Introduction
Gastric cancer (GC) is the fifth most common cancer
and the third most common cause of cancer-related mortality
worldwide (1). Systemic
chemotherapy, including cytotoxic agents, molecular-targeted drugs,
or immune checkpoint inhibitors, has demonstrated a statistically
significant prolongation of survival compared with best supportive
care alone. Therefore, it is recommended as a first-line or later
conventional treatment for advanced GC (2–4). In
addition to other cancers, blockade of program death-1 (PD-1) or
program death ligand-1 (PD-L1) restores T-cell activity and has
emerged as a breakthrough therapy for GC.
Among the specific drugs, nivolumab, a PD-1
inhibitor, was evaluated for its efficacy against GC in a
randomized phase III study, ATTRACTION-2 (5). The ATTRACTION-2 results indicated that
patients treated with nivolumab exhibited significantly prolonged
OS compared with those treated with placebo. Although this
represents an advancement in GC treatment, the outcome of patients
with unresectable GC remains poor. Indeed, the median OS is
approximately one year after initiation of first-line chemotherapy
(6). In the ATTRACTION-2 study, the
patients receiving nivolumab showed a significant increase in
median OS; however, approximately half experienced exhibited
progressive disease at their first radiographical examination.
Thus, biomarkers that can predict the outcome or efficacy of these
agents are needed.
Systemic inflammation is vital in tumor promotion
and progression (7). Several
markers of systemic inflammation, including C reactive protein,
platelet-to-lymphocyte ratio, and neutrophil-to-lymphocyte ratio
(NLR), are associated with clinical outcomes in various cancers.
The peripheral blood NLR, an indicator of systemic inflammation, is
a conventional biomarker in the clinical setting and has been
reported as a prognostic biomarker in solid neoplasms, such as
breast, lung, pancreas, colon, rectum, and stomach (8–12). In
addition to its prognostic role, NLR has been recognized as a
predictive marker for several unresectable solid neoplasm
treatments with cytotoxic chemotherapy and immunotherapy (13,14).
Recently, Valero et al demonstrated that increased NLR is
significantly associated with poorer OS and progression-free
survival and lower response rates and clinical benefit after immune
checkpoint inhibitor (ICI) therapy for multiple cancer types in a
retrospective cohort of 1,714 patients representing 16 different
cancer types, including GC patients treated with ICI (15).
Although the NLR values fluctuate over time, most
studies have used NLR values obtained at a one-time point before
the induction of targeted agent therapy to evaluate clinical
outcomes. In this study, we evaluated NLR values in blood at the
moment before the initiation of each chemotherapy line (e.g.,
before the 1st-, 2nd-, and 3rd-line, respectively) to determine
whether NLR could predict clinical outcome.
Materials and methods
Patients
In this retrospective study, we analyzed 83 patients
with unresectable GC who were histologically diagnosed with
adenocarcinoma and received palliative chemotherapy in the
Department of Clinical Oncology, Kawasaki Medical School Hospital,
between March 2018 and December 2020. All patients-received one or
more lines of chemotherapy and at least one evaluation of
anti-tumor efficacy by computed tomography after the start of
chemotherapy. The duration of follow-up ranged from 1.0 to 58.4
months (median: 10.1 months). OS was calculated from the initiation
of each line of chemotherapy to death. Clinicopathological factors,
e.g., age at the start of chemotherapy, gender, an Eastern
Cooperative Oncology Group (ECOG) performance status, primary tumor
location, human epidermal growth factor receptor type 2 (HER2)
status, histology, metastatic status, and chemotherapy regimens,
were collected from clinical and pathological records. The HER2
status was evaluated using immunohistochemistry (IHC) or
fluorescence in situ hybridization (FISH). Tumors with IHC 3+, IHC
2+, or FISH positive were HER2 positive (16). According to the Japanese
classification of gastric carcinoma (17), the primary tumor location was
classified into three categories (upper, middle, and lower). GCs
were classified according to Lauren's classification (18). Metastatic sites were evaluated by
computed tomography, positron emission tomography, or magnetic
resonance imaging, which were examined before induction of each
chemotherapy.
Chemotherapy
All patients received 1st-line chemotherapy (e.g.,
SOX, XELOX, FOLFOX SP, XP, weekly solvent-based paclitaxel
(sb-paclitaxel) plus ramucirumab, weekly nab-paclitaxel plus
ramucirumab, docetaxel monotherapy, or S-1 monotherapy) according
to the Japanese GC treatment guidelines. Details of the number of
patients using each regimen are presented in Table I. These regimens were administered
as follows: i) SOX: twice daily for the first 2 weeks of a 3-week
cycle of S-1 (80–120 mg/day) with 100 mg/m2 of
oxaliplatin on day one; ii) XELOX: twice daily for the first 2
weeks of a 3-week cycle of capecitabine (2,400 to 4,200 mg/day)
with 130 mg/m2 of oxaliplatin on day one; iii) FOLFOX:
oxaliplatin 85 mg/m2 and LV 200 mg/m2
followed by bolus 5-FU 400 mg/m2 and continuous 5-FU
2,400 mg/m2 were intravenously infused every 2 weeks;
iv) SP regimen: twice daily for the first 3 weeks of a 5-week cycle
of S-1 (80–120 mg/day) with 60 mg/m2 of cisplatin on day
8 of each cycle; v) XP regimen: twice daily for the first 2 weeks
of a 3-week cycle of capecitabine (2,400–4,200 mg/day) with 100
mg/m2 of cisplatin on day one; vi) Sb-paclitaxel plus
ramucirumab regimen: ramucirumab (8 mg/kg intravenously on days 1
and 15) with sb-paclitaxel (80 mg/m2 intravenously on
days 1, 8, and 15) every 4 weeks. vii) Nab-paclitaxel plus
ramucirumab regimen: Ramucirumab (8 mg/kg intravenously on days 1
and 15) with nab-paclitaxel (100 mg/m2 intravenously on
days 1, 8, and 15) every 4 weeks; and viii) Docetaxel regimen:
Docetaxel (60–70 mg/m2) was administered intravenously
on day 1 every 3 weeks. ix) S-1 monotherapy: Twice daily for the
first 2 weeks of a 3-week cycle of S-1 (80–120 mg/day). Dose
reduction and/or cycle delays were permitted according to the
decision of each physician. Fifteen patients that were
HER2-positive received trastuzumab in combination with the SOX or
XP regimen.
 | Table I.Clinicopathological characteristics of
patients with unresectable GC patients. |
Table I.
