Long non-coding RNAs (lncRNAs) are newly discovered
RNAs that are >200 nucleotides in length and are involved in a
variety of molecular regulatory processes, including
transcriptional and posttranscriptional regulation, protein
localisation and RNA interference (1–3).
Although the full function of a number of lncRNAs is unknown, their
role in cancer is becoming increasingly clear (4,5).
The transforming growth factor (TGF)-β signalling
pathway consists of multiple signalling proteins that control a
variety of cell functions, including proliferation,
differentiation, apoptosis and survival (6). Its inactivation leads to a variety of
pathological states, including malignancy, immune system disorder
and inflammatory responses (7).
However, the role of the TGF-β pathway in carcinogenesis is
complex, and it exerts either tumour-suppressive or
tumour-promoting effects depending on the cellular environment
(8). The complex regulatory
mechanisms of the TGF-β pathway in cancer are currently
unknown.
There is growing evidence of the interaction between
the TGF-β signalling pathway and lncRNAs in tumours and several
members of the TGF-β signalling pathway have been identified as
targets of lncRNAs (9–11). The present review summarizes
knowledge of crosstalk between the TGF-β signalling pathway and
lncRNAs in cancer.
LncRNAs that participate in chromatin remodelling,
transcriptional control, posttranscriptional processing, protein
modification and RNA degradation (12–14).
After the discovery of the first lncRNAs in 1990 (15), lncRNAs have received increasing
attention. Numerous lncRNAs participate in the pathogenesis of
different diseases (16); these
include lncRNA CDC6 in breast cancer (17), lncRNA OIN1 in ovarian cancer
(18) and lncRNA RP11-567G11.1 in
pancreatic cancer (19). Owing to
the development of sequencing technology, lncRNAs have been found
to serve an important role in tumour cell proliferation, apoptosis,
differentiation and invasion (11,20).
lncRNAs are considered to be an important component
of cancer, but they play different roles in different types of
cancer. For instance, lncRNA FGD5-AS1 accelerates cell
proliferation in pancreatic cancer by regulating the microRNA
(miRNA or miR)-520a-3p/KIAA1522 axis (21), high expression levels of lncRNA
PCAT1 are associated with drug resistance in colorectal cancer
(CRC) (22) and lncRNA LNMICC
promotes cervical cancer lymph node metastasis by reprogramming
fatty acid metabolism (23). High
or low expression of lncRNAs in tumours contributes to disease via
multiple molecular mechanisms and they have a variety of unique
functions and characteristics. Guide lncRNAs bind enzymatically
active protein complexes and direct them to target gene promoter
regions or genome-specific loci (24). Scaffold lncRNAs build a central
platform to which multiple protein complexes attach, thus guiding
them to their designated locations (24). Decoy lncRNAs activate or silence
downstream target genes by binding and interacting with
transcription factors or repressors (25). In addition, lncRNAs are associated
with a number of key signalling pathways. Regardless of the
position of these lncRNAs in the signalling pathway, they serve
different functions. For example, Wei et al (26) found that lncRNA MEG3 inhibits
proliferation and metastasis of gastric cancer (GC) cells via the
TP53 (a tumour suppressor gene) signalling pathway. High levels of
lncRNA p21 in thoracic aortic aneurysms may be associated with
regulating vascular smooth muscle cell proliferation and apoptosis
by activating the TGF-β signalling pathway (27).
lncRNAs are known to be involved in cellular
physiological and pathological processes (28). Therefore, lncRNAs are also relevant
for diagnosis, treatment and prognosis evaluation (29).
The TGF-β superfamily has numerous members,
including TGF-β isoforms, bone morphogenetic protein, growth
differentiation factors, activators, inhibitors and nodulins
(30,31). TGF has three receptor ligands,
TGF-β1, 2, and 3, which have similar, but not identical, biological
activities in vitro (32).
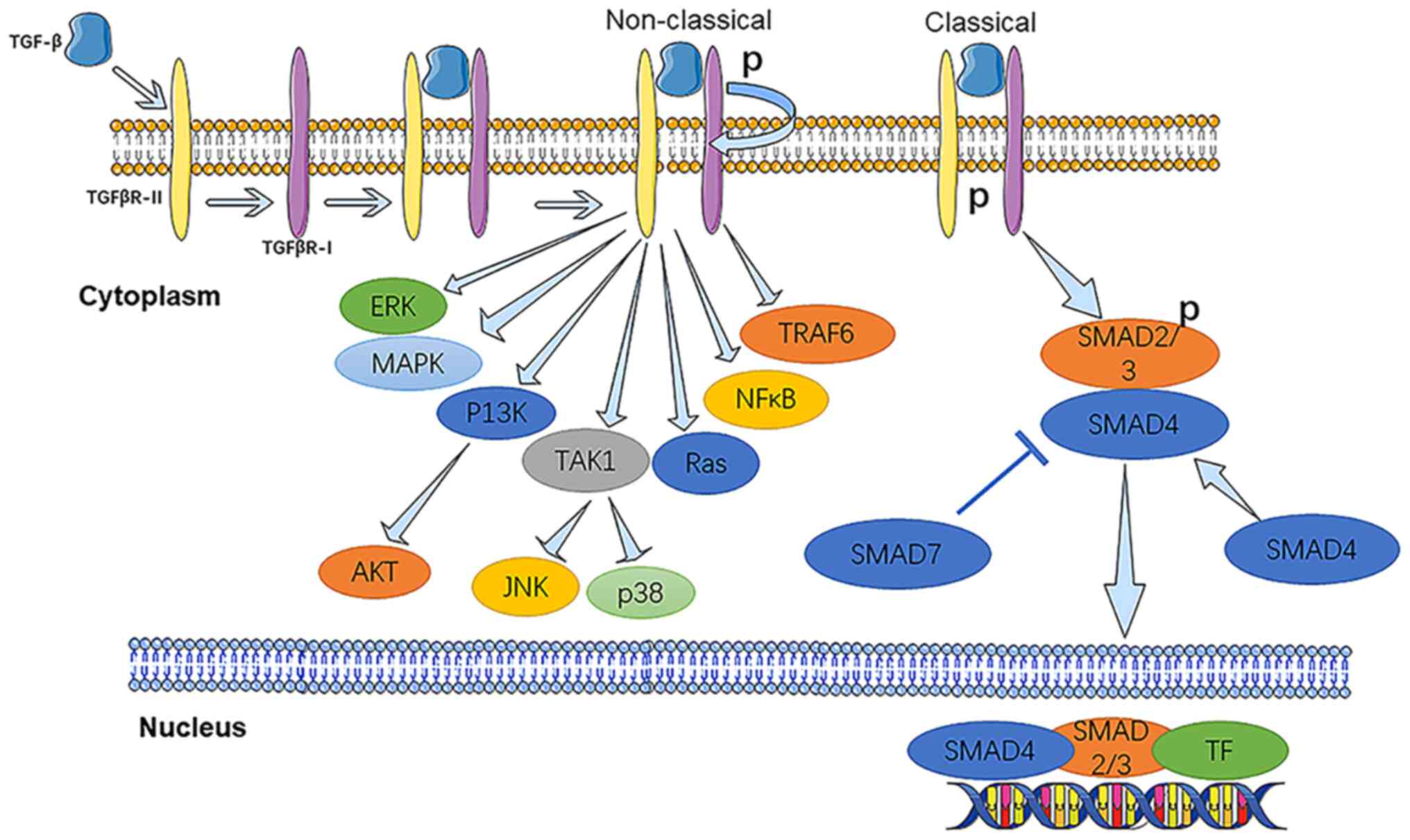
The TGF-β signalling pathway consists of two distinct intracellular
pathways: SMAD-dependent (known as the classical TGF-β pathway) and
non-SMAD-dependent pathway (known as the non-classical TGF-β
pathway; Fig. 1) (32). By contrast with other signalling
pathways, the classical TGF-β pathway is widely evolved and
distributed in a variety of organisms (from drosophila and
nematodes to mice and humans). Activated TGF-β is altered by
binding to TGF-β type II receptor (TGFβR-II), which affects its
structure, then TGFβR-II phosphorylates TGFβR-I on specific serine
and threonine residues (33). In
the classical pathway, the activated receptor complex
phosphorylates receptor-SMADs (R-SMADs; including SMAD2 and SMAD3),
which are primarily responsible for the activation of downstream
signalling pathways (34). The
receptor-activated SMAD anchor recruits R-SMADs into the activated
receptor complex. Finally, the activated receptor complex binds to
SMAD4 (Co-SMAD4 or common mediator SMAD4) in a large complex and
enters the nucleus, where it interacts with transcription factors
and coactivators to regulate expression of target genes (34,35).
