Introduction
Sarcomas are solid tumours accounting for ~1% of all
malignant tumours in adults within Europe and the United States
(1). Superficial soft tissue
sarcomas (SSTS) are a rare group of heterogeneous tumours with at
least 50 histological subtypes (2,3). By
definition, SSTS occur above the superficial fascia and can arise
in a variety of anatomical locations (4).
The mainstay of treatment for these tumours is
surgical resection, however, post-operative radiotherapy or
chemotherapy may also be employed (5,6).
Resection with negative margins is the primary aim of surgical
management in order to minimise the incidence of local recurrence
(1).
Although prognostic factors for other cancers have
been well described, data regarding SSTS outcomes remain
comparatively limited (7). Factors
identified as predictive of local recurrence include resection
margin, tumour size, depth of tumour and histological type
(8–10). Size and grade of SSTS have been
identified as the key factors in predicting survival whilst other
studies have suggested that patient age, tumour grade, lymph node
involvement, and resection margin are important prognostic factors
in determining the risk of distant metastatic disease (11–14).
The biology of SSTS rather than the specific medical or surgical
intervention has been found to be the greatest factor in survival
(2).
NICE (National Institute for Health and Care
Excellence) guidelines for quality standards in the management of
sarcoma, published in January 2015, advise that healthcare
professionals collect and publish data about sarcoma outcomes
including site-specific data (REF) (15).
We report on a 10 year experience in the management
of superficial soft tissue sarcoma in Southeast of Scotland and
describe the epidemiological and prognostic factors related to
disease outcome with regard to overall survival, local recurrence
and metastatic disease.
Materials and methods
Study description
This is a service evaluation project, rather than
research, hence no ethical approval was required. The project was
endorsed by the Scottish Sarcoma Network (SSN) and the key findings
aimed at informing the development of the Scottish cutaneous
sarcoma guidelines. Eligible patients were those referred to our
cancer network with a diagnosis of SSTS. Patients surgically
treated for SSTS within Edinburgh and Lothian Hospital from January
2000 to November 2010, were retrospectively identified through
histopathology coding, patient notes, electronic patient notes and
the institutional pathology database. For each patient, the case
notes or the electronic patient record was reviewed for
demographics, as well as tumour and treatment details. We recorded
the following items: gender, age, histology, tumour site, diameter,
treatment modality (surgery/radiation/chemotherapy), surgical
margin, date of local/metastatic recurrence and death. The Enneking
surgical staging system was used to classify the surgical margins
used (16). If patients had more
than one operation in order to achieve satisfactory resection, then
the re-excision margins were used to classify the resection. Data
were stored securely and accessed only by the clinical team.
Descriptive statistical analysis was performed by the authors.
Statistical analysis
Statistical analysis was performed using SPSS
version 25.0 (IBM Corp.). Continuous variables were expressed as
mean ± standard deviation and categorical variables were expressed
as numbers (percentages). The 5-year survival of 66 patients was
studied. The survival analysis was conducted with the Kaplan-Meier
method. Multivariate Cox regression analyses were utilized to
identify independent risk factors of overall survival (OS) and
relapse free survival (RFS). To determine if a new model-including
explanatory variables-improves upon the baseline model, the Omnibus
Tests of Model Coefficients are utilized. The log-likelihoods of
the baseline model and the new model are compared using chi-square
tests to determine whether there is a significant difference. All
analyses were conducted in the statistical package SPSS 25 (IBM
Corp.). The minimum value of statistical significance (P-value) was
determined at 5%.