Clinicopathological characteristics of
patients with unresectable GC patients.
|
| Line of
chemotherapy |
|---|
|
|
|
|---|
| Characteristic | 1st-line (n=83) | 2nd-line (n=56) | 3rd-line (n=34) |
|---|
| Sex, n (%) |
|
|
|
| Male | 52 (63) | 34 (61) | 20 (59) |
|
Female | 31 (37) | 22 (39) | 14 (41) |
| Median age, years
(range) | 72 (44–86) | 72 (53–79) | 70 (53–77) |
| Age, n (%) |
|
|
|
| <70
years | 36 (43) | 28 (50) | 16 (47) |
| ≥70
years | 47 (57) | 28 (50) | 18 (53) |
| Performance status,
n (%) |
|
|
|
| 0 | 25 (30) | 15 (27) | 7 (21) |
| 1 | 30 (36) | 22 (40) | 16 (47) |
| 2 | 22 (27) | 16 (28) | 10 (29) |
| 3 | 6 (7) | 3 (5) | 1 (3) |
| Primary tumor
location, n (%) |
|
|
|
|
Upper | 29 (35) | 17 (30) | 12 (35) |
|
Middle | 34 (41) | 20 (36) | 13 (38) |
|
Lower | 20 (24) | 19 (34) | 9 (27) |
| HER2 status, n
(%) |
|
|
|
|
Positive | 15 (18) | 11 (20) | 7 (21) |
|
Negative | 68 (72) | 45 (80) | 27 (79) |
| Lauren
classification, n (%) |
|
|
|
|
Intestinal type | 45 (54) | 30 (54) | 16 (47) |
| Diffuse
type | 38 (46) | 26 (46) | 18 (53) |
| Number of
metastatic organs, n (%) |
|
|
|
| ≤1 | 51 (61) | 27 (48) | 17 (50) |
| ≥2 | 32 (39) | 29 (52) | 17 (50) |
| Liver metastasis, n
(%) |
|
|
|
|
Yes | 26 (31) | 21 (38) | 10 (29) |
| No | 57 (69) | 35 (62) | 24 (71) |
| Peritoneal
dissemination, n (%) |
|
|
|
|
Yes | 35 (42) | 23 (41) | 14 (41) |
| No | 48 (58) | 33 (59) | 20 (59) |
| Ascites, n (%) |
|
|
|
|
Yes | 30 (36) | 13 (23) | 14 (41) |
| No | 53 (64) | 43 (77) | 20 (59) |
| Chemotherapy
regimen, n (%) |
|
|
|
|
Platinum-based |
|
|
|
|
No antibody
agent | 61 (73) | 4 (7) | 0 (0) |
|
Trastuzumab | 13 (16) | 0 (0) | 0 (0) |
|
Taxane-based | 5 (6) | 48 (85) | 3 (9) |
|
ICI | 0 (0) | 2 (4) | 24 (70) |
|
Others | 4 (5) | 2 (4) | 7 (21) |
This study summarized i) SOX, ii) XELOX, iii)
FOLFOX, iv) SP, and v) XP regimens as Platinum-based chemotherapy
regimens. The vi) weekly sb-paclitaxel plus ramucirumab, vii)
weekly nab-paclitaxel plus ramucirumab, and viii) docetaxel
monotherapy were Taxane-based chemotherapy regimens. The ix) S1
monotherapy was categorized as others. S1 monotherapy is sometimes
used in the front-line chemotherapy in Japan. Although S1
monotherapy is restricted to use to the patients with PS 3, we
introduced intensive chemotherapy to the patients with PS 3 when we
judged that PS was getting worse due to stomach cancer burden.
Evaluation of NLR in blood
NLR was calculated by dividing the absolute
neutrophil and lymphocyte counts measured in peripheral blood
before each line of chemotherapy. Previous studies varied the
cut-off value of NLR from 2.0 to 5.0. A meta-analysis summarized
the median NLR for OS, cancer-specific OS, and progression-free
survival as 4.0, 3.85, and 3.0, respectively (19). Moreover, the meta-analysis revealed
a consistent effect of an elevated NLR on survival. As the median
NLR at 1st-line initiation in this study was 3.0 (range: 0.7 to
48.9), we used the lowest NLR cut-off value of 3.0 among OS,
cancer-specific OS, and progression-free survival in the
meta-analysis (19) as the cut-off
value throughout the study. Patients were divided into an NLR-high
group (>3.0) and an NLR-low group (<3.0) based on the NLR
value determined before the initiation of each line of
chemotherapy.
Statistical analyses
The primary aim was to evaluate the association
between peripheral blood NLR and clinical outcomes in GC patients.
OS was calculated using the Kaplan-Meier method, and the log-rank
test was used to compare survival between groups. Fisher's exact
test was performed to compare clinical characteristics between two
groups. Logistic regression analysis was used to obtain the risk
ratio of the NLR-high group to the NLR-low group. Cox regression
analysis was used to estimate the hazard ratio (HR) for OS. All
statistical tests were two-sided, and P<0.05 was considered
statistically significant. All statistical analyses were performed
with EZR software (Saitama Medical Centre, Jichi Medical
University, Saitama, Japan, version 1.40), which is a graphical
user interface for R (The R Foundation for Statistical Computing,
Vienna, version 3.5.2) (20).
