Introduction
Adolescent and young adult (AYA) patients with
cancer have become long-term survivors because of improvements in
early diagnosis and treatment. Five-year relative survival rates of
over 80% have been estimated for the AYA cancer population in the
United States (1). Cancer survivors
were found to have fewer pregnancies across all cancer types, and
the chance of achieving a first pregnancy was also lower with the
use of chemotherapy (2–4).
Chow et al conducted a study of 10,938
survivors and 3,949 siblings (2);
38% of survivors and 62% of siblings reported having or siring a
pregnancy and 83 and 90% of these individuals reported at least one
livebirth, respectively. Multivariable analysis showed a decreased
likelihood of siring or having a pregnancy (male survivors: hazard
ratio 0.63, 95% CI 0.58-0.68; female survivors: 0.87, 0.81-0.94) or
of having a livebirth (male survivors: 0.63, 0.58-0.69; female
survivors: 0.82, 0.76-0.89). A recent population-based cohort study
using universal health care databases in Ontario, Canada compared
14,316 AYA cancer survivors and 60,975 unexposed women (4). The overall risk of an infertility
diagnosis was higher in cancer survivors (relative risk 1.30, 95%
CI 1.23-1.37). Among females with brain, breast, thyroid or
colorectal cancer, leukemia, Hodgkin lymphoma, non-Hodgkin
lymphoma, or melanoma, those with breast or thyroid cancer,
hematological malignancies, or melanoma have a higher risk of a
subsequent infertility diagnosis.
Several studies concerning birth outcomes among AYA
cancer survivors have been reported (1,2,5,6).
Anderson et al conducted a survey using the North Carolina
Central Cancer Registry from January 2000 to December 2013, to
examine 2,598 births to AYA cancer survivors and found that the
survivors had a significantly increased prevalence of preterm
birth, low birth weight, and caesarean delivery, but not
small-for-gestational-age (SGA) birth or low Apgar score (<7)
relative to the comparison cohort of 1,299 (1).
Regarding uterine cervical cancer, about 10,000
women suffer from and 3,000 die from it recent every year and the
peak of onset is in the 30s in Japan. Patients with cervical cancer
increased year by year (7). The
current human papilloma virus (HPV) vaccine can prevent 60~70% of
cervical cancers, and the WHO has confirmed its efficacy and
safety. Even in developed countries in Europe and the United States
and Japan, vaccination has shown that HPV infection rates and the
frequency of precancerous lesions are reduced compared to those who
have not been vaccinated. In Japan, the HPV vaccine has been
routinely administered since April 2013, but due to reports about
severe symptoms after vaccination, active encouragement by the
Ministry of Health, Labour and Welfare has been refrained between
June 2013 and November 2021. Cervical cancer must be included as
AYA cancer because it is the second most common cancer after breast
cancer in 20–39 year olds of Japanese population.
However, birth cohort studies examining pregnancy
and perinatal outcomes among AYA cancer survivors with adjustment
of appropriate covariates using multiple imputation have been
limited. We focused on not only pregnancy outcomes but also infant
outcomes because there has been no birth cohort study that included
infant outcomes up to age of one year.
This study aimed to examine the outcomes of
pregnancy and the postpartum period up to the age of one year in
cancer survivors using data obtained from the Japan Environment and
Children's Study (JECS).
Materials and methods
Study design
In the JECS, pregnant women were recruited between
January, 2011 and March, 2014. Eligibility criteria for expectant
mothers were as follows: that they i) resided at the time of
recruitment in any of the study areas selected by 15 Regional JECS
Centers located countrywide; ii) had an expected delivery date
after August 1, 2011; and iii) were capable of comprehending the
Japanese language and completing the self-administered
questionnaire (8–12). Those residing outside the study
areas, even if they visit the cooperating health care providers
within the study areas, were excluded from the study. Excluded were
those who did not consent to the study protocol and could not be
accessed during the pregnancy period. The participants were able to
withdraw from the study at any time.
The sample size has been calculated in the JECS
protocol. In principle, women completed the questionnaires during
the first and the second/third trimester, and at one month, six
months and one year after delivery. Their medical records were
transcribed by physicians, midwives/nurses, and/or trained Research
Co-coordinators at registration, just after delivery and at one
month after delivery.
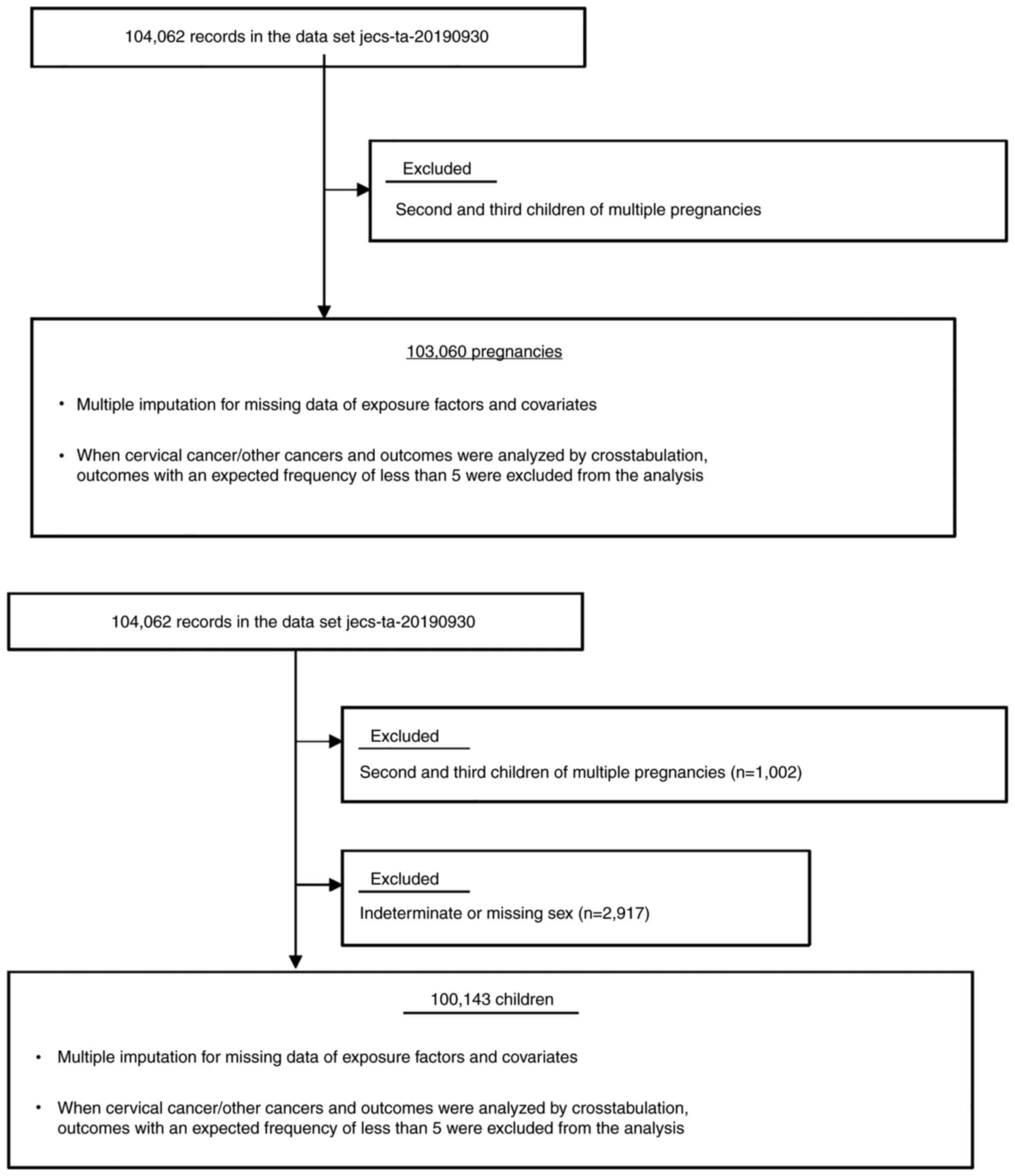
The present study was based on the jecs-ta-20190930
data set, which includes 104,062 registered children (fetuses and
embryos), and was released restrictively to all concerned in
October, 2019. The second and third children of multiple
pregnancies were excluded and these numbered 1,002 (0.96%, Fig. 1). Finally, 103,060 pregnancies were
included in the main analysis. The mean (SD) age and gestational
weeks at registration was 30.7 (5.1) and 14.2 (6.4) weeks.