Results
Overall survival
The study sample consists of 66 patients (20 women
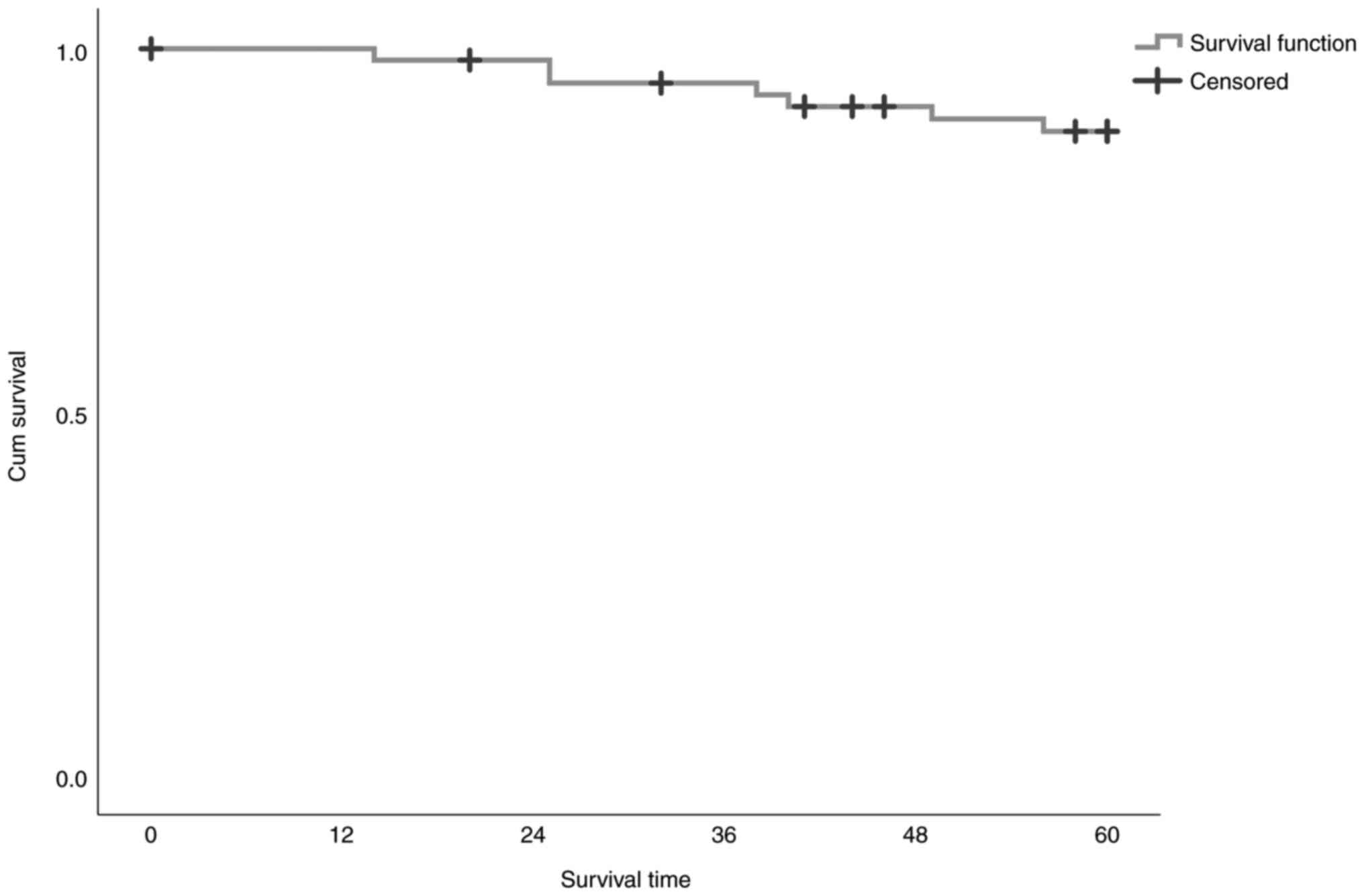
and 46 men) with a mean age of 51.7 years (Table I). The overall survival (OS) as
Kaplan-Meier evaluation is shown in Tables II and III and Fig.
1. The number of terminal events (deaths) and censored
observations (alive people) is presented in Table I. For the total of cases, the
percentage of living observations is 89.4%. In Table III, the descriptive measures (mean
and median value with the equivalent fluctuations) of OS are
reported. Mean estimated survival time is 57.2 months, with a 95%
confidence interval between 55 and 59.5 months. The estimation of
the average survival time is limited to the highest full time thus
ignoring any longer times, but which were censored. Median survival
time cannot be estimated because there is no time point during
which the survival function has a value <50% (See also Table III and Fig. 1).
 | Table I.Sample descriptive
characteristics. |
Table I.
Sample descriptive
characteristics.
| Variable | Value |
|---|
| Mean age ± SD,
years | 51.7±19.1 |
| Sex, n (%) |
|
| Female | 20 (30.3) |
| Male | 46 (69.7) |
 | Table II.Case processing summary. |
Table II.
Case processing summary.
| Total, n | No. of events | Censored, n (%) |
|---|
| 66 | 7 | 59 (89.4) |
 | Table III.Means and medians for overall survival
time. |
Table III.
Means and medians for overall survival
time.