Results
Patient characteristics
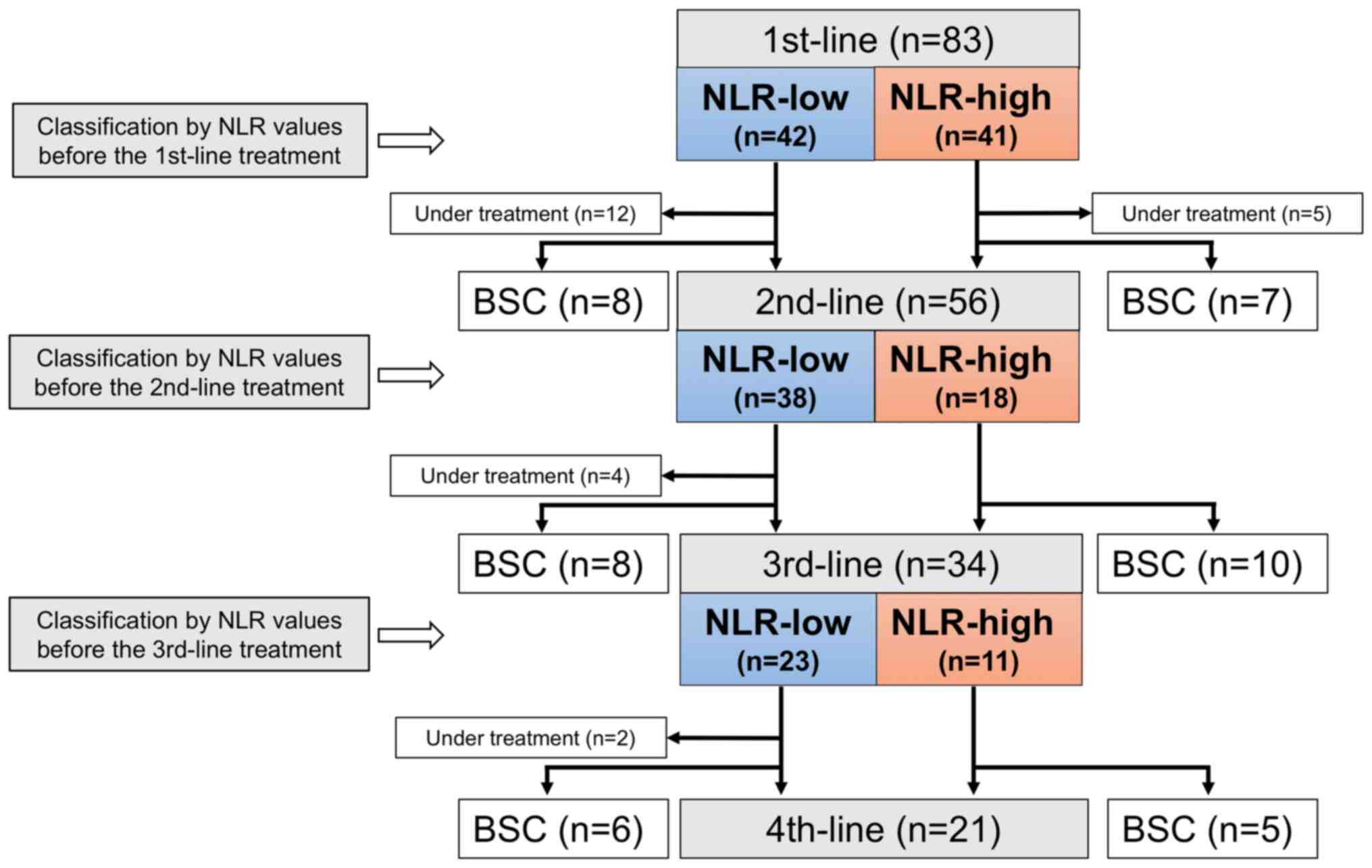
A total of 83 patients were enrolled. Patient
characteristics are shown in Table
I. The median age was 72 (range: 44–86), and 63% (n=52) of the
patients were male. Fifty-five patients (66%) had good performance
status (ECOG performance status: 0–1). Among six patients with PS
3, four patients received S-1 monotherapy. Thirty-two patients
(39%) had two or more metastatic organs, 26 (31%) had liver
metastases, and 35 (42%) confirmed peritoneal dissemination. Based
on the cut-off value, at the induction of the 1st-line
chemotherapy, 42 patients were categorized into the NLR-low group
(NLR <3.0) and 41 patients into the NLR-high group (NLR ≥3.0;
Fig. 1).
At the analysis, 12 patients were still undergoing
the 1st-line treatment, 15 were transferred to best supportive care
(BSC) after the 1st-line treatment, and 56 (79%) received the
2nd-line treatment. Of the 56 patients, 38 and 18 were divided into
the NLR-low and NLR-high groups by blood NLRs before the 2nd-line
treatment.
Among the 2nd-line treatment patients, four were
still undergoing the 2nd-line treatment at the analysis, and 34
(65%) had received the 3rd-line treatment. Of the 34 patients, 23
and 11 were divided into the NLR-low and NLR-high groups by blood
NLRs before the 3rd-line treatment. Finally, of the 34 patients
treated with the 3rd-line chemotherapy, two were still under the
3rd-line treatment at the analysis, 21 (66%) received the 4th-line
chemotherapy, and the remaining 11 patients were transferred to
BSC.
Clinical outcomes in relation to NLR
values before each chemotherapy line
Table II presents
the clinicopathological characteristics of GC patients at each
chemotherapy line concerning the NLR-high and NLR-low groups.
Regarding the patient characteristics, the NLR-high group had
significantly more patients with a poor ECOG performance status
throughout chemotherapy.
 | Table II.The association between
clinicopathological characteristics of unresectable GC patients and
NLR variations obtained before the 1st-, 2nd-, and 3rd-line
chemotherapy. |
Table II.
The association between
clinicopathological characteristics of unresectable GC patients and
NLR variations obtained before the 1st-, 2nd-, and 3rd-line
chemotherapy.