Regarding children, just 100,143 children were included in the main
analysis because 2,917 children with indeterminate or missing sex
whose sex were not ascertained because of immaturity or congenital
anomalies were excluded (Fig. 1).
The JECS population has been recognized as representative of
pregnant women in Japan (11).
The JECS was approved by the Institutional Review
Board of the Japan National Institute for Environmental Studies
(approval no. 100910001), as well as by the ethics committees of
all participating institutions. This study was conducted with the
approval of the Research Ethics Committee of Nagoya City University
Graduate School of Medical Sciences (approval nos. 554 and 554-2).
Written informed consent was obtained from all participants.
Data collection
The first questionnaire included sociodemographic
characteristics, medical histories, details of all previous
pregnancies and exercise habits. The socioeconomic status was
assessed by the education level and annual household income and
lifestyle details were included in the second questionnaire.
The first medical record transcript included
maternal age, gestational weeks at registration, maternal body
weight, height, conception, and details of all previous pregnancies
(vaginal delivery/caesarian delivery/miscarriage/induced
abortion/stillbirth).
The medical record transcript at delivery included
maternal age, gestational weeks at miscarriage and delivery,
single/multiple pregnancies, live birth/stillbirth,
miscarriage/induced abortion, male/female, birth weight,
vaginal/caesarian delivery, pregnancy complications and perinatal
outcome.
The questionnaire at six months and one year after
birth included the presence/absence of infectious disease in the
infant and vaccination.
Outcomes
The maternal and neonatal outcomes of interest were
preterm birth, placenta previa, premature rupture of the membrane
(PROM), oligohydraminios, hypertensive disorders of pregnancy
(HDP), uterine infection, SGA <10th percentile, congenital
anomalies and caesarean section.
The infant outcomes were upper respiratory tract
inflammation and respiratory syncytial (RS) virus infection at six
months and otitis media, upper and lower respiratory tract
inflammation, diarrhea and vomiting, influenza, exanthema subitum,
herpangina, hand, foot and mouth disease, adenovirus, RS virus
infection and chickenpox at one year.
Exposures and covariates
Exposures included a history of cervical cancer and
other cancers. Potential covariates for maternal and neonatal
outcomes were maternal age at registration (<20, 20–29, 30–39,
≥40 years), body mass index (BMI, <18.5, 18.5-25.0, ≥25.0),
smoking status, income level per year [<2, 2-<4, 4-<6,
6-<8, 8-<10, ≥10 JPYx 1 million, (1 US$=114.38 JPY, 21
October 2021)], pregnancy loss history, the presence/absence of in
vitro fertilization and embryo transfer (IVF-ET), and the
presence/absence of previous deliveries.
Covariates for six months were maternal age at
registration, maternal BMI before pregnancy, maternal smoking
status, household income, pregnancy loss history, the
presence/absence of IVF-ET, the presence/absence of previous
deliveries, sex of the child, maternal allergy and ear, nose, and
throat disease, congenital diseases of the child, feeding method at
one month of age, the number of family members living with the
child, the month of questionnaire entry at six months of age, the
period of breast milk intake (until six month of age), the period
of artificial nutrition intake (until six months), attendance at a
nursery facility (at six months), influenza virus vaccination,
rotavirus vaccination, and Haemophilus influenzae type b (Hib) and
pneumococcal vaccination.
To covariates at one year, we added the month of
questionnaire entry at one year, the period of breast milk intake
(until one year), the period of artificial nutrition intake (until
one year), attendance at a nursery facility (at one year), maternal
working pattern, influenza virus vaccination, rotavirus
vaccination, Hib and pneumococcal vaccination, palivizumab
injection, and chickenpox vaccination to the covariates for
six-months of age.
Statistical analysis
The maternal and infant demographic characteristics
of the participants were shown in relation to discrete data.
χ2 tests were performed to compare the association
between the history of cancer and each variable shown as nominal
variables. One-way ANOVA was performed when we compared mean values
between the history of cancer and each variable shown as numerical
variables. If we would obtain significant ANOVA results, Tukey's
pairwise post hoc tests between variables were conducted. Binominal
logistic regression analyses were performed by adding all the
covariates to calculate the adjusted ORs (aORs) for association
between the history of cancer and each outcome. Traditionally,
univariate analyses were performed first to find out the
significant confounders for adjusting confounding effect for
subsequent multivariate analysis. As explained by Vandenbroucke
et al, such procedure is not recommended now (13). Significance tests of univariate
analysis should be avoided as a criterion for selecting confounders
to adjust for. The STROBE statement gives that P-values are not an
appropriate criterion for selecting which confounders to adjust for
in analysis; even small differences in a confounder that has a
strong effect on the outcome can be important.
Since missing data can potentially undermine the
scientific credibility of causal conclusions, we applied a multiple
imputation method to reduce the potential non-response bias created
by the missing data and to improve the precision of the estimates
when calculating the aORs. When cervical cancer/other cancers and
outcomes were analyzed by crosstabulation, outcomes with an
expected frequency of less than 5 were excluded from the analysis.
To prevent multiple comparisons possibly yielding false positive
findings, we adopted the Benjamini-Hochberg method and assessed
statistical significance by obtaining the q-values adjusted for a
false discovery rate.
χ2 test was performed to compare the
association between the mode of delivery and each variable and
Student's t-tests were used for analyzing mean data.
All calculations were conducted using SPSS version
26 (IBM Corp., Japan), and P<0.05 was considered to indicate a
statistically significant difference.
Results
Of 99,816 participants (3,244 were missing), 1,102
(1.1%) had a cancer history including 56 patients with cancer
during pregnancy (Table I). Of
these, 812 participants (0.8%) had a history of cervical cancer and
290 (0.3%) had other cancers. Fifty seven (0.057%) had breast
cancer, 8 (0.008%) had endometrial cancer, 4 (0.004%) had stomach
cancer, 11 (0.01%) had colon cancer, 40 (0.04%) had a blood cancer,
and 176 (0.17%) had other cancers.
 | Table I.Demographic characteristics of
mothers. |
Table I.