| Variable | Meana | Medianb |
|---|
| Estimate | 57.236 | . |
| Std. error | 1.133 | . |
| 95% CI | 55.015-59.457 | . |
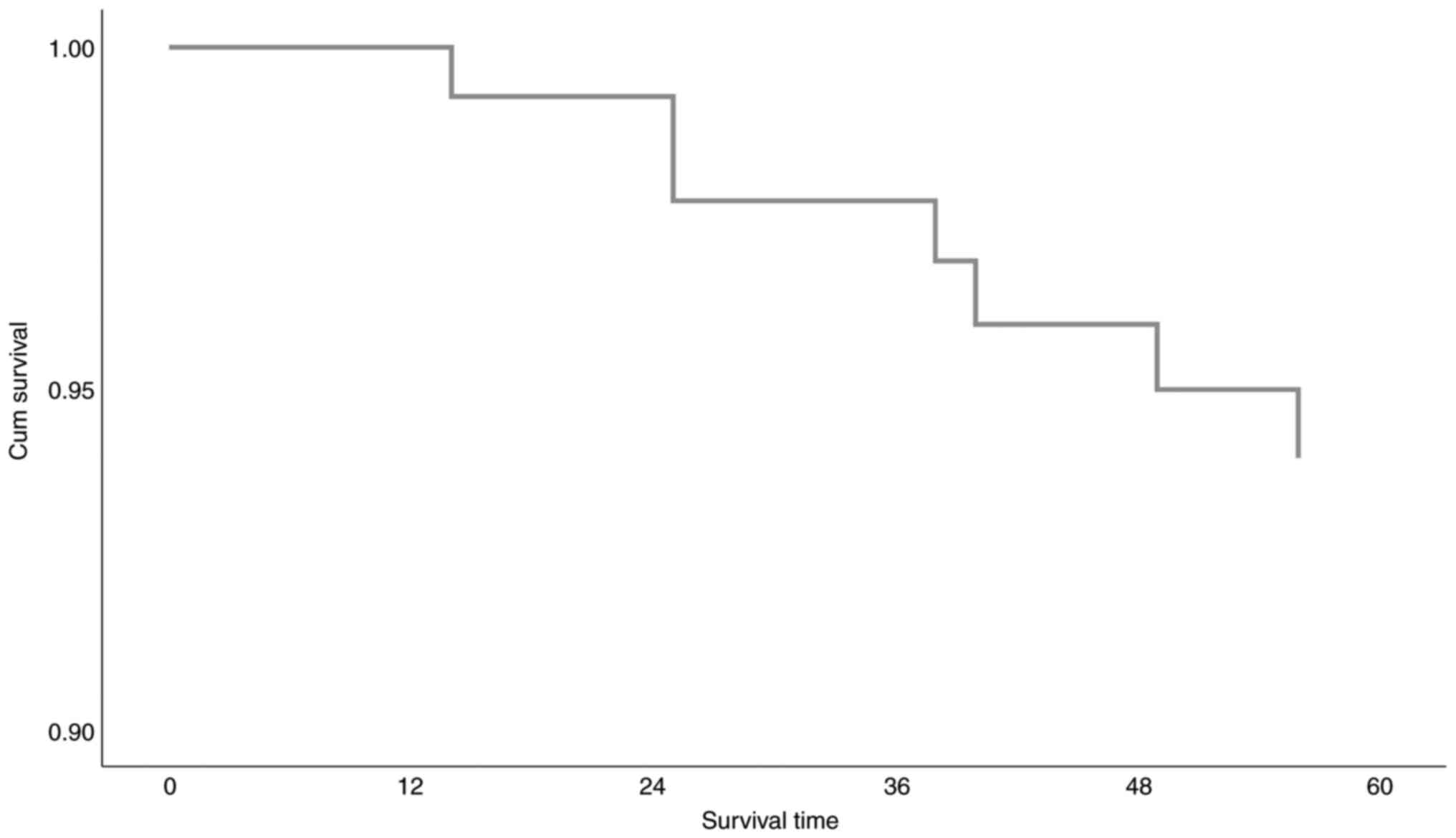
The OS information concerning data (Cox Proportional
Risk Model) is provided in Tables
IV, V and VI, and Fig.
2. There are 5 missing values (3 about age and 2 about SIMD
indicator) (17), no negative time
and 2 censored cases before terminal event (death) in a stratum. 7
out of totally 59 cases (10.6%) presented this contingency while 52
(78.8%) concern censored survival time (Table IV). The independent variables
chosen for Cox proportional risk model (OS) are: a) Age, b) sex,
and c) SIMD indicator. In Table V
there are provided the collective controls for the adjustment of
the reciprocating model. Since χ2 variation is
statistically significant (P=0.046), the model is well adjusted.
Meaning that at least one of the aforementioned independent
variables significantly affects survival time (Table V). In Table VI, the assessment of the model
parameters is recorded. We observe that, out of all the independent
variables being in consideration, only age can statistically
significantly explicate overall survival time. More specifically,
death risk for a person with SSTS (Superficial Soft Tissue
Sarcomas) is increased by 7.3% [Exp(B)=1.073.95% CI(1.012, 1.138)]
for every additional year of life. The estimated survival function,
based on Cox Proportional Risk Model, is graphically presented in
Fig. 2 and Table VI.
 | Table IV.Case processing summary (survival
time). |
Table IV.