SMAD6 and SMAD7, also known as inhibitory SMADs
(I-SMADs), serve an important role in the inhibition of the TGF-β
signalling pathway through multiple mechanisms. Firstly, SMAD6/7
competes with R-SMADs for recruitment to type I receptors and
prevents activation of R-SMADs by phosphorylation (36). SMAD7 induces ubiquitination and
degradation of type I receptors by recruiting the E3 ligases SMURF1
and SMURF2 (37,38). SMAD7 recruits ubiquitin-conjugated
E2 enzyme UbcH7 to stimulate SMURF1/2 activity in the
R-SMAD7SM-URF1/2 complex (39).
SMAD7 induces degradation and inactivation of TGFβR-I by recruiting
two HECT-type E3 ligases (WWP1/Tiul1 and NEDD4-2) (40). This suggests that I-SMADs are
involved in negative feedback regulation in the TGF-β/SMAD pathway.
Although SMAD proteins are the basis of TGF-β regulation of various
cellular signalling pathways, numerous signalling responses are
stimulated by TGF-β, which is not regulated by SMADs (32). For example, in the non-classical
pathway, the activated TGF-β receptor complex promotes or inhibits
downstream cell biological processes through a number of other
transduction factors, such as tumour necrosis factor (TNF), TNF
receptor-associated factor 4 (TRAF4), TRAF6, p38 MAPK, Ras homology
(Rho), phosphoinositide 3 kinase (PI3K)/AKT, extracellular
signal-regulated kinase (ERK) and NF-κB, to promote or inhibit
downstream cell biological processes (32).
TGF-β serves as both an oncogene and an oncogene
promoter. In normal tissue, TGF-β promotes tissue stabilisation and
suppresses inflammatory responses. In premalignant progression,
TGF-β serves as an oncogene to promote apoptosis and cytostasis and
inhibit tumorigenesis. However, in cancer cells, TGF-β serves as a
pro-oncogene, promoting tumour growth and metastasis (41,42).
TGF-β signalling promotes epithelial-mesenchymal transition (EMT)
by increasing expression of mesenchymal markers, such as N-cadherin
and vimentin, and decreasing expression of epithelial markers, such
as E-cadherin (43,44). Since TGF-β acts extensively in
cells, blocking TGF-β and its downstream signals is a therapeutic
tool. Therefore, anti-TGF-β signalling therapy is an additional
therapeutic tool along with the currently used CAR-T (45) and anti-PD-L1 (46) therapy.
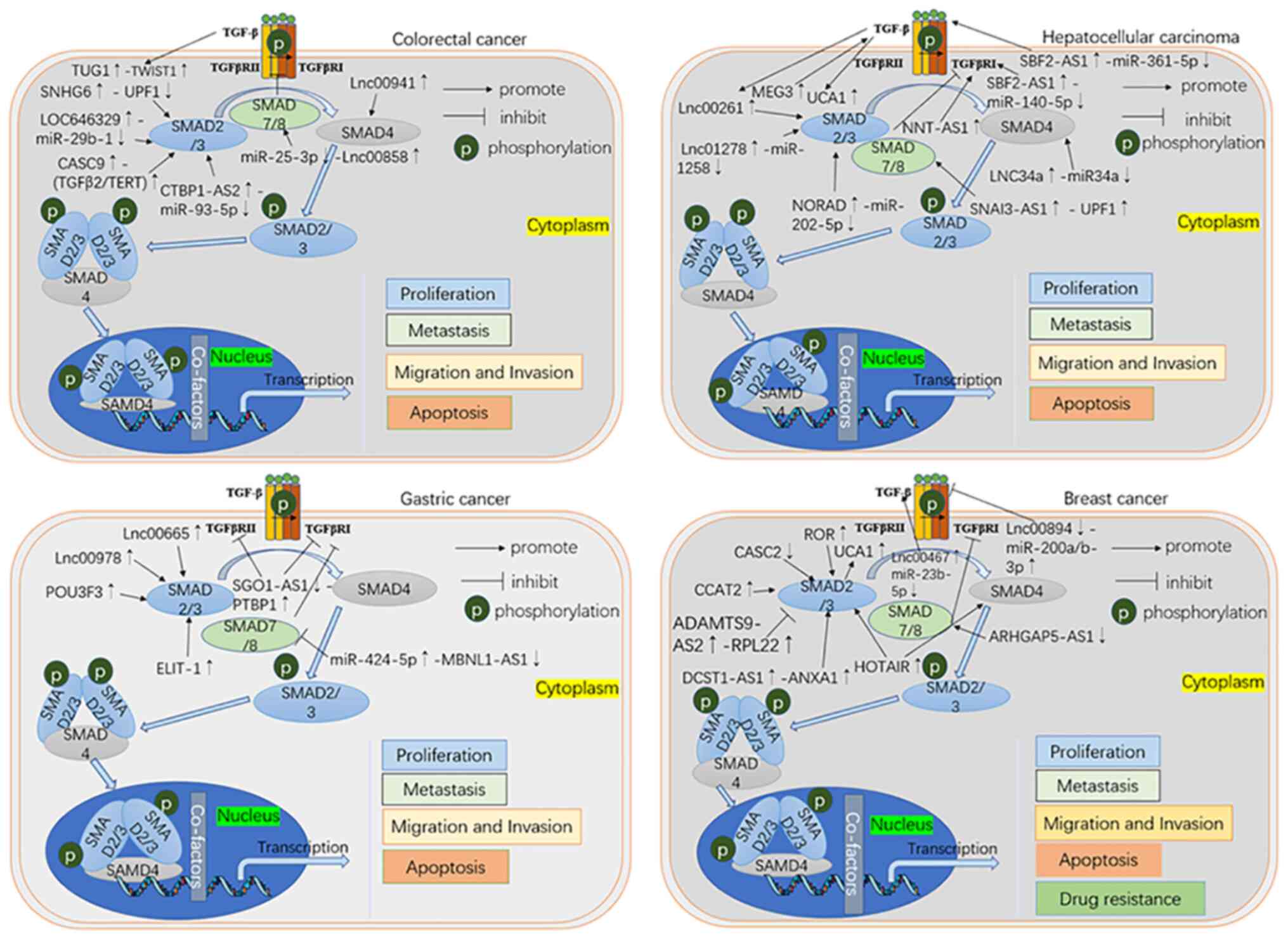
Aberrant lncRNAs in CRC are hypothesized to
contribute to activation or inactivation of the TGF-β pathway to
regulate tumour development. TGF-β pathway-associated CRC lncRNAs
are discussed here, to explore the roles of lncRNAs in the
progression of CRC.
CRC is the third leading cause of cancer-associated
death worldwide and there are 1.85 million new cases and 850,000
CRC-associated deaths each year (47). The majority of CRC tumours arise
from precursor lesions, such as adenoma transforming to
adenocarcinoma (48). Therefore, it
is key to identify useful biomarkers to diagnose CRC at the early
stages of disease. Numerous studies have demonstrated the novel
role and therapeutic potential of lncRNAs in CRC (49,50)
(Fig. 2). lncRNA SNHG6 is
upregulated in CRC and binds UPF1 to activate the downstream
TGF-β/SMAD signalling pathway to promote proliferation, migration
and invasion of CRC cells (51).