Demographic characteristics of
mothers.
|
|
| History of
cancer |
|
|---|
|
|
|
|
|
|---|
| Variable | Total n=99,816 n
(%) | Cervicala n=812 n (%) | Othersb n=290 n (%) | Nothing n=98,714 n
(%) | P-valuec |
|---|
| Age, years |
|
|
|
| <0.001 |
|
<20 | 1,145 (1.1) | 6 (0.7) | 0 (0.0) | 1139 (1.2) |
|
|
20-29 | 39,814 (39.9) | 249 (30.7) | 66 (22.8) | 39,499 (40.0) |
|
|
30-39 | 55,278 (55.4) | 518 (63.8) | 195 (67.2) | 54,565 (55.3) |
|
| ≥40 | 3,500 (3.5) | 39 (4.8) | 29 (10.0) | 3,432 (3.5) |
|
|
Missing | 79 (0.1) | 0 (0.0) | 0 (0.0) | 79 (0.1) |
|
| Pregnancy body mass
index, kg/m2 |
|
|
|
| 0.203 |
|
<18.5 | 16,147 (16.2) | 150 (18.5) | 45 (15.5) | 15,952 (16.2) |
|
|
18.5-24.9 | 72,897 (73.0) | 564 (69.5) | 217 (74.8) | 72,116 (73.1) |
|
|
≥25.0 | 10,724 (10.7) | 98 (12.1) | 28 (9.7) | 10,598 (10.7) |
|
|
Missing | 48 (0.0) | 0 (0.0) | 0 (0.0) | 48 (0.0) |
|
| Smoking status |
|
|
|
| <0.001 |
| Never
smoker | 55,891 (56.0) | 301 (37.1) | 169 (58.3) | 55,421 (56.1) |
|
|
Ex-smoker, stopped before
learning of pregnancy | 23,162 (23.2) | 262 (32.3) | 73 (25.2) | 22,827 (23.1) |
|
|
Ex-smoker, stopped on
awareness of pregnancy | 13,258 (13.3) | 153 (18.8) | 32 (11.0) | 13,073 (13.2) |
|
| Current
smoker | 4,429 (4.4) | 72 (8.9) | 6 (2.1) | 4,351 (4.4) |
|
|
Missing | 3,076 (3.1) | 24 (3.0) | 10 (3.4) | 3,042 (3.1) |
|
| Annual household
income, one million Japanese yen |
|
|
|
| 0.018 |
|
<2 | 5,132 (5.1) | 63 (7.8) | 10 (3.4) | 5,059 (5.1) |
|
|
2-<4 | 31,324 (31.4) | 272 (33.5) | 80 (27.6) | 30,972 (31.4) |
|
|
4-<6 | 29,957 (30.0) | 228 (28.1) | 91 (31.4) | 29,638 (30.0) |
|
|
6-<8 | 14,409 (14.4) | 114 (14.0) | 53 (18.3) | 14,242 (14.4) |
|
|
8-<10 | 5,913 (5.9) | 44 (5.4) | 22 (7.6) | 5,847 (5.9) |
|
|
≥10 | 3,873 (3.9) | 26 (3.2) | 11 (3.8) | 3,836 (3.9) |
|
|
Missing | 9,208 (9.2) | 65 (8.0) | 23 (7.9) | 9,120 (9.2) |
|
| Pregnancy loss
history |
|
|
|
| <0.001 |
| 0 | 76,245 (76.4) | 576 (70.9) | 216 (74.5) | 75,453 (76.4) |
|
| 1 | 17,572 (17.6) | 163 (20.1) | 53 (18.3) | 17,356 (17.6) |
|
| 2 | 3,891 (3.9) | 38 (4.7) | 14 (4.8) | 3,839 (3.9) |
|
| ≥3 | 1,135 (1.1) | 21 (2.6) | 1 (0.3) | 1,113 (1.1) |
|
|
Missing | 973 (1.0) | 14 (1.7) | 6 (2.1) | 953 (1.0) |
|
| In vitro
fertilization and embryo transfer |
|
|
|
| <0.001 |
| No | 96,639 (96.8) | 763 (94.0) | 268 (92.4) | 95,608 (96.9) |
|
|
Yes | 3,177 (3.2) | 49 (6.0) | 22 (7.6) | 3,106 (3.1) |
|
|
Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Previous live
birth |
|
|
|
| 0.018 |
| No | 39,299 (39.4) | 286 (35.2) | 116 (40.0) | 38,897 (39.4) |
|
|
Yes | 58,116 (58.2) | 519 (63.9) | 163 (56.2) | 57,434 (58.2) |
|
|
Missing | 2,401 (2.4) | 7 (0.9) | 11 (3.8) | 2,383 (2.4) |
|
| Maternal allergy
and ear, nose, and throat disease |
|
|
|
| 0.040 |
| No | 42,109 (42.2) | 343 (42.2) | 101 (34.8) | 41,665 (42.2) |
|
|
Yes | 57,707 (57.8) | 469 (57.8) | 189 (65.2) | 57,049 (57.8) |
|
|
Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| The month of
questionnaire entry at 6 months of age |
|
|
|
| 0.963 |
| Spring
(March to May) | 25,016 (25.1) | 203 (25.0) | 67 (23.1) | 24,746 (25.1) |
|
| Summer
(June to August) | 21,339 (21.4) | 174 (21.4) | 63 (21.7) | 21,102 (21.4) |
|
| Autumn
(September to November) | 20,777 (20.8) | 157 (19.3) | 63 (21.7) | 20,557 (20.8) |
|
| Winter
(December to February) | 24,844 (24.9) | 206 (25.4) | 73 (25.2) | 24,565 (24.9) |
|
|
Missing | 7,840 (7.9) | 72 (8.9) | 24 (8.3) | 7,744 (7.8) |
|
| The month of
questionnaire entry at 1 year of age |
|
|
|
| 0.950 |
| Spring
(March to May) | 20,467 (20.5) | 166 (20.4) | 59 (20.3) | 20,242 (20.5) |
|
| Summer
(June to August) | 23,897 (23.9) | 203 (25.0) | 65 (22.4) | 23,629 (23.9) |
|
| Autumn
(September to November) | 24,319 (24.4) | 184 (22.7) | 66 (22.8) | 24,069 (24.4) |
|
| Winter
(December to February) | 20,434 (20.5) | 159 (19.6) | 58 (20.0) | 20,217 (20.5) |
|
|
Missing | 10,699 (10.7) | 100 (12.3) | 42 (14.5) | 10,557 (10.7) |
|
| Current employment
status (when the child is 1-year-old) |
|
|
|
| <0.001 |
|
Full-time homemaker | 42,464 (42.5) | 366 (45.1) | 122 (42.1) | 41,976 (42.5) |
|
|
Unemployed | 3,231 (3.2) | 30 (3.7) | 7 (2.4) | 3,194 (3.2) |
|
|
Student | 202 (0.2) | 2 (0.2) | 1 (0.3) | 199 (0.2) |
|
|
Full-time employee | 24,578 (24.6) | 143 (17.6) | 69 (23.8) | 24,366 (24.7) |
|
|
Part-time employee | 12,294 (12.3) | 105 (12.9) | 25 (8.6) | 12,164 (12.3) |
|
|
Self-employed | 3,238 (3.2) | 46 (5.7) | 15 (5.2) | 3,177 (3.2) |
|
| Part
time work at home | 600 (0.6) | 5 (0.6) | 0 (0.0) | 595 (0.6) |
|
|
Other | 1,235 (1.2) | 8 (1.0) | 3 (1.0) | 1,224 (1.2) |
|
|
Missing | 11,974 (12.0) | 107 (13.2) | 48 (16.6) | 11,819 (12.0) |
|
Characteristics of the 99,816 pregnant women are
shown in Table I. Maternal age,
smoking status, income level, pregnancy loss history, the
presence/absence of IVF-ET, previous deliveries, and employment
status were significantly associated with cervical cancer, other
cancers and no history of any cancer. These variables were analyzed
for covariates of maternal outcomes.
Characteristics of the 99,816 infants and the
association between each variable and the cancer history are shown
in Table II. The period of both
breast milk and artificial nutrition intake (both in months until
six months and one year of age) and palivizumab injection at one
year of age were significantly associated among three groups. These
variables were analyzed for covariates of infant outcomes.
 | Table II.Demographic characteristics of
infants. |
Table II.