Case processing summary (survival
time).
| Variable | No. (%) |
|---|
| Cases available in
analysis |
|
| Eventa | 7 (10.6) |
| Censored | 52 (78.8) |
| Total | 59 (89.4) |
| Cases dropped |
|
| Cases with missing
values | 5 (7.6) |
| Cases with negative
time | 0 (0.0) |
| Censored cases
before the earliest event in a stratum | 2 (3.0) |
| Total | 7 (10.6) |
| Total | 66 (100.0) |
 | Table V.Omnibus tests of model
coefficients. |
Table V.
Omnibus tests of model
coefficients.
|
| Overall
(score) | Change from
previous step | Change from
previous block |
|---|
|
|
|
|
|
|---|
| −2
Loglikelihood | χ2 | df | Sig. | χ2 | df | Sig. | χ2 | df | Sig. |
|---|
| 46.758 | 8.005 | 3 | 0.046 | 9.095 | 3 | 0.028 | 9.095 | 3 | 0.028 |
 | Table VI.Variables in the equation. |
Table VI.
Variables in the equation.
| Variable | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for
Exp(B) |
|---|
| Age | 0.071 | 0.030 | 5.538 | 1 | 0.019 | 1.073 | 1.012-1.138 |
| Sex | 0.164 | 0.842 | 0.038 | 1 | 0.846 | 1.178 | 0.226-6.136 |
| SIMD | −0.513 | 0.451 | 1.294 | 1 | 0.255 | 0.599 | 0.247-1.449 |
Relapse free survival
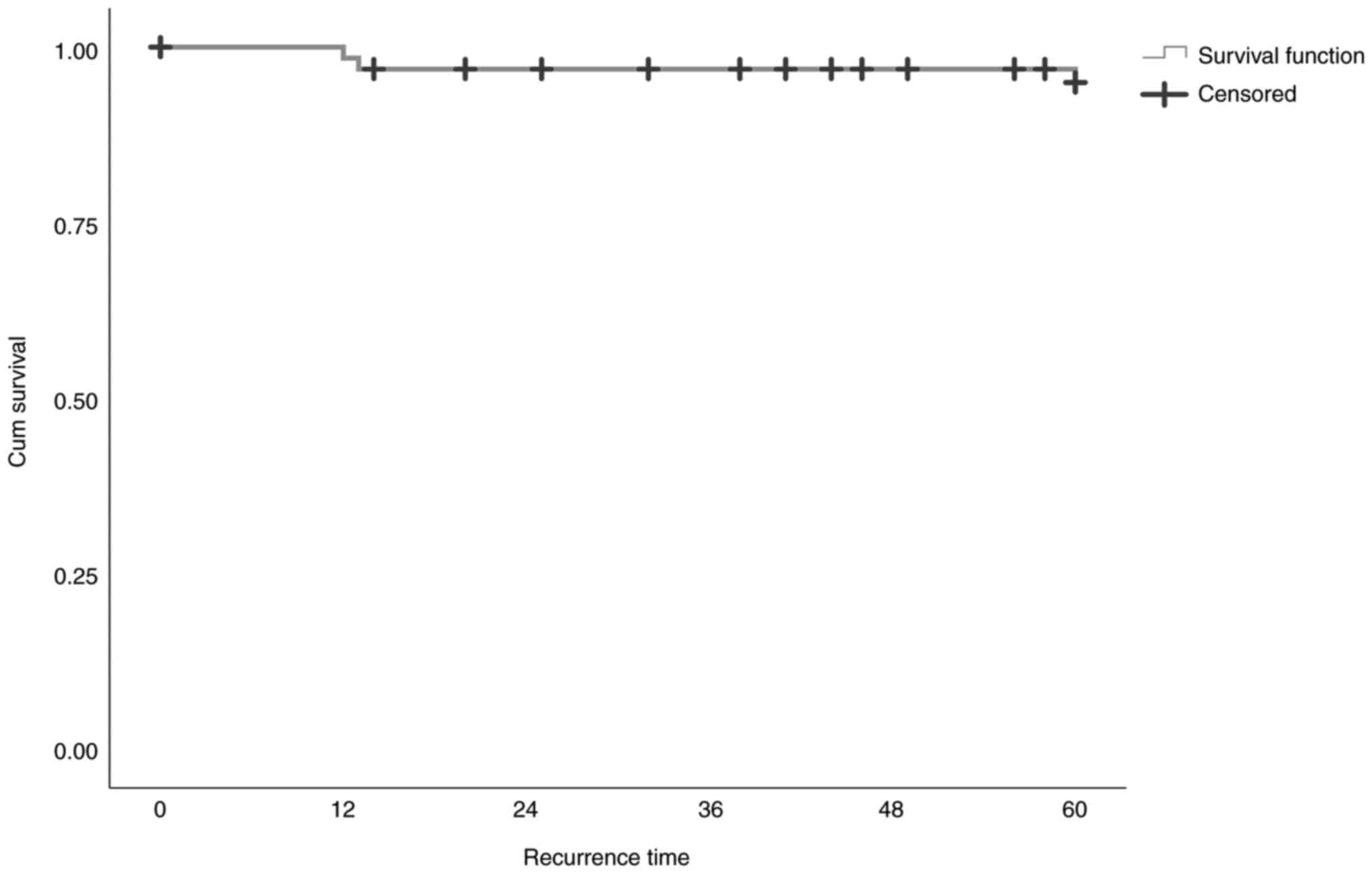
The local relapse free survival (Kaplan-Meier
evaluation) is shown in Tables
VII and VIII, and Figs. 3 and 4, as the number of terminal events (local
relapse) and censored observations (people with no local relapse).
