTTGCTTGAGCTGTGTTC-3′; VEGFR2 forward,
5′-GGCCCAATAATCAGAGTGGCA-3′ and reverse,
5′-CCAGTGTCATTTCCGATCACTTT-3′; VEGFR3 forward,
5′-TGCACGAGGTACATGCCAAC-3′ and reverse,
5′-GCTGCTCAAAGTCTCTCACGAA-3′ and RPL27 forward,
5′-ACCGCTACCCCCGCAAAGTG-3′ and reverse,
5′-CCCGTCGGGCCTTGCGTTTA-3′.
Statistical analysis
All qPCR experiments were performed in triplicate.
Data are presented as the mean ± SEM. Correlation was analyzed
using Pearson's rank test. Overall survival was estimated by
Kaplan-Meier method with log-rank test. To assess diagnostic value
of VEGF and VEGFRs gene expression as candidate
biomarkers was performed receiver operating characteristic (ROC)
analysis. Area under ROC curve (AUC) was used to compare the
discriminatory performance of putative markers to determine their
utility as a novel diagnostic test. Statistical significance was
analyzed using an unpaired two-tailed Student's t test. P<0.05
was considered to indicate a statistically significant difference.
All statistical calculations were performed using GraphPad Prism
for Windows program, Version 5.00 (Trial), 2007 (Dotmatics).
Results
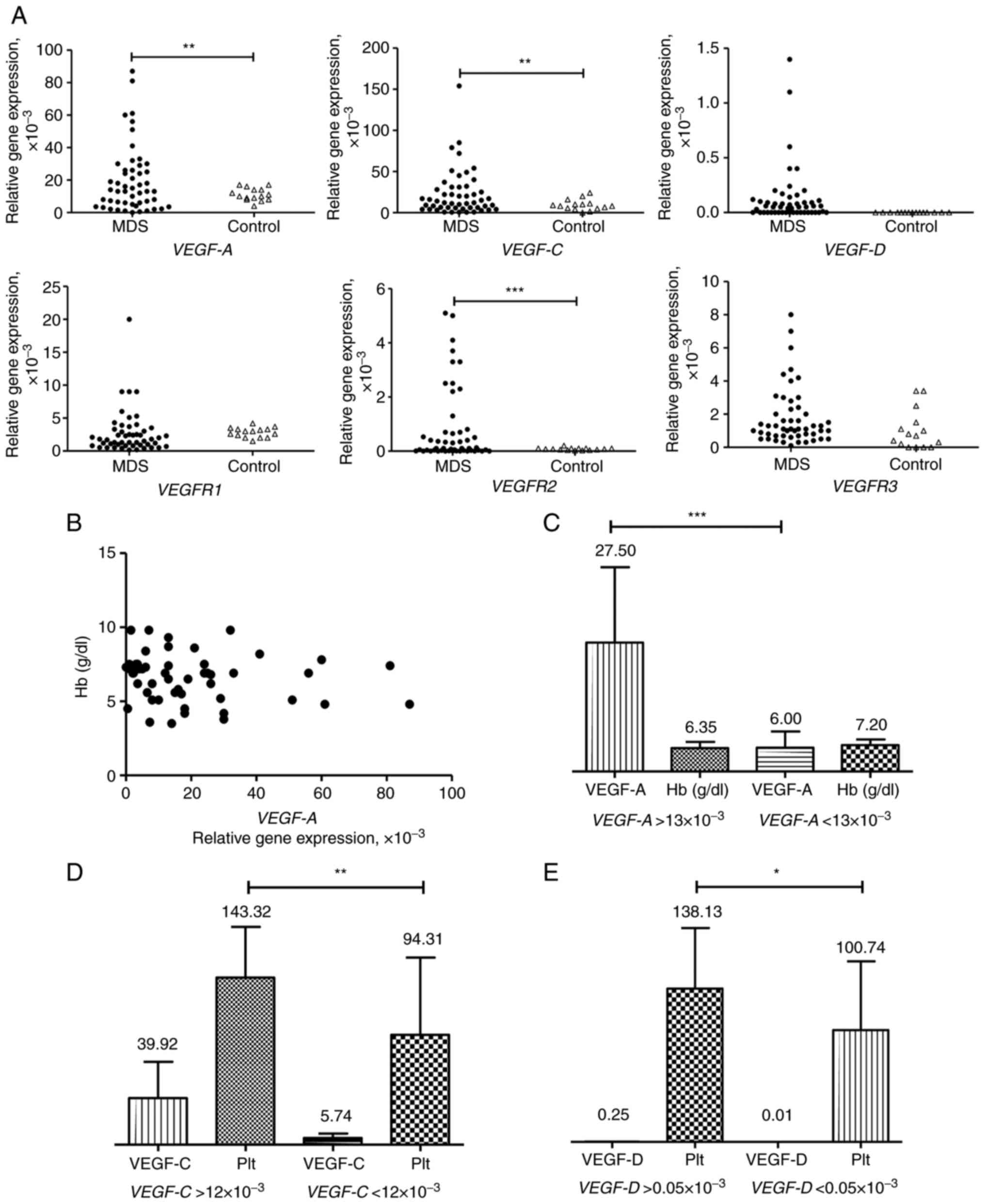
VEGF and VEGFR expression is elevated
in patients with MDS
VEGF (VEGF-A, VEGF-C and
VEGF-D) and VEGFR (VEGFR1, VEGFR2 and
VEGFR3) gene expression levels were studied in the
peripheral blood samples of 51 patients with MDS and 15 healthy
donors. Gene expression varied considerably in patients with MDS
compared with healthy donors (Fig.
1A). Although no statistical difference between patients with
MDS and controls in VEGFR1 and VEGFR3 expression was
found, relative VEGFR1 expression was
0.4-20.0×10−3 in patients with MDS and from
1.5×10−3 to 4.2×10−3 in control group (mean,
2.71±0.46×10−3 vs. 2.82±0.19) ×10−3.