Upregulation of lncRNA LOC646329 promotes CRC cell proliferation by
competing for binding to miR-29b-1 (52). In addition, knockdown of lnc00858
reduces the proliferative capacity of CRC cells by inducing
production of p53 and blocking the G0/G1 phase of CRC cells
(53). lnc00858 upregulation is
negatively correlated with miR-25-3p and SMAD7 is a downstream
target of miR-25-3p (53).
Similarly, miR-93-5p serves as an competing endogenous RNA (ceRNA)
for lncRNA CTBP1-AS2 and activates the TGF-β/SMAD2/3 pathway to
promote proliferation, invasion and resistance to apoptosis in
colon cancer cells (54). Shen
et al (11) demonstrated
that TGF-β promotes CRC metastasis via the lncRNA TUG1/TWIST1/EMT
signalling pathway. TGF-β induces metastasis, and knockdown of
TUG1can inhibit metastasis (11).
However, expression of TGF-β does not increase after TUG1knockdown,
suggesting that TUG1is located downstream of TGF-β. TUG1 may serve
as a drug target to inhibit CRC development by suppressing TGF-β
pathway activation (11).
Furthermore, Wu et al (49)
found that lnc00941 promotes EMT by directly competing with
β-transducin repeats-containing protein to bind to the MH2
structural domain on SMAD4, thereby preventing SMAD4 protein
degradation and activating the TGF-β/SMAD2/3 signalling pathway.
lncRNA CASC9 is upregulated in CRC, and high expression of CASC9
predicts a low prognosis and an association with TNM stage I
(55). Luo et al (55) demonstrated that CASC9 enhances the
function of the telomerase Reverse Transcriptase (TERT) complex in
CRC cells by regulating expression of TGF-β2 mRNA and upregulating
levels of TGF-β2 and TERT, leading to phosphorylation of SMAD3 and
activation of the TGF-β signalling pathway, thereby enhancing its
Tumorigenic ability.
Since colon cancer only shows symptoms in the
advanced stages of disease, it is necessary to improve the early
detection rate of CRC. An increasing number of studies have found
that TGF-β/SMAD signalling pathway involvement with lncRNAs serves
an important role in the development of colon cancer, which
provides a new avenue for early diagnosis and treatment (49,51,55).
SMAD3 (an R-SMAD) and SMAD4 (a co-SMAD) are key
proteins involved in the classical TGF-β signalling pathway. Chen
et al (65) found that
lnc00261 inhibits SMAD3 expression and phosphorylation and that
SMAD3 may be involved in transcriptional regulation in TGF-β1
signalling. lnc00261 inhibits EMT in HCC cells by inactivating the
TGFβ1/SMAD3 signalling pathway. In addition, lncRNAs also
participate in the TGF-β pathway through epigenetic modifications.
Zhang et al (66) found that
lncRNA 34a recruits DNA methyltransferase 3α through prohibitin-2
to methylate promoters of miR-34a and histone deacetylase 1 to
influence histone modification, thereby inhibiting miR-34a
expression. miR-34a targets SMAD4 and downregulation the expression
of downstream genes. In the immune system, activation of TGF-β
signalling suppresses recruitment of tumour-infiltrating
lymphocytes, leading to tumour immune escape. Wang et al
(67) found that relatively high
levels of lncRNA NNT-AS1 are associated with a decrease in the
number of infiltrating CD4+ lymphocytes and that
knockdown of lncRNA NNT-AS1 decreases expression of TGF-β and
TGFβR-I in HCC cells. In conclusion, lncRNA NNT-AS1 impairs
CD4+ T cell infiltration in HCC by activating the TGF-β
signalling pathway through a novel mechanism.
Biomarkers useful for early HCC diagnosis are still
lacking and available serum biomarkers show low sensitivity and
specificity, such as α-fetoprotein and des-gamma-carboxy
prothrombin (68). TGF-β signalling
pathway-associated lncRNAs are typically upregulated in HCC and may
be a novel target for early screening.
lncRNAs regulate gene expression at genomic,
transcriptomic and posttranscriptional levels and are recognized as
biomarkers and therapeutic targets for GC (Fig. 2) (77,78).
The TGF-β signalling pathway is an important pathway that promotes
development of GC and studying the effect of the interaction of
this pathway with lncRNAs in the development of GC may provide an
important target for early diagnosis (71).
Globally, breast cancer is the most frequently
diagnosed cancer in women and ranks second among causes of
cancer-related deaths in women (79). Although breast cancer can be
diagnosed early and there are numerous treatments available, it is
typically lethal once it metastasises (80). Therefore, it is key to find
clinically useful biomarkers present in the early stages of breast
cancer. Certain lncRNAs have been shown to promote the development
of breast cancer via the TGF-β signalling pathway (Fig. 2). For example, CASC2 (81) and CCAT2 (82) have been shown to be tumour
therapeutic targets by participating in TGF-β/SMAD2 signalling and
thus promoting proliferation and metastasis of breast cancer cells.
lncRNA ROR knockdown inhibits SMAD2 and α-SMA expression and thus
inactivates the TGF-β signalling pathway to inhibit tumour growth
(83). ARHGAP5-AS1 induces a
decrease in SMAD7 ubiquitination and degradation by interacting
with SMAD7, leading to a decrease in SMAD7 binding to SMURF1 and
SMURF2 (84). In addition,
ARHGAP5-AS1 may inhibit the TGF-β signalling pathway by stabilising
SMAD7. ADAMTS9-AS2 has been shown to target downstream ribosomal
protein L22 to inhibit SMAD2 expression, thereby regulating the
TGF-β signalling pathway, inhibiting cell cycle arrest in breast
cancer cells in vitro and suppressing tumour growth in
vivo (85). Loss of Merlin in
breast cancer cells affects functional cellular metabolism. Mota
et al (86) found that the
cooperative activity of TGF-β transcriptional effectors results in
upregulation of UCA1, which leads to a decrease in Merlin activity
against STAT3. Similarly, Bo et al (87) predicted that lnc00467 may be
involved in signalling pathways involved in peroxisomal lipid
metabolism and immunity via miR-23b-5p targeting TGF-β2. LncRNAs
can also be involved in drug resistance. Zhang et al
(88) found that knockdown of
lnc00894-002 downregulates miR-200a-3p and miR-200b-3p, upregulates
TGF-β2 and ZEB1 and is involved in the development of tamoxifen
resistance. LncRNA DCST1-AS1 enhances TGF-β/SAMD2 signalling in
BT-549 cells by targeting ANXA1 and promoting EMT (89). Ren et al (90) discovered that SMAD2/3/4 binds to the
promoter site of HOTAIR and is directly transcribed by HOTAIR,
which provides a novel idea for treatment of breast cancer.
Based on the established role of TGF-β-associated
lncRNAs in regulating cell proliferation, cell cycle, apoptosis and
other aspects of cell physiology, future studies should evaluate
the potential of these transcripts as therapeutic targets for
breast cancer.