The percentage of local relapse observations is 95.5%. Table VIII presents the descriptive
measures (mean and median value with the equivalent fluctuations)
of local relapse. The estimated mean local relapse time is 58.5
months, with a 95% confidence interval between 56 and 61 months.
The estimation of mean local relapse time is limited to the maximum
fulltime, thus ignoring any possibly bigger time that has been
censored. Median local relapse time cannot be estimated since there
is no time point during which the local recurrence function has a
value <50%.
 | Table VII.Case processing summary (local
relapse). |
Table VII.
Case processing summary (local
relapse).
| Total, n | No. of events | Censored, n
(%) |
|---|
| 66 | 3 | 63 (95.5) |
 | Table VIII.Means and medians for local
recurrence-free survival time. |
Table VIII.
Means and medians for local
recurrence-free survival time.
| Variable | Meana | Medianb |
|---|
| Estimate | 58.516 | . |
| Std. error | 1.265 | . |
| 95% CI | 56.036-60.996 | . |
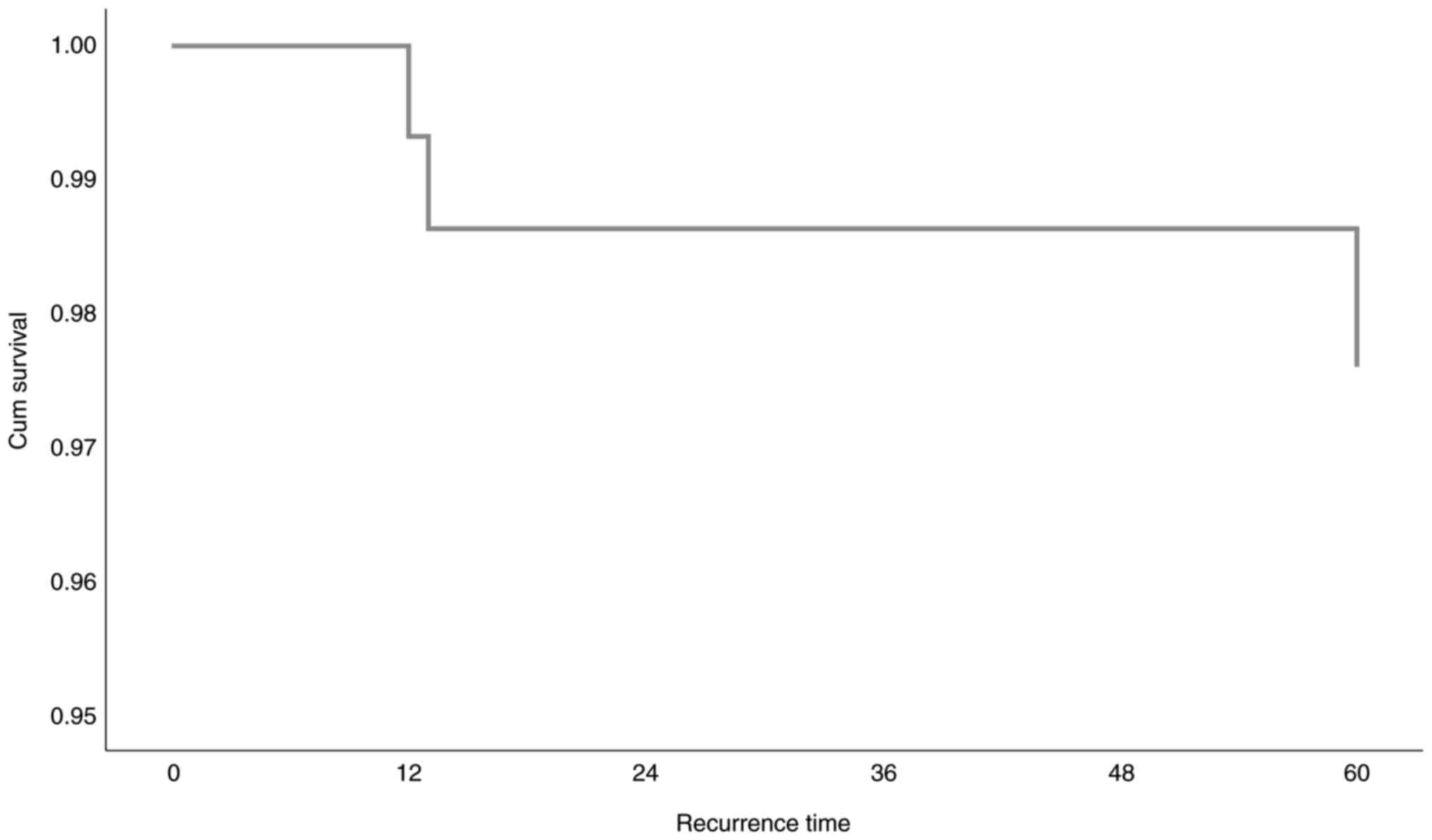
The local relapse free survival information on data
(Cox Proportional Risk Model) is included in Tables IX, X and XI
and Figs. 3 and 4. There are 5 missing values (3 about age
and 2 about SIMD), no negative time and 2 censored cases before
terminal event (local relapse) in a stratum. 3 out of 59 cases in
total (4.5%) presented the contingency (local recurrence) while 56
(84.8%) are censored survival time. For Cox Proportional Risk Model
(Overall Survival), we have chosen these independent variables: a)
Age, b) Gender, and c) SIMD indicator. In Table X, there can be seen the collective
controls for the adjustment of the reciprocating model. Since
χ2 variation is not statistically significant (P=0.321),
the model is not well adjusted. None of the aforementioned
independent variables seem to significantly affect local recurrence
time. In Table XI, the assessment
of the model parameters is recorded. We observed that, out of all
the independent variables being in consideration, none can
statistically significantly explicate local relapse recurrence
time. The estimated survival function, based on Cox Proportional
Risk Model, is graphically presented in Fig. 4.
 | Table IX.Case processing summary (recurrence
time). |
Table IX.
Case processing summary (recurrence
time).
| Variable | No. (%) |
|---|
| Cases available in
analysis |
|
| Eventa | 3 (4.5) |
| Censored | 56 (84.8) |
| Total | 59 (89.4) |
| Cases dropped |
|
| Cases with missing