Demographic characteristics of
infants.
|
|
| History of
cancer |
|
|---|
|
|
|
|
|
|---|
| Variable | Total n=98,627 n
(%) |
Cervicala n=804 n (%) | Othersb n=282 n (%) | Nothing n=97,541 n
(%) | P-value |
|---|
| Sex |
|
|
|
| 0.593c |
|
Male | 50,620 (51.3) | 399 (49.6) | 142 (50.4) | 50,079 (51.3) |
|
|
Female | 48,007 (48.7) | 405 (50.4) | 140 (49.6) | 47,462 (48.7) |
|
|
Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Congenital
diseases |
|
|
|
| 0.427c |
| No | 86,507 (87.7) | 687 (85.4) | 249 (88.3) | 85,571 (87.7) |
|
|
Yes | 8,877 (9.0) | 82 (10.2) | 25 (8.9) | 8,770 (9.0) |
|
|
Missing | 3,243 (3.3) | 35 (4.4) | 8 (2.8) | 3,200 (3.3) |
|
| Feeding method at 1
month |
|
|
|
| 0.056c |
|
Breastfeeding only | 40,449 (41.0) | 311 (38.7) | 96 (34.0) | 40,042 (41.1) |
|
| Mixed
feeding | 54,159 (54.9) | 447 (55.6) | 177 (62.8) | 53,535 (54.9) |
|
| Infant
formula only | 1,427 (1.4) | 16 (2.0) | 4 (1.4) | 1,407 (1.4) |
|
|
Missing | 2,592 (2.6) | 30 (3.7) | 5 (1.8) | 2,557 (2.6) |
|
| Number of family
members currently living together with the child |
|
|
|
| 0.385c |
| 0 | 135 (0.1) | 1 (0.1) | 1 (0.4) | 133 (0.1) |
|
| 1 | 1,598 (1.6) | 18 (2.2) | 5 (1.8) | 1,575 (1.6) |
|
| 2 | 30,683 (31.1) | 225 (28.0) | 95 (33.7) | 30,363 (31.1) |
|
| ≥3 | 59,558 (60.4) | 496 (61.7) | 165 (58.5) | 58,897 (60.4) |
|
|
Missing | 6,626 (6.7) | 64 (8.0) | 16 (5.7) | 6,546 (6.7) |
|
| The period of
breast milk intake, months until 6 months |
|
|
|
| 0.000d; 0.000e; 0.004f; 0.792g |
| Mean ±
SD | 5.17±1.6 | 4.94±1.8 | 4.86±1.9 | 5.18±1.6 |
|
|
Missing | 6,063 | 64 | 16 | 6,573 |
|
| The period of
artificial nutrition intake, months until 6 months |
|
|
|
| 0.000d; 0.003e; 0.002f; 0.443g |
| Mean ±
SD | 2.61±2.6 | 2.92±2.6) | 3.14±2.6 | 2.61±2.6 |
|
|
Missing | 6,063 | 64 | 16 | 6,573 |
|
| Attending a
childcare facility at six months, daycare center/nursery |
|
|
|
| 0.060c |
|
Yes | 6,418 (6.5) | 67 (8.3) | 15 (5.3) | 6,336 (6.5) |
|
| No | 85,365 (86.6) | 672 (83.6) | 250 (88.7) | 84,443 (86.6) |
|
|
Missing | 6,844 (6.9) | 65 (8.1) | 17 (6.0) | 6,762 (6.9) |
|
| Influenza virus
vaccination, at 6 months |
|
|
|
| 0.366c |
| No | 90,059 (91.3) | 730 (90.8) | 261 (92.6) | 89,068 (91.3) |
|
|
Yes | 1,915 (1.9) | 10 (1.2) | 5 (1.8) | 1,900 (1.9) |
|
|
Missing | 6,653 (6.7) | 64 (8.0) | 16 (5.7) | 6,573 (6.7) |
|
| Rotavirus
vaccination, at 6 months |
|
|
|
| 0.284c |
| No | 52,051 (52.8) | 431 (53.6) | 140 (49.6) | 51,480 (52.8) |
|
|
Yes | 39,923 (40.5) | 309 (38.4) | 126 (44.7) | 39,488 (40.5) |
|
|
Missing | 6,653 (6.7) | 64 (8.0) | 16 (5.7) | 6,573 (6.7) |
|
| Haemophilus
influenzae type b vaccination, at 6 months |
|
|
|
| 0.625c |
| No | 6,032 (6.1) | 55 (6.8) | 17 (6.0) | 5,960 (6.1) |
|
|
Yes | 85,942 (87.1) | 685 (85.2) | 249 (88.3) | 85,008 (87.2) |
|
|
Missing | 6,653 (6.7) | 64 (8.0) | 16 (5.7) | 6,573 (6.7) |
|
| Pneumococcal
vaccination, at 6 months |
|
|
|
| 0.927c |
| No | 6,763 (6.9) | 57 (7.1) | 19 (6.7) | 6,687 (6.9) |
|
|
Yes | 85,211 (86.4) | 683 (85.0) | 247 (87.6) | 84,281 (86.4) |
|
|
Missing | 6,653 (6.7) | 64 (8.0) | 16 (5.7) | 6,573 (6.7) |
|
| The period of
breast milk intake, months until 1 year |
|
|
|
| 0.000d; 0.000e; 0.016f; 0.991g |
| Mean ±
SD | 9.35±3.9 | 8.71±4.2 | 8.68±4.3 | 9.36±3.9 |
|
|
Missing | 8,967 | 92 | 34 | 9,386 |
|
| The period of
artificial nutrition intake, months until 1 year |
|
|
|
| 0.000d;
0.001e;
0.015f;
0.833g |
| Mean ±
SD | 5.47±5.1 | 6.14±5.2 | 6.36±5.3 | 5.46±5.1 |
|
|
Missing | 8,967 | 92 | 34 | 9,386 |
|
| Attendance at a
childcare facility at 1 year, daycare center/nursery |
|
|
|
| 0.910c |
|
Yes | 23,786 (24.1) | 193 (24.0) | 64 (22.7) | 23,529 (24.1) |
|
| No | 64,937 (65.8) | 514 (63.9) | 183 (64.9) | 64,240 (65.9) |
|
|
Missing | 9,904 (10.0) | 97 (12.1) | 35 (12.4) | 9,772 (10.0) |
|
| Influenza virus
vaccination, at 1 year |
|
|
|
| 0.991c |
| No | 73,063 (74.1) | 583 (72.5) | 204 (72.3) | 72,276 (74.1) |
|
|
Yes | 16,052 (16.3) | 129 (16.0) | 44 (15.6) | 15,879 (16.3) |
|
|
Missing | 9,512 (9.6) | 92 (11.4) | 34 (12.1) | 9,386 (9.6) |
|
| Rotavirus
vaccination, at 1 year |
|
|
|
| 0.152c |
| No | 50,505 (51.2) | 415 (51.6) | 127 (45.0) | 49,963 (51.2) |
|
|
Yes | 38,610 (39.1) | 297 (36.9) | 121 (42.9) | 38,192 (39.2) |
|
|
Missing | 9,512 (9.6) | 92 (11.4) | 34 (12.1) | 9,386 (9.6) |
|
| Haemophilus
influenzae type b vaccination, at 1 year |
|
|
|
| 0.238c |
| No | 4,520 (4.6) | 45 (5.6) | 10 (3.5) | 4,465 (4.6) |
|
|
Yes | 84,595 (85.8) | 667 (83.0) | 238 (84.4) | 83,690 (85.8) |
|
|
Missing | 9,512 (9.6) | 92 (11.4) | 34 (12.1) | 9,386 (9.6) |
|
| Pneumococcal
vaccination, at 1 year |
|
|
|
| 0.671c |
| No | 6,086 (6.2) | 52 (6.5) | 14 (5.0) | 6,020 (6.2) |
|
|
Yes | 83,029 (84.2) | 660 (82.1) | 234 (83.0) | 82,135 (84.2) |
|
|
Missing | 9,512 (9.6) | 92 (11.4) | 34 (12.1) | 9,386 (9.6) |
|
| Chickenpox
vaccination, at 1 year |
|
|
|
| 0.928c |
| No | 84,793 (86.0) | 676 (84.1) | 235 (83.3) | 83,882 (86.0) |
|
|
Yes | 4,322 (4.4) | 36 (4.5) | 13 (4.6) | 4,273 (4.4) |
|
|
Missing | 9,512 (9.6) | 92 (11.4) | 34 (12.1) | 9,386 (9.6) |
|
| Palivizumab
injection, at 1 year |
|
|
|
|
<0.001c |
|
Yes | 2,624 (2.7) | 51 (6.3.0) | 8 (2.8) | 2,565 (2.6) |
|
| No | 83,318 (84.5) | 632 (78.6) | 231 (81.9) | 82,455 (84.5) |
|
|
Missing | 12,685 (12.9) | 121 (15.0) | 43 (15.2) | 12,521 (12.8) |
|
Cervical cancer, blood cancer and other cancers were
associated with caesarean section (Table SI). Maternal age, BMI, smoking
status, income, the number of previous pregnancy losses, the
presence of IVF-ET, the month of the questionnaire entry at six
months and one year of age were also associated with caesarean
section.