Similarly, VEGFR3 relative expression varied from
0.1-8.0×10−3 in patients with MDS and from 0 to
3.4×10−3 in controls (mean values: (1.78±0.24)
×10−3 vs. 1.02±0.30×10−3. VEGF-A and
VEGF-C expression were higher in patients with MDS than in
controls. The mean values of relative VEGFA expression in
patients with MDS and in control group were (19.73±2.84)
×10−3 and 11.07±0.99×10−3. The mean values of
relative VEGF-C expression in MDS patients and control group
were (22.50±3.88) ×10−3 and 9.23±1.69×10−3.
VEGFR2 expression levels were low in the group of healthy
volunteers (mean value 0.08±0.04) and upregulated in patients with
MDS (mean value 0.82±1.39), indicating that VEGFR2-dependent
signaling was stimulated in patients with MDS. VEGF-D
expression was absent in control group and varied from 0 to
1.4×10−3 in patients with MDS.
VEGF-A is activated under hypoxic conditions
(24). To evaluate the association
between VEGF-A expression and hypoxia, levels of
VEGF-A expression and hemoglobin content in the peripheral
blood samples of patients with MDS was compared. No correlation was
found between these two variables (Fig.
1B). Moreover, the mean value of hemoglobin was almost the same
in patients with MDS with different levels of VEGF-A
expression (above and below the mean level of 19.62×10−3
of relative VEGF-A gene expression; Fig. 1C). Thus, VEGF-A expression
was not dependent on the content of hemoglobin in peripheral blood
of patients with MDS.
To determine if there was an association between
clinical characteristics of patients with MDS and gene expression,
levels of hemoglobin, platelets and leukocytes were compared with
VEGF and VEGFR gene expression levels. Significant
associations were found between levels of platelets and the
expression levels of VEGF-C and VEGF-D (Fig. 1D and E).
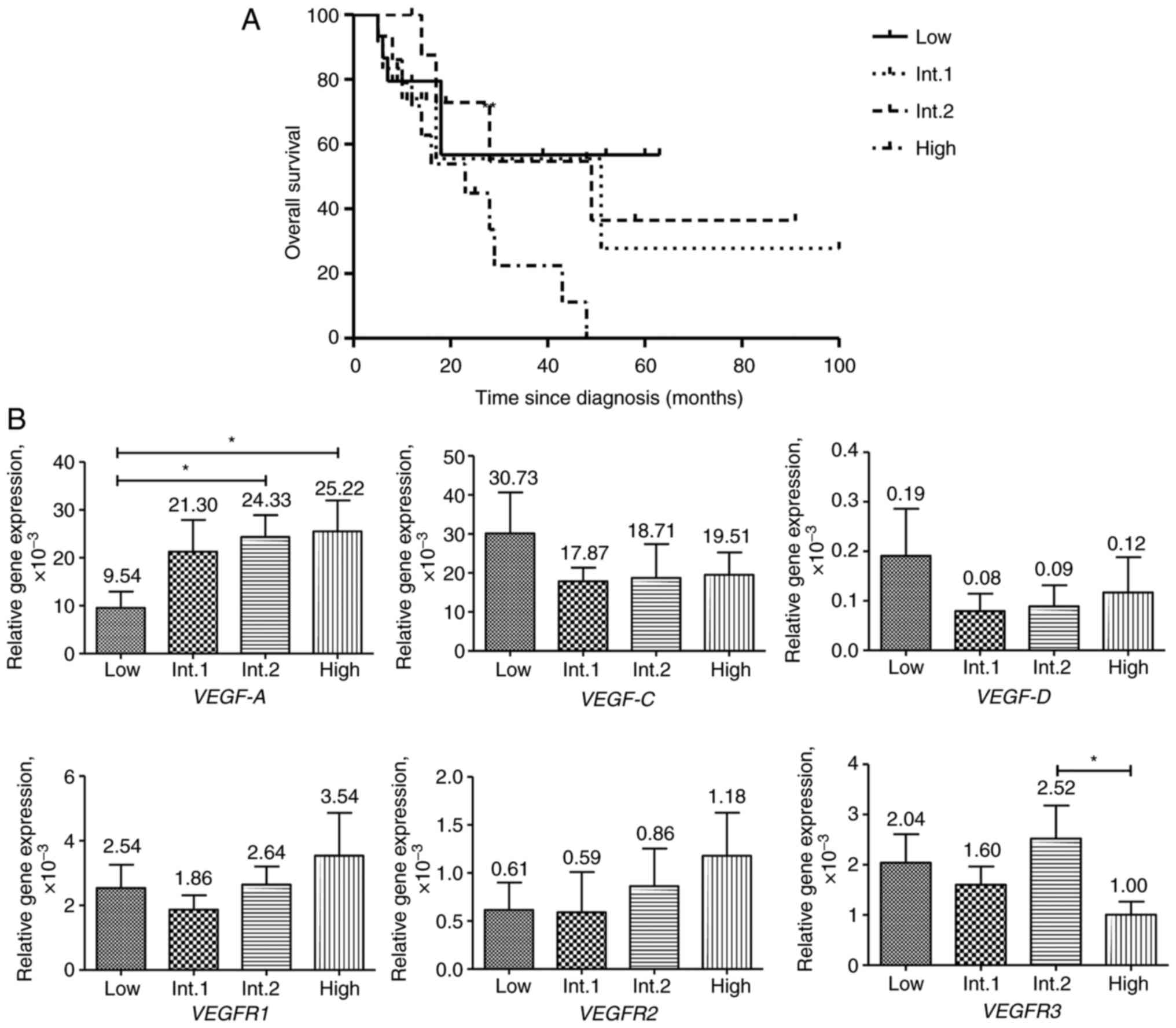
VEGF and VEGFR expression in patients
with MDS with different risk of disease development
All patients with MDS were stratified in the present
study, according to the IPSS. Patients were assigned to low (15
patients), intermediate-1 (12 patients), intermediate-2 (9
patients) and high (15 patients) risk groups and the survival time
and gene expression in these groups were compared. No statistical
difference in survival time between any of these risk groups was
found. Nevertheless, the median survival time diminished from the
intermediate-1 (51 months) and intermediate-2 (49 months) risk
groups to the high-risk group (23 months). The median survival time
in the low-risk group was not reached (Fig. 2A).