values | 5 (7.6) |
| Cases with negative
time | 0 (0.0) |
| Censored cases
before the earliest event in a stratum | 2 (3.0) |
| Total | 7 (10.6) |
| Total | 66 (100.0) |
 | Table X.Omnibus tests of model
coefficients. |
Table X.
Omnibus tests of model
coefficients.
|
| Overall
(score) | Change from
previous step | Change from
previous block |
|---|
|
|
|
|
|
|---|
| −2 Log
Likelihood | χ2 | df | Sig. | χ2 | df | Sig. | χ2 | df | Sig. |
|---|
| 19.944 | 3.497 | 3 | 0.321 | 4.032 | 3 | 0.258 | 4.032 | 3 | 0.258 |
 | Table XI.Variables in the equation. |
Table XI.
Variables in the equation.
| Variable | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for
Exp(B) |
|---|
| Age | 0.078 | 0.049 | 2.495 | 1 | 0.114 | 1.081 | 0.982-1.190 |
| Sex | 0.018 | 1.235 | 0.000 | 1 | 0.988 | 1.019 | 0.091-11.450 |
| SIMD | −0.336 | 0.631 | 0.284 | 1 | 0.594 | 0.714 | 0.207-2.460 |
Discussion
Soft-tissue sarcomas are mesenchymal neoplasms with
an incidence of <1% per year (1). SSTS are relatively rare entities and
differ from deep sarcomas because they are usually smaller. Due to
their small size and superficial location, these lesions are
frequently treated by marginal or intralesional resection before
referral to a sarcoma centre (18).
SSTS present lower rates of distant metastasis and higher rates of
disease-free survival (19).
Treatment of choice in patients with either SSTS or
deep STS is complete surgical removal with wide negative margins
(20). A sufficiently wide excision
and micrographic control of margins, especially in anatomically
challenging locations, should be attempted as they tend to recur
locally. Mohs micrographic surgery (MMS) compared with wide local
excision have shown a favourable outcome regarding local
recurrence. Where available, MMS is currently the surgical
treatment of choice for the majority of SSTS (especially in
dermatofibrosarcoma protuberans).
In hospitals where it is not available, conventional
surgery with deep margins 1 to 3 cm, is recommended (21). If resection margins are found
positive in the final pathology, re-resection to obtain negative
margins should strongly be advised if it will not have a
significant impact upon functionality. Should excision not be
feasible or adequate, radiotherapy should be employed (22). Our study established similar
findings to other larger studies of prognostic factors and outcomes
in SSTS (2,9,23). Age
at diagnosis, tumour size and tumour grade were significantly
associated with 5-year OS and LRFS.