Feeding by infant formula at one month, a short
period of breast feeding and a long period of artificial nutrition
until six months and one year, and a palivizumab injection were
associated with caesarean section (Table SII).
Among the 812 women with a history of cervical
cancer, a preterm birth at both <34 and 37 weeks' gestation and
PROM increased significantly (Table
III). aORs using multiple imputation were as follows: 3.25 (95%
CI, 2.31 to 4.57, q=0.00) for preterm birth <34 weeks'
gestation, 2.82 (2.31 to 3.44, q=0.00) for preterm birth <37
weeks' gestation, and 1.67 (1.36 to 2.06, q=0.00) for PROM. History
of cervical cancer did not increase the risk of congenital
anomalies.
 | Table III.The association of maternal and
neonatal outcomes with maternal cancer histories. |
Table III.
The association of maternal and
neonatal outcomes with maternal cancer histories.
|
| History of cervical
cancer (n=812) | History of other
cancer (n=290) |
|---|
|
|
|
|
|---|
| Maternal and
neonatal outcomeb | Crude ORs (95%
CI) | P-value | Adjusted
ORsc (95% CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value | Crude ORs (95%
CI) | P-value | Adjusted
ORsc (95% CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value |
|---|
| Preterm birth
<34 weeks' | 3.78 | 0.00 | 4.05 | 0.00a | 3.25 | 0.00a |
|
|
|
|
|
|
|
gestationd | (2.69–5.30) |
| (2.81–5.82) |
| (2.31–4.57) |
|
|
|
|
|
|
|
| Preterm birth
<37 weeks’ | 3.07 | 0.00 | 2.87 | 0.00a | 2.82 | 0.00a | 1.37 | 0.17 | 1.38 | 0.53 | 1.27 | 0.53 |
|
gestationd | (2.52–3.75) |
| (2.31–3.56) |
| (2.31–3.44) |
| (0.87–2.17) |
| (0.85–2.24) |
| (0.81–2.01) |
|
| Placenta
previad | 1.32 | 0.47 | 1.30 | 0.69 | 1.15 | 0.90 |
|
|
|
|
|
|
|
| (0.62–2.78) |
| (0.61–2.76) |
| (0.54–2.44) |
|
|
|
|
|
|
|
| Premature
ruptured | 1.62 | 0.00 | 1.62 | 0.00a | 1.67 | 0.00a | 0.87 | 0.52 | 0.83 | 0.67 | 0.81 | 0.58 |
|
| (1.32–1.99) |
| (1.30–2.01) |
| (1.36–2.06) |
| (0.56–1.34) |
| (0.52–1.31) |
| (0.52–1.26) |
|
|
Oligohydramniosd | 1.08 | 0.81 | 0.87 | 0.94 | 1.06 | 0.97 |
|
|
|
|
|
|
|
| (0.59–1.95) |
| (0.43–1.76) |
| (0.58–1.93) |
|
|
|
|
|
|
|
| Mild
hypertensive | 0.81 | 0.41 | 0.71 | 0.55 | 0.75 | 0.53 | 1.70 | 0.08 | 1.40 | 0.67 | 1.52 | 0.50 |
| disorders of
pregnancyd | (0.48–1.34) |
| (0.41–1.23) |
| (0.45–1.26) |
| (0.93–3.12) |
| (0.71–2.75) |
| (0.82–2.81) |
|
| Severe
hypertensive | 1.02 | 0.95 | 0.94 | 0.99 | 0.96 | 0.97 |
|
|
|
|
|
|
| disorders of
pregnancyd | (0.51–2.06) |
| (0.45–2.00) |
| (0.47–1.93) |
|
|
|
|
|
|
|
| Uterine
infection | 1.42 | 0.36 | 1.43 | 0.67 | 1.34 | 0.69 |
|
|
|
|
|
|
|
| (0.67–3.00) |
| (0.63–3.22) |
| (0.63–2.85) |
|
|
|
|
|
|
|
| Small for
gestational | 0.73 | 0.02 | 0.74 | 0.11 | 0.73 | 0.08 | 1.02 | 0.91 | 0.99 | 1.00 | 1.02 | 0.97 |
| age
(<10%)d | (0.56–0.95) |
| (0.56–0.97) |
| (0.56–0.95) |
| (0.70–1.50) |
| (0.65–1.50) |
| (0.70–1.51) |
|
| Physical
anomalies | 1.04 | 0.76 | 1.03 | 0.99 | 1.00 | 0.99 | 1.37 | 0.14 | 1.44 | 0.30 | 1.28 | 0.53 |
| at birth | (0.79–1.38) |
| (0.76–1.38) |
| (0.76–1.33) |
| (0.90–2.09) |
| (0.94–2.22) |
| (0.84–1.96) |
|
| Physical anomalies
at | 1.16 | 0.19 | 1.10 | 0.67 | 1.14 | 0.53 | 0.98 | 0.92 | 0.96 | 0.99 | 0.94 | 0.91 |
| 1 month | (0.93–1.47) |
| (0.86–1.40) |
| (0.91–1.44) |
| (0.65–1.48) |
| (0.62–1.48) |
| (0.62–1.41) |
|
| Caesarean | 1.32 | 0.00 | 1.25 | 0.06 | 1.19 | 0.13 | 1.64 | 0.00 | 1.44 | 0.06 | 1.43 | 0.04a |
|
| (1.12–1.55) |
| (1.05–1.49) |
| (1.01–1.41) |
| (1.27–2.13) |
| (1.09–1.91) |
| (1.10–1.87) |
|
In the 290 women with a history of other cancers,
the incidence of caesarean section was significantly higher (1.43,
1.10 to 1.17, q=0.04).
The associations of infant outcomes with maternal
cancer histories are shown in Table
IV. Lower respiratory tract inflammation in one-year-old
infants born by vaginal delivery, but not caesarean section,
increased significantly in cases with a history of cervical cancer
(1.77, 1.33 to 2.36, q=0.00).
 | Table IV.The associations of infant outcomes
with maternal cancer histories. |
Table IV.