VEGF-A expression was progressively elevated
from the low to the high-risk groups (Fig. 2). A statistically significant
difference was found for VEGF-A expression between the low
and intermediate-2 risk groups, as well as between the low and
high-risk groups. No statistically significant difference between
risk groups in VEGF-C, VEGF-D, VEGFR1 and VEGFR2
expression was found. Expression of VEGFR1 and VEGFR2
genes was notably elevated from the intermediate-1 to the high-risk
group. VEGF-C and VEGF-D expression was notably
higher in the low-risk group of patients compared with the other
groups. VEGFR3 expression was lowest in the high risk group.
The only statistically significant difference in gene expression
was found between the intermediate-2 and high risk groups for
VEGFR3.
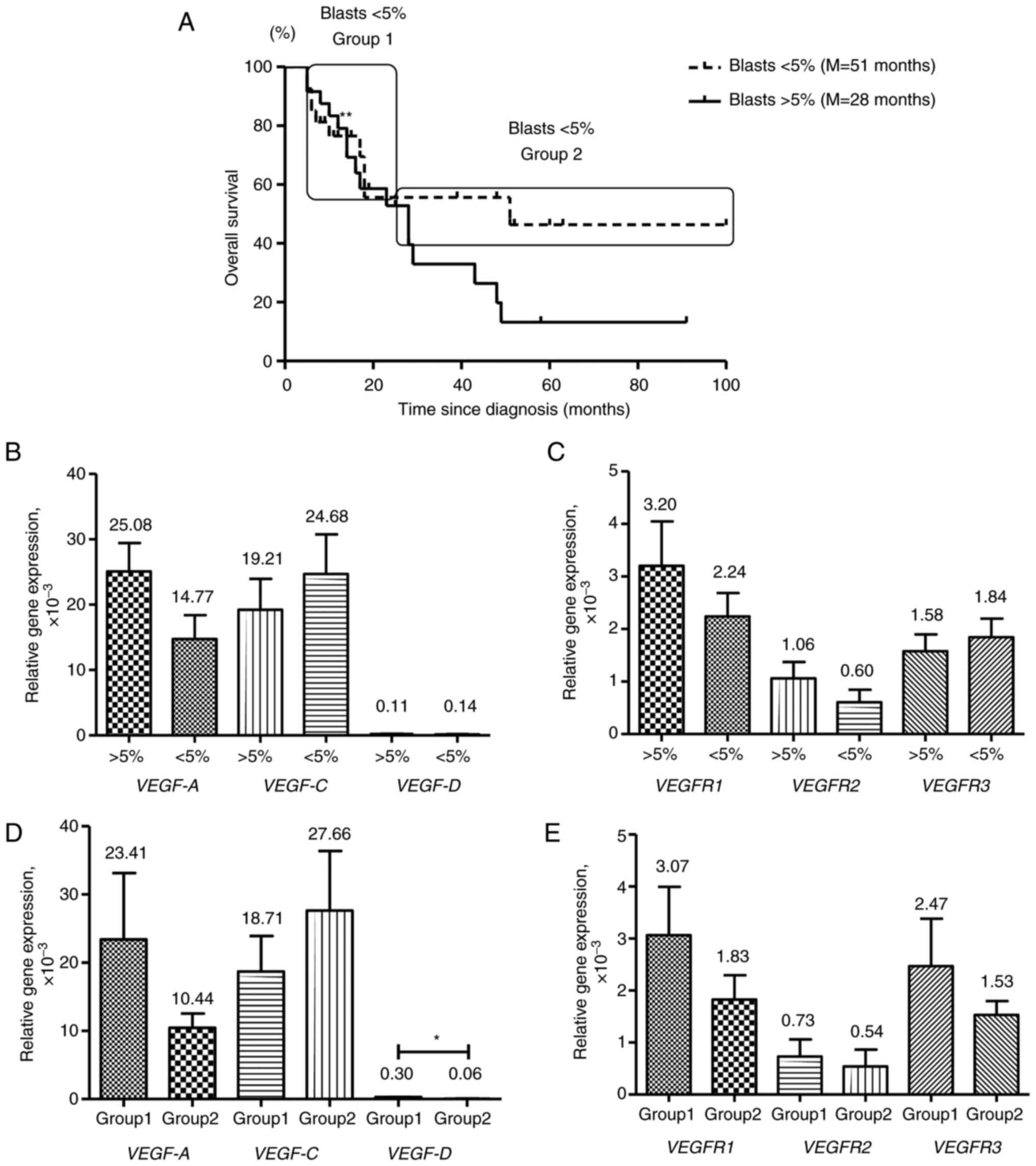
VEGF and VEGFR expression in patients
with MDS with different levels of BM blasts
A key prognostic factor characterizing patients with
MDS is the percentage of BM blast cells. BM blast cells <5% is
usually associated with good prognosis (20). In the present study, all patients
from low and intermediate-1 risk groups had BM blast levels <5%
and patients from intermediate-2 and high-risk groups had BM blast
levels >5%. The survival time and gene expression levels of MDS
patients with BM blast levels <5% (27 patients) and >5% (24
patients) were compared. No significant difference in survival time
between these patients was found, although the median survival
times were different (51 vs. 28 months for <5% and >5% BM
blast levels, respectively; Fig.
3A).
Although there was no statistically significant
difference, the mean VEGF-A, VEGFR1 and VEGFR2
expression levels were elevated in patients with MDS with >5% BM
blast levels (Fig. 3B and C).