|
| NLR classification
before each initiation for chemotherapy, n (%) |
|---|
|
|
|
|---|
|
| 1st-line | 2nd-line | 3rd-line |
|---|
|
|
|
|
|
|---|
|
Characteristics | NLR Low (N=42) | High (N=41) | P-value | NLR Low (N=38) | High (N=18) | P-value | NLR Low (N=23) | High (N=11) | P-value |
|---|
| Sex |
|
| 0.82 |
|
| 0.77 |
|
| 0.46 |
|
Male | 27 (64) | 25 (61) |
| 24 (63) | 10 (56) |
| 15 (65) | 5 (46) |
|
|
Female | 15 (36) | 16 (39) |
| 14 (37) | 8 (44) |
| 8 (35) | 6 (54) |
|
| Age |
|
| 0.38 |
|
| 0.39 |
|
| 0.27 |
| <70
years | 16 (38) | 20 (49) |
| 21 (55) | 7 (39) |
| 9 (39) | 7 (64) |
|
| ≥70
years | 26 (62) | 21 (51) |
| 17 (45) | 11 (61) |
| 14 (61) | 4 (36) |
|
| ECOG PS |
|
| 0.03 |
|
| <0.001 |
|
| 0.02 |
| 0 | 15 (36) | 10 (24) |
| 14 (37) | 1 (6) |
| 6 (26) | 1 (9) |
|
| 1 | 19 (45) | 11 (27) |
| 18 (47) | 4 (22) |
| 13 (57) | 3 (27) |
|
| 2 | 7 (17) | 15 (37) |
| 5 (13) | 11 (61) |
| 3 (13) | 7 (64) |
|
| 3 | 1 (2) | 5 (12) |
| 1 (3) | 2 (11) |
| 1 (4) | 0 (0) |
|
| Primary tumor
location |
|
| 0.41 |
|
| 0.16 |
|
| 0.08 |
|
Upper | 14 (33) | 15 (37) |
| 10 (26) | 7 (39) |
| 5 (22) | 7 (64) |
|
|
Middle | 20 (48) | 14 (34) |
| 12 (32) | 8 (44) |
| 10 (44) | 3 (27) |
|
|
Low | 8 (19) | 12 (29) |
| 16 (42) | 3 (17) |
| 8 (34) | 1 (9) |
|
| HER2 status |
|
| 0.78 |
|
| 0.73 |
|
| 0.38 |
|
Positive | 7 (17) | 8 (20) |
| 7 (18) | 4 (22) |
| 6 (26) | 1 (9) |
|
|
Negative | 35 (83) | 33 (80) |
| 31 (82) | 14 (78) |
| 17 (74) | 10 (91) |
|
| Lauren
classification |
|
| 1.00 |
|
| 0.40 |
|
| 0.72 |
|
Intestinal type | 23 (55) | 22 (54) |
| 22 (58) | 8 (44) |
| 10 (44) | 6 (54) |
|
| Diffuse
type | 19 (45) | 19 (46) |
| 16 (42) | 10 (56) |
| 13 (56) | 5 (46) |
|
| Number of
metastatic organs |
|
| 0.01 |
|
| 0.16 |
|
| 1.00 |
| ≤1 | 32 (76) | 19 (46) |
| 21 (55) | 6 (33) |
| 11 (48) | 6 (55) |
|
| ≥2 | 10 (24) | 22 (54) |
| 17 (45) | 12 (67) |
| 12 (52) | 5 (45) |
|
| Liver
metastasis |
|
| 0.35 |
|
| 0.56 |
|
| 1.00 |
|
Yes | 11 (26) | 15 (37) |
| 13 (34) | 8 (44) |
| 6 (30) | 4 (36) |
|
| No | 31 (74) | 26 (63) |
| 25 (66) | 10 (56) |
| 14 (70) | 7 (64) |
|
| Peritoneal
dissemination |
|
| 0.27 |
|
| 0.04 |
|
| 0.69 |
|
Yes | 15 (36) | 20 (49) |
| 12 (32) | 11 (61) |
| 6 (26) | 4 (36) |
|
| No | 27 (64) | 21 (51) |
| 26 (68) | 7 (39) |
| 17 (74) | 7 (64) |
|
| Ascites |
|
| 0.02 |
|
| 0.09 |
|
| 0.08 |
|
Yes | 10 (24) | 20 (49) |
| 6 (16) | 7 (39) |
| 12 (52) | 2 (18) |
|
| No | 32 (76) | 21 (51) |
| 32 (84) | 11 (61) |
| 11 (48) | 9 (82) |
|
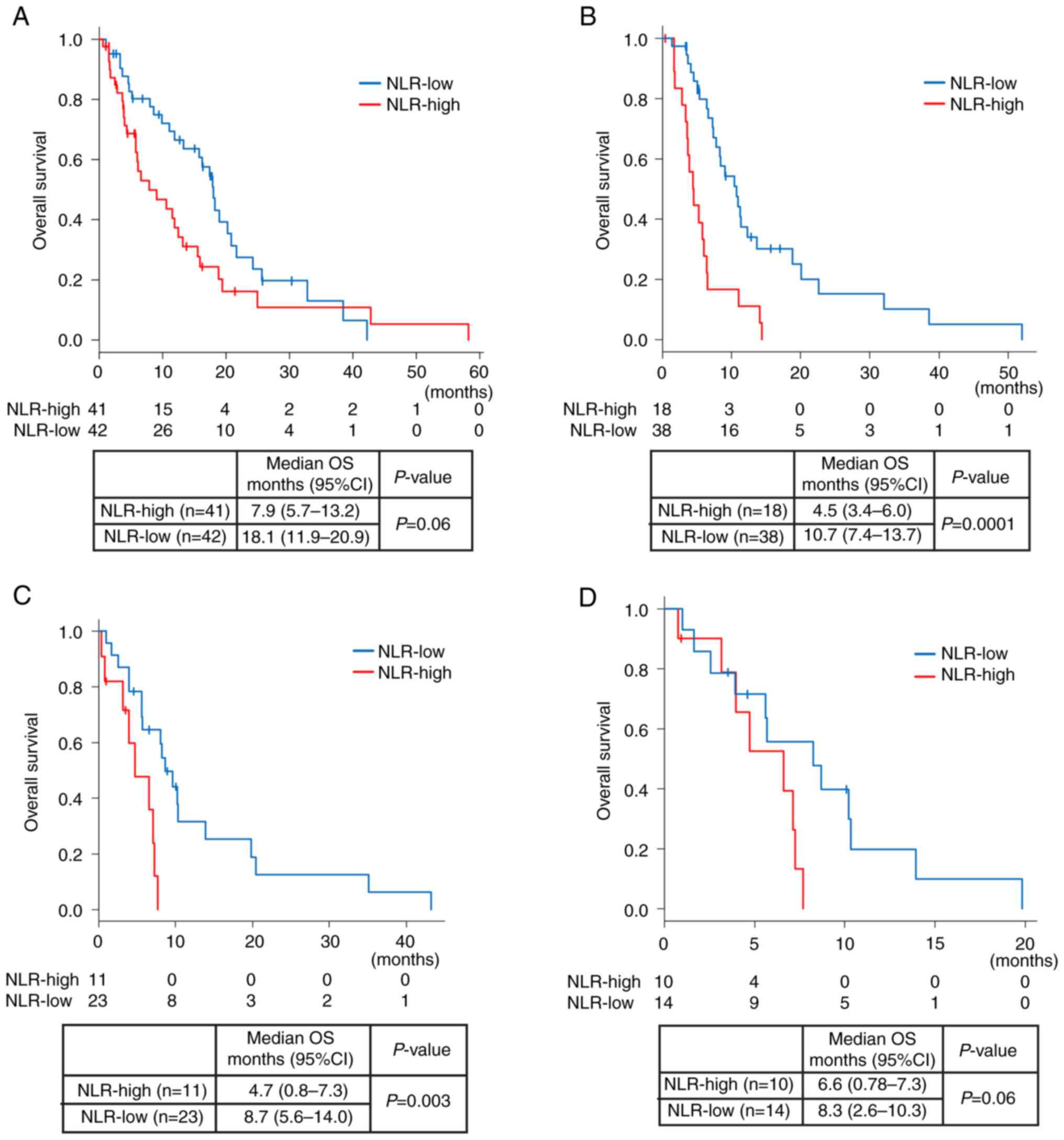
Of all 83 patients, the median OS was 13.2 months
(95% CI: 9.1-17.9). The median OS from the start of the 1st-line
chemotherapy was shorter in the NLR-high group than in the NLR-low
group [OS: 8.0 months (95% CI: 5.7-13.2) vs. 18.1 months
(11.9–20.9), P=0.06; Fig. 2A]. Of
56 patients who received 2nd-line chemotherapy, the median OS from
the start of the 2nd-line chemotherapy was significantly shorter in