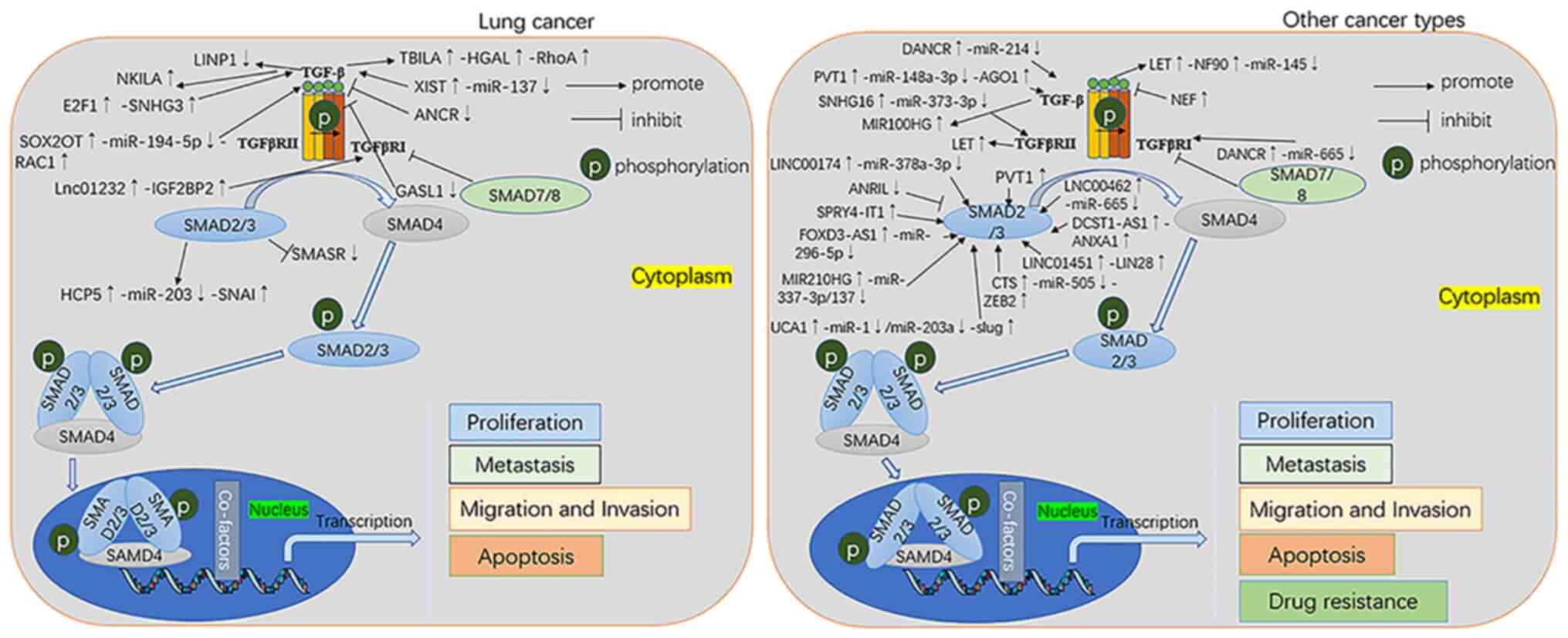
In the aforementioned lung cancer studies, multiple
differentially expressed lncRNAs have been identified, some of
which activate the TGF-β pathway to drive tumorigenesis, while
others inactivate the TGF-β pathway to inhibit tumour progression
(Fig. 3). Further study of the role
of lncRNAs in the TGF-β pathway may help develop molecular markers
for early diagnosis of lung cancer.
In thyroid cancer, lncRNA FOXD3-AS1 serves as a
sponge to adsorb miR-296-5p and upregulate miR-296-5p expression,
which inhibits the migration and invasion of thyroid cancer cells
by inactivating the TGF-β1/SMAD signalling pathway (103). Zhao et al (104) found that ANRIL may decrease
expression of cyclin-dependent kinase 4 by inhibiting the
TGF-β/SMAD signalling pathway and promoting invasion and metastasis
of thyroid cancer cells. Similarly, silencing SPRY4-IT1 inhibits
TGF-β1 and phosphorylated SMAD2/3 levels, thereby inhibiting
proliferation and migratory capacity of thyroid cancer cells;
knockdown of SPRY4-IT1-mediated functions can be rescued by
interference with TGF-β1 (105).
In cervical cancer, knockdown of lncRNA NEF
decreases the expression of TGF-β1, which inhibits the migration
and invasion of cervical cancer cells (106). In addition, miR-665 serves as a
ceRNA for lncRNA DANCR and targets TGFβR-I through the ERK/SMAD
pathway to suppress the malignant phenotype of cervical cancer
cells, which may provide a novel therapeutic strategy for cervical
cancer treatment (107).
Similarly, lncRNA CTS enhances migration and invasive ability of
cervical cancer cells as well as TGF-β1-induced EMT (108). The expression of lncRNA CTS has a
negative correlation with miR-505 expression and ZEB2 may act as
the target of miR-505 (108).
lncRNA CTS promotes cervical cell migration and invasion via the
miR-505/ZEB2/TGF-β/SMAD axis (108).
In lymphoma, knockdown of lncRNA ANRIL may inhibit
proliferation and promote apoptosis of Burkitt's lymphoma cells by
regulating the TGF-β1 signalling pathway (109).
In glioma, UAC1 promotes Slug expression and thus
participates in TGF-β-induced EMT by targeting miR-1 and miR-203a
(110). In addition, p53 inhibits
expression of PVT1and thus inactivates the TGF-β/SMAD pathway,
inhibiting the proliferation, migration and invasion of glioma
cells, inducing cell apoptosis and inhibiting tumour growth
(111).
In endometrial cancer, lncRNAs promote tumorigenesis
and metastasis via the MIR210HG/miR-337-3p/137-HMGA2 axis, which
activates the TGF-β/SMAD3 signalling pathway (112).
In prostate cancer, SNHG16 promotes proliferation
and migration of prostate cancer cells by targeting the
TGF-βRII/SMAD axis (113).
In pancreatic cancer, knockdown of PVT1 inhibits
cell survival, adhesion, migration and invasion by suppressing
TGF-β/SMAD2/3 signalling (114).
These findings reveal that PVT1 may serve an oncogenic role in
pancreatic cancer by regulating EMT via the TGF-β/SMAD pathway
(114). In addition, miR100HG
controls the intensity of TGF-β signalling via the production of
TGFβR-I in tumours (115).
Overexpression of Lnc00462 increases expression levels of TGFβR-I
and TGFβR-II, thereby activating the SMAD2/3 pathway in pancreatic
cancer cells (116). miR-665 is
also a target of lnc00462 (116).
Taken together, these findings indicate that the
lnc00462/miR-665/TGFβR-I/II regulatory network may underlie the
mechanism of pancreatic carcinogenesis.
In ovarian cancer, lncRNA PVT1 promotes tumour
growth and proliferation via the PVT1/miR-148a-3p/AGO1/TGF-β axis
(117). In addition, DANCR is a
sponge for miR-214, while KLF5 is a target of miR-214 (118). Silencing DANCR inhibits
TGF-β-treated ovarian cancer cell viability, migration and invasion
via the miR-214/KLF5 axis and induces apoptosis (118).
In bladder cancer, lnc01451 directly targets LIN28
to activate the TGF-β/SMAD signalling pathway (119). In terms of drug resistance, Zhuang
et al (120) found that
gemcitabine-induced aberrant TGF-β1 regulation of the
LET/NF90/miR-145 axis promotes urothelial bladder cancer
chemoresistance by enhancing cancer cell stemness.
In osteosarcoma (OS), high levels of lnc00174 form a
ceRNA network with miR-378a-3p/SSH2 and activate the TGF-β/SMAD
pathway to promote OS cell proliferation (121).