These findings compare well with other studies in
demonstrating that older age (>55 years), larger tumours and
higher grade of tumour at diagnosis are all correlated to OS and
LRFS (2,23,24).
Other studies have demonstrated similar significance of these
factors in the impact on MFS (2).
As similar studies have concluded, the nature of SSTS tumours
appears to have a significant impact on OS and LRFS (2,13).
Surgical excision ensuring wide clear surgical margins remains the
mainstay οf local disease treatment. Wide excision and negative
surgical margins are still the main goal. Radiotherapy and
chemotherapy in neoadjuvant or adjuvant setting are secondary in
excisable tumours and only in selected patients discussed in the
MDT meeting. Our data showed a lower risk for local recurrence
(6.1%) than has previously been reported (11–23%) and similar rate
of overall metastasis (12.1%) (2).
This may be limited by the smaller number of cases in comparison
with larger studies.
Additionally, metastatic disease at presentation was
not significant in predicting OS, however, this may be skewed due
to the small proportion of deaths in this study caused by SSTS and
a larger case study may provide a more reliable conclusion. Like
other studies, patient gender and tumour location were not
significant in predicting OS or LRFS (1,2,23).
SSTS overall appear to have relatively good outcomes
as demonstrated by our findings and elsewhere in the literature,
with only a 6.1% mortality associated with SSTS at 5 years in this
study (11). Previous studies have
alluded to the reason for this suggesting that the superficial
nature of these tumours in comparison with deeper STS make them
readily detectable and comparatively easier to treat and monitor.
Deeper STS tend to be larger at presentation in comparison to the
average tumour size found in this study (20.7 mm) and elsewhere in
the literature (2). This may be
attributed to the fact that more superficial tumours can be
detected by patients at an earlier stage, perhaps also explaining
their superior outcomes. Other studies have found predominantly
high-grade tumours in SSTS whilst we found the majority were
low-grade tumours (1). However,
data for tumour grade was available for only 66.6% of cases in this
study.
The impact of surveillance in reducing mortality in
SSTS has not been established and follow-up data in this study was
insufficient for analysis. Scottish Sarcoma Network (SSN) has
developed Scottish guidelines and all cases are discussed at the
National Sarcoma MDT to ensure optimal care for these patients. In
keeping with current UK guidelines, prospective collection of
surveillance data may help to better define its significance in
overall mortality in SSTS (7). As
this study and others have demonstrated, significant factors
relating to OS appear primarily related to the nature of the tumour
(size and grade), rather than the surgical management therefore
further investigation of aetiological factors may improve
understanding of prognosis in these rare tumours.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT, AG and IN conceived the study. MT, AG, LH and GG
have made substantial intellectual contributions to the methodology
(logic of study, research setting and participants, methods and
procedures of data collection and analysis). DM and IN wrote the
original draft. SP, AK, DM and IN reviewed and edited the
manuscript. MT, AG and IN supervised the manuscript. IN, LH and GG
confirm the authenticity of all raw data. AK and DM had a
significant contribution in analysis and interpretation of data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STS
|
soft tissue sarcomas
|
|
SSTS
|
superficial soft tissue sarcomas
|
|
OS
|
overall survival
|
|
LR
|
local recurrence
|
|
M
|
metastasis
|
|
NICE
|
National Institute for Health and Care
Excellence
|
|
LRFS
|
local recurrence-free survival
|
|
MFS
|
metastasis-free survival
|
|
MMS
|
Mohs micrographic surgery
|
References
|
1
|
Daigeler A, Harati K, Goertz O, Hirsch T,
Steinau HU and Lehnhardt M: Prognostic factors and surgical tactics
in patients with locally recurrent soft tissue sarcomas. Handchir
Mikrochir Plast Chir. 47:118–127. 2015.(In German). PubMed/NCBI
|
|
2
|
Tsagozis P, Bauer HC, Styring E, Trovik
CS, Zaikova O and Brosjö O: Prognostic factors and follow-up
strategy for superficial soft-tissue sarcomas: Analysis of 622
surgically treated patients from the Scandinavian sarcoma group
register. J Surg Oncol. 111:951–956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singer S, Demetri GD, Baldini EH and
Fletcher CD: Management of soft-tissue sarcomas: An overview and
update. Lancet Oncol. 1:75–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Francescutti V, Sanghera SS, Cheney RT,
Miller A, Salerno K, Burke R, Skitzki JJ and Kane JM III:
Homogenous good outcome in a heterogeneous group of tumors: An
institutional series of outcomes of superficial soft tissue
sarcomas. Sarcoma. 2015:3250492015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Austin JL, Temple WJ, Puloski S, Schachar
NS, Oddone Paolucci E, Kurien E, Sarkhosh K and Mack LA: Outcomes
of surgical treatment alone in patients with superficial soft
tissue sarcoma regardless of size or grade. J Surg Oncol.