VEGF-C, VEGF-D and VEGFR3 expression was decreased in
the group of patients with MDS with BM blast levels >5%.
The prognostic evaluation of potential clinical
outcomes in MDS patients with BM blast levels <5% is
complicated. Some of these patients die within a few months of
developing AML transformation or other complications of bone marrow
failure, while others can survive for a long time (25). Survival time of patients with BM
blast levels <5% (group 1, 9 patients) did not differ from that
of patients with BM blast levels >5% (17 vs. 14 months,
respectively), while patients of a group 2 with BM blast levels
<5% had an improved survival (group 2, 18 patients; Fig. 3A). VEGF and VEGFRs
gene expression levels in groups 1 and 2 were compared (Fig. 3D and E) and VEGF-A, VEGF-C,
VEGFR1 and VEGFR2 expression in these groups was very
similar to that in patients with MDS with BM blast levels >5 and
<5% (Fig. 3B and C).
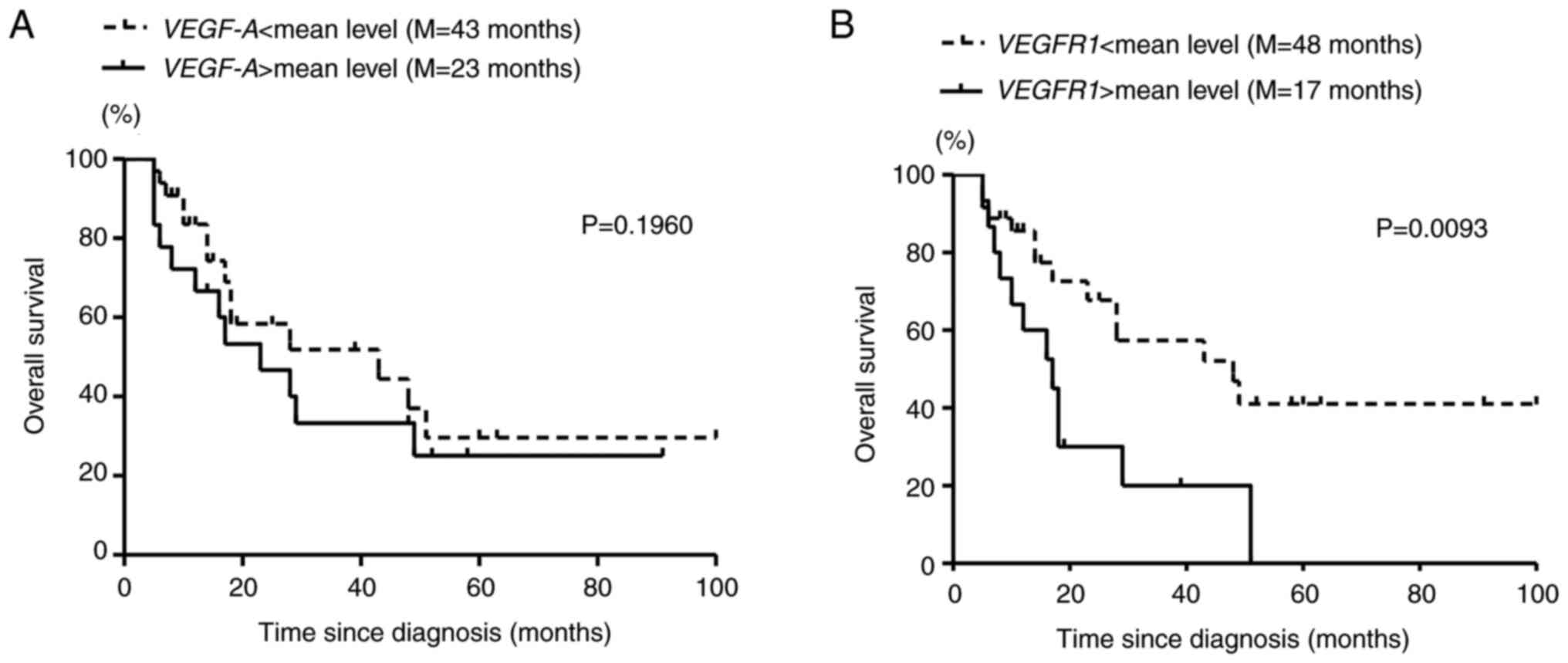
Survival of MDS patients with
different VEGF-A and VEGFR1 expression levels
As both VEGF-A and VEGFR1 gene
expression levels were elevated in patients with MDS with a worse
prognosis, the survival of patients was compared. No statistically
significant difference was found between patients with MDS with
high and low levels of VEGF-A expression, although the
median survival times in patients with VEGF-A expression
levels above (18 patients) and below (33 patients) the mean value
(1.96х10−3) were different (23 and 43 months,
respectively; Fig. 4A). Patients
with MDS with VEGFR1 expression levels exceeding the mean
relative VEGFR1 gene expression (2.69х10−3; 15
patients) had statistically worse survival (log-rank test; Fig. 4B) with a median survival time of 17
months compared with 48 months for the group with low levels of
VEGFR1 expression (36 patients).