The discovery of a large number of TGF-β-associated
lncRNAs, their extensive expression patterns in various types of
cancer (thyroid and cervical cancer, lymphoma, glioma and
endometrial, prostate, pancreatic, ovarian and bladder cancer) and
the biological properties that promote tumour cell proliferation,
migration and invasion provides a novel basis for the development
of cancer diagnosis and therapy.
lncRNAs are differentially expressed in different
tissue and cells and are highly heterogeneous. They regulate gene
expression and intracellular homeostasis via multiple mechanisms,
including tumour cell proliferation, survival, migration and
genomic stability (122). The
present review confirmed that lncRNAs play an important role in
tumour development, similar to protein-coding genes, and are
associated with multiple cellular signalling pathways. Although
there are multiple signalling pathways in tumours by which lncRNAs
may regulate cell proliferation, the TGF-β signalling pathway is
widely distributed in tumours and serves a key role in the
development of different types of cancer (123). lncRNA transcription can activate
or inhibit the TGF-β signalling pathway by interacting with other
molecules in the cell, including DNA, protein and RNA, to provide
malignant transformation signals. Thus, lncRNAs affect the
pathology of different cancer types (124,125). Table
I lists lncRNAs associated with the TGF-β signalling pathway in
cancer. In addition, these lncRNAs may have different methods of
targeting the TGF-β signalling pathway since they have high tissue
and cell specificity. These lncRNAs can also act in different
cancer types through the TGF-β pathway. For example, lncRNA UCA1
promotes tumour cell proliferation and EMT in breast and liver
cancer and glioma (63,110). lncRNA PVT1 promotes tumour cell
proliferation in pancreatic and ovarian cancer and glioma (111,117). Although the same lncRNAs are
involved in the TGF-β pathway in different cancer types, they act
in different ways, either directly targeting SMADs or forming a
ceRNA network with miRNAs, which makes clinical targeting difficult
(126). Overall, TGF-β
pathway-associated lncRNAs are differentially regulated in
different types of cancer and targeted therapy is a potential way
to disrupt key signalling pathways in tumour cells, such as the
Wnt, Notch and TGF-β pathways, without compromising their essential
functions in normal tissue (49,126,127). The lncRNA network and TGF-β
signalling pathway could reveal new cancer diagnosis and treatment
approaches.
Since the TGF-β signalling pathway is related to
tumour development and metastasis, interfering with this cascade
via inhibitors may be a valuable strategy in tumour treatment
approaches. For example, SD-208, an inhibitor of TGFβR-I,
significantly downregulates expression of miR-135b, a key tumour
molecule, in SW-48 colon cells and nude mice implanted with tumours
in situ (128). Han et
al (129) found that
dexamethasone inhibits AKT and ERK phosphorylation in colon cancer
cells, leading to a decrease in cy61 expression, which in turn
blocks TGF-β1-induced migration. Similarly, Koelink et al
(130) found that 5-aminosalicylic
acid eliminates the TGF-β1 cascade in HCT116 CRC cells and
therefore disrupts phosphorylation of downstream SMAD3. These
inhibitors or drugs act on an important molecular target in the
TGF-β pathway, which affects the entire pathway. lncRNAs only
indirectly affect expression of certain related proteins in the
TGF-β pathway and, to the best of our knowledge, little is known
about the potential involvement of lncRNAs in direct regulation.
For TGF-β-induced lncRNAs, inhibition of TGF-β expression may be a
promising therapeutic approach. Identifying these potential lncRNAs
will provide a more comprehensive understanding of regulation of
the TGF-β pathway.
Not applicable.
The present study was supported by the Youth Program of National
Natural Science Foundation of China (grant no. 81500169), the Hunan
Provincial Groundbreaking Platform Open Fund of University of South
China (grant no. 19K080) and the Student Research Learning and
Innovative Experimental Project of the University of South China
(grant nos. 20155760439 and X2019141).
Not applicable.
ZH is responsible for writing the article. YL and ML
revised the manuscript for important intellectual content and
constructed figures. YZ and CW conceived the study. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Dahariya S, Paddibhatla I, Kumar S,
Raghuwanshi S, Pallepati A and Gutti RK: Long non-coding RNA:
Classification, biogenesis and functions in blood cells. Mol
Immunol. 112:82–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhat AA, Younes SN, Raza SS, Zarif L,
Nisar S, Ahmed I, Mir R, Kumar S, Sharawat SK, Hashem S, et al:
Role of non-coding RNA networks in leukemia progression, metastasis
and drug resistance. Mol Cancer. 19:572020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Qiu M, Shen M, Dong S, Ye G, Shi X
and Sun M: The emerging regulatory roles of long non-coding RNAs
implicated in cancer metabolism. Mol Ther. 29:2209–2218. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu D, Shi C, Jiang Y, Zhu K, Wang X and
Feng W: Cisatracurium inhibits the growth and induces apoptosis of
ovarian cancer cells by promoting lincRNA-p21. Bioengineered.
12:1505–1516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikushima H and Miyazono K: TGF-β signal
transduction spreading to a wider field: A broad variety of
mechanisms for context-dependent effects of TGF-β. Cell Tissue Res.
347:37–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao M, Mishra L and Deng CX: The role of
TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 14:111–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Hao Y, Wang Y, Xu J, Teng Y and
Yang X: TGF-β/SMAD4-regulated LncRNA-LINP1 inhibits
epithelial-mesenchymal transition in lung cancer. Int J Biol Sci.
14:1715–1723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Z, Chen Z, Li Y, Wang J, Zhang Z, Che
Y, Huang J, Sun S, Mao S, Lei Y, et al: TGF-β-induced NKILA
inhibits ESCC cell migration and invasion through NF-κB/MMP14
signaling. J Mol Med (Berl). 96:301–313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen X, Hu X, Mao J, Wu Y, Liu H, Shen J,
Yu J and Chen W: The long noncoding RNA TUG1 is required for
TGF-β/TWIST1/EMT-mediated metastasis in colorectal cancer cells.
Cell Death Dis. 11:652020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan YT, Lin JF, Li T, Li JJ, Xu RH and Ju
HQ: LncRNA-mediated posttranslational modifications and
reprogramming of energy metabolism in cancer. Cancer Commun (Lond).
41:109–120. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou B, Yang H, Yang C, Bao YL, Yang SM,
Liu J and Xiao YF: Translation of noncoding RNAs and cancer. Cancer
Lett. 497:89–99. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brannan CI, Dees EC, Ingram RS and
Tilghman SM: The product of the H19 gene may function as an RNA.
Mol Cell Biol. 10:28–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong X, Duan Y, Sang Y, Li Y, Zhang H,
Liang Y, Liu Y, Zhang N and Yang Q: LncRNA-CDC6 promotes breast
cancer progression and function as ceRNA to target CDC6 by sponging
microRNA-215. J Cell Physiol. 234:9105–9117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeiwa T, Mitobe Y, Ikeda K, Hasegawa K,
Horie K and Inoue S: Long intergenic noncoding RNA promotes ovarian
cancer growth by modulating apoptosis-related gene expression. Int
J Mol Sci. 22:112422021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang R, Nie W, Yao K and Chou J:
Depletion of the lncRNA RP11-567G11.1 inhibits pancreatic cancer
progression. Biomed Pharmacother. 112:1086852019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu K, Cai Y, Zhang M, Zou H, Chang Z, Li
D, Bai J, Xu J and Li Y: Pan-cancer characterization of expression
and clinical relevance of m6A-related tissue-elevated
long non-coding RNAs. Mol Cancer. 20:312021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Liao S, Liu Z, Li E, Wu X and Zeng
W: LncRNA FGD5-AS1 accelerates cell proliferation in pancreatic
cancer by regulating miR-520a-3p/KIAA1522 axis. Cancer Biol Ther.
22:257–266. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun H, Sun X, Zhang H, Yue A and Sun M:
LncRNA-PCAT1 controls the growth, metastasis and drug resistance of
human colon cancer cells. J BUON. 25:2180–2185. 2020.PubMed/NCBI
|
|
23
|
Shang C, Wang W, Liao Y, Chen Y, Liu T, Du
Q, Huang J, Liang Y, Liu J, Zhao Y, et al: LNMICC promotes nodal
metastasis of cervical cancer by reprogramming fatty acid
metabolism. Cancer Re. 78:877–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ransohoff JD, Wei Y and Khavari PA: The
functions and unique features of long intergenic non-coding RNA.
Nat Rev Mol Cell Biol. 19:143–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
27
|
Hu W, Wang Z, Li Q, Wang J, Li L and Jiang
G: Upregulation of lincRNA-p21 in thoracic aortic aneurysms is
involved in the regulation of proliferation and apoptosis of
vascular smooth muscle cells by activating TGF-β1 signaling
pathway. J Cell Biochem. 120:4113–4120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han M, Liao Z, Liu F, Chen X and Zhang B:
Modulation of the TGF-β signaling pathway by long noncoding RNA in
hepatocellular carcinoma. Biomark Res. 8:702020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goyal B, Yadav SRM, Awasthee N, Gupta S,
Kunnumakkara AB and Gupta SC: Diagnostic, prognostic, and
therapeutic significance of long non-coding RNA MALAT1 in cancer.