113:108–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murray PM: Soft tissue sarcoma of the
upper extremity. Hand Clin. 20:325–327. vii2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brennan MF, Antonescu CR, Moraco N and
Singer S: Lessons learned from the study of 10,000 patients with
soft tissue sarcoma. Ann Surg. 260:416–421; discussion 421-2. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutierrez JC, Perez EA, Franceschi D,
Moffat FL Jr, Livingstone AS and Koniaris LG: Outcomes for
soft-tissue sarcoma in 8249 cases from a large state cancer
registry. J Surg Res. 141:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbas JS, Holyoke ED, Moore R and
Karakousis CP: The surgical treatment and outcome of soft-tissue
sarcoma. Arch Surg. 116:765–769. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu CY, Yen CC, Chen WM, Chen TH, Chen PC,
Wu HT, Shiau CY, Wu YC, Liu CL and Tzeng CH: Soft tissue sarcoma of
extremities: The prognostic significance of adequate surgical
margins in primary operation and reoperation after recurrence. Ann
Surg Oncol. 17:2102–2111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brooks AD, Heslin MJ, Leung DH, Lewis JJ
and Brennan MF: Superficial extremity soft tissue sarcoma: An
analysis of prognostic factors. Ann Surg Oncol. 5:41–47. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lachenmayer A, Yang Q, Eisenberger CF,
Boelke E, Poremba C, Heinecke A, Ohmann C, Knoefel WT and Peiper M:
Superficial soft tissue sarcomas of the extremities and trunk.
World J Surg. 33:1641–1649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trovik CS, Bauer HC, Alvegård TA, Anderson
H, Blomqvist C, Berlin O, Gustafson P, Saeter G and Wallöe A:
Surgical margins, local recurrence and metastasis in soft tissue
sarcomas: 559 surgically-treated patients from the Scandinavian
Sarcoma Group Register. Eur J Cancer. 36:710–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujimoto M, Aozasa K, Ueda T, Morimura Y,
Komatsubara Y and Doi T: Multivariate analysis for histologic
prognostic factors in soft tissue sarcomas. Cancer. 62:994–998.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grimer R, Judson I, Peake D and Seddon B:
Guidelines for the management of soft tissue sarcomas. Sarcoma.
2010:5061822010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. 1980.
Clin Orthop Relat Res. (415):4–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scottish Government: Scottish Index of
Multiple Deprivation 2020v2 postcode lookup file. https://www.gov.scot/publications/scottish-index-of-multiple-deprivation-2020v2-postcode-look-up/24–July.
2022
|
|
18
|
Pisters PW, Leung DH, Woodruff J, Shi W
and Brennan MF: Analysis of prognostic factors in 1,041 patients
with localized soft tissue sarcomas of the extremities. J Clin
Oncol. 14:1679–1689. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eward WC, Lazarides AL, Griffin AM,
O'Donnell PW, Sternheim A, O'Neill A, Hofer SO, Ferguson PC and
Wunder JS: Superficial soft-tissue sarcomas rarely require advanced
soft-tissue reconstruction following resection. Plast Reconstr Surg
Glob Open. 5:e15532017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diamantis A, Baloyiannis I, Magouliotis
DE, Tolia M, Symeonidis D, Bompou E, Polymeneas G and Tepetes K:
Perioperative radiotherapy versus surgery alone for retroperitoneal
sarcomas: A systematic review and meta-analysis. Radiol Oncol.
54:14–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veronese F, Boggio P, Tiberio R, Gattoni
M, Fava P, Caliendo V, Colombo E and Savoia P: Wide local excision
vs. Mohs Tübingen technique in the treatment of dermatofibrosarcoma
protuberans: A two-centre retrospective study and literature
review. J Eur Acad Dermatol Venereol. 31:2069–2076. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Comprehensive Cancer Network
(2020), . Soft Tissue Sarcoma version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdfAugust
1–2022
|
|
23
|
Lintz F, Moreau A, Odri GA, Waast D,
Maillard O and Gouin F: Critical study of resection margins in
adult soft-tissue sarcoma surgery. Orthop Traumatol Surg Res. 98 (4
Suppl):S9–S18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teixeira LE, Araújo ID, de Andrade MA,
Gomes RA, Salles PG and Ghedini DF: Local recurrence in soft tissue
sarcoma: Prognostic factors. Rev Col Bras Cir. 36:377–381. 2009.(In
Portuguese). View Article : Google Scholar : PubMed/NCBI
|