To evaluate the diagnostic value of VEGF-A
and VEGFR1 gene expression levels as candidate biomarkers of
MDS, receiver operating characteristic (ROC) curve analysis was
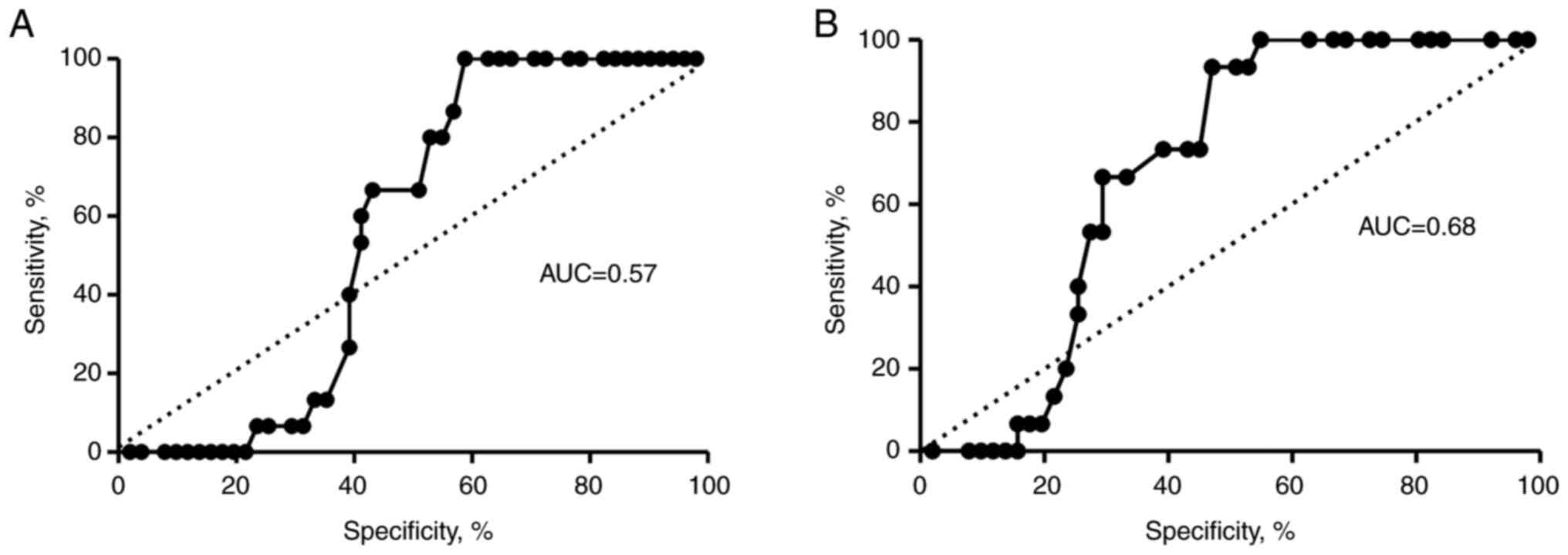
applied. Area under the curve (AUC) was 0.57 for the VEGF-A
gene, but the result was not statistically significant (Fig. 5A). However, VEGFR1 expression
levels discriminated between patients with MDS and the healthy
controls (AUC=0.684), suggesting its potential diagnostic value
(Fig. 5B).
Discussion
Due to the heterogeneity of MDSs at morphological,
clinical and molecular levels, the accurate diagnosis of these
diseases has certain problems (subjectivity of morphological
assessment of bone marrow aspirate and biopsy, as well as
heterogeneity of cytogenetic alterations) and the expected clinical
outcome for patients with MDS is different. Whereas some patients
transform to AML or die from complications of BM transplant failure
within a few months, other patients with MDS survive for years
without major hematological problems (26). A precise diagnosis is important for
the prediction of patient survival and risk of AML transformation
and selection of appropriate therapy.
Several classification systems have been presented
to evaluate MDS diagnosis, the most commonly used being the
French-American-British (FAB) (19)
and IPSS (20) classification. In
the present study, patients with MDS were stratified according to
IPSS scoring. The diagnosis of MDS is often based on morphological
characteristics, and due to the subjectivity of this evaluation,
discrepancy in MDS diagnoses exists. Naqvi et al (27) analyzed discordance between the
diagnosis of 915 patients with MDS referred according to FAB or
IPSS and final diagnosis, and found that 12% of diagnoses (109/915
patients) were reclassified. Most of the reclassified patients (67%
classified according to FAB and 77% classified by IPSS) had higher
risk disease. According to the WHO 2017 classification, a correct
diagnosis of MDS requires the integration of not only morphological
but also clinical, cytogenetic and, potentially, molecular biology
parameters, thus providing an improved classification of MDS
diagnosis (19). Diagnosis is more
obvious in patients with excess BM blasts: Increased blast
percentages (>5–10%) indicate an increase in risk of leukemic
transformation and of death (28).
The patients with early low-risk disease need additional diagnostic
tools, including cytogenetic evaluation, flow cytometry and DNA
sequencing, to define the diagnosis and predict outcomes (29). New biological markers may also be
helpful to stratify patients with MDS.
VEGF and one of its receptors, VEGFR2, are
prognostic factors for a number of solid tumors such as primary
(30) and non-small cell lung
cancer (31), neuroblastoma
(32) and bladder (33), colon (34) and breast cancer (35). To the best of our knowledge,
however, the data on VEGF-dependent signaling and its role in MDS
development are scarce. A study showed an increase in BM
microvessel density (MVD) in refractory anemia with excess blasts
in transformation (RAEB-T), chronic myelomonocytic leukemia and AML
compared with patients with RA, RA with ring sideroblasts and RAEB
(36). VEGF expression in
MDS specimens is especially high in RAEB ± T and AML samples
(14). VEGFR1 and, to a
lesser extent, VEGFR2 are expressed in patients with MDS and
exhibit autocrine cytokine interaction (14,17).
Based on expression data of VEGF and its receptors
in MDS, it was suggested that VEGF-dependent signaling could be a
potential therapeutic target (7,17). A
number of agents interfering with VEGF signaling, such as
bevacizumab (37), SU5416 (38), AG-013736 (39), Vatalanib (40), Aflibercept/NSC 724770 (41), have been tested in patients with
MDS, but yielded no or small clinical responses and clinical
applicability is limited by toxicity and side effects.
The results of investigations on the VEGF prognostic
impact in patients with MDS are also not encouraging. The
prognostic significance of VEGF plasma levels in patients with AML
and MDS has been studied (16):
VEGF plasma levels are associated with a shorter survival in
patients with AML, but not MDS. By contrast, elevated BM cellular
VEGF levels are significantly associated with shorter survival in
patients with MDS (17). Cheng
et al (42) studied the
potential prognostic value of VEGF-A, VEGF-C, angiopoietin-1
(Ang-1), Ang-2 and receptor Tie-2 expression
in the BM of patients with MDS. Ang-1, but not Ang-2,
VEGFs or Tie-2, was shown to be an independent poor
prognostic factor for patients with MDS.