Biochim Biophys Acta Rev Cancer. 1875:1885022021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β Family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol. 8:a0218732016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heldin CH and Moustakas A: Signaling
receptors for TGF-β family members. Cold Spring Harb Perspect Biol.
8:a0220532016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wrana JL, Attisano L, Wieser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Attisano L and Wrana JL: Smads as
transcriptional co-modulators. Curr Opin Cell Biol. 12:235–243.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyazawa K and Miyazono K: Regulation of
TGF-β family signaling by inhibitory smads. Cold Spring Harb
Perspect Biol. 9:a0220952017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kavsak P, Rasmussen RK, Causing CG, Bonni
S, Zhu H, Thomsen GH and Wrana JL: Smad7 binds to Smurf2 to form an
E3 ubiquitin ligase that targets the TGF beta receptor for
degradation. Mol Cell. 6:1365–1375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ebisawa T, Fukuchi M, Murakami G, Chiba T,
Tanaka K, Imamura T and Miyazono K: Smurf1 interacts with
transforming growth factor-beta type I receptor through Smad7 and
induces receptor degradation. J Biol Chem. 276:12477–12480. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ogunjimi AA, Briant DJ, Pece-Barbara N, Le
Roy C, Di Guglielmo GM, Kavsak P, Rasmussen RK, Seet BT, Sicheri F
and Wrana JL: Regulation of Smurf2 ubiquitin ligase activity by
anchoring the E2 to the HECT domain. Mol Cell. 19:297–308. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Komuro A, Imamura T, Saitoh M, Yoshida Y,
Yamori T, Miyazono K and Miyazawa K: Negative regulation of
transforming growth factor-beta (TGF-beta) signaling by WW
domain-containing protein 1 (WWP1). Oncogene. 23:6914–6923. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Alexander PB and Wang XF: TGF-β
family signaling in the control of cell proliferation and survival.
Cold Spring Harb Perspect Biol. 9:a0221452017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Molecular Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie F, Ling L, van Dam H, Zhou F and Zhang
L: TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin
(Shanghai). 50:121–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang
C, Ye Z and Qian Q: Current progress in CAR-T cell therapy for
solid tumors. Int J Biol Sci. 15:2548–2560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen X and Zhao B: Efficacy of PD-1 or
PD-L1 inhibitors and PD-L1 expression status in cancer:
Meta-analysis. BMJ. 362:k35292018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Harada S and Morlote D: Molecular
pathology of colorectal cancer. Adv Anat Pathol. 27:20–26. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu N, Jiang M, Liu H, Chu Y, Wang D, Cao
J, Wang Z, Xie X, Han Y and Xu B: LINC00941 promotes CRC metastasis
through preventing SMAD4 protein degradation and activating the
TGF-β/SMAD2/3 signaling pathway. Cell Death Differ. 28:219–232.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schwarzmueller L, Bril O, Vermeulen L and
Léveillé N: Emerging role and therapeutic potential of lncRNAs in
colorectal cancer. Cancers (Basel). 12:38432020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Lai Q, He J, Li Q, Ding J, Lan Z,
Gu C, Yan Q, Fang Y, Zhao X and Liu S: LncRNA SNHG6 promotes
proliferation, invasion and migration in colorectal cancer cells by
activating TGF-beta/Smad signaling pathway via targeting UPF1 and
inducing EMT via regulation of ZEB1. Int J Med Sci. 16:51–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Javanmard AR, Dokanehiifard S, Bohlooli M
and Soltani BM: LOC646329 long non-coding RNA sponges miR-29b-1 and
regulates TGFβ signaling in colorectal cancer. J Cancer Res Clin
Oncol. 146:1205–1215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhan J, Tong J and Fu Q: Long non-coding
RNA LINC00858 promotes TP53-wild-type colorectal cancer progression
by regulating the microRNA-25-3p/SMAD7 axis. Oncol Rep.
43:1267–1277. 2020.PubMed/NCBI
|
|
54
|
Li Q, Yue W, Li M, Jiang Z, Hou Z, Liu W,
Ma N, Gan W, Li Y, Zhou T, et al: Downregulating Long Non-coding
RNAs CTBP1-AS2 inhibits colorectal cancer development by modulating
the miR-93-5p/TGF-β/SMAD2/3 pathway. Front Oncol. 11:6266202021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo K, Geng J, Zhang Q, Xu Y, Zhou X,
Huang Z, Shi KQ, Pan C and Wu J: LncRNA CASC9 interacts with CPSF3
to regulate TGF-β signaling in colorectal cancer. J Exp Clin Cancer
Res. 38:2492019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Laube R, Sabih AH, Strasser SI, Lim L,
Cigolini M and Liu K: Palliative care in hepatocellular carcinoma.
J Gastroenterol Hepatol. 36:618–628. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang WJ, Tian XP, Bi SX, Zhang SR, He TS,
Song LY, Yun JP, Zhou ZG, Yu RM and Li M: The
β-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes
hepatocellular carcinoma metastasis. Oncogene. 39:4538–4550. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu YH, Yu B, Chen WX, Ai X, Zhang W, Dong
W and Shao YJ: Downregulation of lncRNA SBF2-AS1 inhibits
hepatocellular carcinoma proliferation and migration by regulating
the miR-361-5p/TGF-β1 signaling pathway. Aging (Albany NY).
13:19260–19271. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Y, Liu G, Li X, Dong H, Xiao W and Lu
S: Long non-coding RNA SBF2-AS1 promotes hepatocellular carcinoma
progression through regulation of miR-140-5p-TGFBR1 pathway.
Biochem Biophys Res Commun. 503:2826–2832. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li Y, Guo D, Ren M, Zhao Y, Wang X, Chen
Y, Liu Y, Lu G and He S: Long non-coding RNA SNAI3-AS1 promotes the
proliferation and metastasis of hepatocellular carcinoma by
regulating the UPF1/Smad7 signalling pathway. J Cell Mol Med.
23:6271–6282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang X, Cai JB, Peng R, Wei CY, Lu JC, Gao
C, Shen ZZ, Zhang PF, Huang XY, Ke AW, et al: The long noncoding
RNA NORAD enhances the TGF-β pathway to promote hepatocellular
carcinoma progression by targeting miR-202-5p. J Cell Physiol.
234:12051–12060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hu ML, Wang XY and Chen WM: TGF-β1
upregulates the expression of lncRNA UCA1 and its downstream HXK2
to promote the growth of hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 22:4846–4854. 2018.PubMed/NCBI
|
|
64
|
Dong H, Zhang Y, Xu Y, Ma R, Liu L, Luo C
and Jiang W: Downregulation of long non-coding RNA MEG3 promotes
proliferation, migration, and invasion of human hepatocellular
carcinoma cells by upregulating TGF-β1. Acta Biochim Biophys Sin
(Shanghai). 51:645–652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen Z, Xiang L, Li L, Ou H, Fang Y, Xu Y,
Liu Q, Hu Z, Huang Y, Li X and Yang D: TGF-β1 induced deficiency of
linc00261 promotes epithelial-mesenchymal-transition and stemness
of hepatocellular carcinoma via modulating SMAD3. J Transl Med.
20:752022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang L, Niu H, Ma J, Yuan BY, Chen YH,
Zhuang Y, Chen GW, Zeng ZC and Xiang ZL: The molecular mechanism of
LncRNA34a-mediated regulation of bone metastasis in hepatocellular
carcinoma. Mol Cancer. 18:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Yang L, Dong X, Yang X, Zhang X,
Liu Z, Zhao X and Wen T: Overexpression of NNT-AS1 activates TGF-β
signaling to decrease tumor CD4 lymphocyte infiltration in
hepatocellular carcinoma. Biomed Res Int. 2020:82165412020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tayob N, Kanwal F, Alsarraj A, Hernaez R
and El-Serag HB: The performance of AFP, AFP-3, DCP as biomarkers
for detection of hepatocellular carcinoma (HCC): A Phase 3
Biomarker Study in the United States. Clin Gastroenterol Hepatol.