the NLR-high group than in the NLR-low group [OS: 4.5 months (95%
CI: 3.4-6.0) vs. 10.7 months (95% CI: 7.4-13.7), P<0.05;
Fig. 2B]. Of the 34 patients who
received 3rd-line chemotherapy, the median OS from the start of the
3rd-line chemotherapy was significantly shorter in the NLR-high
group than in the NLR-low group [OS: 4.7 months (95% CI: 0.8-7.3)
vs. 8.7 months (95% CI: 5.6-14.0), P<0.05; Fig. 2C].
Next, we focused on the 24 patients who received
nivolumab monotherapy at the 3rd-line treatment. According to the
NLR value before nivolumab therapy, 14 patients (55%) were
stratified into the NLR-low group and ten patients into the
NLR-high group. Patient characteristics are shown in Table SI. The median OS was shorter in the
NLR-high group than that in the NLR-low group [median OS: 6.6
months (95% CI: 0.8-7.3 months) vs. 8.3 months (2.6–10.3 months),
P=0.06; Fig. 2D].
Finally, we examined which factor affected NLR
values in each chemotherapy line by logistic regression analysis
(Table III). By this multivariate
analysis, poor ECOG performance status was significantly associated
with the NLR-high group throughout the 1st- to 3rd-line. In
contrast, regarding prognosis, Cox regression analysis revealed
that the NLR high group was significantly associated with poor
prognosis only in the 3rd-line OS (risk ratio=4.33, P=0.03;
Table IV).
 | Table III.Logistic regression analysis
estimates the risk ratio for NLR-high among explanatory clinical
variables. |
Table III.
Logistic regression analysis
estimates the risk ratio for NLR-high among explanatory clinical
variables.
|
| 1st-line
pretreatment NLR-high | 2nd-line
pretreatment NLR-high | 3rd-line
pretreatment NLR-high |
|---|
|
|
|
|
|
|---|
| Characteristic | Risk ratio
(95%CI) | P-value | Risk ratio
(95%CI) | P-value | Risk ratio
(95%CI) | P-value |
|---|
| Sex
(Male/Female) | 0.84
(0.29–2.38) | 0.74 | 1.34
(0.26–6.85) | 0.72 | 0.06
(0.01–1.36) | 0.08 |
| Age
(≥70/<70) | 0.46
(0.16–1.32) | 0.15 | 1.36
(0.22–8.33) | 0.74 | 0.30
(0.02–4.41) | 0.38 |
| Performance status
(≥2/≤1) | 5.17
(1.59–16.8) | 0.01a | 19.2
(2.76–133.6) |
<0.01a | 70.7
(1.98–2526.4) | 0.02a |
| HER2 status
(Positive/Negative) | 1.40
(0.33–5.87) | 0.64 | 1.06
(0.08–13.5) | 0.96 | 0.007
(2.27e-5-2.59) | 0.10 |
| Lauren
classification (Diffuse/Intestinal) | 0.92
(0.31–2.68) | 0.87 | 7.11
(0.91–55.6) | 0.06 | 0.09
(0.004–1.87) | 0.12 |
| Number of
metastatic organs (≥2/≤1) | 3.64
(1.14–11.6) | 0.03a | 1.57
(0.24–10.2) | 0.64 | 0.45
(0.03–7.49) | 0.58 |
| Liver metastasis
(Yes/No) | 1.68
(0.48–5.92) | 0.42 | 3.77
(0.41–34.9) | 0.24 | 1.82
(0.12–26.5) | 0.66 |
| Peritoneal
dissemination (Yes/No) | 1.92
(0.45–8.20) | 0.38 | 5.43
(0.47–62.5) | 0.17 | 1.29
(0.04–39.2) | 0.88 |
| Ascites
(Yes/No) | 0.67
(0.14–3.06) | 0.60 | 1.04
(0.09–11.6) | 0.97 | 0.02
(0.00–1.62) | 0.08 |
 | Table IV.Cox regression analyses estimate the
Hazard ratio for OS among explanatory clinical variables. |
Table IV.