Despite elevated BM MVD in patients with MDS, the
treatment with anti-VEGF or its receptor agents does not produce
any appreciable therapeutic effects. The attempts to evaluate the
potential of VEGF or its receptors to be prognostic factors for
patients with MDS also were not fruitful.
In the present study, relative mRNA expression
levels of VEGF (VEGF-A, VEGF-C and VEGF-D) and VEGF
receptors (VEGFR1, VEGFR2 and VEGFR3) in peripheral
blood samples of patients with MDS were augmented compared with
healthy control samples, suggesting that VEGF-dependent signaling
was activated in patients with MDS. Although VEGF-A is
activated under hypoxia, no association between VEGF-A
expression and hemoglobin content in peripheral blood samples was
found. The comparison of VEGF and VEGFR gene
expression levels in patients with MDS subdivided according to IPSS
risk revealed increased VEGF-A, VEGFR1 and VEGFR2
expression in higher risk groups, with the most significant
difference for VEGFR1 expression.
A key predictor of MDS development is levels of BM
blast cells in patients (28)
Patients with MDS with BM blast levels <5% included all patients
from the IPSS system low and intermediate-1-risk groups; patients
with MDS with BM blast levels >5% comprised the intermediate-2
and high-risk groups.
The difference in overall survival of patients
stratified by BM blast levels (patients with >5% vs. patients
with <5% blasts) was not statistically significant, but median
survival time varied considerably (51 vs. 28 months, respectively).
VEGF-A, VEGFR1 and VEGFR2 gene expression was
upregulated in patients with MDS with BM blast levels >5%
compared with patients with BM blast levels <5%. By contrast,
VEGF-C, VEGF-D and VEGFR3 gene expression levels were
elevated in patients with MDS with BM blast levels <5%. This
suggested that VEGF-A/VEGFR1 and VEGFR2 signaling was activated and
VEGF-C and VEGF-D/VEGFR3 signaling was suppressed in patients with
BM blast levels >5%.
In the present study, according to the survival
curve of patients with MDS with BM blast levels <5%, two groups
of patients were discriminated. The survival curve of a subgroup of
these patients (group 1) largely coincided with the survival curve
of patients with BM blast levels >5%, while the survival curve
of patients with BM blast levels <5% (group 2) differed. The
comparison of VEGF and VEGFR gene expression levels
in groups 1 and 2 revealed similar gene expression, as in groups of
patients with BM blast levels >5 and <5%, with the exception
of VEGF-D and VEGFR3 genes. As such, the elevation of
certain VEGF (VEGF-A) and VEGFR (VEGFR1
and VEGFR2) gene expression levels in patients with MDS with
BM blast levels <5% may indicate the intensification of
disease.
As the most prominent difference in gene expression
levels between groups concerned VEGF-A and VEGFR1,
the survival of patients with MDS subdivided by these gene
expression levels was analyzed. A significant difference in
survival was found for subgroups by VEGFR1 expression. The
survival rate of patients with MDS with VEGFR1 expression
below the mean, but not the median level of expression, was higher
than in patients with higher levels of this gene expression. The
difference in survival of patients subdivided by VEGF-A
expression was not significant, but the median survival times in
groups with higher VEGF-A expression differed significantly
from those in groups with lower expression (23 vs. 43 months in
groups subdivided by the mean expression and 17 vs. 48 months in
groups subdivided by the median level of expression).
VEGF-A exerts its function through binding with its
two specific receptors-VEGFR1 and VEGFR2, where VEGFR2 is the
primary mediator of such VEGF-A biological functions as
embryogenesis and hematopoiesis (43,44).
According to the present study, expression of VEGFR2, the
known negative prognostic factor for many solid tumors, (45–47) is
not important in MDS. It was hypothesized that VEGF-A-dependent
signaling may be preferentially realized through another receptor
for VEGF-A, VEGFR1, as VEGFR1 expression is higher in the
peripheral blood samples of patients with MDS compared with
VEGFR2 expression. Previous data on VEGF and
VEGFR expression levels in BM samples of patients with MDS
have shown increased expression of VEGFR1, but not
VEGFR2 (48). Only
VEGFR1 expression in the present study had prognostic impact
for patients with MDS. The elevated VEGF-A/VEGFR1
expression in patients with MDS with BM blast levels >5% and
patients with BM blast levels <5% with worse survival (group 1)
indicated that the progression of the disease was accompanied by
VEGF-A/VEGFR1 activation. ROC analysis showed that
VEGFR1 expression rather than VEGF-A expression could
serve as a potential prognostic marker in MDS with low and
intermediate-1 risk.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NKa, GD, NKo, AS and AK contributed to study
conception and design. Clinical material preparation and data
collection were performed by GD. RT-qPCR analysis was performed by
NKa, NKo and AS. AK and NKa analyzed gene expression data and wrote
the manuscript. GD, NKa, NKo, AS and AK confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
6/2016) by the ethics commission of the A.S. Loginov Moscow
Clinical Scientific Center (Moscow, Russia). All patients and
volunteers provided written informed consent to participate in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papaemmanuil E, Gerstung M, Malcovati L,
Tauro S, Gundem G, Van Loo P, Yoon CJ, Ellis P, Wedge DC,
Pellagatti A, et al: Clinical and biological implications of driver
mutations in myelodysplastic syndromes. Blood. 122:3616–3627.
36992013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Odenike O, Anastasi J and Le Beau MM:
Myelodysplastic Syndromes. Clin Lab Med. 31:763–784. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foucar K:
Myelodysplastic/myeloproliferative neoplasms. Am J Clin Pathol.