Feb 3–2022.doi: 10.1016/j.cgh.2022.01.047 (Epub ahead of
print).
|
|
69
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X and Wu J: LINC00665 promotes cell
proliferation, invasion, and metastasis by activating the TGF-β
pathway in gastric cancer. Pathol Res Pract. 224:1534922021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fu M, Huang Z, Zang X, Pan L, Liang W,
Chen J, Qian H, Xu W, Jiang P and Zhang X: Long noncoding RNA
LINC00978 promotes cancer growth and acts as a diagnostic biomarker
in gastric cancer. Cell Prolif. 51:e124252018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Moreau JM, Velegraki M, Bolyard C,
Rosenblum MD and Li Z: Transforming growth factor-β1 in regulatory
T cell biology. Sci Immunol. 7:eabi46132022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xiong G, Yang L, Chen Y and Fan Z:
Linc-POU3F3 promotes cell proliferation in gastric cancer via
increasing T-reg distribution. Am J Transl Res. 7:2262–2269.
2015.PubMed/NCBI
|
|
75
|
Huang D, Zhang K, Zheng W, Zhang R, Chen
J, Du N, Xia Y, Long Y, Gu Y, Xu J and Deng M: Long noncoding RNA
SGO1-AS1 inactivates TGFβ signaling by facilitating TGFB1/2 mRNA
decay and inhibits gastric carcinoma metastasis. J Exp Clin Cancer
Res. 40:3422021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sakai S, Ohhata T, Kitagawa K, Uchida C,
Aoshima T, Niida H, Suzuki T, Inoue Y, Miyazawa K and Kitagawa M:
Long Noncoding RNA ELIT-1 Acts as a Smad3 cofactor to facilitate
TGFβ/Smad signaling and promote epithelial-mesenchymal transition.
Cancer Res. 79:2821–2838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Su J, Chen D, Ruan Y, Tian Y, Lv K, Zhou
X, Ying D and Lu Y: LncRNA MBNL1-AS1 represses gastric cancer
progression via the TGF-β pathway by modulating miR-424-5p/Smad7
axis. Bioengineered. 13:6978–6995. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li H, Wang M, Zhou H, Lu S and Zhang B:
Long Noncoding RNA EBLN3P promotes the progression of liver cancer
via alteration of microRNA-144-3p/DOCK4 signal. Cancer Manag Res.
12:9339–9349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fahad Ullah M: Breast cancer: Current
perspectives on the disease status. Adv Exp Med Biol. 1152:51–64.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Januškevičienė I and Petrikaitė V:
Heterogeneity of breast cancer: The importance of interaction
between different tumor cell populations. Life Sci. 239:1170092019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang Y, Zhu M, Sun Y, Li W, Wang Y and Yu
W: Upregulation of lncRNA CASC2 suppresses cell proliferation and
metastasis of breast cancer via inactivation of the TGF-β signaling
pathway. Oncol Res. 27:379–387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu ZJ, Li Y, Wu YZ, Wang Y, Nian WQ, Wang
LL, Li LC, Luo HL and Wang DL: Long non-coding RNA CCAT2 promotes
the breast cancer growth and metastasis by regulating TGF-β
signaling pathway. Eur Rev Med Pharmacol Sci. 21:706–714.
2017.PubMed/NCBI
|
|
83
|
Hou L, Tu J, Cheng F, Yang H, Yu F, Wang
M, Liu J, Fan J and Zhou G: Long noncoding RNA ROR promotes breast
cancer by regulating the TGF-β pathway. Cancer Cell International.
18:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang CL, Li JC, Zhou CX, Ma CN, Wang DF,
Wo LL, He M, Yin Q, He JR and Zhao Q: Long non-coding RNA
ARHGAP5-AS1 inhibits migration of breast cancer cell via
stabilizing SMAD7 protein. Breast Cancer Res Treat. 189:607–619.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ni K, Huang Z, Zhu Y, Xue D, Jin Q, Zhang
C and Gu C: The lncRNA ADAMTS9-AS2 regulates RPL22 to modulate TNBC
progression controlling the TGF-β signaling pathway. Front Oncol.
11:6544722021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mota MSV, Jackson WP, Bailey SK, Vayalil
P, Landar A, Rostas JW III, Mulekar MS, Samant RS and Shevde LA:
Deficiency of tumor suppressor Merlin facilitates metabolic
adaptation by co-operative engagement of SMAD–Hippo signaling in
breast cancer. Carcinogenesis. 39:1165–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bo H, Zhang W, Zhong X, Chen J, Liu Y,
Cheong KL, Fan R and Tang S: LINC00467, driven by copy number
amplification and DNA demethylation, is associated with oxidative
lipid metabolism and immune infiltration in breast cancer. Oxid Med
Cell Longev. 2021:45863192021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang X, Wang M, Sun H, Zhu T and Wang X:
Downregulation of LINC00894-002 contributes to tamoxifen resistance
by enhancing the TGF-β signaling pathway. Biochemistry (Mosc).
83:603–611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tang L, Chen Y, Chen H, Jiang P, Yan L, Mo
D, Tang X and Yan F: DCST1-AS1 Promotes TGF-β-induced
epithelial-mesenchymal transition and enhances chemoresistance in
triple-negative breast cancer cells via ANXA1. Front Oncol.
10:2802020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ren Y, Jia HH, Xu YQ, Zhou X, Zhao XH,
Wang YF, Song X, Zhu ZY, Sun T, Dou Y, et al: Paracrine and
epigenetic control of CAF-induced metastasis: The role of HOTAIR
stimulated by TGF-ß1 secretion. Mol Cancer. 17:52018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Toumazis I, Bastani M, Han SS and
Plevritis SK: Risk-based lung cancer screening: A systematic
review. Lung Cancer. 147:154–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Nooreldeen R and Bach H: Current and
future development in lung cancer diagnosis. Int J Mol Sci.
22:86612021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang S, Lan F and Xia Y: lncRA ANCR
inhibits non-small cell lung cancer cell migration and invasion by
inactivating TGF-β pathway. Med Sci Monit. 24:6002–6009. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Su WZ and Yuan X: LncRNA GASL1 inhibits
tumor growth of non-small cell lung cancer by inactivating TGF-β
pathway. Eur Rev Med Pharmacol Sci. 22:7282–7288. 2018.PubMed/NCBI
|
|
95
|
Lu Z, Li Y, Wang J, Che Y, Sun S, Huang J,
Chen Z and He J: Long non-coding RNA NKILA inhibits migration and
invasion of non-small cell lung cancer via NF-κB/Snail pathway. J
Exp Clin Cancer Res. 36:542017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xu L, Liu W, Li T, Hu Y, Wang Y, Huang L,
Wang Y, Shao S, Liu X and Zhan Q: Long non-coding RNA SMASR
inhibits the EMT by negatively regulating TGF-β/Smad signaling
pathway in lung cancer. Oncogene. 40:3578–3592. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang X, Zhang G, Cheng Z, Dai L, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of
LncRNA-XIST suppresses proliferation and TGF-β1-induced EMT in
NSCLC through the Notch-1 pathway by regulation of miR-137. Genet
Test Mol Biomarkers. 220:333–342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ni J, Zhang X, Li J, Zheng Z, Zhang J,
Zhao W and Liu L: Tumour-derived exosomal lncRNA-SOX2OT promotes
bone metastasis of non-small cell lung cancer by targeting the
miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis.