Cox regression analyses estimate the
Hazard ratio for OS among explanatory clinical variables.
|
| 1st-line OS | 2nd-line OS | 3rd-line OS |
|---|
|
|
|
|
|
|---|
| Characteristic | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Sex
(Male/Female) | 1.44
(0.79–2.61) | 0.23 | 1.52
(0.74–3.09) | 0.26 | 3.05
(0.80–11.7) | 0.10 |
| Age
(≥70/<70) | 1.31
(0.71–2.42) | 0.38 | 0.57
(0.25–1.31) | 0.19 | 0.93
(0.36–2.42) | 0.89 |
| Performance status
(≥2/≤1) | 2.91
(1.52–5.29) |
<0.01a | 6.30
(2.34–16.9) |
<0.001a | 4.57
(0.90–23.1) | 0.06 |
| HER2 status
(Positive/Negative) | 1.14
(0.54–2.42) | 0.72 | 0.69
(0.27–1.80) | 0.46 | 1.18
(0.25–5.50) | 0.84 |
| Lauren
classification (Diffuse/Intestinal) | 1.02
(0.54–1.93) | 0.94 | 0.85
(0.41–1.73) | 0.67 | 6.72
(1.59–28.4) | <0.01a |
| Number of
metastatic organs (≥2/≤1) | 1.15
(0.56–2.38) | 0.70 | 1.39
(0.62–3.14) | 0.42 | 2.79
(1.00–7.74) | 0.04a |
| Liver metastasis
(Yes/No) | 0.65
(0.29–1.41) | 0.27 | 0.76
(0.32–1.72) | 0.50 | 1.83
(0.57–5.87) | 0.31 |
| Peritoneal
dissemination (Yes/No) | 0.60
(0.27–1.31) | 0.20 | 0.79
(0.31–2.02) | 0.62 | 0.35
(0.08–1.47) | 0.15 |
| Ascites
(Yes/No) | 2.87
(1.21–6.80) | 0.02a | 0.72
(0.22–2.36) | 0.59 | 3.73
(0.86–16.2) | 0.08 |
| 1st-line
pretreatment NLR (High/Low) | 1.65
(0.85–3.20) | 0.14 |
|
|
|
|
| 2nd-line
pretreatment NLR (High/Low) |
|
| 2.35
(0.99–5.61) | 0.05 |
|
|
| 3rd-line
pretreatment NLR (High/Low) |
|
|
|
| 5.28
(1.43–19.6) | 0.01a |
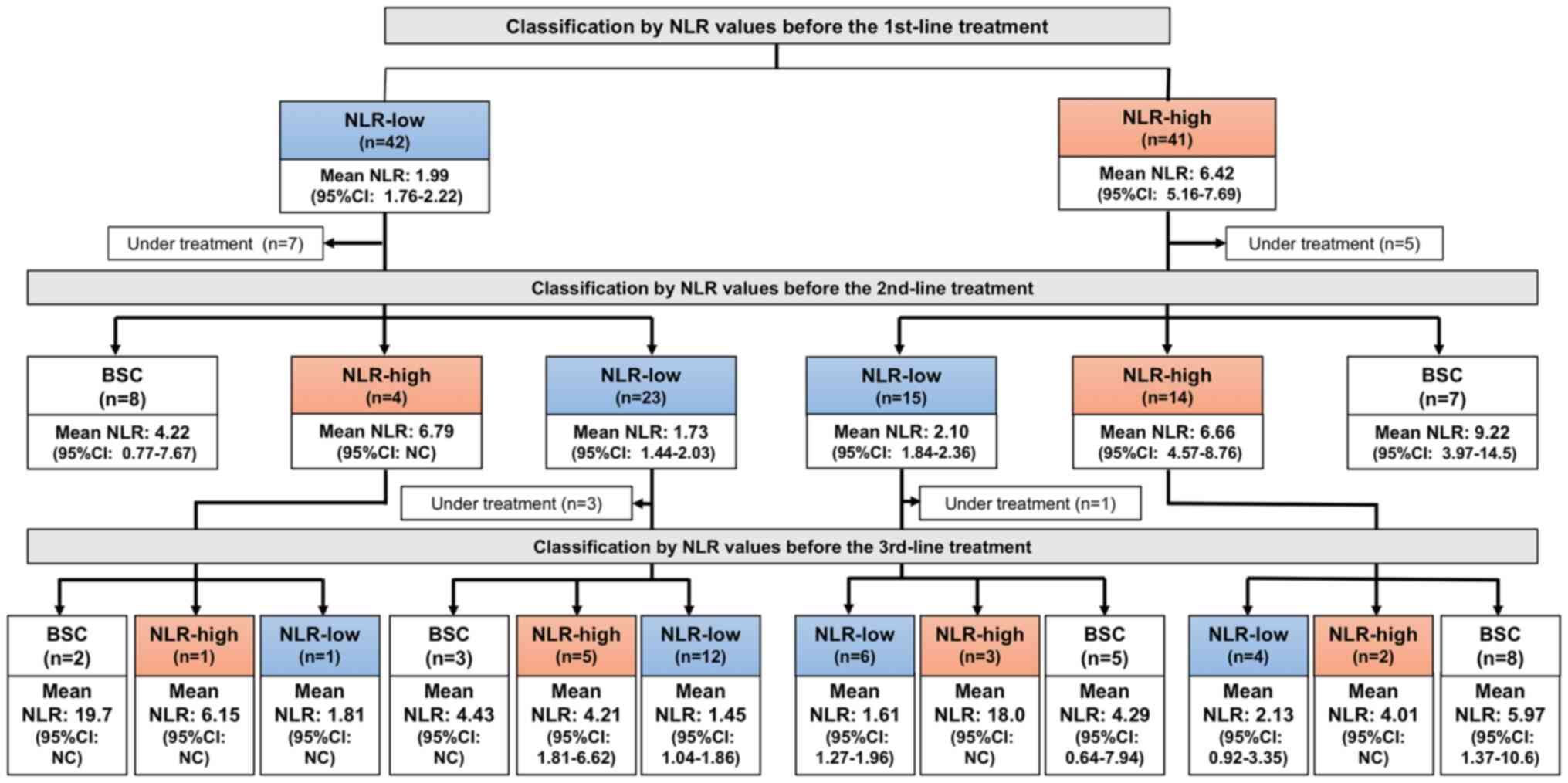
Changes in NLR value throughout
chemotherapeutic drug treatment in unresectable GC patients
We examined fluctuations of NLR values in each case
throughout chemotherapy (Fig. S1).