132:281–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dayyani F, Conley AP, Strom SS, Stevenson
W, Cortes JE, Borthakur G, Faderl S, O'Brien S, Pierce S,
Kantarjian H and Garcia-Manero G: Cause of death in patients with
lower-risk myelodysplastic syndrome. Cancer. 116:2174–2179.
2010.PubMed/NCBI
|
|
5
|
Garcia-Manero G: Myelodysplastic
syndromes: 2014 Update on diagnosis, risk-stratification, and
management. Am J Hematol. 89:97–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Price DJ, Miralem T, Jiang S, Steinberg R
and Avraham H: Role of vascular endothelial growth factor in the
stimulation of cellular invasion and signaling of breast cancer
cells. Cell Growth Differ. 12:129–135. 2001.PubMed/NCBI
|
|
7
|
Podar K and Anderson KC: The
pathophysiologic role of VEGF in hematologic malignancies:
Therapeutic implications. Blood. 105:1383–1395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee TH, Seng S, Sekine M, Hinton C, Fu Y,
Avraham HK and Avraham S: Vascular endothelial growth factor
mediates intracrine survival in human breast carcinoma cells
through internally expressed VEGFR1/FLT1. PLoS Med. 4:e1862007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veikkola T, Karkkainen M, Claesson-Welsh L
and Alitalo K: Regulation of angiogenesis via vascular endothelial
growth factor receptors. Cancer Res. 60:203–212. 2000.PubMed/NCBI
|
|
11
|
Vincent L, Jin DK, Karajannis MA, Shido K,
Hooper AT, Rashbaum WK, Pytowski B, Wu Y, Hicklin DJ, Zhu Z, et al:
Fetal stromal-dependent paracrine and intracrine vascular
endothelial growth factor-a/vascular endothelial growth factor
receptor-1 signaling promotes proliferation and motility of human
primary myeloma cells. Cancer Res. 65:3185–3192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z,
Hicklin DJ and Moore MA: Targeting autocrine and paracrine VEGF
receptor pathways inhibits human lymphoma xenografts in vivo.
Blood. 104:2893–2902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boiocchi L, Vener C, Savi F, Bonoldi E,
Moro A, Fracchiolla NS, Iurlo A, Deliliers GL, Coggi G, Bosari S
and Gianelli U: Increased expression of vascular endothelial growth
factor receptor 1 correlates with VEGF and microvessel density in
Philadelphia chromosomenegative myeloproliferative neoplasms. J
Clin Pathol. 64:226–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellamy WT, Richter L, Sirjani D, Roxas C,
Glinsmann-Gibson B, Frutiger Y, Grogan TM and List AF: Vascular
endothelial cell growth factor is an autocrine promoter of abnormal
localized immature myeloid precursors and leukemia progenitor
formation in myelodysplastic syndromes. Blood. 97:1427–1434. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wimazal F, Krauth MT, Vales A, Böhm A,
Agis H, Sonneck K, Aichberger KJ, Mayerhofer M, Simonitsch-Klupp I,
Müllauer L, et al: Immunohistochemical detection of vascular
endothelial growth factor (VEGF) in the bone marrow in patients
with myelodysplastic syndromes: Correlation between VEGF expression
and the FAB category. Leuk Lymphoma. 47:451–460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aguayo A, Kantarjian HM, Estey EH, Giles
FJ, Verstovsek S, Manshouri T, Gidel C, O'Brien S, Keating MJ and
Albitar M: Plasma vascular endothelial growth factor levels have
prognostic significance in patients with acute myeloid leukemia but
not in patients with myelodysplastic syndromes. Cancer.
95:1923–1930. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verstovsek S, Estey E, Manshouri T, Giles
FJ, Cortes J, Beran M, Rogers A, Keating M, Kantarjian H and
Albitar M: Clinical relevance of vascular endothelial growth factor
receptors 1 and 2 in acute myeloid leukaemia and myelodysplastic
syndrome. Br J Haematol. 118:151–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO Classification of Tumors of
Haematopoietic and Lymphoid Tissues. Revised 4th edition. IARC;
Lyon: 2017
|
|
20
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaffer LG, Slovak ML and Campbell LJ:
ISCN 2009: An international system for human cytogenetic
nomenclature (2009). Basel, Switzerland: S. Karger; 2009
|
|
22
|
Kalitin NN and Buravtsova IV:
Transcription factor RARa expression correlates with
VEGFR3-dependent signaling system components expression in multiple
myeloma. Klinicheskaya Onkogematologiya. 8:31–36. 2015.(In
Russian).
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dor Y, Porat R and Keshet E: Vascular
endothelial growth factor and vascular adjustments to perturbations
in oxygen homeostasis. Am J Physiol Cell Physiol. 280:C1367–C1374.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greenberg P, Cox C, LeBeau MM, Fenaux P,
Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al:
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wimazal F, Sperr WR, Kundi M, Meidlinger
P, Fonatsch C, Jordan JH, Thalhammer-Scherrer R, Schwarzinger I,
Geissler K, Lechner K and Valent P: Prognostic value of lactate
dehydrogenase activity in myelodysplastic syndromes. Leuk Res.
25:287–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naqvi K, Jabbour E, Bueso-Ramos C, Pierce
S, Borthakur G, Estrov Z, Ravandi F, Faderl S, Kantarjian H and
Garcia-Manero G: Implications of discrepancy in morphologic
diagnosis of myelodysplastic syndrome between referral and tertiary
care centers. Blood. 118:4690–4693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mufti GJ, Bennett JM, Goasguen J, Bain BJ,
Baumann I, Brunning R, Cazzola M, Fenaux P, Germing U,
Hellström-Lindberg E, et al: Diagnosis and classification of
myelodysplastic syndrome: International working group on morphology
of myelodysplastic syndrome (IWGM-MDS) consensus proposals for the
definition and enumeration of myeloblasts and ring sideroblasts.