12:6622021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shi J, Li J, Yang S, Hu X, Chen J, Feng J,
Shi T, He Y, Mei Z, He W, et al: LncRNA SNHG3 is activated by E2F1
and promotes proliferation and migration of non-small-cell lung
cancer cells through activating TGF-β pathway and IL-6/JAK2/STAT3
pathway. J Cell Physiol. 235:2891–2900. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhu L, Liu Y, Tang H and Wang P: FOXP3
activated-LINC01232 accelerates the stemness of non-small cell lung
carcinoma by activating TGF-β signaling pathway and recruiting
IGF2BP2 to stabilize TGFBR1. Exp Cell Res. 413:1130242022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lu Z, Li Y, Che Y, Huang J, Sun S, Mao S,
Lei Y, Li N, Sun N and He J: The TGFβ-induced lncRNA TBILA promotes
non-small cell lung cancer progression in vitro and in vivo via
cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer
Lett. 432:156–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jiang L, Wang R, Fang L, Ge X, Chen L,
Zhou M, Zhou Y, Xiong W, Hu Y, Tang X, et al: HCP5 is a
SMAD3-responsive long non-coding RNA that promotes lung
adenocarcinoma metastasis via miR-203/SNAI axis. Theranostics.
9:2460–2474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen Y, Gao H and Li Y: Inhibition of
LncRNA FOXD3-AS1 suppresses the aggressive biological behaviors of
thyroid cancer via elevating miR-296-5p and inactivating
TGF-β1/Smads signaling pathway. Mol Cell Endocrinol.
500:1106342020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S,
Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG and Luo DL: Long
non-coding RNA ANRIL promotes the invasion and metastasis of
thyroid cancer cells through TGF-β/Smad signaling pathway.
Oncotarget. 7:57903–57918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhou H, Sun Z, Li S, Wang X and Zhou X:
LncRNA SPRY4-IT was concerned with the poor prognosis and
contributed to the progression of thyroid cancer. Cancer Gene Ther.
25:39–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ju W, Luo X and Zhang N: LncRNA NEF
inhibits migration and invasion of HPV-negative cervical squamous
cell carcinoma by inhibiting TGF-β pathway. Biosci Rep.
39:BSR201808782019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cao L, Jin H, Zheng Y, Mao Y, Fu Z, Li X
and Dong L: DANCR-mediated microRNA-665 regulates proliferation and
metastasis of cervical cancer through the ERK/SMAD pathway. Cancer
Sci. 110:913–925. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Feng S, Liu W, Bai X, Pan W, Jia Z, Zhang
S, Zhu Y and Tan W: LncRNA-CTS promotes metastasis and
epithelial-to-mesenchymal transition through regulating
miR-505/ZEB2 axis in cervical cancer. Cancer Lett. 465:105–117.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mao S, Jin J, Li Z and Yang W: Knockdown
of long non-coding RNA ANRIL inhibits the proliferation and
promotes the apoptosis of Burkitt lymphoma cells through the TGF-β1
signaling pathway. Mol Med Rep. 23:1462021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li Z, Liu H, Zhong Q, Wu J and Tang Z:
LncRNA UCA1 is necessary for TGF-β-induced epithelial-mesenchymal
transition and stemness via acting as a ceRNA for Slug in glioma
cells. FEBS Open Bio. 8:1855–1865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Z, Li M, Xia P, Wang L and Lu Z:
Targeting long non-coding RNA PVT1/TGF-β/Smad by p53 prevents
glioma progression. Cancer Biol Ther. 23:225–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ma J, Kong FF, Yang D, Yang H, Wang C,
Cong R and Ma XX: lncRNA MIR210HG promotes the progression of
endometrial cancer by sponging miR-337-3p/137 via the
HMGA2-TGF-β/Wnt pathway. Mol Ther Nucleic Acids. 24:905–922. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Weng W, Liu C, Li G, Ruan Q, Li H, Lin N
and Chen G: Long non-coding RNA SNHG16 functions as a tumor
activator by sponging miR-373-3p to regulate the TGF-β-R2/SMAD
pathway in prostate cancer. Mol Med Rep. 24:8432021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang X, Feng W, Zhang J, Ge L, Zhang Y,
Jiang X, Peng W, Wang D, Gong A and Xu M: Long non-coding RNA PVT1
promotes epithelial-mesenchymal transition via the TGF-β/Smad
pathway in pancreatic cancer cells. Oncol Rep. 40:1093–1102.
2018.PubMed/NCBI
|
|
115
|
Papoutsoglou P, Rodrigues-Junior DM, Morén
A, Bergman A, Pontén F, Coulouarn C, Caja L, Heldin CH and
Moustakas A: The noncoding MIR100HG RNA enhances the autocrine
function of transforming growth factor β signaling. Oncogene.
40:3748–3765. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou B, Guo W, Sun C, Zhang B and Zheng F:
Linc00462 promotes pancreatic cancer invasiveness through the
miR-665/TGFBR1-TGFBR2/SMAD2/3 pathway. Cell Death Dis. 9:7062018.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wu Y, Gu W, Han X and Jin Z: LncRNA PVT1
promotes the progression of ovarian cancer by activating TGF-β
pathway via miR-148a-3p/AGO1 axis. J Cell Mol Med. 25:8229–8243.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang P, Qi B, Yao H, Zhang L, Li Y and Li
Q: Knockdown of DANCR suppressed the biological behaviors of
ovarian cancer cells treated with transforming growth factor-β
(TGF-β) by sponging MiR-214. Med Sci Monit. 26:e9227602020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shi H, Xie J, Wang K, Li W, Yin L, Wang G,
Wu Z, Ni J, Mao W, Guo C and Peng B: LINC01451 drives
epithelial-mesenchymal transition and progression in bladder cancer
cells via LIN28/TGF-β/Smad pathway. Cell Signal. 81:1099322021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhuang J, Shen L, Yang L, Huang X, Lu Q,
Cui Y, Zheng X, Zhao X, Zhang D, Huang R, et al: TGFβ1 promotes
gemcitabine resistance through regulating the
LncRNA-LET/NF90/miR-145 signaling axis in bladder cancer.
Theranostics. 7:3053–3067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zheng C, Li R, Zheng S, Fang H, Xu M and
Zhong L: LINC00174 facilitates cell proliferation, cell migration
and tumor growth of osteosarcoma regulating the TGF-β/SMAD
signaling pathway and upregulating SSH2 expression. Front Mol
Biosci. 8:6977732021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tzavlaki K and Moustakas A: TGF-β
signaling. Biomolecules. 10:4872020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lai XN, Li J, Tang LB, Chen WT, Zhang L
and Xiong LX: MiRNAs and LncRNAs: Dual roles in TGF-β
signaling-regulated metastasis in lung cancer. Int J Mol Sci.
21:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan
C, Xu M, Sun H, Liu C, Wei P and Du X: The lncRNA NEAT1 activates
Wnt/β-catenin signaling and promotes colorectal cancer progression
via interacting with DDX5. J Hematol Oncol. 11:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tao S, Chen Q, Lin C and Dong H: Linc00514
promotes breast cancer metastasis and M2 polarization of
tumor-associated macrophages via Jagged1-mediated notch signaling
pathway. J Exp Clin Cancer Res. 39:1912020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Akbari A, Ghahremani MH, Mobini GR,
Abastabar M, Akhtari J, Bolhassani M and Heidari M: Down-regulation
of miR-135b in colon adenocarcinoma induced by a TGF-β receptor I
kinase inhibitor (SD-208). Iran J Basic Med Sci. 18:856–861.
2015.PubMed/NCBI
|
|
129
|
Han S, Bui NT, Ho MT, Kim YM, Cho M and
Shin DB: Dexamethasone inhibits TGF-β1-induced cell migration by
regulating the ERK and AKT pathways in human colon cancer cells via
CYR61. Cancer Res Treat. 48:1141–1153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Koelink PJ, Hawinkels LJAC, Wiercinska E,
Sier CF, Ten Dijke P, Lamers CB, Hommes DW and Verspaget HW:
5-Aminosalicylic acid inhibits TGF-beta1 signalling in colorectal
cancer cells. Cancer Lett. 287:82–90. 2010. View Article : Google Scholar : PubMed/NCBI
|