The associations of infant outcomes
with maternal cancer histories.
|
|
History of
cervical cancer |
History of
other cancer |
|---|
|
|
|
|
|---|
|
| Transvaginal
(n=609) | Caesarean
(n=192) | Transvaginal
(n=202) | Caesarean
(n=80) |
|---|
|
|
|
|
|
|
|---|
|
Outcomesb | Crude ORs (95%
CI) | P-value | Adjusted ORs (95%
CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value | Crude ORs (95%
CI) | P-value | Adjusted ORs (95%
CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value | Crude ORs (95%
CI) | P-value | Adjusted ORs (95%
CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value | Crude ORs (95%
CI) | P-value | Adjusted ORs (95%
CI) | q-value | Adjusted ORs using
multiple imputation (95% CI) | q-value |
|---|
| At
6-month-oldc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Otitis
media | 0.89 | 0.70 | 0.69 | 0.89 | 0.82 | 0.95 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
| (0.50–1.58) |
| (0.35–1.34) |
| (0.46–1.47) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Upper
respiratory | 1.12 | 0.35 | 1.14 | 0.89 | 1.09 | 0.93 | 1.05 | 0.80 | 0.99 | 1.00 | 0.97 | 1.00 | 1.07 | 0.75 | 0.96 | 1.00 | 1.01 | 1.00 | 1.18 | 0.61 | 1.13 | 1.00 | 1.14 | 1.00 |
|
inflammation | (0.89–1.41) |
| (0.89–1.46) |
| (0.86–1.38) |
| (0.69–1.60) |
| (0.63–1.55) |
| (0.63–1.49) |
| (0.71–1.59) |
| (0.62–1.50) |
| (0.67–1.52) |
| (0.63–2.19) |
| (0.56–2.25) |
| (0.61–2.15) |
|
|
Respiratory | 0.89 | 0.58 | 0.96 | 1.00 | 0.83 | 0.90 | 1.70 | 0.06 | 1.63 | 0.71 | 1.59 | 0.75 | 0.87 | 0.69 | 1.02 | 1.00 | 0.93 | 1.00 | - | - | - | - | - | - |
|
syncytial virus | (0.59–1.34) |
| (0.63–1.46) |
| (0.55–1.26) |
| (0.98–2.95) |
| (0.91–2.93) |
| (0.91–2.79) |
| (0.43–1.76) |
| (0.50–2.1) |
| (0.46–1.91) |
|
|
|
|
|
|
|
|
Exanthema | 1.04 | 0.90 | 0.89 | 1.00 | 0.96 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
subitum | (0.57–1.89) |
| (0.46–1.74) |
| (0.53–1.75) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Influenza | 1.40 | 0.27 | 0.90 | 1.00 | 1.27 | 0.93 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
| (0.77–2.55) |
| (0.42–1.92) |
| (0.69–2.32) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diarrhea and | 1.58 | 0.11 | 1.57 | 0.71 | 1.36 | 0.90 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
vomiting
(gastroenteritis) | (0.91–2.74) |
| (0.90–2.76) |
| (0.78–2.38) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| At
1-year-oldd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Otitis
media | 0.91 | 0.50 | 0.83 | 0.89 | 0.89 | 0.93 | 1.10 | 0.67 | 1.11 | 1.00 | 1.05 | 1.00 | 0.83 | 0.46 | 0.88 | 1.00 | 0.87 | 1.00 | 1.24 | 0.53 | 1.89 | 0.71 | 1.45 | 0.90 |
|
| (0.69–1.20) |
| (0.60–1.15) |
| (0.67–1.18) |
| (0.70–1.73) |
| (0.67–1.83) |
| (0.65–1.68) |
| (0.50–1.37) |
| (0.51–1.53) |
| (0.52–1.45) |
| (0.63–2.42) |
| (0.88–4.02) |
| (0.72–2.92) |
|
| Upper
respiratory | 0.99 | 0.96 | 1.10 | 0.92 | 1.02 | 1.00 | 1.31 | 0.11 | 1.08 | 1.00 | 1.31 | 0.75 | 1.27 | 0.15 | 1.22 | 0.89 | 1.22 | 0.90 | 1.80 | 0.02 | 1.77 | 0.71 | 1.72 | 0.41 |
|
inflammation | (0.81–1.22) |
| (0.88–1.38) |
| (0.83–1.25) |
| (0.94–1.82) |
| (0.73–1.59) |
| (0.93–1.85) |
| (0.91–1.77) |
| (0.84–1.75) |
| (0.87–1.71) |
| (1.10–2.93) |
| (0.99–3.16) |
| (1.04–2.85) |
|
| Lower
respiratory | 1.84 | 0.00 | 1.74 | 0.02a | 1.77 | 0.00a | 1.10 | 0.76 | 1.00 | 1.00 | 1.01 | 1.00 | 0.73 | 0.39 | 0.64 | 0.89 | 0.77 | 0.93 | - | - | - | - | - | - |
|
inflammation | (1.40–2.43) |
| (1.27–2.39) |
| (1.33–2.36) |
| (0.61–1.98) |
| (0.51–1.93) |
| (0.55–1.85) |
| (0.36–1.49) |
| (0.28–1.47) |
| (0.38–1.58) |
|
|
|
|
|
|
|
|
Diarrhea and | 0.86 | 0.35 | 0.79 | 0.89 | 0.80 | 0.75 | 1.22 | 0.40 | 0.91 | 1.00 | 1.14 | 1.00 | 1.05 | 0.84 | 1.03 | 1.00 | 1.10 | 1.00 | 1.53 | 0.21 | 0.88 | 1.00 | 1.41 | 1.00 |
|
vomiting
(gastroenteritis) | (0.63–1.18) |
| (0.56–1.12) |
| (0.58–1.10) |
| (0.76–1.95) |
| (0.52–1.61) |
| (0.70–1.84) |
| (0.64–1.74) |
| (0.59–1.82) |
| (0.66–1.84) |
| (0.78–3.00) |
| (0.34–2.28) |
| (0.71–2.83) |
|
|
Influenza | 1.26 | 0.18 | 1.11 | 1.00 | 1.17 | 0.90 | 1.44 | 0.21 | 1.04 | 1.00 | 1.32 | 0.90 | 1.05 | 0.88 | 0.96 | 1.00 | 1.06 | 1.00 | - | - | - | - | - | - |
|
| (0.90–1.77) |
| (0.75– |
| (0.83–1.66) |
| (0.82–2.55) |
| (0.52–2.08) |
| (0.74–2.36) |
| (0.55–1.99) |
| (0.47–1.98) |
| (0.56–2.03) |
|
|
|
|
|
|
|
|
Exanthema | 1.00 | 0.97 | 1.03 | 1.00 | 1.00 | 1.00 | 1.08 | 0.70 | 0.97 | 1.00 | 1.04 | 1.00 | 1.08 | 0.68 | 1.26 | 0.89 | 1.18 | 0.90 | 1.55 | 0.10 | 1.69 | 0.71 | 1.99 | 0.25 |
|
subitum | (0.81–1.24) |
| (0.81–1.30) |
| (0.81–1.24) |
| (0.74–1.56) |
| (0.64–1.48) |
| (0.71–1.52) |
| (0.75–1.54) |
| (0.85–1.86) |
| (0.82–1.71) |
| (0.92–2.63) |
| (0.88–3.25) |
| (1.15–3.45) |
|
|
Herpangina | 1.18 | 0.44 | 1.12 | 1.00 | 1.18 | 0.93 | 1.58 | 0.16 | 2.01 | 0.71 | 1.63 | 0.75 | 0.58 | 0.28 | 0.70 | 1.00 | 0.59 | 0.90 | - | - | - | - | - | - |
|
| (0.78–1.78) |
| (0.70–1.79) |
| (0.78–1.8) |
| (0.83–3.00) |
| (1.03–3.93) |
| (0.84–3.16) |
| (0.22–1.57) |
| (0.26–1.91) |
| (0.22–1.61) |
|
|
|
|
|
|
|
| Hand,
foot and | 0.80 | 0.23 | 0.70 | 0.71 | 0.79 | 0.90 | 0.33 | 0.03 | 0.41 | 0.71 | 0.33 | 0.41 | 0.98 | 0.95 | 1.11 | 1.00 | 1.02 | 1.00 | - | - | - | - | - | - |
| mouth
disease | (0.55–1.15) |
| (0.45–1.08) |
| (0.54–1.16) |
| (0.12–0.90) |
| (0.15–1.11) |
| (0.12–0.9) |
| (0.55–1.76) |
| (0.59–2.07) |
| (0.56–1.86) |
|
|
|
|
|
|
|
|
Adenovirus | 1.06 | 0.82 | 1.25 | 0.92 | 1.01 | 1.00 | 0.77 | 0.60 | 0.56 | 0.92 | 0.64 | 1.00 | 0.20 | 0.10 | 0.25 | 0.89 | 0.21 | 0.75 | - | - | - | - | - | - |
|
| (0.64–1.75) |
| (0.75–2.08) |
| (0.60–1.67) |
| (0.28–2.08) |
| (0.18–1.81) |
| (0.23–1.78) |
| (0.03–1.40) |
| (0.03–1.77) |
| (0.03–1.53) |
|
|
|
|
|
|
|
|
Respiratory | 0.84 | 0.27 | 0.75 | 0.71 | 0.79 | 0.75 | 1.40 | 0.14 | 1.31 | 0.89 | 1.37 | 0.75 | 0.92 | 0.74 | 1.05 | 1.00 | 0.97 | 1.00 | 1.00 | 0.99 | 1.47 | 0.92 | 1.06 | 1.00 |
|
syncytial virus | (0.62–1.14) |
| (0.53–1.06) |
| (0.58–1.08) |
| (0.90–2.17) |
| (0.79–2.16) |
| (0.87–2.17) |
| (0.55–1.53) |
| (0.61–1.80) |
| (0.58–1.64) |
| (0.46–2.18) |
| (0.65–3.33) |
| (0.47–2.36) |
|
|
Chickenpox | 0.82 | 0.43 | 0.97 | 1.00 | 0.78 | 0.90 | 0.49 | 0.22 | 0.53 | 0.89 | 0.44 | 0.75 | 0.94 | 0.87 | 0.96 | 1.00 | 1 | 1.00 | - | - | - | - | - | - |
|
| (0.50–1.35) |
| (0.58–1.61) |
| (0.47–1.30) |
| (0.16–1.53) |
| (0.17–1.69) |
| (0.14–1.39) |
| (0.41–2.11) |
| (0.39–2.37) |
| (0.44–2.27) |
|
|
|
|
|
|
|
Discussion
We found for the first time that lower respiratory
tract inflammation in one-year-old infants born by vaginal delivery
but not caesarean section was significantly higher in cervical
cancer survivors. No risk of other infant inflammations was found
in any of the cancer survivors.