Among 35 patients in the NLR-low group at 1st-line who were
eligible for 2nd-line treatment, 23 (66%) retained their NLR within
3.0, and only 4 cases (13%) showed an increase in NLR by 3.0 or
more. In contrast, of 36 patients in the NLR-high group at 1st-line
who were eligible for 2nd-line treatment, 15 patients (42%)
recovered their NLR within 3.0, but 14 (39%) retained the NLR as
3.0 or more at the initiation of the 2nd-line therapy (P=0.026;
Fig. 3).
Discussion
This study examined whether the NLR value in blood
obtained before initiation of the 1st-, 2nd-, and 3rd-line
chemotherapy could represent a prognostic biomarker for
unresectable GC.
Several predictive and prognostic factors were
recently evaluated in various cancer types to accurately define
patient groups that may benefit from anticancer therapy and predict
their survival. As systemic inflammation plays an essential role in
tumor promotion and progression (7), systemic inflammatory markers,
including C-reactive protein, Platelet-to-Lymphocyte ratio, and
NLR, have attracted attention as putative prognostic markers in
various cancer types (10–12). Peripheral blood NLR, an indicator of
systemic inflammation, is a simple and conventional biomarker that
has been considered a prognostic factor in multiple solid
neoplasms, including GC (9,21). According to a recent report by Zhou
et al, NLR was a significant independent prognostic factor
for progression-free survival (PFS) and OS, and elevated NLR was
associated with poor PFS and OS in unresectable GC patients treated
with first-line chemotherapy (22).
However, in this study, a multivariate analysis revealed that only
3rd-line pretreatment NLR was associated with the clinical
outcomes. Additionally, as shown in Fig
2, the Kaplan-Mayer's curves estimated from the start of the
2nd-line and the 3rd-line demonstrated that the survival curves by
the NLR-low group were significantly better than those by the
NLR-high group. This finding may be due to the NLR-high group at
late lines possessed more patients with poor PS than the NLR-low
groups.
A series of studies measured NLR before initiating
the 1st-line chemotherapy (22,23),
but NLR is not a fixed value and fluctuates with the patient's
condition. Wang et al reported that the OS of unresectable
GC patients whose NLR levels increased after the 1st-line treatment
was nine months, whereas that of patients with decreased NLR was 20
months (24). Changes in NLR
following chemotherapy were reported to predict prognosis in many
types of carcinomas (24–26). Our results also demonstrate that the
mean NLR value significantly decreased in patients who were
transferred to the 2nd-line treatment, whereas it increased in
patients who could not be transferred to the 2nd-line treatment.
Together with another result in this study that, throughout the
1st- to 3rd- line treatment, patients with each NLR-high (>3.0)
value were associated with poor EGOG performance status, NLR might
be an objective surrogate marker for EGOG performance status.
High NLR was associated with poor prognosis at the
3rd-line by multivariate analysis in this study. Thus, we focused
on 3rd-line nivolumab monotherapy patients, consisting of 70% of
patients who received 3rd-line chemotherapy. Nivolumab, a PD-1
inhibitor, was evaluated in a randomized phase III study in GC
patients with >2 prior chemotherapy regimens and significantly
prolonged OS compared with the placebo group (5). However, approximately half of the
patients treated with nivolumab did not receive a survival benefit
compared with the placebo (27). In
recent years, numerous biomarkers, including genomic tumor markers,
neoantigens, the tumor immune microenvironment phenotype, and
liquid biopsy markers, have been evaluated in association with
immune checkpoint inhibitors. Kumagai et al showed that in
cancer patients, including GC, the frequency of PD-1+CD8+T cells
relative to that of PD-1+ regulatory T cells in the tumor
microenvironment predicts the clinical outcome of PD-1 blockade
therapies and is superior to other biomarkers, including PD-L1
expression or tumor mutational burden (28). Recently, Kawakami et al
demonstrated that baseline soluble PD-L1 levels in plasma were
found to be a potential biomarker for predicting the efficacy of
nivolumab in advanced GC (29). In
addition, they showed that the combination of the Glasgow
Prognostic Score (GPS) to soluble PD-L1, a prognostic indicator of
inflammation and nutritional status, may be a more accurate
predictive biomarker. However, such PD-L1 expression soluble PD-L1
are often challenging to measure and introduce in clinical
practice. In contrast, the advantage of peripheral blood NLR is
that it is routinely measured in cancer patients, so this parameter
is readily available to physicians. Ogata et al reported
that in 26 advanced GC patients treated with nivolumab, the median
OS was significantly longer in GC patients with lower NLR values
(30). NLR of patients treated with
3rd-line nivolumab may predict OS. Similarly, in our analysis of
patients treated with 3rd-line nivolumab monotherapy, NLR before
nivolumab induction could predict the prognosis of unresectable GC
patients.
This study has some limitations. It is a
single-center, retrospective study with a small sample size.
Additionally, with concern to biomarkers, we did not examine or
compare well-known prognostic biomarkers for GC patients, such as
GPS (29), Prognostic Nutritional
Index (31), or C-reactive
protein/albumin ratio (32), to
NLR. However, we identified associations between NLR and clinical
outcomes in each chemotherapy line for the first time. Future
prospective analyses will be required to confirm the results. In
conclusion, NLR obtained before the initiation of each chemotherapy
line might be an objective surrogate marker to predict prognosis
for performance status.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Toru Nakai and
Mrs. Tae Yamanishi (Department of Gastroenterological Surgery,
Okayama University Graduate School of Medicine) for their technical
assistance in pathological findings.
Funding
This work was supported by KAKENHI (grant no. 21K07185 to HT and
grant no. 21K19934 to TN) and a Research Project Grant (grant nos.