Haematologica. 93:1712–1717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montalban-Bravo G and Garcia-Manero G:
Myelodysplastic syndromes: 2018 Update on diagnosis,
risk-stratification and management. Am J Hematol. 93:129–147. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda
M, Hayashi Y, Watanabe Y and Sasaki T: Significance of vascular
endothelial growth factor messenger RNA expression in primary lung
cancer. Clin Cancer Res. 2:1411–1416. 1996.PubMed/NCBI
|
|
31
|
Fontanini G, Bigini D, Vignati S, Basolo
F, Mussi A, Lucchi M, Chine S, Angeletti CA, Harris AL and
Bevilacqua G: Microvessel count predicts metastatic disease and
survival in non-small cell lung cancer. J Pathol. 177:57–63. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meitar D, Crawford SE, Rademaker AW and
Cohn SL: Tumor angiogenesis correlates with metastatic disease,
N-myc amplification, and poor outcome in human neuroblastoma. J
Clin Oncol. 14:405–414. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Brien T, Cranston D, Fuggle S, Bicknell
R and Harris AL: Different angiogenic pathways characterize
superficial and invasive bladder cancer. Cancer Res. 55:510–513.
1995.PubMed/NCBI
|
|
34
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
35
|
Anan K, Morisaki T, Katano M, Ikubo A,
Kitsuki H, Uchiyama A, Kuroki S, Tanaka M and Torisu M: Vascular
endothelial growth factor and platelet-derived growth factor are
potential angiogenic and metastatic factors in human breast cancer.
Surgery. 119:333–339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pruneri G, Bertolini F, Soligo D, Carboni
N, Cortelezzi A, Ferrucci PF, Buffa R, Lambertenghi-Deliliers G and
Pezzella F: Angiogenesis in myelodysplastic syndromes. Br J Cancer.
81:1398–1401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Legros L, Slama B, Karsenti JM, Vey N,
Natarajan-Amé S, Watel E, Richard B, Bouabdallah K, Mannone L,
Benchetrit M, et al: Treatment of myelodysplastic syndromes with
excess of blasts by bevacizumab is well tolerated and is associated
with a decrease of VEGF plasma level. Ann Hematol. 91:39–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giles FJ, Stopeck AT, Silverman LR, Lancet
JE, Cooper MA, Hannah AL, Cherrington JM, O'Farrell AM, Yuen HA,
Louie SG, et al: SU5416, a small molecule tyrosine kinase receptor
inhibitor, has biologic activity in patients with refractory acute
myeloid leukemia or myelodysplastic syndromes. Blood. 102:795–801.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giles FJ, Bellamy WT, Estrov Z, O'Brien
SM, Verstovsek S, Ravandi F, Beran M, Bycott P, Pithavala Y,
Steinfeldt H, et al: The anti-angiogenesis agent, AG-013736, has
minimal activity in elderly patients with poor prognosis acute
myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Leuk Res.
30:801–811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta P, Mulkey F, Hasserjian RP, Sanford
BL, Vij R, Hurd DD, Odenike OM, Bloomfield CD, Owzar K, Stone RM,
et al: A phase II study of the oral VEGF receptor tyrosine kinase
inhibitor vatalanib (PTK787/ZK222584) in myelodysplastic syndrome:
Cancer and leukemia group B study 10105 (alliance). Invest New
Drugs. 31:1311–1320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kirschbaum MH, Frankel P, Synold TW, Zain
J, Claxton D, Tuscano J, Newman EM, Gandara DR and Lara PN Jr: A
phase II study of vascular endothelial growth factor trap
(Aflibercept, NSC 724770) in patients with myelodysplastic
syndrome: A California cancer consortium study. Br J Haematol.
180:445–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng CL, Hou HA, Jhuang JY, Lin CW, Chen
CY, Tang JL, Chou WC, Tseng MH, Yao M, Huang SY, et al: High bone
marrow angiopoietin-1 expression is an independent poor prognostic
factor for survival in patients with myelodysplastic syndromes. Br
J Cancer. 105:975–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shalaby F, Rossant J, Yamaguchi T,
Gertsenstein M, Wu XF, Breitman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Eichmann A, Corbel C, Nataf V, Vaigot P,
Bréant C and Le Douarin NM: Ligand-dependent development of the
endothelial and hemopoietic lineages from embryonic mesodermal
cells expressing vascular endothelial growth factor receptor 2.
Proc Natl Acad Sci USA. 94:5141–5146. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carrillo de Santa PE, Arias FC, Caso
Peláez E, Muñoz Molina GM, Sánchez Hernández I, Muguruza Trueba I,
Moreno Balsalobre R, Sacristán López S, Gómez Pinillos A and del
Val Toledo Lobo M: Prognostic significance of the expression of
vascular endothelial growth factors A, B, C, and D and their
receptors R1, R2, and R3 in patients with non-small cell lung
cancer. Cancer. 115:1701–1712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ock CY, Nam AR, Bang JH, Kim TY, Lee KH,
Han SW, Im SA, Kim TY, Bang YJ and Oh DY: The distinct signatures
of VEGF and soluble VEGFR2 increase prognostic implication in
gastric cancer. Am J Cancer Res. 5:3376–3388. 2015.PubMed/NCBI
|
|
47
|
Dhakal HP, Naume B, Synnestvedt M, Borgen
E, Kaaresen R, Schlichting E, Wiedswang G, Bassarova A, Holm R,
Giercksky KE and Nesland JM: Expression of vascular endothelial
growth factor and vascular endothelial growth factor receptors 1
and 2 in invasive breast carcinoma: Prognostic significance and
relationship with markers for aggressiveness. Histopathology.
61:350–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kalitin NN, Dudina GA, Semochkin SV and
Karamysheva AF: Analysis of VEGF-A/VEGFR1/VEGFR2 genes expression
analyses in patients with myelodysplastic syndromes. Ther Arch.
89:39–44. 2017.(In Russian). View Article : Google Scholar : PubMed/NCBI
|