Newborn babies experience rapid colonization mainly
by passage through the maternal vagina, and the difference in the
gut microbiota of infants between vaginal delivery and caesarean
section may result from significant differences between vaginal and
endometrial microbiota (14). Shao
et al conducted a whole-genome shotgun metagenomic analysis
of 1,679 gut microbiota samples during the neonatal period and in
infancy from 596 full-term babies born in UK hospitals and found
disrupted transmission of maternal Bacteroides strains, and
high-level colonization by opportunistic pathogens associated with
the hospital environment (including Enterococcus,
Enterobacter and Klebsiella species), in babies
delivered by caesarean section (15). These effects were also seen in
vaginally delivered babies whose mothers underwent antibiotic
prophylaxis and in babies who were not breastfed during the
neonatal period. This analysis demonstrated that the mode of
delivery is a significant factor that affects the composition of
the gut microbiota throughout the neonatal period and into
infancy.
A meta-analysis revealed that caesarean section is a
risk factor for respiratory tract infections (pooled OR 1.30, 95%
CI 1.06-1.60), asthma (1.23, 1.14-1.33) as well as obesity (1.35,
1.29-1.41) in offspring (16). The
risk of severe lower respiratory inflammation during infancy was
moderately elevated in infants born by planned caesarean, compared
to those born vaginally, in the general population (17). Thus, rapid colonization by maternal
vaginal microbiota might be important for protecting the infant
from infectious disease.
In the present study however, lower respiratory
tract inflammation was higher in infants born by vaginal delivery
from cervical cancer survivors. Cervical cancer is well-known to be
caused by high-risk human papillomavirus (HPV) infections. HPV
infection induces vaginal microbial taxonomic shifts and may
influence the maintenance of microbial homeostasis (18). Sims et al demonstrated that
the diversity of gut microbiota was associated with a favorable
response to chemoradiation in patients with cervical cancer and
that compositional variation correlated with short term and
long-term survival (19).
Regarding advance pregnancy outcomes in AYA cancer
survivors, the first birth cohort study compared outcomes of 1,894
AYA survivors diagnosed in Western Australia during the period from
1982 to 2007 with those of controls matched by maternal age, parity
and year of delivery (5). Female
survivors had an increased risk of threatened abortion, gestational
diabetes, pre-eclampsia, post-partum hemorrhage, caesarean
delivery, maternal postpartum hospitalization >5 days, premature
birth (<37 weeks), low birth weight, fetal growth restriction,
neonatal distress indicated by low Apgar score (<7) at 1 min,
and the need for resuscitation or special care nursery admission.
Our present study found no risk of HDP and SGA. The limitation of
Haggar's study was that covariates were adjusted for only age and
parity (5).
Anderson et al showed a significantly
increased risk of preterm birth (prevalence ratio 1.52, 95% CI
1.34-1.71), low birth weight (1.59, 1.38-1.83), and caesarean
delivery (1.08, 1.01-1.14) (1). The
higher prevalence of these outcomes was most concentrated among
births to women diagnosed during pregnancy. Other factors
associated with a preterm birth and low birth weight included
treatment with chemotherapy and a diagnosis of breast cancer,
non-Hodgkin's lymphoma, or gynecological cancers. The prevalence of
SGA and a low Apgar score (<7) did not differ significantly
between groups. The results were in line with our findings.
Ji et al found a risk of stillbirth among
children of female cancer survivors who were born within three
years after a cancer diagnosis (1.92, 1.03-3.57) and suggested that
the risk of stillbirth was negatively associated with the time
after the diagnosis, providing evidence that the adverse effect
associated with cancer treatment may diminish with time (20).
Cervical cancer was the most frequent and women with
a prior cervical cancer had a higher risk of preterm birth in the
present study. This is in line with previous study (21,22).
Nitecki et al found that 2.9% (118/4087) of patients
conceived at least 3 months after fertility-sparing surgery for
stage I cervical cancer and had higher odds of preterm birth and
neonatal morbidity (21). There was
no difference in rates of SGA, stillbirth, caesarean delivery and
maternal morbidity. The cervical intraepithelial neoplasia grade 3
(CIN 3) is speculated to be included in the present study because
fertility rate after surgery for Stage I cervical cancer is very
low. He et al found that 78450 patients with a prior CIN 3
had an increase risk of preterm birth, chorioamnionitis, infant
sepsis and neonatal death compared to 784500 matched controls
(22). We should pay attention to
chorioamnionitis, infant sepsis and neonatal death though we could
not examine the risk. Persistent vaginal HPV-16/18 detection was
reported to be significantly associated with preterm birth
(23). The study to examine whether
HPV vaccination has an effect to prevent it is also necessary.
In our present study, 56 women had cancer as a
complication during pregnancy, however, details regarding treatment
and age at cancer diagnosis were not available. This was one
limitation of our study. The type of cancer was not taken into
account in analysis since the number of women with a history of
each specific cancer was small. The CIN 3 might be included in
cervical cancer because questionnaires were self-administered.
These were other limitations.
In conclusion, lower respiratory tract inflammation
in one-year-old infants born by vaginal delivery increased
significantly in cervical cancer survivors. No risk of other infant
inflammations was found in the total group of survivors. Infants
delivered from women with prior cervical cancer should be taken
care till one year old. Further study concerning an association
between HPV and lower respiratory inflammation is necessary. It was
ascertained that preterm births increased in women with a history
of cervical cancer, that caesarean section increased in all cancer
survivors and that there was no increase in congenital anomalies in
the cancer survivors as a whole. It is important to provide this
information to cancer survivors before they become pregnant.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank all of the JECS
participants and all obstetricians in the 15 Regional Centers.