R01B066 and R02B048) from Kawasaki Medical School.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HT performed all analyses, drafted the manuscript,
and secured funding. MO, SY, and YY treated patients, collected
clinical data and assisted with data interpretation. TN designed
the project, assisted with interpreting all data, secured funding
and drafted the manuscript. TY, KT and AN confirm the authenticity
of all the raw data and performed statistical analyses. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Kawasaki Medical School (IRB no.: 3939-01). The patients provided
written informed consent, and all studies were conducted in
accordance with The Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glimelius B, Ekström K, Hoffman K, Graf W,
Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H
and Heuman R: Randomized comparison between chemotherapy plus best
supportive care with best supportive care in advanced gastric
cancer. Ann Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thuss-Patience PC, Kretzschmar A, Bichev
D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer-a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naive patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diem S, Schmid S, Krapf M, Flatz L, Born
D, Jochum W, Templeton AJ and Früh M: Neutrophil-to-Lymphocyte
ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic
markers in patients with non-small cell lung cancer (NSCLC) treated
with nivolumab. Lung Cancer. 111:176–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami Y, Saito H, Shimizu S, Kono Y,
Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y and Fujiwara Y:
Neutrophil-to-lymphocyte ratio as a prognostic indicator in
patients with unresectable gastric cancer. Anticancer Res.
39:2583–2589. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19:22017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwai N, Okuda T, Sakagami J, Harada T,
Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, et al:
Neutrophil to lymphocyte ratio predicts prognosis in unresectable
pancreatic cancer. Sci Rep. 10:187582020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ciocan A, Bolboacă SD, Drugan C, Ciocan
RA, Graur F and Al Hajjar N: Pattern of calculated inflammation
ratios as prognostic values in patients with colorectal cancer.
Comb Chem High Throughput Screen. 24:1428–1435. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagley SJ, Kothari S, Aggarwal C, Bauml
JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson
JC, et al: Pretreatment neutrophil-to-lymphocyte ratio as a marker
of outcomes in nivolumab-treated patients with advanced
non-small-cell lung cancer. Lung Cancer. 106:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caziuc A, Schlanger D, Amarinei G and
Dindelegan GC: Neutrophils-to-lymphocytes, lymphocytes to-monocytes
and platelets-to-lymphocytes ratios-predictive biomarkers for
response to neoadjuvant chemotherapy in breast cancer. J BUON.
25:182–187. 2020.PubMed/NCBI
|
|
15
|
Valero C, Lee M, Hoen D, Weiss K, Kelly
DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al:
Pretreatment neutrophil-to-lymphocyte ratio and mutational burden
as biomarkers of tumor response to immune checkpoint inhibitors.
Nat Commun. 12:7292021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartley AN, Washington MK, Ventura CB,
Ismaila N, Colasacco C, Benson AB III, Carrato A, Gulley ML, Jain
D, Kakar S, et al: HER2 testing and clinical decision making in
gastroesophageal adenocarcinoma: Guideline from the college of
American pathologists, American society for clinical pathology, and
american society of clinical oncology. Arch Pathol Lab Med.
140:1345–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ock CY, Nam AR, Lee J, Bang JH, Lee KH,
Han SW, Kim TY, Im SA, Kim TY, Bang YJ and Oh DY: Prognostic
implication of antitumor immunity measured by the
neutrophil-lymphocyte ratio and serum cytokines and angiogenic
factors in gastric cancer. Gastric Cancer. 20:254–262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou D, Wu Y, Zhu Y, Lin Z, Yu D and Zhang
T: The prognostic value of neutrophil-to-lymphocyte ratio and
monocyte-to-lymphocyte ratio in metastatic gastric cancer treated
with systemic chemotherapy. J Cancer. 11:4205–4212. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Song M, Fang F, Gao X, Zhang Z and
Wang S: Prediction of chemotherapeutic efficacy using the ratio of
neutrophils to lymphocytes in patients with unresectable or
recurrent gastric cancer. Oncol Lett. 10:2244–2248. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Liu ZY, Xia YY, Zhou C, Shen XM,
Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, et al: Changes in
neutrophil/lymphocyte and platelet/lymphocyte ratios after
chemotherapy correlate with chemotherapy response and prediction of
prognosis in patients with unresectable gastric cancer. Oncol Lett.
10:3411–3418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLellan P, Henriques J, Ksontini F, Doat
S, Hammel P, Desrame J, Trouilloud I, Louvet C, Pietrasz D,
Vernerey D and Bachet JB: Prognostic value of the early change in
neutrophil-to-lymphocyte ratio in metastatic pancreatic
adenocarcinoma. Clinics Res Hepatol Gastroenterol. 45:1015412020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JY, Jung EJ, Kim JM, Lee HS, Kwag SJ,
Park JH, Park T, Jeong SH, Jeong CY and Ju YT: Dynamic changes of
neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio
predicts breast cancer prognosis. BMC Cancer. 20:12062020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen LT, Satoh T, Ryu MH, Chao Y, Kato K,
Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, et al: A phase 3 study
of nivolumab in previously treated advanced gastric or
gastroesophageal junction cancer (ATTRACTION-2): 2-year update
data. Gastric Cancer. 23:510–519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumagai S, Togashi Y, Kamada T, Sugiyama
E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y,
Matsui S, et al: The PD-1 expression balance between effector and
regulatory T cells predicts the clinical efficacy of PD-1 blockade
therapies. Nat Immunol. 21:1346–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami H, Sunakawa Y, Inoue E, Matoba R,
Noda K, Sato T, Suminaka C, Sakamoto Y, Kawabata R, Ishiguro A, et
al: SO-8 Soluble programmed cell death ligand 1 associated with
clinical outcome in gastric cancer patients treated with nivolumab:
Blood based biomarker analysis of DELIVER trial (JACCRO-GC08AR).
Ann Oncol. 33:S3592022. View Article : Google Scholar
|
|
30
|
Ogata T, Satake H, Ogata M, Hatachi Y,
Inoue K, Hamada M and Yasui H: Neutrophil-to-lymphocyte ratio as a
predictive or prognostic factor for gastric cancer treated with
nivolumab: A multicenter retrospective study. Oncotarget.
9:34520–34527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao
J, Ma B and Wang Z: The prognostic nutritional index is a
predictive indicator of prognosis and postoperative complications
in gastric cancer: A meta-analysis. Eur J Surg Oncol. 42:1176–1182.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu J, Liu H, Zeng X, Zhao Y, Jiang D, Lu H
and Qian J: Prognostic and clinicopathological significance of
C-reactive protein/albumin ratio (CAR) in patients with gastric
cancer: A meta-analysis. PLoS One. 16:e02502952021. View Article : Google Scholar : PubMed/NCBI
|