Members of the JECS Group as of 2021 are: Michihiro Kamijima
(principal investigator, Nagoya City University, Nagoya, Japan),
Shin Yamazaki (National Institute for Environmental Studies,
Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health
and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University,
Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan),
Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan),
Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito
(Yokohama City University, Yokohama, Japan), Zentaro Yamagata
(University of Yamanashi, Chuo, Japan), Hidekuni Inadera
(University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto
University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita,
Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya,
Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan),
Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi
Kusuhara (University of Occupational and Environmental Health,
Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University,
Kumamoto, Japan).
Funding
This study was funded by the Ministry of the Environment,
Government of Japan.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the Act on the
Protection of Personal Information (Act No. 57 of 30 May 2003,
amendment on 9 September 2015) but are available from the
corresponding author on reasonable request. Ethical Guidelines for
Epidemiological Research enforced by the Japan Ministry of
Education, Culture, Sports, Science and Technology and the Ministry
of Health, Labour and Welfare also restricts the open sharing of
the epidemiologic data. All inquiries about access to data should
be sent to: jecs-en@nies.go.jp. The person
responsible for handling enquiries sent to this e-mail address is
Dr. Shoji F. Nakayama, JECS Programme Office, National Institute
for Environmental Studies.
Authors' contributions
The JECS group conducted the nationwide study
project. RN wrote the first draft of the manuscript. MSO designed
the present study and analyzed the data. TM and HT analyzed the
data. TE organized the study team, and was responsible for
obtaining and analyzing the data. MK was responsible for data
acquisition and supervision of the study. RN, SK, KK and SS were
responsible for data acquisition. MK and TE confirm the
authenticity of all the raw data. All authors interpreted the data,
contributed to the writing of the manuscript and revised it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The JECS was approved by the Institutional Review
Board of the Japan National Institute for Environmental Studies
(approval no. 100910001), as well as by the ethics committees of
all participating institutions. This study was conducted with the
approval of the Research Ethics Committee of Nagoya City University
Graduate School of Medical Sciences (approval nos. 554 and 554-2).
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson C, Engel SM, Mersereau JE, Black
KZ, Wood WA, Anders CK and Nichols HB: Birth outcomes among
adolescent and young adult cancer survivors. JAMA Oncol.
3:1078–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow EJ, Stratton KL, Leisenring WM,
Oeffinger KC, Sklar CA, Donaldson SS, Ginsberg JP, Kenney LB,
Levine JM, Robison LL, et al: Pregnancy after chemotherapy in male
and female survivors of childhood cancer treated between 1970 and
1999: A report from the childhood cancer survivor study cohort.
Lancet Oncol. 17:567–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson RA, Brewster DH, Wood R, Nowell
S, Fischbacher C, Kelsey TW and Wallace WHB: The impact of cancer
on subsequent chance of pregnancy: A population-based analysis. Hum
Reprod. 33:1281–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velez MP, Richardson H, Baxter NN,
McClintock C, Greenblatt E, Barr R and Green M: Risk of infertility
in female adolescents and young adults with cancer: A
population-based cohort study. Hum Reprod. 36:1981–1988. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haggar FA, Pereira G, Preen D, Holman CD
and Einarsdottir K: Adverse obstetric and perinatal outcomes
following treatment of adolescent and young adult cancer: A
population-based cohort study. PLoS One. 9:e1132922014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armuand G, Svanberg AS, Bladh M and Sydsjö
G: Adverse obstetric outcomes among female childhood and adolescent
cancer survivors in Sweden: A population-based matched cohort
study. Acta Obstet Gynecol Scand. 98:1603–1611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Japan Society of Obstetrics and
Gynecology, . Cervical Cancer. https://www.jsog.or.jp/modules/diseases/index.php?content_id=10
|
|
8
|
Kawamoto T, Nitta H, Murata K, Toda E,
Tsukamoto N, Hasegawa M, Yamagata Z, Kayama F, Kishi R, Ohya Y, et
al: Rationale and study design of the Japan environment and
children's study (JECS). BMC Public Health. 14:252014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki K, Shinohara R, Sato M, Otawa S and
Yamagata Z: Association between maternal smoking during pregnancy
and birth weight: An appropriately adjusted model from the Japan
environment and children's study. J Epidemiol. 26:371–377. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishitsuka K, Nakayama SF, Kishi R, Mori C,
Yamagata Z, Ohya Y, Kawamoto T and Kamijima M: Japan environment
and children's study: Backgrounds, activities, and future
directions in global perspectives. Environ Health Prev Med.
22:612017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michikawa T, Nitta H, Nakayama SF,
Yamazaki S, Isobe T, Tamura K, Suda E, Ono M, Yonemoto J,
Iwai–Shimada M, et al: Japan environment and children's study
group. Baseline profile of participants in the Japan environment
and children's study (JECS). J Epidemiol. 28:99–104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugiura-Ogasawara M, Ebara T, Matsuki T,
Yamada Y, Omori T, Matsumoto Y, Kato S, Kano H, Kurihara T, Saitoh
S, et al: Endometriosis and recurrent pregnancy loss as new risk
factors for venous thromboembolism during pregnancy and
post–partum: The JECS birth cohort. Thromb Haemost. 119:606–617.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vandenbroucke JP, von Elm E, Altman DG,
Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ and
Egger M: Strengthening the reporting of observational studies in
epidemiology (STROBE): Explanation and elaboration. PLoS Med.
4:e2972007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreno I and Simon C: Relevance of
assessing the uterine microbiota in infertility. Fertil Steril.
110:337–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao Y, Forster SC, Tsaliki E, Vervier K,
Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P,
et al: Stunted microbiota and opportunistic pathogen colonization
in caesarean–section birth. Nature. 574:117–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Słabuszewska-Jóźwiak A, Szymański JK,
Ciebiera M, Sarecka-Hujar B and Jakiel G: Pediatrics consequences
of caesarean section–a systematic review and meta–analysis. Int J
Environ Res Public Health. 17:80312020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alterman N, Kurinczuk JJ and Quigley MA:
Caesarean section and severe upper and lower respiratory tract
infections during infancy: Evidence from two UK cohorts. PLoS One.
16:e02468322021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chorna N, Romaguera J and Godoy-Vitorino
F: Cervicovaginal microbiome and urine metabolome paired analysis
reveals niche partitioning of the microbiota in patients with human
papilloma virus infections. Metabolites. 10:362020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sims TT, El Alam MB, Karpinets TV,
Dorta-Estremera S, Hegde VL, Nookala S, Yoshida-Court K, Wu X,
Biegert GWG, Medrano AY, et al: Gut microbiome diversity is an
independent predictor of survival in cervical cancer patients
receiving chemoradiation. Commun Biol. 4:2372021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji J, Sundquist J and Sundquist K:
Stillbirth and neonatal death among female cancer survivors: A
national cohort study. Int J Cancer. 139:1046–1052. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nitecki R, Floyd J, Lamiman K, Clapp MA,
Fu S, Jorgensen K, Melamed A, Brady PC, Kaimal A, Del Carmen MG, et
al: Outcomes of the first pregnancy after fertility-sparing surgery
for early-stage cervical cancer. Obstet Gynecol. 138:565–573. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Sparén P, Fang F, Sengpiel V,
Strander B and Czene K: Pregnancy outcomes in women with a prior
cervical intraepithelial neoplasia grade 3 diagnosis: A nationwide
population-based cohort study with sibling comparison design. Ann
Intern Med. 175:210–218. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niyibizi J, Mayrand MH, Audibert F,
Monnier P, Brassard P, Laporte L, Lacaille J, Zahreddine M, Bédard
MJ, Girard I, et al: Association between human papillomavirus
infection among pregnant women and preterm birth. JAMA Netw Open.
4:e21253082021. View Article : Google Scholar : PubMed/NCBI
|